Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting
Abstract
1. Introduction
2. Results
2.1. Study Population Characteristics
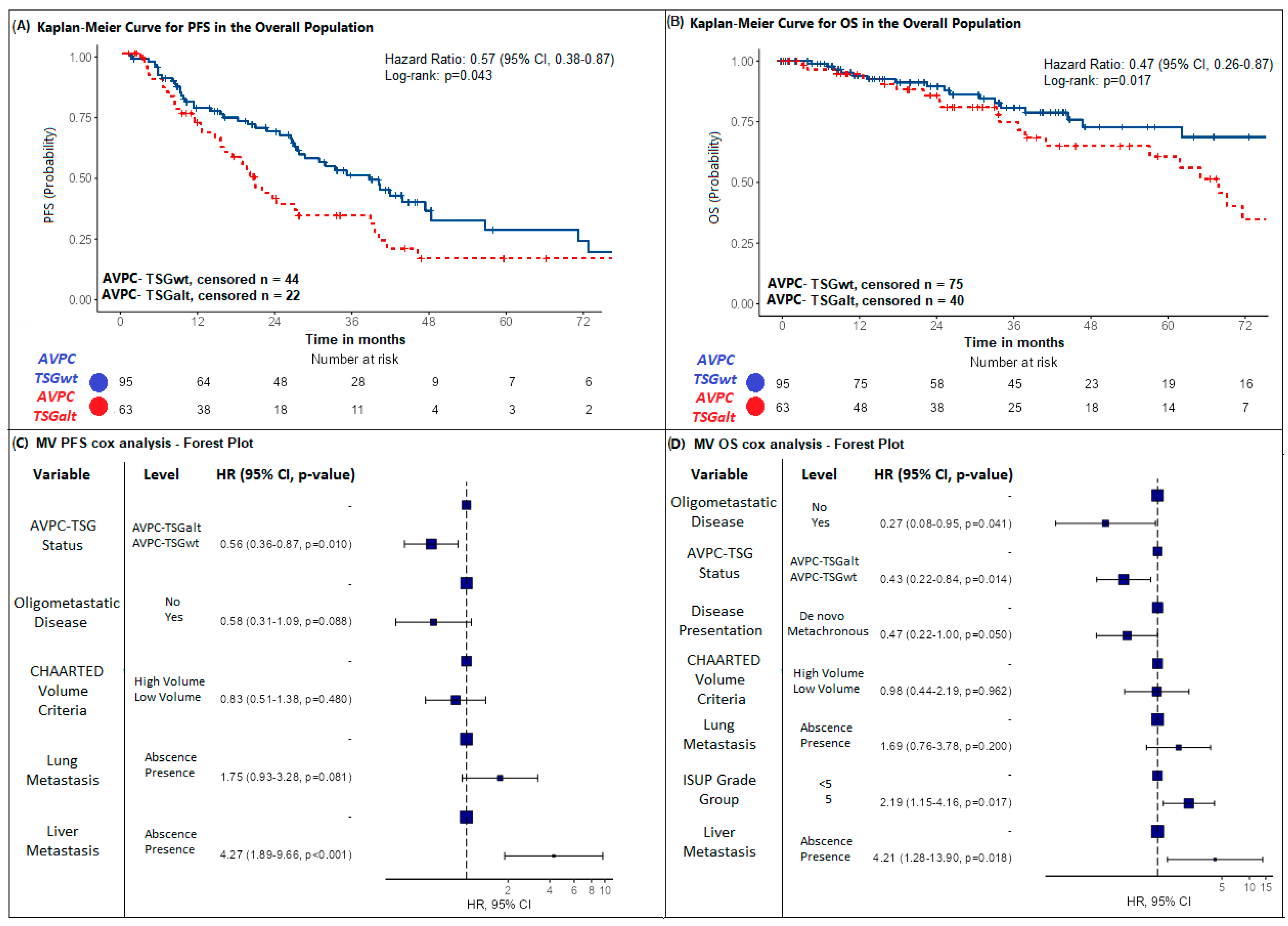
2.2. Impact of AVPC-TSG Alteration on Survival Outcomes
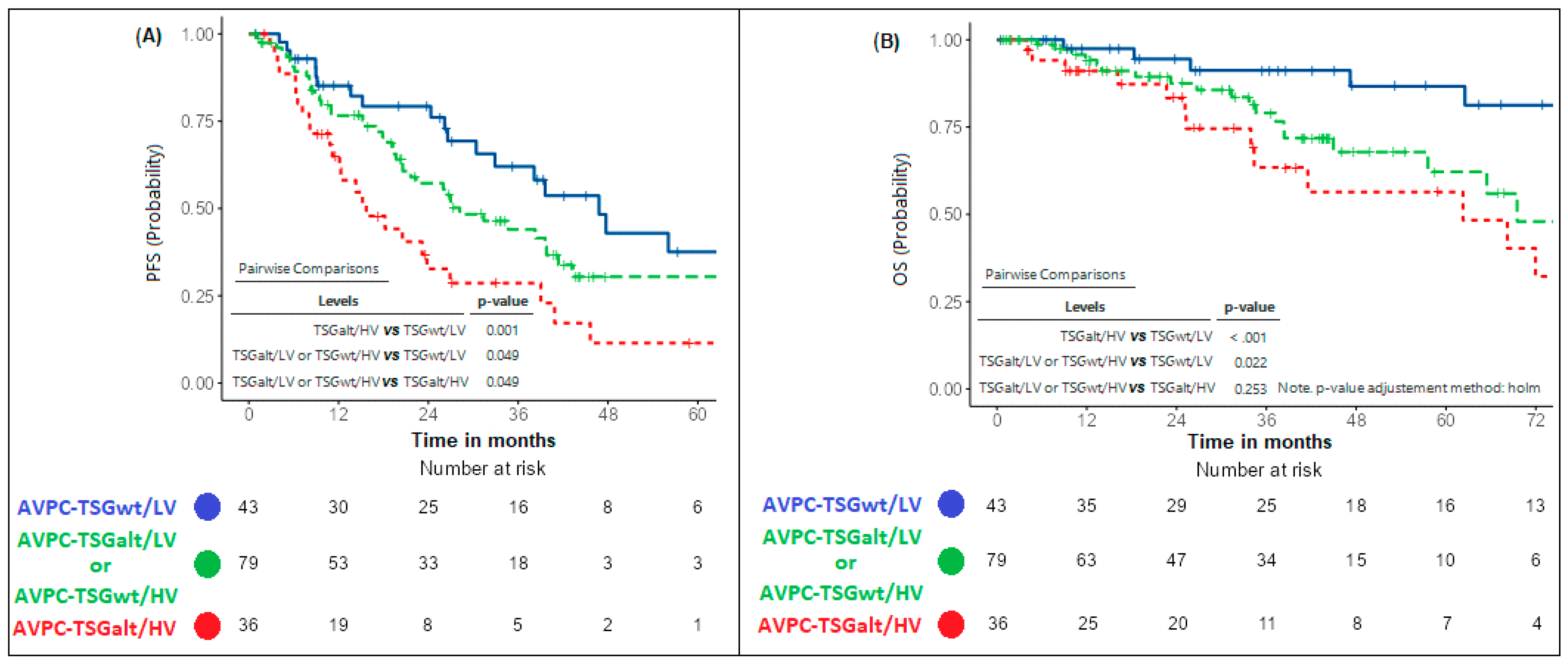
2.3. Refining CHAARTED Criteria Including AVPC-TSG Status
- -
- “AVPC-TSGwt/LV” (absence of AVPC-TSG alterations and presence of low-volume disease).
- -
- “AVPC-TSGalt/HV” (presence of both AVPC-TSG alterations and high-volume disease).
- -
- “AVPC-TSGalt/LV or AVPC-TSGwt/HV” (presence of either AVPC-TSG alteration or high-volume disease).
2.4. Predictive Value of AVPC-TSG Alteration Status
2.5. Quality Assessment
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Hamid, A.A.; Sayegh, N.; Tombal, B.; Hussain, M.; Sweeney, C.J.; Graff, J.N.; Agarwal, N. Metastatic Hormone-Sensitive Prostate Cancer: Toward an Era of Adaptive and Personalized Treatment. Am. Soc. Clin. Oncol. Educ. B. 2023, 43, e390166. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.J.; Godolphin, P.J.; Rydzewska, L.H.; Boher, J.-M.; Burdett, S.; Chen, Y.-H.; Clarke, N.W.; Fizazi, K.; Gravis, G.; et al. Which patients with metastatic hormone-sensitive prostate cancer benefit from docetaxel: A systematic review and meta-analysis of individual participant data from randomised trials. Lancet Oncol. 2023, 24, 783–797. [Google Scholar] [CrossRef]
- Corn, P.G.; Heath, E.I.; Zurita, A.; Ramesh, N.; Xiao, L.; Sei, E.; Li-Ning-Tapia, E.; Tu, S.-M.; Subudhi, S.K.; Wang, J.; et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: A randomised, open-label, phase 1–2 trial. Lancet Oncol. 2019, 20, 1432–1443. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.-C.; Wongvipat, J.; Ku, S.-Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef]
- Cronauer, M.V.; Schulz, W.A.; Burchardt, T.; Ackermann, R.; Burchardt, M. Inhibition of p53 function diminishes androgen receptor-mediated signaling in prostate cancer cell lines. Oncogene 2004, 23, 3541–3549. [Google Scholar] [CrossRef]
- Shenk, J.L.; Fisher, C.J.; Chen, S.-Y.; Zhou, X.-F.; Tillman, K.; Shemshedini, L. p53 Represses Androgen-induced Transactivation of Prostate-specific Antigen by Disrupting hAR Amino- to Carboxyl-terminal Interaction. J. Biol. Chem. 2001, 276, 38472–38479. [Google Scholar] [CrossRef]
- Gurova, K.V.; Roklin, O.W.; Krivokrysenko, V.I.; Chumakov, P.M.; Cohen, M.B.; Feinstein, E.; Gudkov, A. V Expression of prostate specific antigen (PSA) is negatively regulated by p53. Oncogene 2002, 21, 153–157. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.; Comstock, C.E.S.; Knudsen, E.S.; Cao, K.H.; Hess-Wilson, J.K.; Morey, L.M.; Barrera, J.; Knudsen, K.E. Retinoblastoma tumor suppressor status is a critical determinant of therapeutic response in prostate cancer cells. Cancer Res. 2007, 67, 6192–6203. [Google Scholar] [CrossRef]
- Sharma, A.; Yeow, W.-S.; Ertel, A.; Coleman, I.; Clegg, N.; Thangavel, C.; Morrissey, C.; Zhang, X.; Comstock, C.E.S.; Witkiewicz, A.K.; et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Investig. 2010, 120, 4478–4492. [Google Scholar] [CrossRef]
- Knudsen, K.E.; Arden, K.C.; Cavenee, W.K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998, 273, 20213–20222. [Google Scholar] [CrossRef]
- Maughan, B.L.; Guedes, L.B.; Boucher, K.; Rajoria, G.; Liu, Z.; Klimek, S.; Zoino, R.; Antonarakis, E.S.; Lotan, T.L. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 260–268. [Google Scholar] [CrossRef]
- De Laere, B.; Oeyen, S.; Mayrhofer, M.; Whitington, T.; van Dam, P.-J.; Van Oyen, P.; Ghysel, C.; Ampe, J.; Ost, P.; Demey, W.; et al. TP53 Outperforms Other Androgen Receptor Biomarkers to Predict Abiraterone or Enzalutamide Outcome in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 1766–1773. [Google Scholar] [CrossRef]
- Ferraldeschi, R.; Nava Rodrigues, D.; Riisnaes, R.; Miranda, S.; Figueiredo, I.; Rescigno, P.; Ravi, P.; Pezaro, C.; Omlin, A.; Lorente, D.; et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur. Urol. 2015, 67, 795–802. [Google Scholar] [CrossRef]
- Pedrani, M.; Barizzi, J.; Salfi, G.; Nepote, A.; Testi, I.; Merler, S.; Castelo-Branco, L.; Mestre, R.P.; Turco, F.; Tortola, L.; et al. The Emerging Predictive and Prognostic Role of Aggressive-Variant-Associated Tumor Suppressor Genes Across Prostate Cancer Stages. Int. J. Mol. Sci. 2025, 26, 318. [Google Scholar] [CrossRef]
- Attard, G.; Parry, M.; Grist, E.; Mendes, L.; Dutey-Magni, P.; Sachdeva, A.; Brawley, C.; Murphy, L.; Proudfoot, J.; Lall, S.; et al. Clinical testing of transcriptome-wide expression profiles in high-risk localized and metastatic prostate cancer starting androgen deprivation therapy: An ancillary study of the STAMPEDE abiraterone Phase 3 trial. Res. Sq. 2023, rs.3, rs-2488586. [Google Scholar] [CrossRef]
- Velez, M.G.; Kosiorek, H.E.; Egan, J.B.; McNatty, A.L.; Riaz, I.B.; Hwang, S.R.; Stewart, G.A.; Ho, T.H.; Moore, C.N.; Singh, P.; et al. Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 479–483. [Google Scholar] [CrossRef]
- Fizazi, K.; Gillessen, S. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann. Oncol. 2023, 34, 557–563. [Google Scholar] [CrossRef]
- EAU Guidelines Office. EAU Guidelines. Edn. In Proceedings of the Presented at the EAU Annual Congress Paris 2024, Paris, France, 5–8 April 2024. [Google Scholar]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; Desai, N.; Dorff, T.; et al. Prostate Cancer, Version 3.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 140–150. [Google Scholar] [CrossRef]
- Gill, J.K.; Sperandio, R.C.; Nguyen, T.H.; Emmenegger, U. Toxicity-benefit analysis of advanced prostate cancer trials using weighted toxicity scoring. J. Clin. Oncol. 2024, 42 (Suppl. S4), 110. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Lou, W.; Nadiminty, N.; Chen, X.; Zhou, Q.; Shi, X.B.; deVere White, R.W.; Gao, A.C. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate 2013, 73, 418–427. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Tan, H.-L.; Sood, A.; Rahimi, H.A.; Wang, W.; Gupta, N.; Hicks, J.; Mosier, S.; Gocke, C.D.; Epstein, J.I.; Netto, G.J.; et al. Rb Loss Is Characteristic of Prostatic Small Cell Neuroendocrine Carcinoma. Clin. Cancer Res. 2014, 20, 890–903. [Google Scholar] [CrossRef]
- Aparicio, A.M.; Shen, L.; Tapia, E.L.N.; Lu, J.-F.; Chen, H.-C.; Zhang, J.; Wu, G.; Wang, X.; Troncoso, P.; Corn, P.; et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin. Cancer Res. 2016, 22, 1520–1530. [Google Scholar] [CrossRef]
- Zou, M.; Toivanen, R.; Mitrofanova, A.; Floch, N.; Hayati, S.; Sun, Y.; Le Magnen, C.; Chester, D.; Mostaghel, E.A.; Califano, A.; et al. Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov. 2017, 7, 736–749. [Google Scholar] [CrossRef]
- Jiménez, N.; Garcia de Herreros, M.; Reig, Ò.; Marín-Aguilera, M.; Aversa, C.; Ferrer-Mileo, L.; García-Esteve, S.; Rodríguez-Carunchio, L.; Trias, I.; Font, A.; et al. Development and Independent Validation of a Prognostic Gene Expression Signature Based on RB1, PTEN, and TP53 in Metastatic Hormone-sensitive Prostate Cancer Patients. Eur. Urol. Oncol. 2024, 7, 954–964. [Google Scholar] [CrossRef]
- Pedrani, M.; Salfi, G.; Merler, S.; Testi, I.; Cani, M.; Turco, F.; Trevisi, E.; Tortola, L.; Treglia, G.; Di Tanna, G.L.; et al. Prognostic and Predictive Role of SPOP Mutations in Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2024, 7, 1199–1215. [Google Scholar] [CrossRef]
- de Jong, A.C.; Danyi, A.; van Riet, J.; de Wit, R.; Sjöström, M.; Feng, F.; de Ridder, J.; Lolkema, M.P. Predicting response to enzalutamide and abiraterone in metastatic prostate cancer using whole-omics machine learning. Nat. Commun. 2023, 14, 1968. [Google Scholar] [CrossRef]
- Nava Rodrigues, D.; Casiraghi, N.; Romanel, A.; Crespo, M.; Miranda, S.; Rescigno, P.; Figueiredo, I.; Riisnaes, R.; Carreira, S.; Sumanasuriya, S.; et al. RB1 Heterogeneity in Advanced Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 687–697. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef]
- Castelo-Branco, L.; Pellat, A.; Martins-Branco, D.; Valachis, A.; Derksen, J.W.G.; Suijkerbuijk, K.P.M.; Dafni, U.; Dellaporta, T.; Vogel, A.; Prelaj, A.; et al. ESMO Guidance for Reporting Oncology real-World evidence (GROW). Ann. Oncol. 2023, 34, 1097–1112. [Google Scholar] [CrossRef]
- Sonpavde, G.; Pond, G.R.; Armstrong, A.J.; Galsky, M.D.; Leopold, L.; Wood, B.A.; Wang, S.; Paolini, J.; Chen, I.; Chow-Maneval, E.; et al. Radiographic progression by Prostate Cancer Working Group (PCWG)-2 criteria as an intermediate endpoint for drug development in metastatic castration-resistant prostate cancer. BJU Int. 2014, 114, E25–E31. [Google Scholar] [CrossRef]
- Tilki, D.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II—2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024, 86, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Stat. Methodol. 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]

| Prognostic Variable | All Patients n = 158 (%) | TSG Alt | TSG wt | p Value | |
|---|---|---|---|---|---|
| n = 63 (39.9%) | n = 95 (60.1%) | ||||
| Median age, years (Min, Max) | 73 (47, 91) | 72.8 (54, 89) | 73.1 (47, 91) | p = 0.72 1 | |
| Age—categorical | p = 0.53 2 | ||||
| <75 yo | 93 (58.9%) | 39 (61.9%) | 54 (56.8%) | ||
| ≥75 yo | 65 (41.1%) | 24 (38.1%) | 41(33.2%) | ||
| Median PSA (Min-Max) | p = 0.961 | ||||
| 29.1 [Min: 0.038–Max: 6231] | 28 [Min: 0.770–Max: 5609] | 30 [Min: 0.038–Max: 6231] | |||
| PSA at mHSPC diagnosis | p = 0.96 2 | ||||
| PSA < 10 | 36 (22.8%) | 15 (23.8%) | 21 (22.1%) | ||
| PSA 10–100 | 86 (55.1%) | 34 (54%) | 53 (55.8%) | ||
| PSA ≥ 100 | 35(22.2%) | 14 (2.2%) | 21 (22.1%) | ||
| mHSPC treatment | p = 0.64 2 | ||||
| ADT alone | 55 (34.8%) | 23 (36.5%) | 32 (33.6%) | ||
| ADT + ARPI | 77 (48.7%) | 28 (44.5%) | 49 (51.6%) | ||
| ADT + Docetaxel | 15 (9.5%) | 8 (12.7%) | 7 (7.4%) | ||
| ADT + ARPI + Docetaxel | 11 (7%) | 4 (6.3%) | 7 (7.4%) | ||
| De Novo or Relapsed | p = 0.29 2 | ||||
| De Novo | 100 (63.3%) | 43 (68.3%) | 57 (60%) | ||
| Relapsed | 58 (36.7%) | 20 (31.7%) | 38 (40%) | ||
| Chaarted Volume | p = 0.77 2 | ||||
| High Volume | 88 (55.7%) | 36 (57.1%) | 52 (54.7%) | ||
| Low Volume | 70 (44.3%) | 27 (42.9%) | 43 (45.3%) | ||
| ISUP grade | p = 0.77 2 | ||||
| <5 | 78 (49.4%) | 32 (50.1%) | 46 (48.4%) | ||
| 5 | 80 (50.6%) | 31 (49.9%) | 49 (51.6%) | ||
| Oligometastatic disease | p = 0.55 2 | ||||
| No | 129 (81.6%) | 50 (79.4%) | 79 (83.2%) | ||
| Yes | 29 (18.4%) | 13 (20.6%) | 16 (16.8%) | ||
| Bone met | p = 0.14 2 | ||||
| No | 38 (24.1%) | 19 (30.2%) | 19 (20%) | ||
| Yes | 120 (75.9%) | 44 (69.8%) | 76 (80%) | ||
| Liver met | p = 0.53 2 | ||||
| No | 151 (95.6%) | 61 (96.8%) | 90 (94.7%) | ||
| Yes | 7 (4.4%) | 2 (3.2%) | 5 (5.3%) | ||
| Lung met | p = 0.01 2 | ||||
| No | 140 (88.6%) | 51(80.1%) | 89 (93.7%) | ||
| Yes | 18 (11.4%) | 12 (19.9%) | 6 (6.3%) | ||
| PTEN/PI3K/AKT pathway alteration status | |||||
| PTEN/PI3K/AKT wt | 138 (87.3%) | 43 (68.3%) | 95 (100%) | ||
| PTEN/PI3K/AKT alt | 20 (12.7%) | 20 (31.7%) | 0(0%) | ||
| RB1 alteration status | |||||
| RB1 wt | 155 (98.1%) | 60 (95.2%) | 95 (100%) | ||
| RB1 alt | 3 (1.9%) | 3 (4.8%) | 0 (0%) | ||
| TP53 alteration status | |||||
| P53 wt | 111 (70.3%) | 16 (25.4%) | 95 (100%) | ||
| P53 alt | 47 (29.7%) | 47 (74.6%) | 0(0%) | ||
| Number of AVPC-TSG alterations | |||||
| AVPC-TSG wt | 95 (60.1%) | 0(%) | 95 (100%) | ||
| AVPC-TSG 1 alt | 56 (35.4%) | 56 (88.9%) | 0 (0%) | ||
| AVPC-TSG 2–3 alt | 7 (4.4%) | 7 (11.1%) | 0 (0%) | ||
| Prostate RT in met setting | p = 0.74 2 | ||||
| No | 131 (82.9%) | 53 (84.1%) | 78 (82.1%) | ||
| Yes | 27 (17.1%) | 10 (25.9%) | 17 (17.9%) | ||
| median PFS (months Min–Max) | 28.2 (IC95% 23.8–39.6) [Min: 1.13–Max: 129] | 20.5 (IC95% 15.7–38.3) | 39.6 (IC95% 28.2–56.1) | p = 0.010 3 | |
| PFS-censored patients | 66 (41.8%) | 22 (34.9%) | 44 (46.3%) | ||
| median follow-up for PFS censored (months 95%CI) | 40.7 (IC95% 33.7–45.2) [Min: 1.00–Max: 104] | 33.7 (IC95% 27.3-NA) | 41.2 (IC95% 34.2–45.2) | ||
| median OS (months Min–Max) | 87.5 (IC95% 68.2-NR) [Min: 3.90–Max: 128] | 68.2 (IC95% 57.6-NR) | NR (IC95% 94.6-NR) | p = 0.017 3 | |
| OS-censored patients | 115 (72.8%) | 40 (63.5%) | 75 (78.9%) | ||
| median follow-up for OS censored (months 95%CI) | 41(34.2–44.6) [Min: 1–Max: 129] | 72.5 (60.9-not reached) | 80.5 (59.9-not reached) | ||
| Prognostic Variable | Levels | Univariate Analysis | Multivariate Analysis |
|---|---|---|---|
| Total N. 158 | HR (95%CI), p-Value | HR (95%CI), p-Value | |
| Chaarted Volume | High Volume | - | - |
| Low Volume | 0.57 (0.37–0.88) p = 0.012 ** | 0.58 (0.37–0.90) p = 0.014 ** | |
| De Novo/Metacronous | De novo | - | - |
| Metacronous | 0.89 (0.58–1.36) p = 0.576 | ||
| AVPC-TSG status | AVPC-TSGalt | - | - |
| AVPC-TSGwt | 0.57 (0.38–0.87) p = 0.010 ** | 0.58 (0.38–0.89) p = 0.012 ** | |
| ISUP Grade | <5 | - | - |
| 5 | 1.22 (0.43–3.44) p = 0.703 | ||
| Age at mHSPC | <75 | - | - |
| ≥75 | 0.87(0.57–1.32) p = 0.506 | ||
| Test for interaction | |||
| Chaarted Volume * TSG status | Df, Chi-square, p-value 1, 0.0013, 0.971 | ||
| Prognostic Variable | Levels | Univariate Analysis | Multivariate Analysis |
|---|---|---|---|
| Total N. 158 | HR (95%CI), p-Value | HR (95%CI), p-Value | |
| Chaarted Volume | High Volume | - | - |
| Low Volume | 0.37 (0.19–0.72) p = 0.003 ** | 0.49 (0.24–1.01) p = 0.052 | |
| Disease Presentation | De novo | - | - |
| Metacronous | 0.40 (0.21–0.77) p = 0.004 ** | 0.59 (0.29–1.23) p = 0.158 | |
| AVPC-TSG status | AVPC-TSGalt | - | - |
| AVPC-TSGwt | 0.47 (0.26–0.87) p = 0.017 ** | 0.48 (0.26–0.91) p = 0.025 ** | |
| ISUP Grade | <5 | - | - |
| 5 | 1.76 (0.95–3.25) p = 0.072 * | 2.10 (1.11–3.94) p = 0.022 ** | |
| Age at mHSPC | <75 | - | - |
| ≥75 | 0.96 (0.52–1.78) p = 0.9 | ||
| Test for interaction | |||
| Chaarted Volume * TSG status | Df, Chi-square, p-value 1, 0.9338, 0.3339 | ||
| Disease Presentation * TSG status | Df, Chi-square, p-value 1, 0.2731, 0.6013 | ||
| ISUP Grade * TSG status | Df, Chi-square, p-value 1, 0.0118, 0.9134 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrani, M.; Salfi, G.; Merler, S.; Testi, I.; Agrippina Clerici, C.M.; Pecoraro, G.; Castelo-Branco, L.; Turco, F.; Tortola, L.; Vogl, U.; et al. Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting. Int. J. Mol. Sci. 2025, 26, 5309. https://doi.org/10.3390/ijms26115309
Pedrani M, Salfi G, Merler S, Testi I, Agrippina Clerici CM, Pecoraro G, Castelo-Branco L, Turco F, Tortola L, Vogl U, et al. Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting. International Journal of Molecular Sciences. 2025; 26(11):5309. https://doi.org/10.3390/ijms26115309
Chicago/Turabian StylePedrani, Martino, Giuseppe Salfi, Sara Merler, Irene Testi, Chiara Maria Agrippina Clerici, Giovanna Pecoraro, Luis Castelo-Branco, Fabio Turco, Luigi Tortola, Ursula Vogl, and et al. 2025. "Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting" International Journal of Molecular Sciences 26, no. 11: 5309. https://doi.org/10.3390/ijms26115309
APA StylePedrani, M., Salfi, G., Merler, S., Testi, I., Agrippina Clerici, C. M., Pecoraro, G., Castelo-Branco, L., Turco, F., Tortola, L., Vogl, U., Gillessen, S., Theurillat, J.-P., Zilli, T., & Mestre, R. P. (2025). Integrating Aggressive-Variant Prostate Cancer-Associated Tumor Suppressor Gene Status with Clinical Variables to Refine Prognosis and Predict Androgen Receptor Pathway Inhibitor Response in Metastatic Hormone-Sensitive Setting. International Journal of Molecular Sciences, 26(11), 5309. https://doi.org/10.3390/ijms26115309





