Functionalization of Two-Component Gelatinous Peptide/Reactive Oligomer Hydrogels with Small Molecular Amines for Enhanced Cellular Interaction
Abstract
:1. Introduction
2. Results and Discussion
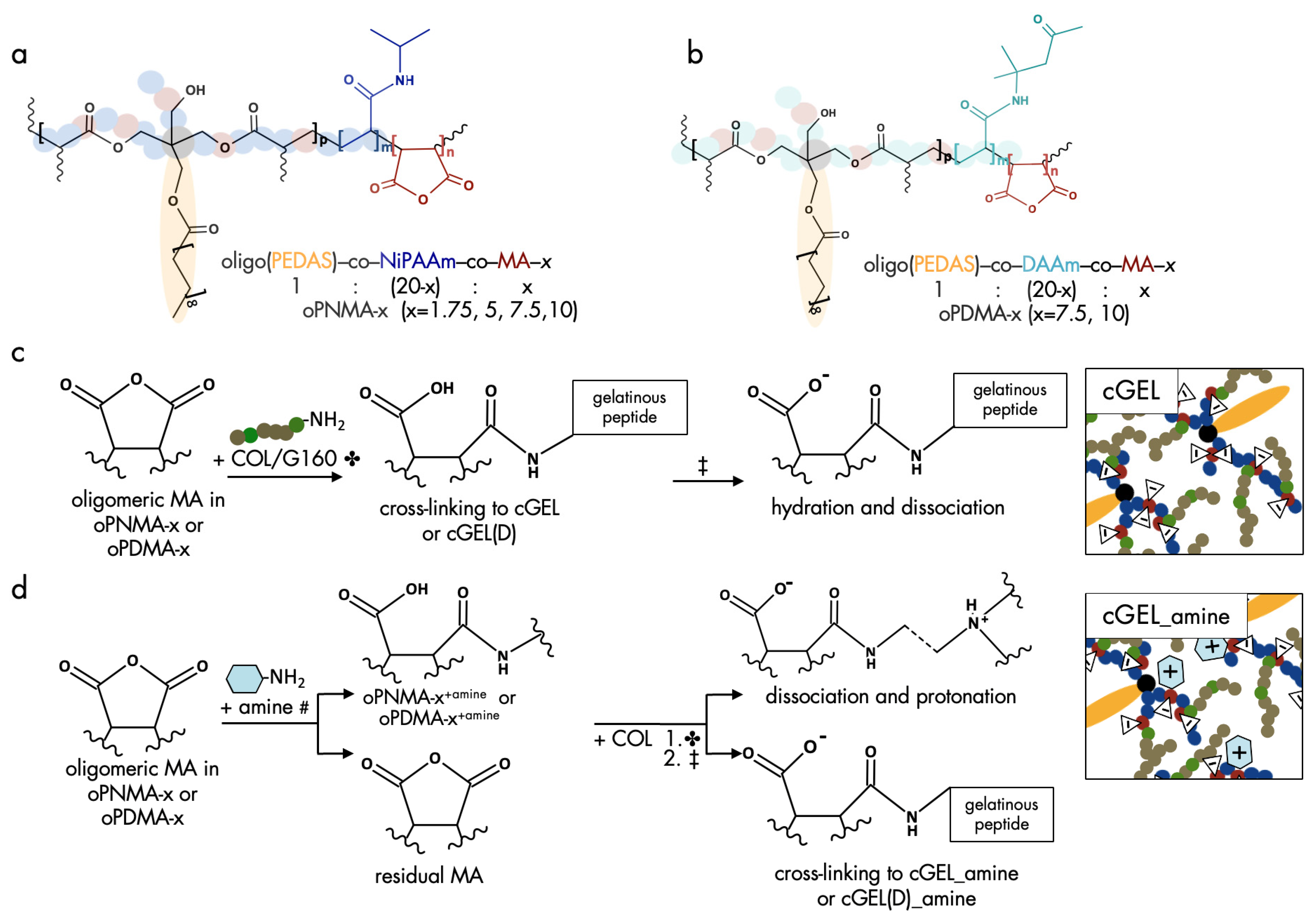
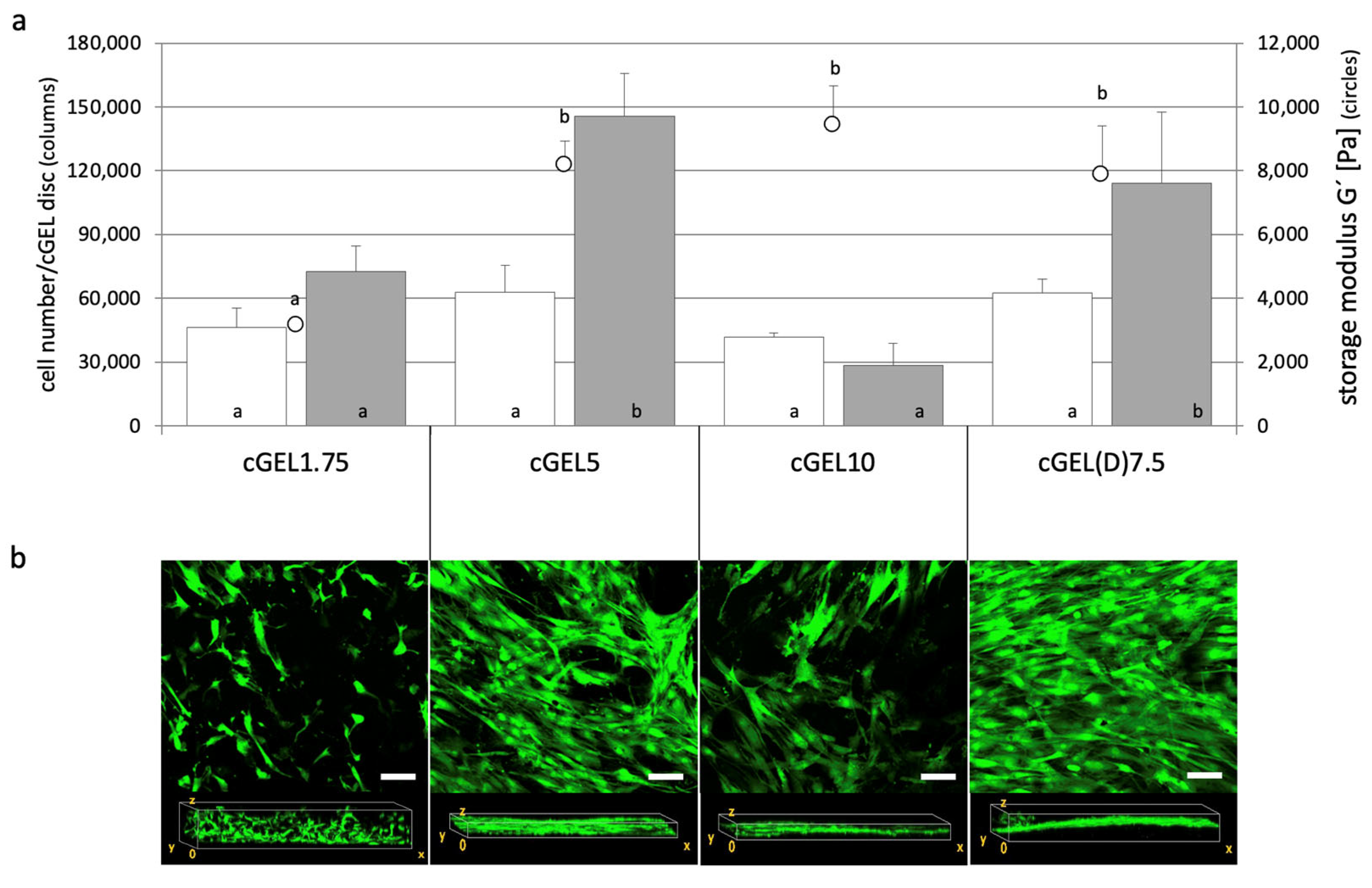
2.1. Oligomeric Building Blocks, Functionalization and Material Characterization of cGEL/cGEL(D) Matrices
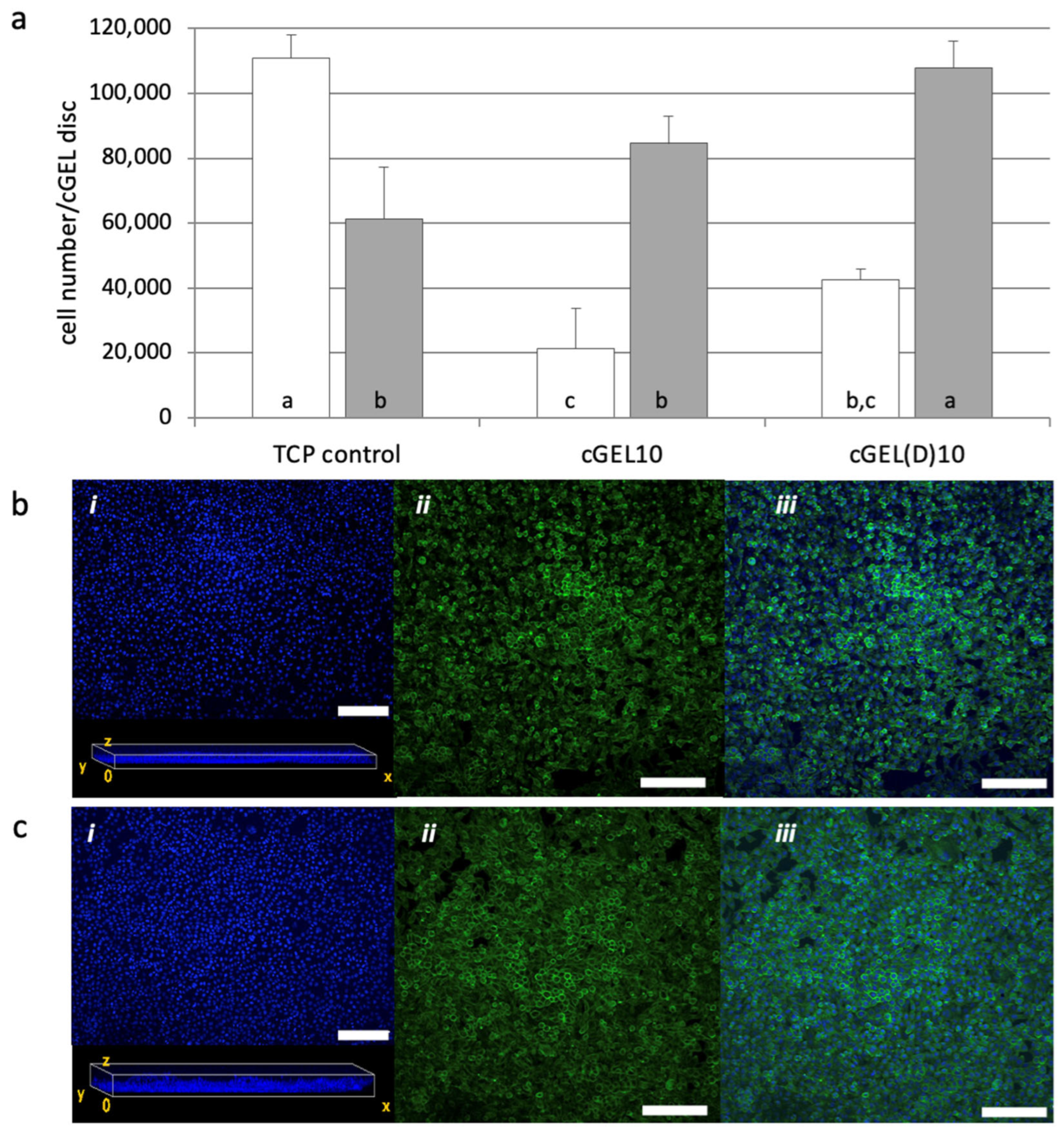
2.2. Cellular Response and Interaction of Fibroblasts and hASCs with cGEL and cGEL(D) Matrices
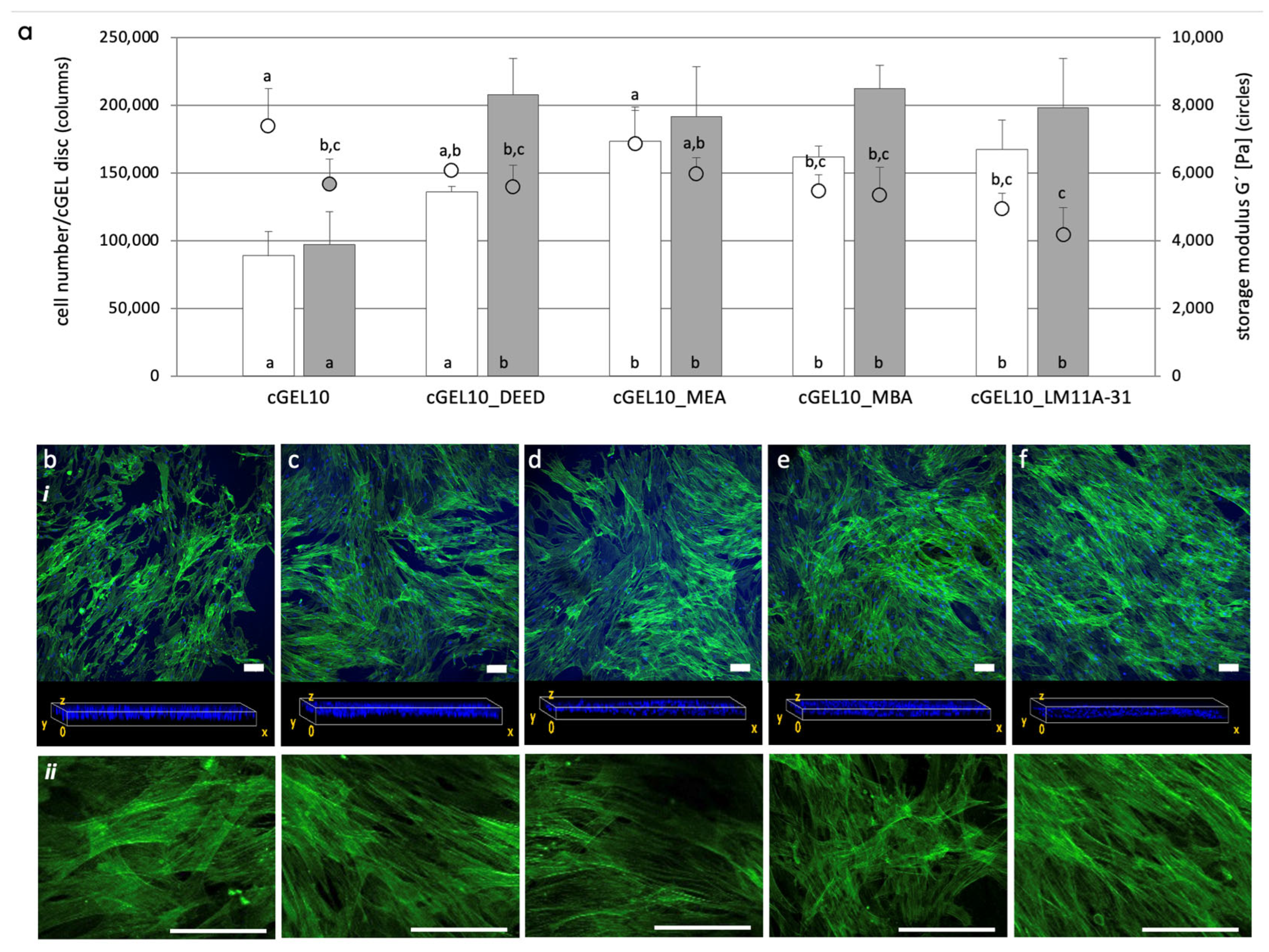
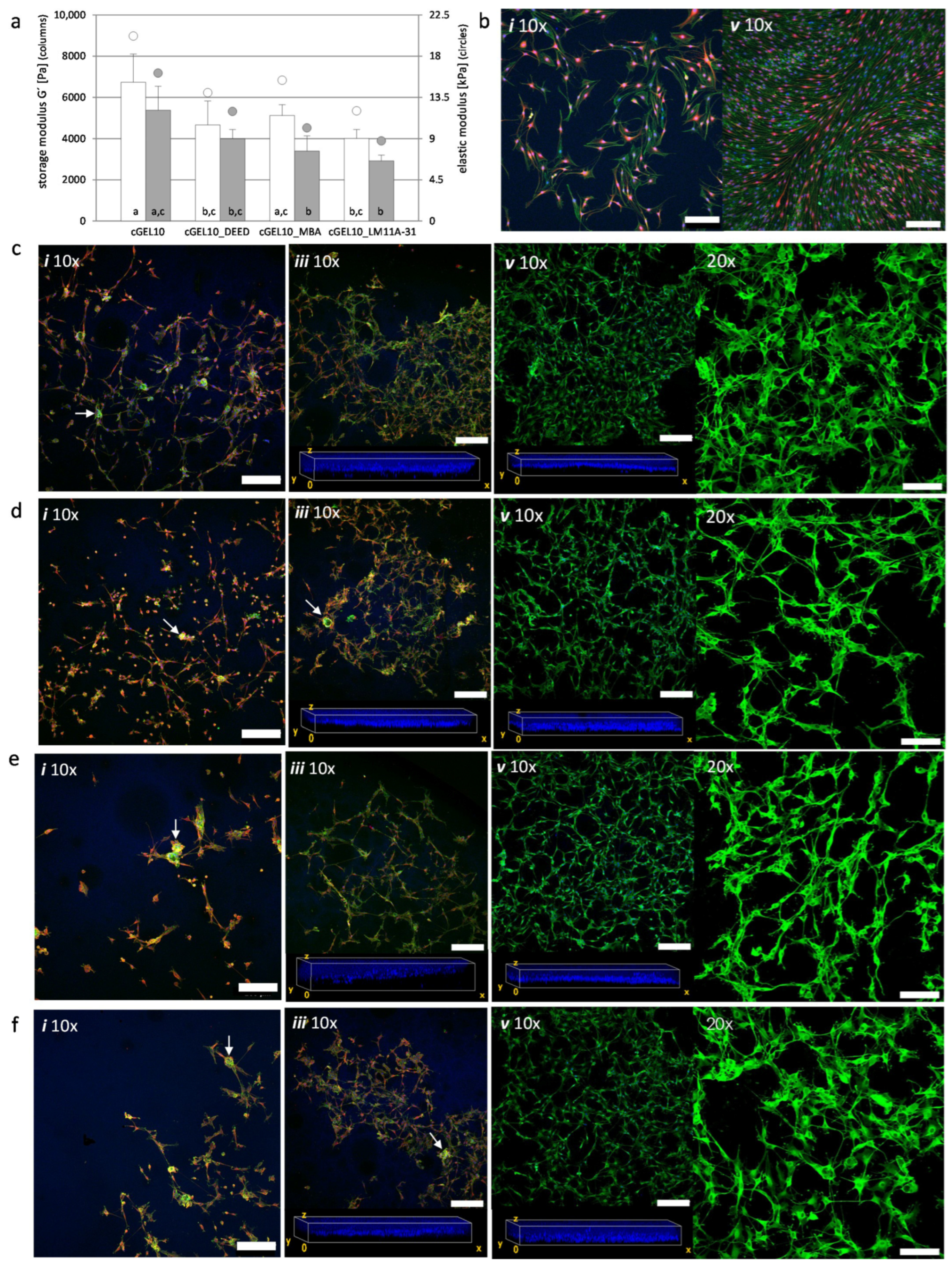
2.3. Cellular Response of hASCs with Amine Functionalized cGEL and cGEL(D) Matrices
2.4. Cellular Response and Interaction of Neonatal Schwann Cells with cGEL Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Oligomeric Cross-Linker Synthesis and Characterization
3.3. Functionalization of oPNMA-x and oPDMA-x with Small Molecular Amines
3.4. Fabrication of Cross-Linked Hydrogel cGEL and cGEL(D)
3.5. Mechanical Characterization
3.6. Cross-Linking Degree and Water Content
3.7. In Vitro Cell Proliferation of Fibroblasts and Adipose Tissue-Derived Stem Cells on Pristine and Amine-Functionalized cGEL and cGEL(D)
3.8. Neuron-Free Culture and Migration of Neonatal Schwann Cells (nSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.F. Hydrogels in Regenerative Medicine. In Principles of Regenerative Medicine; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 627–650. ISBN 9780128098806. [Google Scholar]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of Different Cross-Linking Approaches on 3D Gelatin Scaffolds for Tissue Engineering Application: A Comparative Analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine. Adv. Healthc. Mater. 2018, 7, e1700939. [Google Scholar] [CrossRef] [PubMed]
- Scadden, D.T. The Stem-Cell Niche as an Entity of Action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as Stem Cell Regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Chen, B.; Ji, B.; Gao, H. Modeling Active Mechanosensing in Cell-Matrix Interactions. Annu. Rev. Biophys. 2015, 44, 1–32. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin Stress Fibers—Assembly, Dynamics and Biological Roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Rizwan, M.; Tse, J.W.; Nori, A.; Leong, K.W.; Yim, E.K.F. Cell–Substrate Interactions. In Principles of Regenerative Medicine; Nerem, R., Lanza, R., Mikos, A.G., Atala, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 437–468. ISBN 9780128098806. [Google Scholar]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The Stiffness of Living Tissues and Its Implications for Tissue Engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Marquez-Posadas, M.D.C.; Araujo, A.R.; Yang, Y.; Merino, S.; Groth, T.; Reis, R.L.; Pashkuleva, I. Adhesion of Adipose-Derived Mesenchymal Stem Cells to Glycosaminoglycan Surfaces with Different Protein Patterns. ACS Appl. Mater. Interfaces 2015, 7, 10034–10043. [Google Scholar] [CrossRef]
- Moore, S.W.; Roca-Cusachs, P.; Sheetz, M.P. Stretchy Proteins on Stretchy Substrates: The Important Elements of Integrin-Mediated Rigidity Sensing. Dev. Cell 2010, 19, 194–206. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Karimi, F.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Integrin Clustering Matters: A Review of Biomaterials Functionalized with Multivalent Integrin-Binding Ligands to Improve Cell Adhesion, Migration, Differentiation, Angiogenesis, and Biomedical Device Integration. Adv. Healthc. Mater. 2018, 7, e1701324. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent Advances in the Use of Gelatin in Biomedical Research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-Based Biomaterial Engineering with Anhydride-Containing Oligomeric Cross-Linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef]
- Kohn-Polster, C.; Bhatnagar, D.; Woloszyn, D.J.; Richtmyer, M.; Starke, A.; Springwald, A.H.; Franz, S.; Schulz-Siegmund, M.; Kaplan, H.M.; Kohn, J.; et al. Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 1104–1132. [Google Scholar] [CrossRef]
- Massa, S.M.; Xie, Y.M.; Longo, F.M. Alzheimer’s Therapeutics—Neurotrophin Small Molecule Mimetics. J. Mol. Neurosci. MN 2002, 19, 107–111. [Google Scholar] [CrossRef]
- Loth, T.; Hennig, R.; Kascholke, C.; Hötzel, R.; Hacker, M.C. Reactive and Stimuli-Responsive Maleic Anhydride Containing Macromers—Multi-Functional Cross-Linkers and Building Blocks for Hydrogel Fabrication. React. Funct. Polym. 2013, 73, 1480–1492. [Google Scholar] [CrossRef]
- Kascholke, C.; Loth, T.; Kohn-Polster, C.; Möller, S.; Bellstedt, P.; Schulz-Siegmund, M.; Schnabelrauch, M.; Hacker, M.C. Dual-Functional Hydrazide-Reactive and Anhydride Containing Oligomeric Hydrogel Building Blocks. Biomacromolecules 2017, 18, 683–694. [Google Scholar] [CrossRef]
- Unal, A.Z.; West, J.L. Synthetic ECM: Bioactive Synthetic Hydrogels for 3D Tissue Engineering. Bioconjugate Chem. 2020, 31, 2253–2271. [Google Scholar] [CrossRef]
- Kohn, C.; Klemens, J.M.; Kascholke, C.; Murthy, N.S.; Kohn, J.; Brandenburger, M.; Hacker, M.C. Dual-Component Collagenous Peptide/Reactive Oligomer Hydrogels as Potential Nerve Guidance Materials-From Characterization to Functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef]
- Krieghoff, J.; Rost, J.; Kohn-Polster, C.; Müller, B.M.; Koenig, A.; Flath, T.; Schulz-Siegmund, M.; Schulze, F.-P.; Hacker, M.C. Extrusion-Printing of Multi-Channeled Two-Component Hydrogel Constructs from Gelatinous Peptides and Anhydride-Containing Oligomers. Biomedicines 2021, 9, 370. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Jaramillo, M.; Singh, S.S.; Velankar, S.; Kumta, P.N.; Banerjee, I. Inducing Endoderm Differentiation by Modulating Mechanical Properties of Soft Substrates. J. Tissue Eng. Regen. Med. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Grover, C.N.; Gwynne, J.H.; Pugh, N.; Hamaia, S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Crosslinking and Composition Influence the Surface Properties, Mechanical Stiffness and Cell Reactivity of Collagen-Based Films. Acta Biomater. 2012, 8, 3080–3090. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Mazzara, P.G.; Massimino, L.; Pellegatta, M.; Ronchi, G.; Ricca, A.; Iannielli, A.; Giannelli, S.G.; Cursi, M.; Cancellieri, C.; Sessa, A.; et al. Two Factor-Based Reprogramming of Rodent and Human Fibroblasts into Schwann Cells. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- He, Q.; Shen, M.; Tong, F.; Cong, M.; Zhang, S.; Gong, Y.; Ding, F. Differential Gene Expression in Primary Cultured Sensory and Motor Nerve Fibroblasts. Front. Neurosci. 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Sowa, Y.; Kishida, T.; Tomita, K.; Yamamoto, K.; Numajiri, T.; Mazda, O. Direct Conversion of Human Fibroblasts into Schwann Cells That Facilitate Regeneration of Injured Peripheral Nerve In Vivo. Stem Cells Transl. Med. 2017, 6, 1207–1216. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2019, 226, 119536. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of Cell Adhesion, Proliferation and Differentiation on Materials Designed for Body Implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Osada, S.; Narita, T.; Oishi, Y.; Kodama, H. Promotion of Cell Adhesion by Low-Molecular-Weight Hydrogel by Lys Based Amphiphile. Mater. Sci. Eng. C 2015, 47, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of Stem Cells in Peripheral Nerve Regeneration. Burn. Trauma 2020, 8, tkaa002. [Google Scholar] [CrossRef]
- Yang, E.; Liu, N.; Tang, Y.; Hu, Y.; Zhang, P.; Pan, C.; Dong, S.; Zhang, Y.; Tang, Z. Generation of Neurospheres from Human Adipose-Derived Stem Cells. Biomed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Allbright, K.O.; Bliley, J.M.; Havis, E.; Kim, D.Y.; Dibernardo, G.A.; Grybowski, D.; Waldner, M.; James, I.B.; Sivak, W.N.; Rubin, J.P.; et al. Delivery of Adipose-derived Stem Cells in Poloxamer Hydrogel Improves Peripheral Nerve Regeneration. Muscle Nerve 2018, 58, 251–260. [Google Scholar] [CrossRef]
- Adams, A.M.; Arruda, E.M.; Larkin, L.M. Use of Adipose-Derived Stem Cells to Fabricate Scaffoldless Tissue-Engineered Neural Conduits in Vitro. Neuroscience 2012, 201, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered Neural Tissue with Aligned, Differentiated Adipose-Derived Stem Cells Promotes Peripheral Nerve Regeneration across a Critical Sized Defect in Rat Sciatic Nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef]
- Mirsky, R.; Jessen, K.R.; Brennan, A.; Parkinson, D.; Dong, Z.; Meier, C.; Parmantier, E.; Lawson, D. Schwann Cells as Regulators of Nerve Development. J. Physiol.-Paris 2002, 96, 17–24. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Repair Schwann Cell and Its Function in Regenerating Nerves: Repair Schwann Cell and Its Function in Regenerating Nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Gordon, T. Neurotrophic Factor Expression in Denervated Motor and Sensory Schwann Cells: Relevance to Specificity of Peripheral Nerve Regeneration. Exp. Neurol. 2014, 254, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of Mechanical Stimuli on Schwann Cell Biology. Front. Cell. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Xu, Q.; Liu, W. An Overview of Substrate Stiffness Guided Cellular Response and Its Applications in Tissue Regeneration. Bioact. Mater. 2021, 15, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-M.; LeDuc, P.R.; Lin, Y.-W. Localized Bimodal Response of Neurite Extensions and Structural Proteins in Dorsal-Root Ganglion Neurons with Controlled Polydimethylsiloxane Substrate Stiffness. J. Biomech. 2011, 44, 856–862. [Google Scholar] [CrossRef]
- Kniazeva, E.; Kachgal, S.; Putnam, A.J. Effects of Extracellular Matrix Density and Mesenchymal Stem Cells on Neovascularization in Vivo. Tissue Eng. Part A 2011, 17, 905–914. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Liang, R.; Chen, Y.; Li, S.; Li, S.; Sun, Z.; Wang, Y.; Li, G.; Ming, A.; et al. Fabrication of Alignment Polycaprolactone Scaffolds by Combining Use of Electrospinning and Micromolding for Regulating Schwann Cells Behavior. J. Biomed. Mater. Res. Part A 2018, 106, 3123–3134. [Google Scholar] [CrossRef]
- Poitelon, Y.; Lopez-Anido, C.; Catignas, K.; Berti, C.; Palmisano, M.; Williamson, C.; Ameroso, D.; Abiko, K.; Hwang, Y.; Gregorieff, A.; et al. YAP and TAZ Control Peripheral Myelination and the Expression of Laminin Receptors in Schwann Cells. Nat. Neurosci. 2016, 19, 879–887. [Google Scholar] [CrossRef]
- López-Fagundo, C.; Bar-Kochba, E.; Livi, L.L.; Hoffman-Kim, D.; Franck, C. Three-Dimensional Traction Forces of Schwann Cells on Compliant Substrates. J. R. Soc. Interface 2014, 11, 20140247. [Google Scholar] [CrossRef]
- Zhou, W.; Stukel, J.M.; Cebull, H.L.; Willits, R.K. Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation. Macromol. Biosci. 2016, 16, 535–544. [Google Scholar] [CrossRef]
- Evans, E.B.; Brady, S.W.; Tripathi, A.; Hoffman-Kim, D. Schwann Cell Durotaxis Can Be Guided by Physiologically Relevant Stiffness Gradients. Biomater. Res. 2018, 22, 14. [Google Scholar] [CrossRef]
- Huang, Z.; Kankowski, S.; Ertekin, E.; Almog, M.; Nevo, Z.; Rochkind, S.; Haastert-Talini, K. Modified Hyaluronic Acid-Laminin-Hydrogel as Luminal Filler for Clinically Approved Hollow Nerve Guides in a Rat Critical Defect Size Model. Int. J. Mol. Sci. 2021, 22, 6554. [Google Scholar] [CrossRef] [PubMed]
- Vleggeert-Lankamp, C.L.A.M.; Pêgo, A.P.; Lakke, E.A.J.F.; Deenen, M.; Marani, E.; Thomeer, R.T.W.M. Adhesion and Proliferation of Human Schwann Cells on Adhesive Coatings. Biomaterials 2004, 25, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Hodde, D.; Gerardo-Nava, J.; Woehlk, V.; Weinandy, S.; Jockenhoevel, S.; Kriebel, A.; Altinova, H.; Steinbusch, H.W.M.; Moeller, M.; Weis, J.; et al. Characterisation of Cell-Substrate Interactions between Schwann Cells and Three-Dimensional Fibrin Hydrogels Containing Orientated Nanofibre Topographical Cues. Eur. J. Neurosci. 2016, 43, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, M.S.; Cheon, Y.-P. Decreased Contact Inhibition in Mouse Adipose Mesenchymal Stem Cells. Dev. Reprod. 2012, 16, 329–338. [Google Scholar] [CrossRef]
- Ort, C.; Chen, Y.; Ghagre, A.; Ehrlicher, A.; Moraes, C. Bioprintable, Stiffness-Tunable Collagen-Alginate Microgels for Increased Throughput 3D Cell Culture Studies. ACS Biomater. Sci. Eng. 2021, 7, 2814–2822. [Google Scholar] [CrossRef]
- Dewitt, D.D.; Kaszuba, S.N.; Thompson, D.M.; Stegemann, J.P. Collagen I-Matrigel Scaffolds for Enhanced Schwann Cell Survival and Control of Three-Dimensional Cell Morphology. Tissue Eng. Part A 2009, 15, 2785–2793. [Google Scholar] [CrossRef]
- Meyer, C.; Wrobel, S.; Raimondo, S.; Rochkind, S.; Heimann, C.; Shahar, A.; Ziv-Polat, O.; Geuna, S.; Grothe, C.; Haastert-Talini, K. Peripheral Nerve Regeneration through Hydrogel-Enriched Chitosan Conduits Containing Engineered Schwann Cells for Drug Delivery. Cell Transplant. 2016, 25, 159–182. [Google Scholar] [CrossRef]
- Cornejo, M.; Nambi, D.; Walheim, C.; Somerville, M.; Walker, J.; Kim, L.; Ollison, L.; Diamante, G.; Vyawahare, S.; de Bellard, M.E. Effect of NRG1, GDNF, EGF and NGF in the Migration of a Schwann Cell Precursor Line. Neurochem. Res. 2010, 35, 1643–1651. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Kawasoe, J.; Hoyng, S.A.; Kumar, R.; van Minnen, J.; Verhaagen, J.; Midha, R. Early Regenerative Effects of NGF-Transduced Schwann Cells in Peripheral Nerve Repair. Mol. Cell. Neurosci. 2012, 50, 103–112. [Google Scholar] [CrossRef]
- Massa, S.M.; Xie, Y.; Yang, T.; Harrington, A.W.; Kim, M.L.; Yoon, S.O.; Kraemer, R.; Moore, L.A.; Hempstead, B.L.; Longo, F.M. Small, Nonpeptide P75NTR Ligands Induce Survival Signaling and Inhibit ProNGF-Induced Death. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 5288–5300. [Google Scholar] [CrossRef]
- Seo, S.Y.; Min, S.K.; Bae, H.K.; Roh, D.; Kang, H.K.; Roh, S.; Lee, S.; Chun, G.S.; Chung, D.J.; Min, B.M. A Laminin-2-Derived Peptide Promotes Early-Stage Peripheral Nerve Regeneration in a Dual-Component Artificial Nerve Graft. J. Tissue Eng. Regen. Med. 2013, 7, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Tonazzini, I.; Moffa, M.; Pisignano, D.; Cecchini, M. Neuregulin 1 Functionalization of Organic Fibers for Schwann Cell Guidance. Nanotechnology 2017, 28, 155303. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, M.M.; Kingsbury, L.; Moussouros, D.; Kassim, I.; Mehjabeen, S.; Paknejad, N.; Melendez-Vasquez, C.V. Myelinating Glia Differentiation Is Regulated by Extracellular Matrix Elasticity. Sci. Rep. 2016, 6, 33751. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, M.R.; Sundararaghavan, H.G. Biomaterial Cues to Direct a Pro-Regenerative Phenotype in Macrophages and Schwann Cells. Neuroscience 2018, 376, 172–187. [Google Scholar] [CrossRef]
- Dietzmeyer, N.; Förthmann, M.; Grothe, C.; Haastert-Talini, K. Modification of Tubular Chitosan-Based Peripheral Nerve Implants: Applications for Simple or More Complex Approaches. Neural Regen. Res. 2020, 15, 1421–1431. [Google Scholar] [CrossRef]
- Haastert, K.; Grosskreutz, J.; Jaeckel, M.; Laderer, C.; Bufler, J.; Grothe, C.; Claus, P. Rat Embryonic Motoneurons in Long-Term Co-Culture with Schwann Cells—A System to Investigate Motoneuron Diseases on a Cellular Level in Vitro. J. Neurosci. Methods 2005, 142, 275–284. [Google Scholar] [CrossRef]
- Ravelo, K.M.; Andersen, N.D.; Monje, P.V. Magnetic-Activated Cell Sorting for the Fast and Efficient Separation of Human and Rodent Schwann Cells from Mixed Cell Populations. In Mammalian Cell Viability; Humana Press: New York, NY, USA, 2018; Volume 1739, pp. 87–109. ISBN 978-1-4939-7648-5. [Google Scholar]


| Hydrogel Specimens | CLD [%] | Water Content [w/w] | Storage Modulus G’ [Pa] |
|---|---|---|---|
| cGEL1.75 | 88.9 ± 0.7 | 7.6 ± 0.1 | 1320 ± 210 * |
| cGEL5 | 92.1 ± 1.4 | 6.3 ± 0.5 | 8170 ± 760 * |
| cGEL7.5 | 90.7 ± 0.9 | 6.8 ± 0.8 | 5630 ± 310 |
| cGEL10 | 92.4 ± 1.0 | 5.2 ± 0.2 | 9420 ± 1240 * 7370 ± 1120 |
| cGEL10_DEED | 82.0 ± 2.3 | 7.8 ± 0.3 | 6050 ± 140 |
| cGEL10_MBA | 82.5 ± 1.5 | 7.7 ± 0.5 | 5450 ± 480 |
| cGEL10_LM11A-31 | 80.8 ± 1.3 | 8.2 ± 0.6 | 4930 ± 460 |
| cGEL(D)7.5 | 93.1 ± 0.8 | 5.8 ± 0.3 | 7870 ± 1530 * 3350 ± 430 |
| cGEL(D)10 | 80.4 ± 2.2 | 10.2 ± 0.8 | 3160 ± 470 |
| cGEL(D)10_DEED | 83.4 ± 2.1 | 13.7 ± 0.7 | 2250 ± 440 |
| cGEL(D)10_MBA | 74.2 ± 1.5 | 14.9 ± 1.3 | 1940 ± 130 |
| cGEL(D)10_LM11A-31 | 77.3 ± 2.2 | 11.9 ± 0.4 | 2460 ± 200 |
| Peptide | Gelation Base | Oligomeric Cross-Linker | Small Molecular Monovalent Amine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G160 | COL | TEA | NMPO | oPNMA-x | oPDMA-x | DEED | MEA | MBA | LM11A-31 | |||
| [%] 1 | [%] 1 | [%] 1 | [%] 1 | x | [%] 2 | x | [%] 2 | [MAeq] 3 | ||||
| cGEL1.75 | 3.25 | 10 | 1.75 | 3.5 | ||||||||
| cGEL5 | 15 | 10 | 5 | 5.5 | ||||||||
| cGEL7.5 | 15 | 2 | 7.5 | 3.5 | ||||||||
| cGEL10 | 15 | 2 | 10 | 3.5 | 2.5 | 2.5 | 2.5 | 2.5 | ||||
| cGEL(D)7.5 | 15 | 2 | 7.5 | 3.5 | 2.5 | 2.5 | 2.5 | 2.5 | ||||
| cGEL(D)10 | 15 | 2 | 10 | 3.5 | 2.5 | 2.5 | 2.5 | 2.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohn-Polster, C.; Müller, B.M.; Krieghoff, J.; Nawaz, A.; Maqsood, I.; Starke, A.; Haastert-Talini, K.; Schulz-Siegmund, M.; Hacker, M.C. Functionalization of Two-Component Gelatinous Peptide/Reactive Oligomer Hydrogels with Small Molecular Amines for Enhanced Cellular Interaction. Int. J. Mol. Sci. 2025, 26, 5316. https://doi.org/10.3390/ijms26115316
Kohn-Polster C, Müller BM, Krieghoff J, Nawaz A, Maqsood I, Starke A, Haastert-Talini K, Schulz-Siegmund M, Hacker MC. Functionalization of Two-Component Gelatinous Peptide/Reactive Oligomer Hydrogels with Small Molecular Amines for Enhanced Cellular Interaction. International Journal of Molecular Sciences. 2025; 26(11):5316. https://doi.org/10.3390/ijms26115316
Chicago/Turabian StyleKohn-Polster, Caroline, Benno M. Müller, Jan Krieghoff, Awais Nawaz, Iram Maqsood, Annett Starke, Kirsten Haastert-Talini, Michaela Schulz-Siegmund, and Michael Christian Hacker. 2025. "Functionalization of Two-Component Gelatinous Peptide/Reactive Oligomer Hydrogels with Small Molecular Amines for Enhanced Cellular Interaction" International Journal of Molecular Sciences 26, no. 11: 5316. https://doi.org/10.3390/ijms26115316
APA StyleKohn-Polster, C., Müller, B. M., Krieghoff, J., Nawaz, A., Maqsood, I., Starke, A., Haastert-Talini, K., Schulz-Siegmund, M., & Hacker, M. C. (2025). Functionalization of Two-Component Gelatinous Peptide/Reactive Oligomer Hydrogels with Small Molecular Amines for Enhanced Cellular Interaction. International Journal of Molecular Sciences, 26(11), 5316. https://doi.org/10.3390/ijms26115316







