The Hypoxia–Retinoid Axis in Idiopathic Pulmonary Fibrosis: Multifaceted Etiology and Therapeutic Potential
Abstract
1. Introduction
2. Metabolic Changes in Idiopathic Pulmonary Fibrosis
3. Hypoxia and Progression of Idiopathic Pulmonary Fibrosis
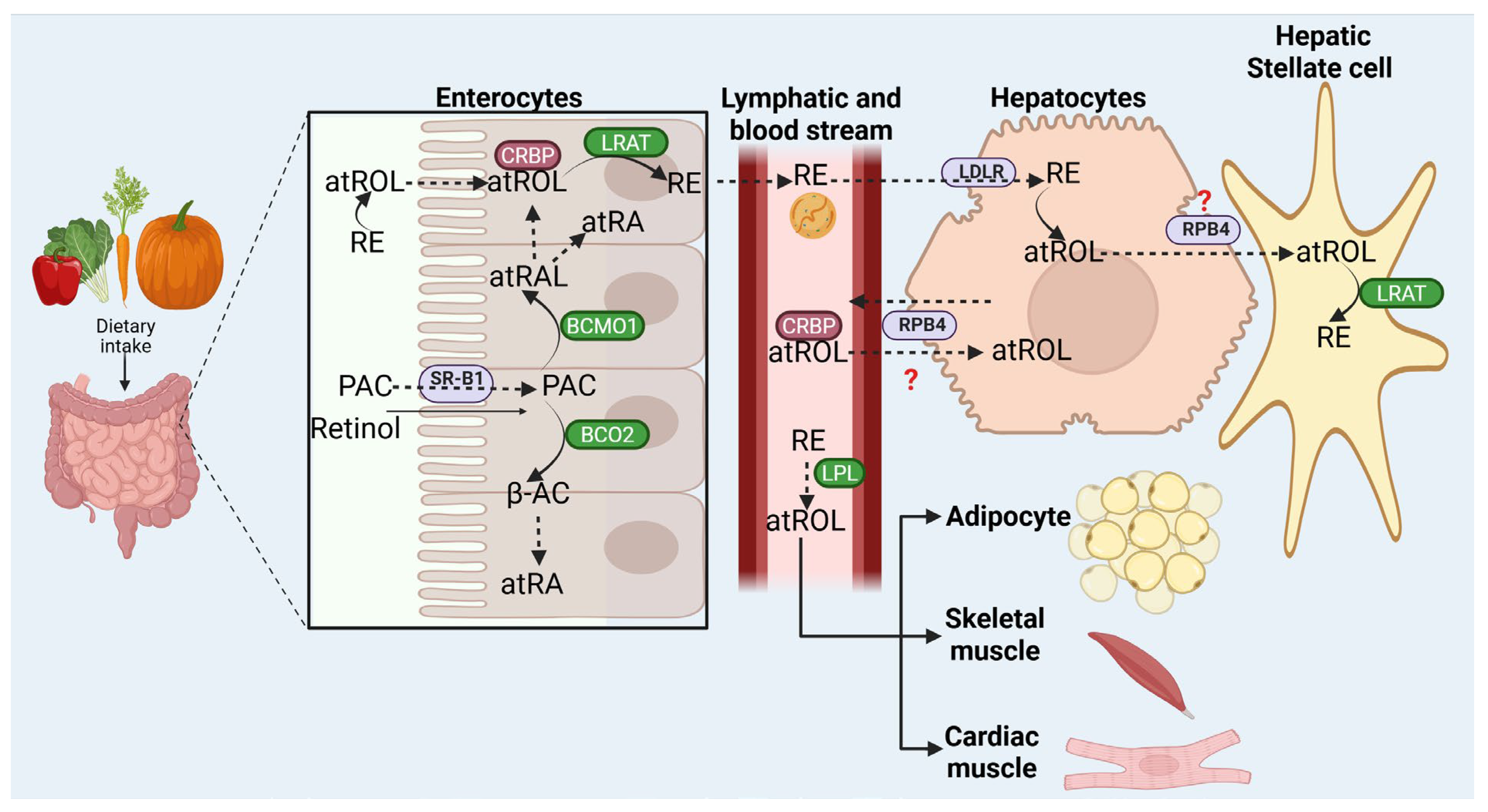
4. Retinoid Uptake, Metabolism, and Storage
5. Retinoic Acid Receptors
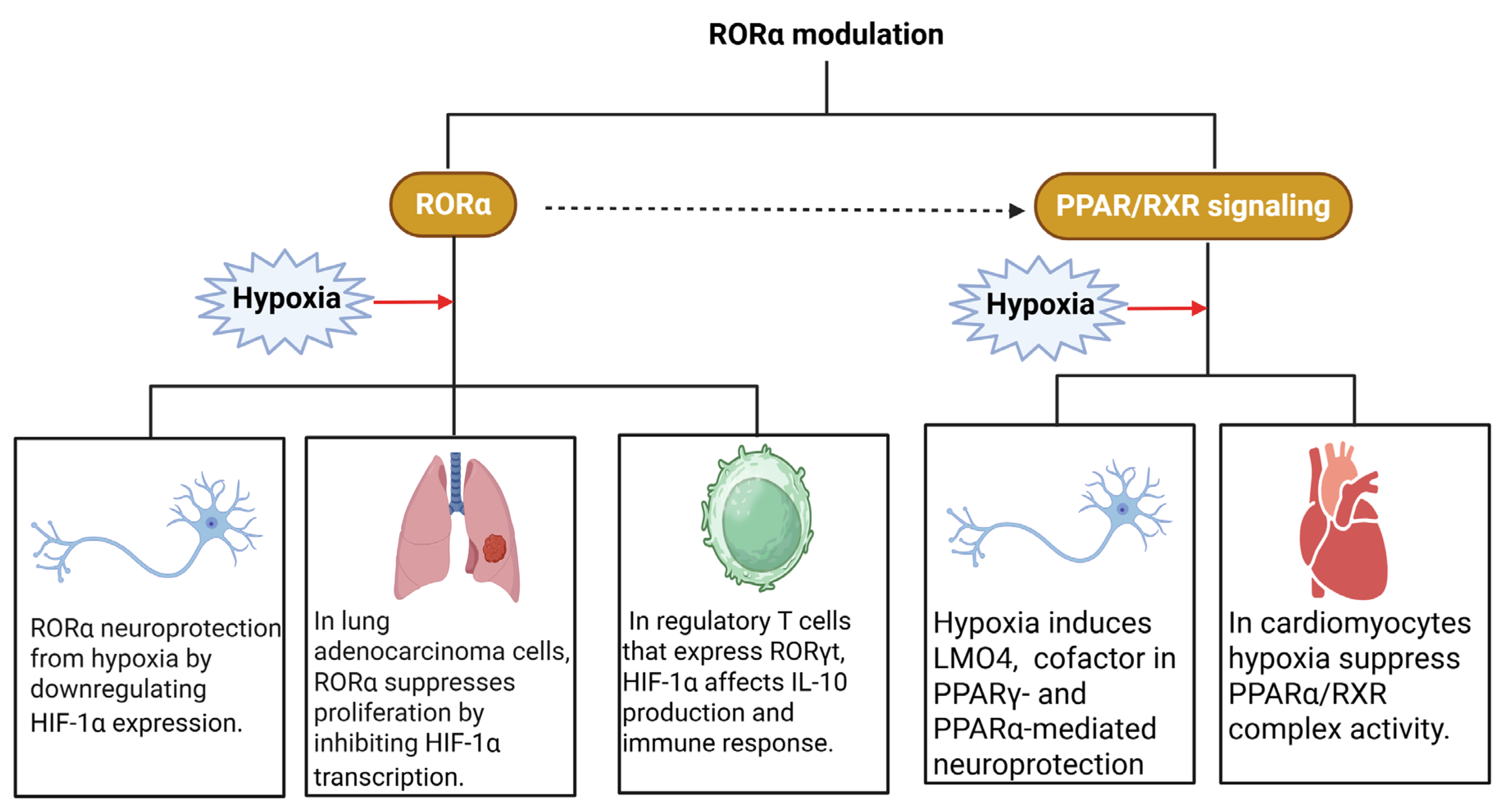
6. Retinoid Receptors and Hypoxia
7. Retinoids in Lung Regeneration and Fibrosis: A Delicate Balance
8. All-Trans Retinoic Acid in Lung Fibrosis
9. Hypoxia–Retinoid Interaction in Disease
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | alcohol dehydrogenase |
| AE2 | epithelial type II cells |
| Akt | serine/threonine-protein kinase |
| ApoE | apoprotein-E |
| atRAL | all-trans-retinal |
| atRA | all-trans-retinoic acid |
| atROL | all-trans-retinol |
| BCO2 | β,β carotene 9′,10′-dioxygenase |
| BCMO1 | β-carotene-15,15′-monooxygenase 1 |
| BLM | Bleomycin |
| CMR | chylomicrons remnants |
| CRABP2 | retinoic acid-binding protein 2 |
| CRBP | cellular retinol binding proteins |
| CYP26 | cytochrome P 450 subfamily 26 |
| DGAT1 | diacylglycerol acyltransferase 1 |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| FABP5 | fatty acid-binding protein 5 |
| FGF | fibroblast growth factor |
| FGF10 | Fibroblast Growth Factor 10 |
| FSK | Forskolin |
| Hh | hedgehog signaling |
| HIF | hypoxia-inducible factor |
| HSL | hormone sensitive lipase |
| HSC | hepatic stellate cells |
| IPF | idiopathic pulmonary fibrosis |
| LDLR | LDL receptor |
| lncRNAs | long non-coding RNAs |
| LPL | lipoprotein lipase |
| LRAT | lecithin:retinol acyl transferase |
| MUC5B | Mucin 5B |
| PI3K | phosphatidylinositol 3-kinase |
| PPARβ/δ | peroxisome proliferator-activated receptors be-ta/delta |
| RADH | retinaldehyde dehydrogenases |
| RARE | retinoic acid response element |
| RBP4 | retinoid-binding protein 4 |
| RDH | retinol dehydrogenase |
| RE | retinyl esters |
| ROR | retinoic acid-related orphan receptors |
| RORE | ROR response elements |
| RNA-Seq | single-cell RNA sequencing |
| RXR | Retinoid X receptors |
| RXRE | RXR responsive elements |
| SR-B1 | scavenger receptor class B |
| TGF-β | transforming growth factor-beta |
| TRF | tocotrienol-rich fraction |
| UIP | usual interstitial pneumonia |
References
- Zhang, Y.; Wang, J. Cellular and Molecular Mechanisms in Idiopathic Pulmonary Fibrosis. Adv. Respir. Med. 2023, 91, 26–48. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-Based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The Role of Aging in Idiopathic Pulmonary Fibrosis. Lung 2015, 193, 605–610. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X. Identification and Validation of Aging-Related Genes in Idiopathic Pulmonary Fibrosis. Front. Genet. 2022, 13, 780010. [Google Scholar] [CrossRef]
- Patel, H.; Shah, J.R.; Patel, D.R.; Avanthika, C.; Jhaveri, S.; Gor, K. Idiopathic Pulmonary Fibrosis: Diagnosis, Biomarkers and Newer Treatment Protocols. Dis. Mon. 2023, 69, 101484. [Google Scholar] [CrossRef]
- King, T.E.; Pardo, A.; Selman, M. Idiopathic Pulmonary Fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Thomson, C.C.; Remy-Jardin, M.; Richeldi, L.; Martinez, F.J.; Kolb, M.; Raghu, G. Idiopathic Pulmonary Fibrosis: State of the Art for 2023. Eur. Respir. J. 2023, 61, 2200957. [Google Scholar] [CrossRef]
- Lawson, W.E.; Loyd, J.E. The Genetic Approach in Pulmonary Fibrosis: Can It Provide Clues to This Complex Disease? Proc. Am. Thorac. Soc. 2006, 3, 345–349. [Google Scholar] [CrossRef][Green Version]
- Klay, D.; Grutters, J.C.; van der Vis, J.J.; Platenburg, M.G.J.P.; Kelder, J.C.; Tromp, E.; van Moorsel, C.H.M. Progressive Disease with Low Survival in Adult Patients With Pulmonary Fibrosis Carrying Surfactant-Related Gene Mutations: An Observational Study. Chest 2023, 163, 870–880. [Google Scholar] [CrossRef]
- Seibold, M.A.; Wise, A.L.; Speer, M.C.; Steele, M.P.; Brown, K.K.; Loyd, J.E.; Fingerlin, T.E.; Zhang, W.; Gudmundsson, G.; Groshong, S.D.; et al. A Common MUC5B Promoter Polymorphism and Pulmonary Fibrosis. N. Engl. J. Med. 2011, 364, 1503–1512. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Cogan, J.D.; Mitchell, D.B.; Sheng, Q.; Zhao, S.; Bai, Y.; Ciombor, K.K.; Sabusap, C.M.; Malabanan, M.M.; et al. The Genetic Landscape of Familial Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2023, 207, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Groen, K.; van der Vis, J.J.; van Batenburg, A.A.; Kazemier, K.M.; Grutters, J.C.; van Moorsel, C.H.M. Genetic Variant Overlap Analysis Identifies Established and Putative Genes Involved in Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 2790. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T. Epigenetics Approaches toward Precision Medicine for Idiopathic Pulmonary Fibrosis: Focus on DNA Methylation. Biomedicines 2023, 11, 1047. [Google Scholar] [CrossRef]
- Cui, F.; Sun, Y.; Xie, J.; Li, D.; Wu, M.; Song, L.; Hu, Y.; Tian, Y. Air Pollutants, Genetic Susceptibility and Risk of Incident Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2023, 61, 2200777. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cox, I.A.; Leigh, L.; de Graaff, B.; Johnston, F.H.; Corte, T.J.; Knibbs, L.D.; Otahal, P.; Navaratnam, V.; Campbell, J.A.; et al. Long-Term Exposure to Low Concentrations of Air Pollution and Decline in Lung Function in People with Idiopathic Pulmonary Fibrosis: Evidence from Australia. Respirology 2023, 28, 916–924. [Google Scholar] [CrossRef]
- Mariscal-Aguilar, P.; Gómez-Carrera, L.; Carpio, C.; Zamarrón, E.; Bonilla, G.; Fernández-Velilla, M.; Torres, I.; Esteban, I.; Regojo, R.; Díaz-Almirón, M.; et al. Relationship between Air Pollution Exposure and the Progression of Idiopathic Pulmonary Fibrosis in Madrid: Chronic Respiratory Failure, Hospitalizations, and Mortality. A Retrospective Study. Front. Public Health 2023, 11, 1135162. [Google Scholar] [CrossRef]
- Yoon, H.-Y.; Kim, S.-Y.; Kim, O.-J.; Song, J.W. Nitrogen Dioxide Increases the Risk of Disease Progression in Idiopathic Pulmonary Fibrosis. Respirology 2023, 28, 254–261. [Google Scholar] [CrossRef]
- Wu, W.; Li, C.; Zhu, X.; Liu, X.; Li, P.; Wan, R.; Wu, X.; Chen, S. Genetic Association of Telomere Length, Obesity and Tobacoo Smoking with Idiopathic Pulmonary Fibrosis Risk. BMC Public Health 2023, 23, 868. [Google Scholar] [CrossRef]
- Edwards, G.D.; Polgar, O.; Patel, S.; Barker, R.E.; Walsh, J.A.; Harvey, J.; Man, W.D.-C.; Nolan, C.M. Mood Disorder in Idiopathic Pulmonary Fibrosis: Response to Pulmonary Rehabilitation. ERJ Open Res. 2023, 9, 3. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, F.; Li, D.; Wang, J.; Tang, L.; Xie, J.; Hu, Y.; Tian, Y. Lifestyle, Genetic Susceptibility, and the Risk of Idiopathic Pulmonary Fibrosis: A Large Prospective Cohort Study. Chest 2023, 164, 929–938. [Google Scholar] [CrossRef]
- Mochizuka, Y.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Inoue, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Geriatric Nutritional Risk Index Is a Predictor of Tolerability of Antifibrotic Therapy and Mortality Risk in Patients with Idiopathic Pulmonary Fibrosis. Respirology 2023, 28, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Hahn, K.; Duraisamy, S.K.; Salathe, M.A.; Huang, S.K.; Burris, T.P.; Sundar, I.K. Rev-Erbα Agonists Suppresses TGFβ1-Induced Fibroblast-to-Myofibroblast Transition and pro-Fibrotic Phenotype in Human Lung Fibroblasts. Biochem. Biophys. Res. Commun. 2023, 669, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Myall, K.J.; West, A.G.; Martinovic, J.L.; Lam, J.L.; Roque, D.; Wu, Z.; Maher, T.M.; Molyneaux, P.L.; Suh, E.-S.; Kent, B.D. Nocturnal Hypoxemia Associates with Symptom Progression and Mortality in Patients with Progressive Fibrotic Interstitial Lung Disease. Chest 2023, 164, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shi, J.; Zhang, D.; Li, C.; Xu, H.; He, J.; Liang, W. Assessing the Genetic Relationship between Gastroesophageal Reflux Disease and Chronic Respiratory Diseases: A Mendelian Randomization Study. BMC Pulm. Med. 2023, 23, 243. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Del Greco, M.F.; Allen, R.J.; Flores, C.; Jenkins, R.G.; Maher, T.M.; Molyneaux, P.L.; Noth, I.; Oldham, J.M.; Wain, L.V.; et al. The Causal Relationship between Gastro-Oesophageal Reflux Disease and Idiopathic Pulmonary Fibrosis: A Bidirectional Two-Sample Mendelian Randomisation Study. Eur. Respir. J. 2023, 61, 2201585. [Google Scholar] [CrossRef]
- Senoo, S.; Higo, H.; Taniguchi, A.; Kiura, K.; Maeda, Y.; Miyahara, N. Pulmonary Fibrosis and Type-17 Immunity. Respir. Investig. 2023, 61, 553–562. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, M.; Li, Y.; Yan, P.; Li, Z.; Chen, X.; Yang, J.; Pan, X.; Zhao, H.; Wang, S.; et al. Serum Proteomics Identifies Biomarkers Associated with the Pathogenesis of Idiopathic Pulmonary Fibrosis. Mol. Cell. Proteom. 2023, 22, 100524. [Google Scholar] [CrossRef]
- Liang, J.; Huang, G.; Liu, X.; Liu, N.; Taghavifar, F.; Dai, K.; Yao, C.; Deng, N.; Wang, Y.; Chen, P.; et al. Reciprocal Interactions between Alveolar Progenitor Dysfunction and Aging Promote Lung Fibrosis. eLife 2023, 12, e85415. [Google Scholar] [CrossRef]
- Lowery, E.M.; Brubaker, A.L.; Kuhlmann, E.; Kovacs, E.J. The Aging Lung. Clin. Interv. Aging 2013, 8, 1489–1496. [Google Scholar] [CrossRef]
- Klee, S.; Picart-Armada, S.; Wenger, K.; Birk, G.; Quast, K.; Veyel, D.; Rist, W.; Violet, C.; Luippold, A.; Haslinger, C.; et al. Transcriptomic and Proteomic Profiling of Young and Old Mice in the Bleomycin Model Reveals High Similarity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L245–L258. [Google Scholar] [CrossRef]
- Sun, W.; Yang, X.; Chen, L.; Guo, L.; Huang, H.; Liu, X.; Yang, Y.; Xu, Z. FSTL1 Promotes Alveolar Epithelial Cell Aging and Worsens Pulmonary Fibrosis by Affecting SENP1-Mediated DeSUMOylation. Cell Biol. Int. 2023, 47, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, F.; Prats, N.; Ramponi, V.; López-Domínguez, J.A.; Meyer, K.; Aguilera, M.; Muñoz Martín, M.I.; Martínez, D.; Agusti, A.; Faner, R.; et al. Human Senescent Fibroblasts Trigger Progressive Lung Fibrosis in Mice. Aging 2023, 15, 6641–6657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.D.; Yin, L.; Archer, S.; Lu, C.; Zhao, G.; Yao, Y.; Wu, L.; Hsin, M.; Waddell, T.K.; Keshavjee, S.; et al. Metabolic Heterogeneity of Idiopathic Pulmonary Fibrosis: A Metabolomic Study. BMJ Open Respir. Res. 2017, 4, e000183. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; He, J. A Novel Prognostic Index Based on the Analysis of Glycolysis-Related Genes in Idiopathic Pulmonary Fibrosis. Medicine 2023, 102, e33330. [Google Scholar] [CrossRef]
- Yang, C.; Wang, G.; Zhan, W.; Wang, Y.; Feng, J. The Identification of Metabolism-Related Subtypes and Potential Treatments for Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2023, 14, 1173961. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, S.; Clynick, B.; How, B.S.; King, A.; Walters, E.H.; Goh, N.S.; Corte, T.J.; Trengove, R.; Tan, D.; Moodley, Y. There Is Detectable Variation in the Lipidomic Profile between Stable and Progressive Patients with Idiopathic Pulmonary Fibrosis (IPF). Respir. Res. 2021, 22, 105. [Google Scholar] [CrossRef]
- Nambiar, S.; Tan, D.B.A.; Clynick, B.; Bong, S.H.; Rawlinson, C.; Gummer, J.; Corte, T.J.; Glaspole, I.; Moodley, Y.P.; Trengove, R. Untargeted Metabolomics of Human Plasma Reveal Lipid Markers Unique to Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Proteom. Clin. Appl. 2021, 15, e2000039. [Google Scholar] [CrossRef]
- Chung, K.-P.; Hsu, C.-L.; Fan, L.-C.; Huang, Z.; Bhatia, D.; Chen, Y.-J.; Hisata, S.; Cho, S.J.; Nakahira, K.; Imamura, M.; et al. Mitofusins Regulate Lipid Metabolism to Mediate the Development of Lung Fibrosis. Nat. Commun. 2019, 10, 3390. [Google Scholar] [CrossRef]
- Shin, H.; Park, S.; Hong, J.; Baek, A.-R.; Lee, J.; Kim, D.-J.; Jang, A.-S.; Chin, S.S.; Jeong, S.H.; Park, S.-W. Overexpression of Fatty Acid Synthase Attenuates Bleomycin Induced Lung Fibrosis by Restoring Mitochondrial Dysfunction in Mice. Sci. Rep. 2023, 13, 9044. [Google Scholar] [CrossRef]
- Zhu, W.; Tan, C.; Zhang, J. Alveolar Epithelial Type 2 Cell Dysfunction in Idiopathic Pulmonary Fibrosis. Lung 2022, 200, 539–547. [Google Scholar] [CrossRef]
- El Husseini, K.; Poté, N.; Jaillet, M.; Mordant, P.; Mal, H.; Frija-Masson, J.; Borie, R.; Cazes, A.; Crestani, B.; Mailleux, A. Adipocytes, adipokines and metabolic alterations in pulmonary fibrosis. Rev. Mal. Respir. 2023, 40, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.-M.; et al. An Atlas of the Aging Lung Mapped by Single Cell Transcriptomics and Deep Tissue Proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Pierre-Louis Odoom, J.; Freeberg, M.A.T.; Camus, S.V.; Toft, R.; Szomju, B.B.; Sanchez Rosado, R.M.; Jackson, P.D.; Allegood, J.C.; Silvey, S.; Liu, J.; et al. Exhaled Breath Condensate Identifies Metabolic Dysregulation in Patients with Radiation-Induced Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L863–L869. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Duan, C.; Feng, J.; Liao, J.; Yang, Y.; Sun, W. Roles of Lipid Metabolism and Its Regulatory Mechanism in Idiopathic Pulmonary Fibrosis: A Review. Int. J. Biochem. Cell Biol. 2023, 155, 106361. [Google Scholar] [CrossRef]
- Aquino-Gálvez, A.; González-Ávila, G.; Jiménez-Sánchez, L.L.; Maldonado-Martínez, H.A.; Cisneros, J.; Toscano-Marquez, F.; Castillejos-López, M.; Torres-Espíndola, L.M.; Velázquez-Cruz, R.; Rodríguez, V.H.O.; et al. Dysregulated Expression of Hypoxia-Inducible Factors Augments Myofibroblasts Differentiation in Idiopathic Pulmonary Fibrosis. Respir. Res. 2019, 20, 130. [Google Scholar] [CrossRef]
- Yang, L.; Gilbertsen, A.; Xia, H.; Benyumov, A.; Smith, K.; Herrera, J.; Racila, E.; Bitterman, P.B.; Henke, C.A. Hypoxia Enhances IPF Mesenchymal Progenitor Cell Fibrogenicity via the Lactate/GPR81/HIF1α Pathway. JCI Insight 2023, 8, e163820. [Google Scholar] [CrossRef]
- Delbrel, E.; Soumare, A.; Naguez, A.; Label, R.; Bernard, O.; Bruhat, A.; Fafournoux, P.; Tremblais, G.; Marchant, D.; Gille, T.; et al. HIF-1α Triggers ER Stress and CHOP-Mediated Apoptosis in Alveolar Epithelial Cells, a Key Event in Pulmonary Fibrosis. Sci. Rep. 2018, 8, 17939. [Google Scholar] [CrossRef]
- Burman, A.; Kropski, J.A.; Calvi, C.L.; Serezani, A.P.; Pascoalino, B.D.; Han, W.; Sherrill, T.; Gleaves, L.; Lawson, W.E.; Young, L.R.; et al. Localized Hypoxia Links ER Stress to Lung Fibrosis through Induction of C/EBP Homologous Protein. JCI Insight 2018, 3, e99543. [Google Scholar] [CrossRef]
- Delbrel, E.; Uzunhan, Y.; Soumare, A.; Gille, T.; Marchant, D.; Planès, C.; Boncoeur, E. ER Stress Is Involved in Epithelial-To-Mesenchymal Transition of Alveolar Epithelial Cells Exposed to a Hypoxic Microenvironment. Int. J. Mol. Sci. 2019, 20, 1299. [Google Scholar] [CrossRef]
- Zhao, T.; Gong, B.; Luo, S.; Zhang, R.; Zhang, L.; Huang, Y.; Gao, H.; Gong, T. A Fibroblastic Foci-Targeting and Hypoxia-Cleavable Delivery System of Pirfenidone for the Treatment of Idiopathic Pulmonary Fibrosis. Acta Biomater. 2023, 167, 574–582. [Google Scholar] [CrossRef]
- Miozzo, A.P.; Watte, G.; Hetzel, G.M.; Altmayer, S.; Nascimento, D.Z.; Cadore, E.; Florian, J.; Machado, S.d.C.; Plentz, R.D.M. Ambulatory Oxygen Therapy in Lung Transplantation Candidates with Idiopathic Pulmonary Fibrosis Referred for Pulmonary Rehabilitation. J. Bras. Pneumol. 2023, 49, e20220280. [Google Scholar] [CrossRef] [PubMed]
- Matsunashi, A.; Nagata, K.; Morimoto, T.; Tomii, K. Mechanical Ventilation for Acute Exacerbation of Fibrosing Interstitial Lung Diseases. Respir. Investig. 2023, 61, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qiao, G.; Zhou, J.; Zhou, Y.; Li, Y.; Li, X.; Jiang, Z.; Wang, Y. Integrated Analysis Reveals the Protective Mechanism and Therapeutic Potential of Hyperbaric Oxygen against Pulmonary Fibrosis. Genes Dis. 2023, 10, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, K.; Zhang, X.; Huang, G.; Lynn, H.; Rabata, A.; Liang, J.; Noble, P.W.; Jiang, D. Multiple Fibroblast Subtypes Contribute to Matrix Deposition in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2023, 69, 45–56. [Google Scholar] [CrossRef]
- Huang, Y.; Guzy, R.; Ma, S.-F.; Bonham, C.A.; Jou, J.; Schulte, J.J.; Kim, J.S.; Barros, A.J.; Espindola, M.S.; Husain, A.N.; et al. Central Lung Gene Expression Associates with Myofibroblast Features in Idiopathic Pulmonary Fibrosis. BMJ Open Respir. Res. 2023, 10, e001391. [Google Scholar] [CrossRef]
- Torres-Soria, A.K.; Romero, Y.; Balderas-Martínez, Y.I.; Velázquez-Cruz, R.; Torres-Espíndola, L.M.; Camarena, A.; Flores-Soto, E.; Solís-Chagoyán, H.; Ruiz, V.; Carlos-Reyes, Á.; et al. Functional Repercussions of Hypoxia-Inducible Factor-2α in Idiopathic Pulmonary Fibrosis. Cells 2022, 11, 2938. [Google Scholar] [CrossRef]
- Romero, Y.; Aquino-Gálvez, A. Hypoxia in Cancer and Fibrosis: Part of the Problem and Part of the Solution. Int. J. Mol. Sci. 2021, 22, 8335. [Google Scholar] [CrossRef]
- McCall, A.S.; Gutor, S.; Tanjore, H.; Burman, A.; Sherrill, T.; Chapman, M.; Calvi, C.L.; Han, D.; Camarata, J.; Hunt, R.P.; et al. Hypoxia-Inducible Factor 2 Regulates Alveolar Regeneration after Repetitive Injury in Three-Dimensional Cellular and in Vivo Models. Sci. Transl. Med. 2025, 17, eadk8623. [Google Scholar] [CrossRef]
- Jia, M.; Rosas, L.; Kapetanaki, M.G.; Tabib, T.; Sebrat, J.; Cruz, T.; Bondonese, A.; Mora, A.L.; Lafyatis, R.; Rojas, M.; et al. Early Events Marking Lung Fibroblast Transition to Profibrotic State in Idiopathic Pulmonary Fibrosis. Respir. Res. 2023, 24, 116. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of Retinoid Metabolism and Function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef]
- Blaner, W.S.; Li, Y. Vitamin A Metabolism, Storage and Tissue Delivery Mechanisms. In The Retinoids: Biology, Biochemistry, and Disease; Dollé, P., Neiderreither, K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–34. ISBN 978-1-118-62798-3. [Google Scholar]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of Chemical Carcinogenesis by Vitamin A and Its Synthetic Analogs (Retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar] [PubMed]
- Sporn, M.B.; Roberts, A.B. What Is a Retinoid? Ciba Foundation Symposium; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1985; Volume 113, pp. 1–5. [Google Scholar]
- During, A.; Harrison, E.H. Mechanisms of Provitamin A (Carotenoid) and Vitamin A (Retinol) Transport into and out of Intestinal Caco-2 Cells. J. Lipid Res. 2007, 48, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Andersson, S. Biochemical Properties of Purified Recombinant Human Beta-Carotene 15,15’-Monooxygenase. J. Biol. Chem. 2002, 277, 23942–23948. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and Characterization of a Mammalian Enzyme Catalyzing the Asymmetric Oxidative Cleavage of Provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef]
- Wang, X.D.; Krinsky, N.I.; Tang, G.W.; Russell, R.M. Retinoic Acid Can Be Produced from Excentric Cleavage of Beta-Carotene in Human Intestinal Mucosa. Arch. Biochem. Biophys. 1992, 293, 298–304. [Google Scholar] [CrossRef]
- Wang, X.D.; Russell, R.M.; Liu, C.; Stickel, F.; Smith, D.E.; Krinsky, N.I. Beta-Oxidation in Rabbit Liver in Vitro and in the Perfused Ferret Liver Contributes to Retinoic Acid Biosynthesis from Beta-Apocarotenoic Acids. J. Biol. Chem. 1996, 271, 26490–26498. [Google Scholar] [CrossRef]
- Ziouzenkova, O.; Orasanu, G.; Sukhova, G.; Lau, E.; Berger, J.P.; Tang, G.; Krinsky, N.I.; Dolnikowski, G.G.; Plutzky, J. Asymmetric Cleavage of Beta-Carotene Yields a Transcriptional Repressor of Retinoid X Receptor and Peroxisome Proliferator-Activated Receptor Responses. Mol. Endocrinol. 2007, 21, 77–88. [Google Scholar] [CrossRef]
- Harrison, E.H.; dela Sena, C.; Eroglu, A.; Fleshman, M.K. The Formation, Occurrence, and Function of β-Apocarotenoids: β-Carotene Metabolites That May Modulate Nuclear Receptor Signaling. Am. J. Clin. Nutr. 2012, 96, 1189S–1192S. [Google Scholar] [CrossRef]
- MacDonald, P.N.; Ong, D.E. Evidence for a Lecithin-Retinol Acyltransferase Activity in the Rat Small Intestine. J. Biol. Chem. 1988, 263, 12478–12482. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Wongsiriroj, N.; Libien, J.; Vogel, S.; Goldberg, I.J.; Baehr, W.; Palczewski, K.; Blaner, W.S. Retinoid Absorption and Storage Is Impaired in Mice Lacking Lecithin:Retinol Acyltransferase (LRAT). J. Biol. Chem. 2005, 280, 35647–35657. [Google Scholar] [CrossRef]
- Wongsiriroj, N.; Piantedosi, R.; Palczewski, K.; Goldberg, I.J.; Johnston, T.P.; Li, E.; Blaner, W.S. The Molecular Basis of Retinoid Absorption: A Genetic Dissection. J. Biol. Chem. 2008, 283, 13510–13519. [Google Scholar] [CrossRef]
- Helgerud, P.; Petersen, L.B.; Norum, K.R. Retinol Esterification by Microsomes from the Mucosa of Human Small Intestine. Evidence for Acyl-Coenzyme A Retinol Acyltransferase Activity. J. Clin. Investig. 1983, 71, 747–753. [Google Scholar] [CrossRef]
- Nayak, N.; Harrison, E.H.; Hussain, M.M. Retinyl Ester Secretion by Intestinal Cells: A Specific and Regulated Process Dependent on Assembly and Secretion of Chylomicrons. J. Lipid Res. 2001, 42, 272–280. [Google Scholar] [CrossRef]
- Blaner, W.S.; Li, Y.; Brun, P.-J.; Yuen, J.J.; Lee, S.-A.; Clugston, R.D. Vitamin A Absorption, Storage and Mobilization. Subcell. Biochem. 2016, 81, 95–125. [Google Scholar] [CrossRef]
- Blaner, W.S.; Obunike, J.C.; Kurlandsky, S.B.; al-Haideri, M.; Piantedosi, R.; Deckelbaum, R.J.; Goldberg, I.J. Lipoprotein Lipase Hydrolysis of Retinyl Ester. Possible Implications for Retinoid Uptake by Cells. J. Biol. Chem. 1994, 269, 16559–16565. [Google Scholar] [CrossRef]
- van Bennekum, A.M.; Kako, Y.; Weinstock, P.H.; Harrison, E.H.; Deckelbaum, R.J.; Goldberg, I.J.; Blaner, W.S. Lipoprotein Lipase Expression Level Influences Tissue Clearance of Chylomicron Retinyl Ester. J. Lipid Res. 1999, 40, 565–574. [Google Scholar] [CrossRef]
- Goodman, D.W.; Huang, H.S.; Shiratori, T. Tissue Distribution and Metabolism of Newly Absorbed Vitamin A in the Rat. J. Lipid Res. 1965, 6, 390–396. [Google Scholar] [CrossRef]
- Blaner, W.S.; Hendriks, H.F.; Brouwer, A.; de Leeuw, A.M.; Knook, D.L.; Goodman, D.S. Retinoids, Retinoid-Binding Proteins, and Retinyl Palmitate Hydrolase Distributions in Different Types of Rat Liver Cells. J. Lipid Res. 1985, 26, 1241–1251. [Google Scholar] [CrossRef]
- Soprano, D.R.; Pickett, C.B.; Smith, J.E.; Goodman, D.S. Biosynthesis of Plasma Retinol-Binding Protein in Liver as a Larger Molecular Weight Precursor. J. Biol. Chem. 1981, 256, 8256–8258. [Google Scholar] [CrossRef]
- Soprano, D.R.; Soprano, K.J.; Goodman, D.S. Retinol-Binding Protein Messenger RNA Levels in the Liver and in Extrahepatic Tissues of the Rat. J. Lipid Res. 1986, 27, 166–171. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef]
- Quadro, L.; Hamberger, L.; Gottesman, M.E.; Wang, F.; Colantuoni, V.; Blaner, W.S.; Mendelsohn, C.L. Pathways of Vitamin A Delivery to the Embryo: Insights from a New Tunable Model of Embryonic Vitamin A Deficiency. Endocrinology 2005, 146, 4479–4490. [Google Scholar] [CrossRef]
- Isken, A.; Golczak, M.; Oberhauser, V.; Hunzelmann, S.; Driever, W.; Imanishi, Y.; Palczewski, K.; von Lintig, J. RBP4 Disrupts Vitamin A Uptake Homeostasis in a STRA6-Deficient Animal Model for Matthew-Wood Syndrome. Cell Metab. 2008, 7, 258–268. [Google Scholar] [CrossRef]
- Nagy, N.E.; Holven, K.B.; Roos, N.; Senoo, H.; Kojima, N.; Norum, K.R.; Blomhoff, R. Storage of Vitamin A in Extrahepatic Stellate Cells in Normal Rats. J. Lipid Res. 1997, 38, 645–658. [Google Scholar] [CrossRef]
- Wu, L.; Ross, A.C. Acidic Retinoids Synergize with Vitamin A to Enhance Retinol Uptake and STRA6, LRAT, and CYP26B1 Expression in Neonatal Lung. J. Lipid Res. 2010, 51, 378–387. [Google Scholar] [CrossRef]
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing Effects of Retinoic Acid on Cell Growth Result from Alternate Activation of Two Different Nuclear Receptors. Cell 2007, 129, 723–733. [Google Scholar] [CrossRef]
- Noy, N. Between Death and Survival: Retinoic Acid in Regulation of Apoptosis. Annu. Rev. Nutr. 2010, 30, 201–217. [Google Scholar] [CrossRef]
- Stevison, F.; Jing, J.; Tripathy, S.; Isoherranen, N. Role of Retinoic Acid-Metabolizing Cytochrome P450s, CYP26, in Inflammation and Cancer. Adv. Pharmacol. 2015, 74, 373–412. [Google Scholar] [CrossRef]
- Stehlin-Gaon, C.; Willmann, D.; Zeyer, D.; Sanglier, S.; Van Dorsselaer, A.; Renaud, J.-P.; Moras, D.; Schüle, R. All-Trans Retinoic Acid Is a Ligand for the Orphan Nuclear Receptor ROR Beta. Nat. Struct. Biol. 2003, 10, 820–825. [Google Scholar] [CrossRef]
- Huq, M.D.M.; Tsai, N.-P.; Gupta, P.; Wei, L.-N. Regulation of Retinal Dehydrogenases and Retinoic Acid Synthesis by Cholesterol Metabolites. EMBO J. 2006, 25, 3203–3213. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism. Nucl. Recept. Signal 2009, 7, e003. [Google Scholar] [CrossRef]
- Cook, D.N.; Kang, H.S.; Jetten, A.M. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl. Recept. Res. 2015, 2, 101185. [Google Scholar] [CrossRef]
- Choi, H.; Oh, D.; Kim, H.-J.; Chambugong, M.; Kim, M.-H.; Lee, M.-O.; Park, H.-G. An RORα Agonist, ODH-08, Inhibits Fibrogenic Activation of Hepatic Stellate Cells via Suppression of SMAD3. Life Sci. 2024, 340, 122443. [Google Scholar] [CrossRef]
- Lo, B.C.; Gold, M.J.; Hughes, M.R.; Antignano, F.; Valdez, Y.; Zaph, C.; Harder, K.W.; McNagny, K.M. The Orphan Nuclear Receptor ROR Alpha and Group 3 Innate Lymphoid Cells Drive Fibrosis in a Mouse Model of Crohn’s Disease. Sci. Immunol. 2016, 1, eaaf8864. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.-P.; Gong, W.-W.; Zheng, Y.-Y.; Shen, J.-R.; Liu, X.; Gu, Y.-H.; Shi, J.-H.; Meng, G.-L. Novel Therapeutic Potential of Retinoid-Related Orphan Receptor α in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 3462. [Google Scholar] [CrossRef]
- Jolly, S.; Journiac, N.; Vernet-der Garabedian, B.; Mariani, J. RORalpha, a Key to the Development and Functioning of the Brain. Cerebellum 2012, 11, 451–452. [Google Scholar] [CrossRef]
- Jolly, S.; Journiac, N.; Naudet, F.; Gautheron, V.; Mariani, J.; Vernet-der Garabedian, B. Cell-Autonomous and Non-Cell-Autonomous Neuroprotective Functions of RORα in Neurons and Astrocytes during Hypoxia. J. Neurosci. 2011, 31, 14314–14323. [Google Scholar] [CrossRef]
- Xiao, W.; Geng, W.; Zhou, M.; Xu, J.; Wang, S.; Huang, Q.; Sun, Y.; Li, Y.; Yang, G.; Jin, Y. POU6F1 Cooperates with RORA to Suppress the Proliferation of Lung Adenocarcinoma by Downregulation HIF1A Signaling Pathway. Cell Death Dis. 2022, 13, 427. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Q.; Chen, N.; Zheng, A.; Abbasi, N.; Wang, G.; Patel, P.R.; Cho, B.S.; Yee, B.A.; Zhang, L.; et al. RNA Binding Protein DDX5 Restricts RORγt+ Treg Suppressor Function to Promote Intestine Inflammation. Sci. Adv. 2023, 9, eadd6165. [Google Scholar] [CrossRef]
- Schock, S.C.; Xu, J.; Duquette, P.M.; Qin, Z.; Lewandowski, A.J.; Rai, P.S.; Thompson, C.S.; Seifert, E.L.; Harper, M.-E.; Chen, H.-H. Rescue of Neurons from Ischemic Injury by Peroxisome Proliferator-Activated Receptor-Gamma Requires a Novel Essential Cofactor LMO4. J. Neurosci. 2008, 28, 12433–12444. [Google Scholar] [CrossRef]
- Belanger, A.J.; Luo, Z.; Vincent, K.A.; Akita, G.Y.; Cheng, S.H.; Gregory, R.J.; Jiang, C. Hypoxia-Inducible Factor 1 Mediates Hypoxia-Induced Cardiomyocyte Lipid Accumulation by Reducing the DNA Binding Activity of Peroxisome Proliferator-Activated Receptor Alpha/Retinoid X Receptor. Biochem. Biophys. Res. Commun. 2007, 364, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Huss, J.M.; Levy, F.H.; Kelly, D.P. Hypoxia Inhibits the Peroxisome Proliferator-Activated Receptor Alpha/Retinoid X Receptor Gene Regulatory Pathway in Cardiac Myocytes: A Mechanism for O2-Dependent Modulation of Mitochondrial Fatty Acid Oxidation. J. Biol. Chem. 2001, 276, 27605–27612. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Langmann, T. Transcriptional Regulatory Networks in Lipid Metabolism Control ABCA1 Expression. Biochim. Biophys. Acta 2005, 1735, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef]
- Kotton, D.N.; Morrisey, E.E. Lung Regeneration: Mechanisms, Applications and Emerging Stem Cell Populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef]
- Massaro, G.D.; Massaro, D. Postnatal Treatment with Retinoic Acid Increases the Number of Pulmonary Alveoli in Rats. Am. J. Physiol. 1996, 270, L305–L310. [Google Scholar] [CrossRef]
- Massaro, G.D.; Massaro, D. Retinoic Acid Treatment Abrogates Elastase-Induced Pulmonary Emphysema in Rats. Nat. Med. 1997, 3, 675–677. [Google Scholar] [CrossRef]
- Stolk, J.; Stockley, R.A.; Stoel, B.C.; Cooper, B.G.; Piitulainen, E.; Seersholm, N.; Chapman, K.R.; Burdon, J.G.W.; Decramer, M.; Abboud, R.T.; et al. Randomised Controlled Trial for Emphysema with a Selective Agonist of the γ-Type Retinoic Acid Receptor. Eur. Respir. J. 2012, 40, 306–312. [Google Scholar] [CrossRef]
- Gao, R.; Kong, X.; Zhu, X.; Zhu, G.; Ma, J.; Liu, X. Retinoic Acid Promotes Primary Fetal Alveolar Epithelial Type II Cell Proliferation and Differentiation to Alveolar Epithelial Type I Cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 479–487. [Google Scholar] [CrossRef]
- Dirami, G.; Massaro, G.D.; Clerch, L.B.; Ryan, U.S.; Reczek, P.R.; Massaro, D. Lung Retinol Storing Cells Synthesize and Secrete Retinoic Acid, an Inducer of Alveolus Formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L249–L256. [Google Scholar] [CrossRef]
- Zacharias, W.J.; Frank, D.B.; Zepp, J.A.; Morley, M.P.; Alkhaleel, F.A.; Kong, J.; Zhou, S.; Cantu, E.; Morrisey, E.E. Regeneration of the Lung Alveolus by an Evolutionarily Conserved Epithelial Progenitor. Nature 2018, 555, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-Cell Wnt Signaling Niches Maintain Stemness of Alveolar Type 2 Cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Ng-Blichfeldt, J.-P.; Schrik, A.; Kortekaas, R.K.; Noordhoek, J.A.; Heijink, I.H.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. Retinoic Acid Signaling Balances Adult Distal Lung Epithelial Progenitor Cell Growth and Differentiation. EBioMedicine 2018, 36, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Shmarakov, I.O.; Gusarova, G.A.; Islam, M.N.; Marhuenda-Muñoz, M.; Bhattacharya, J.; Blaner, W.S. Retinoids Stored Locally in the Lung Are Required to Attenuate the Severity of Acute Lung Injury in Male Mice. Nat. Commun. 2023, 14, 851. [Google Scholar] [CrossRef]
- Perl, A.-K.T.; Gale, E. FGF Signaling Is Required for Myofibroblast Differentiation during Alveolar Regeneration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L299–L308. [Google Scholar] [CrossRef]
- McGowan, S.E.; Grossmann, R.E.; Kimani, P.W.; Holmes, A.J. Platelet-Derived Growth Factor Receptor-Alpha-Expressing Cells Localize to the Alveolar Entry Ring and Have Characteristics of Myofibroblasts during Pulmonary Alveolar Septal Formation. Anat. Rec. 2008, 291, 1649–1661. [Google Scholar] [CrossRef]
- Liebeskind, A.; Srinivasan, S.; Kaetzel, D.; Bruce, M. Retinoic Acid Stimulates Immature Lung Fibroblast Growth via a PDGF-Mediated Autocrine Mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L81–L90. [Google Scholar] [CrossRef]
- Hind, M.; Maden, M. Retinoic Acid Induces Alveolar Regeneration in the Adult Mouse Lung. Eur. Respir. J. 2004, 23, 20–27. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar] [CrossRef]
- Chen, F.; Desai, T.J.; Qian, J.; Niederreither, K.; Lü, J.; Cardoso, W.V. Inhibition of Tgf Beta Signaling by Endogenous Retinoic Acid Is Essential for Primary Lung Bud Induction. Development 2007, 134, 2969–2979. [Google Scholar] [CrossRef]
- Rankin, S.A.; Han, L.; McCracken, K.W.; Kenny, A.P.; Anglin, C.T.; Grigg, E.A.; Crawford, C.M.; Wells, J.M.; Shannon, J.M.; Zorn, A.M. A Retinoic Acid-Hedgehog Cascade Coordinates Mesoderm-Inducing Signals and Endoderm Competence during Lung Specification. Cell Rep. 2016, 16, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, J.; Rodríguez-Fernández, L.; Zaragozá, R.; Marín, M.P.; Cabezuelo, M.T.; Torres, L.; Viña, J.R.; Barber, T. Vitamin A Deficiency and the Lung. Nutrients 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Habermann, A.C.; Gutierrez, A.J.; Bui, L.T.; Yahn, S.L.; Winters, N.I.; Calvi, C.L.; Peter, L.; Chung, M.-I.; Taylor, C.J.; Jetter, C.; et al. Single-Cell RNA Sequencing Reveals Profibrotic Roles of Distinct Epithelial and Mesenchymal Lineages in Pulmonary Fibrosis. Sci. Adv. 2020, 6, eaba1972. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tata, A.; Konkimalla, A.; Katsura, H.; Lee, R.F.; Ou, J.; Banovich, N.E.; Kropski, J.A.; Tata, P.R. Persistence of a Regeneration-Associated, Transitional Alveolar Epithelial Cell State in Pulmonary Fibrosis. Nat. Cell Biol. 2020, 22, 934–946. [Google Scholar] [CrossRef]
- Gokey, J.J.; Snowball, J.; Green, J.; Waltamath, M.; Spinney, J.J.; Black, K.E.; Hariri, L.P.; Xu, Y.; Perl, A.K. Pretreatment of Aged Mice with Retinoic Acid Supports Alveolar Regeneration via Upregulation of Reciprocal PDGFA Signalling. Thorax 2021, 76, 456–467. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; Wells, A.U. Usual Interstitial Pneumonia as a Stand-Alone Diagnostic Entity: The Case for a Paradigm Shift? Lancet Respir. Med. 2023, 11, 188–196. [Google Scholar] [CrossRef]
- Kropotova, E.S.; Zinov’eva, O.L.; Zyrianova, A.F.; Choĭnzonov, E.L.; Afanas’ev, S.G.; Cherdyntseva, N.V.; Beresten’, S.F.; Oparina, N.I.; Mashkova, T.D. Expression of genes involved in retinoic acid biosynthesis in human gastric cancer. Mol. Biol. 2013, 47, 317–330. [Google Scholar] [CrossRef]
- Kropotova, E.S.; Zinovieva, O.L.; Zyryanova, A.F.; Dybovaya, V.I.; Prasolov, V.S.; Beresten, S.F.; Oparina, N.Y.; Mashkova, T.D. Altered Expression of Multiple Genes Involved in Retinoic Acid Biosynthesis in Human Colorectal Cancer. Pathol. Oncol. Res. 2014, 20, 707–717. [Google Scholar] [CrossRef]
- Kuznetsova, E.S.; Zinovieva, O.L.; Oparina, N.Y.; Prokofjeva, M.M.; Spirin, P.V.; Favorskaya, I.A.; Zborovskaya, I.B.; Lisitsyn, N.A.; Prassolov, V.S.; Mashkova, T.D. Abnormal expression of genes that regulate retinoid metabolism and signaling in non-small-cell lung cancer. Mol. Biol. 2016, 50, 255–265. [Google Scholar] [CrossRef]
- Villéger, R.; Chulkina, M.; Mifflin, R.C.; Markov, N.S.; Trieu, J.; Sinha, M.; Johnson, P.; Saada, J.I.; Adegboyega, P.A.; Luxon, B.A.; et al. Loss of Alcohol Dehydrogenase 1B in Cancer-Associated Fibroblasts: Contribution to the Increase of Tumor-Promoting IL-6 in Colon Cancer. Br. J. Cancer 2023, 128, 537–548. [Google Scholar] [CrossRef]
- Langhi, C.; Pedraz-Cuesta, E.; Haro, D.; Marrero, P.F.; Rodríguez, J.C. Regulation of Human Class I Alcohol Dehydrogenases by Bile Acids. J. Lipid Res. 2013, 54, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.K.; Gaedigk, A.; Pearce, R.E.; Leeder, J.S.; Prasad, B. Age-Dependent Protein Abundance of Cytosolic Alcohol and Aldehyde Dehydrogenases in Human Liver. Drug Metab. Dispos. 2017, 45, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Tabata, C.; Kubo, H.; Tabata, R.; Wada, M.; Sakuma, K.; Ichikawa, M.; Fujita, S.; Mio, T.; Mishima, M. All-Trans Retinoic Acid Modulates Radiation-Induced Proliferation of Lung Fibroblasts via IL-6/IL-6R System. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L597–L606. [Google Scholar] [CrossRef] [PubMed]
- Tabata, C.; Kadokawa, Y.; Tabata, R.; Takahashi, M.; Okoshi, K.; Sakai, Y.; Mishima, M.; Kubo, H. All-Trans-Retinoic Acid Prevents Radiation- or Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1352–1360. [Google Scholar] [CrossRef]
- Song, X.; Liu, W.; Xie, S.; Wang, M.; Cao, G.; Mao, C.; Lv, C. All-Transretinoic Acid Ameliorates Bleomycin-Induced Lung Fibrosis by Downregulating the TGF-Β1/Smad3 Signaling Pathway in Rats. Lab. Investig. 2013, 93, 1219–1231. [Google Scholar] [CrossRef]
- Redlich, C.A.; Delisser, H.M.; Elias, J.A. Retinoic Acid Inhibition of Transforming Growth Factor-Beta-Induced Collagen Production by Human Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 1995, 12, 287–295. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, J.H.; Shin, M.H.; Kim, Y.S.; Chung, K.S.; Song, J.H.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. The Effects of Retinoic Acid and MAPK Inhibitors on Phosphorylation of Smad2/3 Induced by Transforming Growth Factor Β1. Tuberc. Respir. Dis. 2019, 82, 42–52. [Google Scholar] [CrossRef]
- Mert, H.; Yoruk, I.; Ertekin, A.; Dede, S.; Deger, Y.; Yur, F.; Mert, N. Vitamin Levels in Lung Tissue of Rats with Bleomycin Induced Pulmonary Fibrosis. J. Nutr. Sci. Vitaminol. 2009, 55, 186–190. [Google Scholar] [CrossRef]
- Leem, A.Y.; Shin, M.H.; Douglas, I.S.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Chang, J.; Kim, Y.S.; et al. All-Trans Retinoic Acid Attenuates Bleomycin-Induced Pulmonary Fibrosis via Downregulating EphA2-EphrinA1 Signaling. Biochem. Biophys. Res. Commun. 2017, 491, 721–726. [Google Scholar] [CrossRef]
- Dong, Z.; Tai, W.; Yang, Y.; Zhang, T.; Li, Y.; Chai, Y.; Zhong, H.; Zou, H.; Wang, D. The Role of All-Trans Retinoic Acid in Bleomycin-Induced Pulmonary Fibrosis in Mice. Exp. Lung Res. 2012, 38, 82–89. [Google Scholar] [CrossRef]
- Eleraky, A.F.; Helal, G.K.; Elshafie, M.F.; Ismail, R.S. Concomitant Inhibition of Hedgehog Signalling and Activation of Retinoid Receptors Abolishes Bleomycin-Induced Lung Fibrosis. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1024–1040. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Pan, Z.; Yang, C.; Chen, L.; Wang, Y.; Xu, D.; Xia, H.; Wang, S.; Chen, S.; et al. Potential “Therapeutic” Effects of Tocotrienol-Rich Fraction (TRF) and Carotene “Against” Bleomycin-Induced Pulmonary Fibrosis in Rats via TGF-β/Smad, PI3K/Akt/mTOR and NF-κB Signaling Pathways. Nutrients 2022, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Al-Qassab, Y.; Grassilli, S.; Brugnoli, F.; Vezzali, F.; Capitani, S.; Bertagnolo, V. Protective Role of All-Trans Retinoic Acid (ATRA) against Hypoxia-Induced Malignant Potential of Non-Invasive Breast Tumor Derived Cells. BMC Cancer 2018, 18, 1194. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.U.; Yoon, J.-H.; Lee, Y.-J.; Lee, J.-H.; Kim, B.H.; Yu, S.J.; Myung, S.J.; Kim, Y.J.; Lee, H.-S. Hypoxia and Retinoic Acid-Inducible NDRG1 Expression Is Responsible for Doxorubicin and Retinoic Acid Resistance in Hepatocellular Carcinoma Cells. Cancer Lett. 2010, 298, 9–15. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoon, J.-H.; Yu, S.J.; Chung, G.E.; Jung, E.U.; Kim, H.Y.; Kim, B.H.; Choi, D.H.; Myung, S.J.; Kim, Y.J.; et al. Retinoic Acid and Its Binding Protein Modulate Apoptotic Signals in Hypoxic Hepatocellular Carcinoma Cells. Cancer Lett. 2010, 295, 229–235. [Google Scholar] [CrossRef]
- Liu, X.-W.; Su, Y.; Zhu, H.; Cao, J.; Ding, W.-J.; Zhao, Y.-C.; He, Q.-J.; Yang, B. HIF-1α-Dependent Autophagy Protects HeLa Cells from Fenretinide (4-HPR)-Induced Apoptosis in Hypoxia. Pharmacol. Res. 2010, 62, 416–425. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wang, J.-J.; Lee, H.-C.; Chi, C.-W.; Lee, C.-H.; Hsu, Y.-C. Combination of Peroxisome Proliferator-Activated Receptor Gamma and Retinoid X Receptor Agonists Induces Sodium/Iodide Symporter Expression and Inhibits Cell Growth of Human Thyroid Cancer Cells. J. Chin. Med. Assoc. 2020, 83, 923–930. [Google Scholar] [CrossRef]
- Bauer, R.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Santos, I.M.; Taverna, F.; Kuhlencord, M.; Gensch, V.; Päsler, S.; Vinckier, S.; et al. Blockade of Myeloid-Derived Suppressor Cell Expansion with All-Trans Retinoic Acid Increases the Efficacy of Antiangiogenic Therapy. Cancer Res. 2018, 78, 3220–3232. [Google Scholar] [CrossRef]
- Yu, J.; Perri, M.; Jones, J.W.; Pierzchalski, K.; Ceaicovscaia, N.; Cione, E.; Kane, M.A. Altered RBP1 Gene Expression Impacts Epithelial Cell Retinoic Acid, Proliferation, and Microenvironment. Cells 2022, 11, 792. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Wang, Z.; Yang, Y.; Tian, J.; Liu, G.; Guan, D.; Cao, X.; Zhang, Y.; Hao, A. GRIM-19 Opposes Reprogramming of Glioblastoma Cell Metabolism via HIF1α Destabilization. Carcinogenesis 2013, 34, 1728–1736. [Google Scholar] [CrossRef][Green Version]
- Gainer, J.L.; Sheehan, J.P.; Larner, J.M.; Jones, D.R. Trans Sodium Crocetinate with Temozolomide and Radiation Therapy for Glioblastoma Multiforme. J. Neurosurg. 2017, 126, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Sherman, J.; Cifarelli, C.; Jagannathan, J.; Dassoulas, K.; Olson, C.; Rainey, J.; Han, S. Effect of Trans Sodium Crocetinate on Brain Tumor Oxygenation. Laboratory Investigation. J. Neurosurg. 2009, 111, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Guo, S.; Yang, L. Effects of All-trans Retinoic Acid on VEGF and HIF-1α Expression in Glioma Cells under Normoxia and Hypoxia and Its Anti-angiogenic Effect in an Intracerebral Glioma Model. Mol. Med. Rep. 2014, 10, 2713–2719. [Google Scholar] [CrossRef][Green Version]
- Liang, C.; Guo, S.; Yang, L. All-Trans Retinoic Acid Upregulates VEGF Expression in Glioma Cells in Vitro. J. Biomed. Res. 2013, 27, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kikukawa, Y.; Fujiwara, S.; Wada, N.; Okuno, Y.; Mitsuya, H.; Hata, H. Hypoxia Reduces CD138 Expression and Induces an Immature and Stem Cell-like Transcriptional Program in Myeloma Cells. Int. J. Oncol. 2013, 43, 1809–1816. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.-P.; Huang, Y.; Zhao, Q.; Zhao, K.-W.; Chen, G.-Q. Accumulation of Hypoxia-Inducible Factor-1 Alpha Protein and Its Role in the Differentiation of Myeloid Leukemic Cells Induced by All-Trans Retinoic Acid. Haematologica 2008, 93, 1480–1487. [Google Scholar] [CrossRef][Green Version]
- Magliulo, D.; Simoni, M.; Caserta, C.; Fracassi, C.; Belluschi, S.; Giannetti, K.; Pini, R.; Zapparoli, E.; Beretta, S.; Uggè, M.; et al. The Transcription Factor HIF2α Partakes in the Differentiation Block of Acute Myeloid Leukemia. EMBO Mol. Med. 2023, 15, e17810. [Google Scholar] [CrossRef]
- Gery, S.; Park, D.J.; Vuong, P.T.; Virk, R.K.; Muller, C.I.; Hofmann, W.-K.; Koeffler, H.P. RTP801 Is a Novel Retinoic Acid-Responsive Gene Associated with Myeloid Differentiation. Exp. Hematol. 2007, 35, 572–578. [Google Scholar] [CrossRef]
- Lancet, J.E.; Moseley, A.B.; Coutre, S.E.; DeAngelo, D.J.; Othus, M.; Tallman, M.S.; Litzow, M.R.; Komrokji, R.S.; Erba, H.P.; Appelbaum, F.R. A Phase 2 Study of ATRA, Arsenic Trioxide, and Gemtuzumab Ozogamicin in Patients with High-Risk APL (SWOG 0535). Blood Adv. 2020, 4, 1683–1689. [Google Scholar] [CrossRef]
- Yang, B.; Fan, L.; Fang, L.; He, Q. Hypoxia-Mediated Fenretinide (4-HPR) Resistance in Childhood Acute Lymphoblastic Leukemia Cells. Cancer Chemother. Pharmacol. 2006, 58, 540–546. [Google Scholar] [CrossRef]
- Coltella, N.; Valsecchi, R.; Ponente, M.; Ponzoni, M.; Bernardi, R. Synergistic Leukemia Eradication by Combined Treatment with Retinoic Acid and HIF Inhibition by EZN-2208 (PEG-SN38) in Preclinical Models of PML-RARα and PLZF-RARα-Driven Leukemia. Clin. Cancer Res. 2015, 21, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Peng, Z.-G.; Wu, Y.-L.; Jiang, Y.; Yu, Y.; Huang, Y.; Zhu, Y.-S.; Zhao, Q.; Chen, G.-Q. Hypoxia-Simulating Agents and Selective Stimulation of Arsenic Trioxide-Induced Growth Arrest and Cell Differentiation in Acute Promyelocytic Leukemic Cells. Haematologica 2005, 90, 1607–1616. [Google Scholar] [PubMed]
- Bhaskara, V.K.; Mohanam, I.; Rao, J.S.; Mohanam, S. Intermittent Hypoxia Regulates Stem-like Characteristics and Differentiation of Neuroblastoma Cells. PLoS ONE 2012, 7, e30905. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, F.; Pezone, L.; Avitabile, M.; Acierno, G.; Andolfo, I.; Capasso, M.; Iolascon, A. Inhibition of Hypoxia Inducible Factors Combined with All-Trans Retinoic Acid Treatment Enhances Glial Transdifferentiation of Neuroblastoma Cells. Sci. Rep. 2015, 5, 11158. [Google Scholar] [CrossRef]
- Westerlund, I.; Shi, Y.; Toskas, K.; Fell, S.M.; Li, S.; Surova, O.; Södersten, E.; Kogner, P.; Nyman, U.; Schlisio, S.; et al. Combined Epigenetic and Differentiation-Based Treatment Inhibits Neuroblastoma Tumor Growth and Links HIF2α to Tumor Suppression. Proc. Natl. Acad. Sci. USA 2017, 114, E6137–E6146. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, P.; Wang, S.; Hong, L.; Yu, S.; Li, B.; Zeng, H.; Yang, X.; Shao, L. lncRNA FGD5 Antisense RNA 1 Upregulates RORA to Suppress Hypoxic Injury of Human Cardiomyocyte Cells by Inhibiting Oxidative Stress and Apoptosis via miR-195. Mol. Med. Rep. 2020, 22, 4579–4588. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, J.; Zhao, X.; Yang, K.; Lu, L.; Zhang, F.; Shen, W.; Zhang, R. All-Trans Retinoic Acid Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Cardiomyocyte Apoptosis. PLoS ONE 2015, 10, e0133414. [Google Scholar] [CrossRef]
- Shan, P.-R.; Xu, W.-W.; Huang, Z.-Q.; Pu, J.; Huang, W.-J. Protective Role of Retinoid X Receptor in H9c2 Cardiomyocytes from Hypoxia/Reoxygenation Injury in Rats. World J. Emerg. Med. 2014, 5, 122–127. [Google Scholar] [CrossRef]
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early Inhibition of Endothelial Retinoid Uptake upon Myocardial Infarction Restores Cardiac Function and Prevents Cell, Tissue, and Animal Death. J. Mol. Cell. Cardiol. 2019, 126, 105–117. [Google Scholar] [CrossRef]
- Guntner, A.S.; Doppler, C.; Wechselberger, C.; Bernhard, D.; Buchberger, W. HPLC-MS/MS Shows That the Cellular Uptake of All-Trans-Retinoic Acid under Hypoxia Is Downregulated by the Novel Active Agent 5-Methoxyleoligin. Cells 2020, 9, 2048. [Google Scholar] [CrossRef]
- Bilbija, D.; Haugen, F.; Sagave, J.; Baysa, A.; Bastani, N.; Levy, F.O.; Sirsjö, A.; Blomhoff, R.; Valen, G. Retinoic Acid Signalling Is Activated in the Postischemic Heart and May Influence Remodelling. PLoS ONE 2012, 7, e44740. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Diano, L.; Campagnolo, L.; Vecchione, L.; Cipollone, D.; Bueno, S.; Prosperini, G.; Desideri, A.; Siracusa, G.; Chillemi, G.; et al. Hif1α Down-Regulation Is Associated with Transposition of Great Arteries in Mice Treated with a Retinoic Acid Antagonist. BMC Genom. 2010, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jiao, X.; Fang, Y.; Yu, X.; Ding, X. The Orphan Nuclear Receptor RORα Is a Potential Endogenous Protector in Renal Ischemia/Reperfusion Injury. FASEB J. 2019, 33, 5704–5715. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, N.; Hitomi, H.; Mae, S.-I.; Kotaka, M.; Lei, L.; Yamamoto, T.; Nishiyama, A.; Osafune, K. Retinoic Acid Regulates Erythropoietin Production Cooperatively with Hypoxia-Inducible Factors in Human iPSC-Derived Erythropoietin-Producing Cells. Sci. Rep. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Neumcke, I.; Schneider, B.; Fandrey, J.; Pagel, H. Effects of Pro- and Antioxidative Compounds on Renal Production of Erythropoietin. Endocrinology 1999, 140, 641–645. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, A.-M.; Ji, L.-J.; Li, X.; Zhong, L.-L.; Li, H.-L.; Zheng, D.-H. All-Trans Retinoic Acid Attenuates Hypoxia-Induced Injury in NRK52E Cells via Inhibiting NF-x03BA;B/VEGF and TGF-Β2/VEGF Pathway. Cell. Physiol. Biochem. 2016, 38, 229–236. [Google Scholar] [CrossRef]
- Zhou, T.-B.; Ou, C.; Jiang, Z.-P.; Xiong, M.-R.; Zhang, F. Potential Signal Pathway between All-Trans Retinoic Acid and LMX1B in Hypoxia-Induced Renal Tubular Epithelial Cell Injury. J. Recept. Signal Transduct. Res. 2016, 36, 53–56. [Google Scholar] [CrossRef]
- Zhou, T.-B.; Ou, C.; Rong, L.; Drummen, G.P.C. Effect of All-Trans Retinoic Acid Treatment on Prohibitin and Renin-Angiotensin-Aldosterone System Expression in Hypoxia-Induced Renal Tubular Epithelial Cell Injury. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 243–249. [Google Scholar] [CrossRef]
- Wan, X.; Li, X.; Bo, H.; Zhao, Y.; Liu, L.; Chen, W.; Yin, Z.; Cao, C. All-Trans Retinoic Acid Protects Renal Tubular Epithelial Cells against Hypoxia Induced Injury in Vitro. Transplant. Proc. 2013, 45, 497–502. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.B.; Jiménez, M.I.A.; Manzano, V.M.; Lucio-Cazaña, F.J. Intracrine Prostaglandin E(2) Signalling Regulates Hypoxia-Inducible Factor-1α Expression through Retinoic Acid Receptor-β. Int. J. Biochem. Cell Biol. 2012, 44, 2185–2193. [Google Scholar] [CrossRef]
- Fernández-Martínez, A.B.; Arenas Jiménez, M.I.; Lucio Cazaña, F.J. Retinoic Acid Increases Hypoxia-Inducible Factor-1α through Intracrine Prostaglandin E(2) Signaling in Human Renal Proximal Tubular Cells HK-2. Biochim. Biophys. Acta 2012, 1821, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, A.B.; Jiménez, M.I.A.; Hernández, I.S.; García-Bermejo, M.L.; Manzano, V.M.; Fraile, E.A.; de Lucio-Cazaña, F.J. Mutual Regulation of Hypoxic and Retinoic Acid Related Signalling in Tubular Proximal Cells. Int. J. Biochem. Cell Biol. 2011, 43, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- van der Mijn, J.C.; Chen, Q.; Laursen, K.B.; Khani, F.; Wang, X.; Dorsaint, P.; Sboner, A.; Gross, S.S.; Nanus, D.M.; Gudas, L.J. Transcriptional and Metabolic Remodeling in Clear Cell Renal Cell Carcinoma Caused by ATF4 Activation and the Integrated Stress Response (ISR). Mol. Carcinog. 2022, 61, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.-S.; Gao, Y.-H.; Yao, X.-D. VHL Enhances 9-Cis-Retinoic Acid Treatment by down-Regulating Retinoid X Receptor α in Renal Cell Carcinomas. Biochem. Biophys. Res. Commun. 2020, 523, 535–541. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Chen, L.-C.; Kim, D.H.; Hsu, S.H.; Chung, H.J. Racial Differences in Tolerability of Topical Retinoids: A 15-Year Single-Center Retrospective Cohort Study. JAAD Int. 2024, 16, 122–124. [Google Scholar] [CrossRef]
- Söderlund, M.B.; Sjöberg, A.; Svärd, G.; Fex, G.; Nilsson-Ehle, P. Biological Variation of Retinoids in Man. Scand. J. Clin. Lab. Investig. 2002, 62, 511–519. [Google Scholar] [CrossRef]
- Clark, S. Retinoids. In xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–2. ISBN 978-0-08-055232-3. [Google Scholar]

| Model | Retinoid | Observed Effects | References |
|---|---|---|---|
| Human embryonic lung fibroblastic cell | atRA | Reduced the increment of IL-6 levels | [135] |
| LF-BLM in mice | atRA | Increased the overall survival rate and attenuated the increase in IL-6, TGFβ1, and collagen AI mRNA levels | [136] |
| LF-BLM in rats | atRA | Reduced the expression of EMT molecules (α-SMA and E-cadherin) | [137] |
| Fibroblasts derived from normal lung | atRA | Collagen production was inhibited by nuclear retinoic acid receptor activation | [138] |
| A549 cells | RA | Completely inhibited the phosphorylation of Smad2/3 | [139] |
| LF-BLM in rats | atRA | Attenuated in the expression of IL-17A, IL-10, IL-6, and TGFβ1 | [140,142] |
| LF-BLM in mice | atRA | Attenuated the upregulation of EphA2, EphriA1, PI3K 110γ, Akt, IL-6, and TNF-α | [141] |
| LF-BLM in rats | atRA | Ameliorated oxidative stress and inflammation, reduced TGF-1 levels, and reversed the effect on the expression of Ptch-1, Smo, and Gli-2 expression | [143] |
| LF-BLM in rats | carotene | Downregulation of the TGFβ/Smad signaling pathway via downregulation of TGFβ1, Smad2/3, and collagen I in lung tissue and by inhibiting the release of the downstream cytokines TNF-458 α, INF-γ, and IL-13 | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz-Gomez, D.; Castillejos-López, M.; Romero, Y.; Flores-Soto, E.; Romero-Martinez, B.S.; Vázquez-Pérez, J.A.; Gonzalez-Avila, G.; Ruiz, V.; Carlos-Reyes, Á.; Velázquez-Cruz, R.; et al. The Hypoxia–Retinoid Axis in Idiopathic Pulmonary Fibrosis: Multifaceted Etiology and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 5302. https://doi.org/10.3390/ijms26115302
Paz-Gomez D, Castillejos-López M, Romero Y, Flores-Soto E, Romero-Martinez BS, Vázquez-Pérez JA, Gonzalez-Avila G, Ruiz V, Carlos-Reyes Á, Velázquez-Cruz R, et al. The Hypoxia–Retinoid Axis in Idiopathic Pulmonary Fibrosis: Multifaceted Etiology and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(11):5302. https://doi.org/10.3390/ijms26115302
Chicago/Turabian StylePaz-Gomez, Daniel, Manuel Castillejos-López, Yair Romero, Edgar Flores-Soto, Bianca S. Romero-Martinez, Joel Armando Vázquez-Pérez, Georgina Gonzalez-Avila, Victor Ruiz, Ángeles Carlos-Reyes, Rafael Velázquez-Cruz, and et al. 2025. "The Hypoxia–Retinoid Axis in Idiopathic Pulmonary Fibrosis: Multifaceted Etiology and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 11: 5302. https://doi.org/10.3390/ijms26115302
APA StylePaz-Gomez, D., Castillejos-López, M., Romero, Y., Flores-Soto, E., Romero-Martinez, B. S., Vázquez-Pérez, J. A., Gonzalez-Avila, G., Ruiz, V., Carlos-Reyes, Á., Velázquez-Cruz, R., Choreño-Parra, J. A., Lara-Lemus, R., Rojas-Duran, F., Martínez Briseño, D., Zuñiga, J., Torres-Espíndola, L. M., & Aquino-Gálvez, A. (2025). The Hypoxia–Retinoid Axis in Idiopathic Pulmonary Fibrosis: Multifaceted Etiology and Therapeutic Potential. International Journal of Molecular Sciences, 26(11), 5302. https://doi.org/10.3390/ijms26115302







