Mechanism of Ginsenosides in the Treatment of Diabetes Mellitus Based on Network Pharmacology and Molecular Docking
Abstract
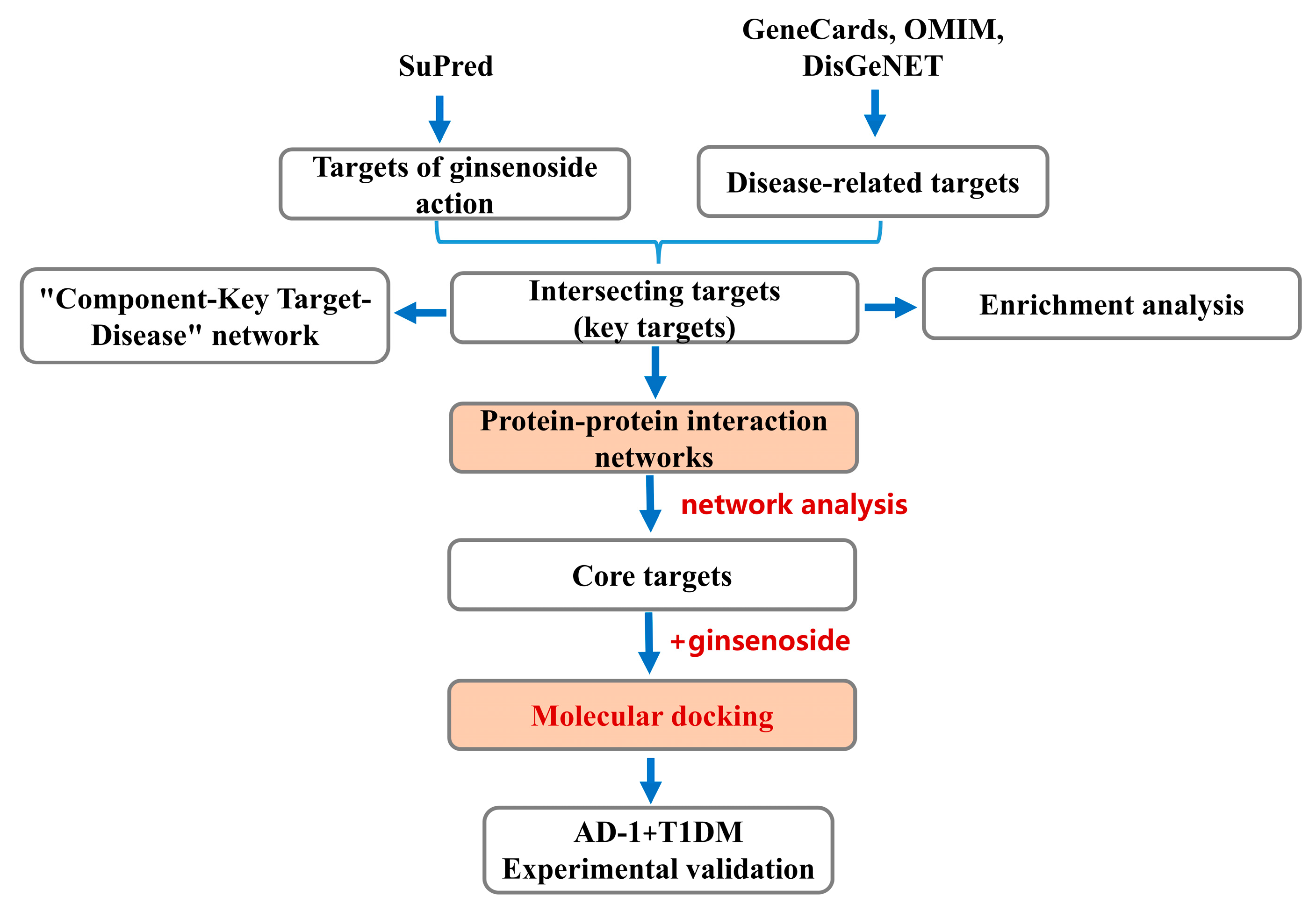
1. Introduction
2. Results
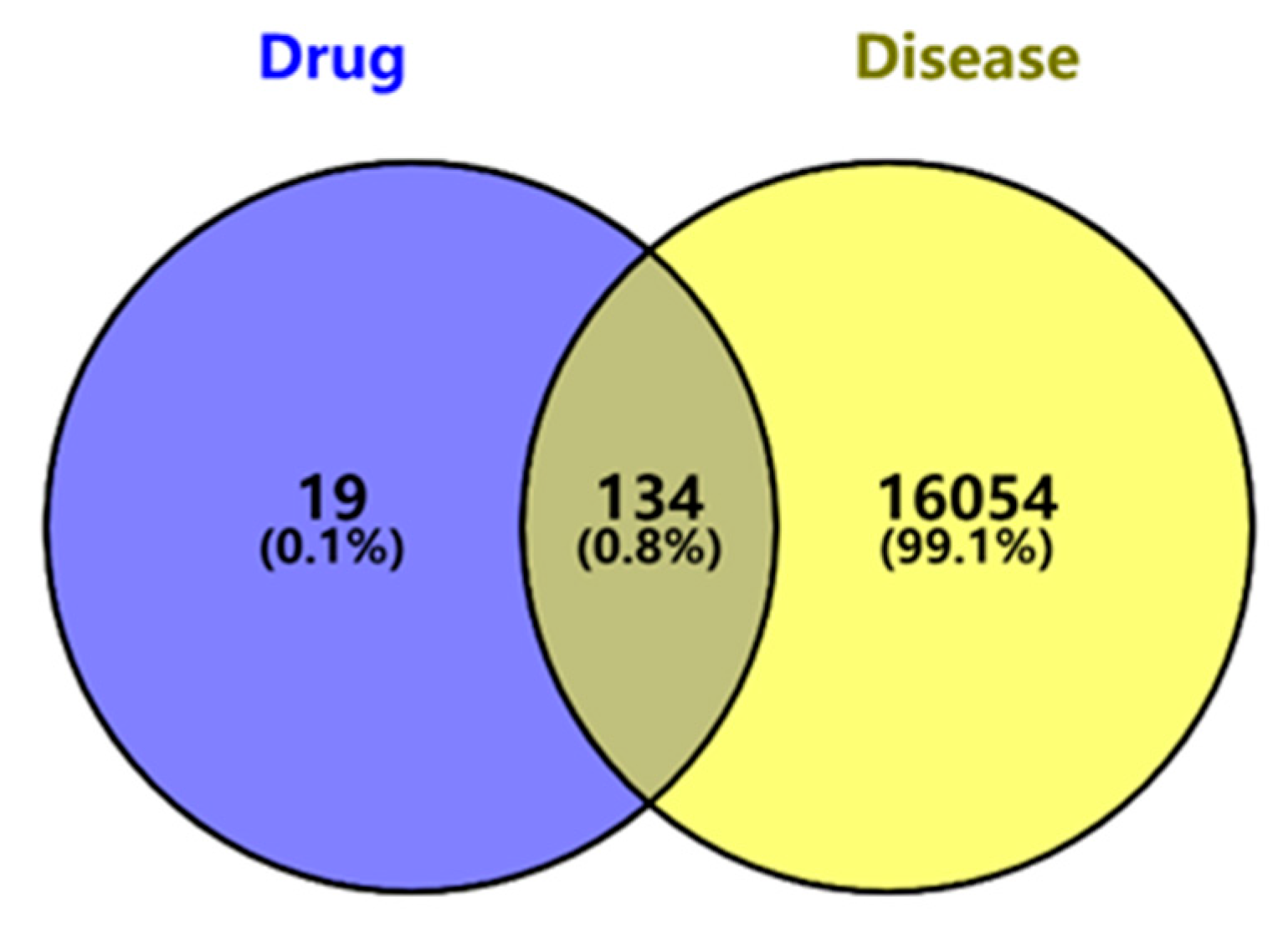
2.1. Screening of Potential Targets of Ginsenosides Against Diabetes
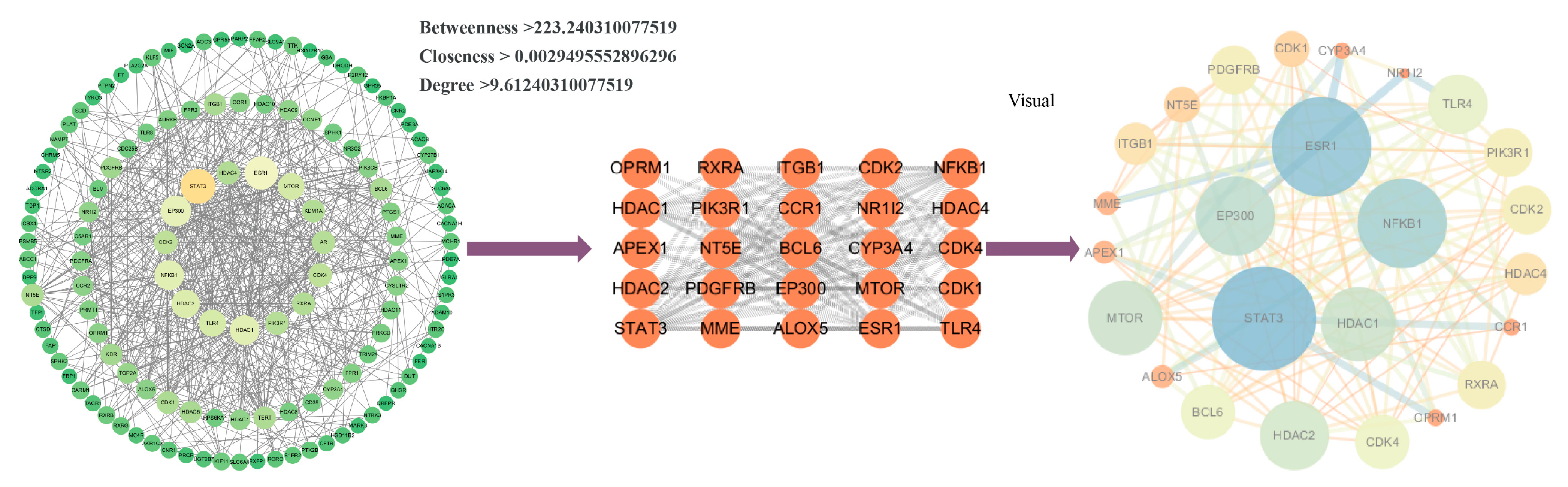
2.2. Protein–Protein Interaction (PPI) Network Analysis and Screening of Key Targets
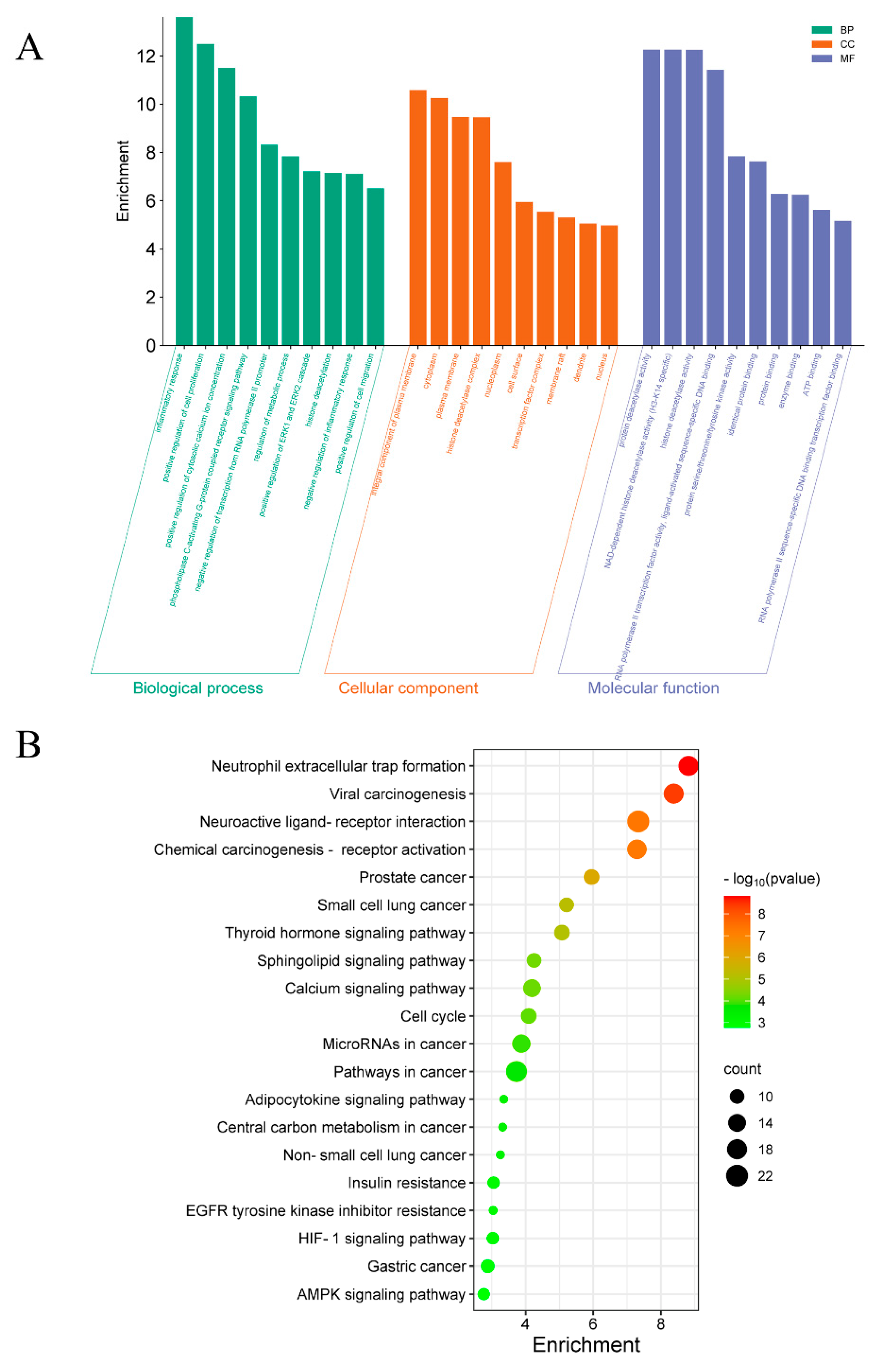
2.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway-Enrichment Analysis
2.4. Molecular Docking of Key Target Proteins
2.5. Molecular Dynamics (MD) Analysis of Complex Stability of Ginsenosides and Predicted Core Targets
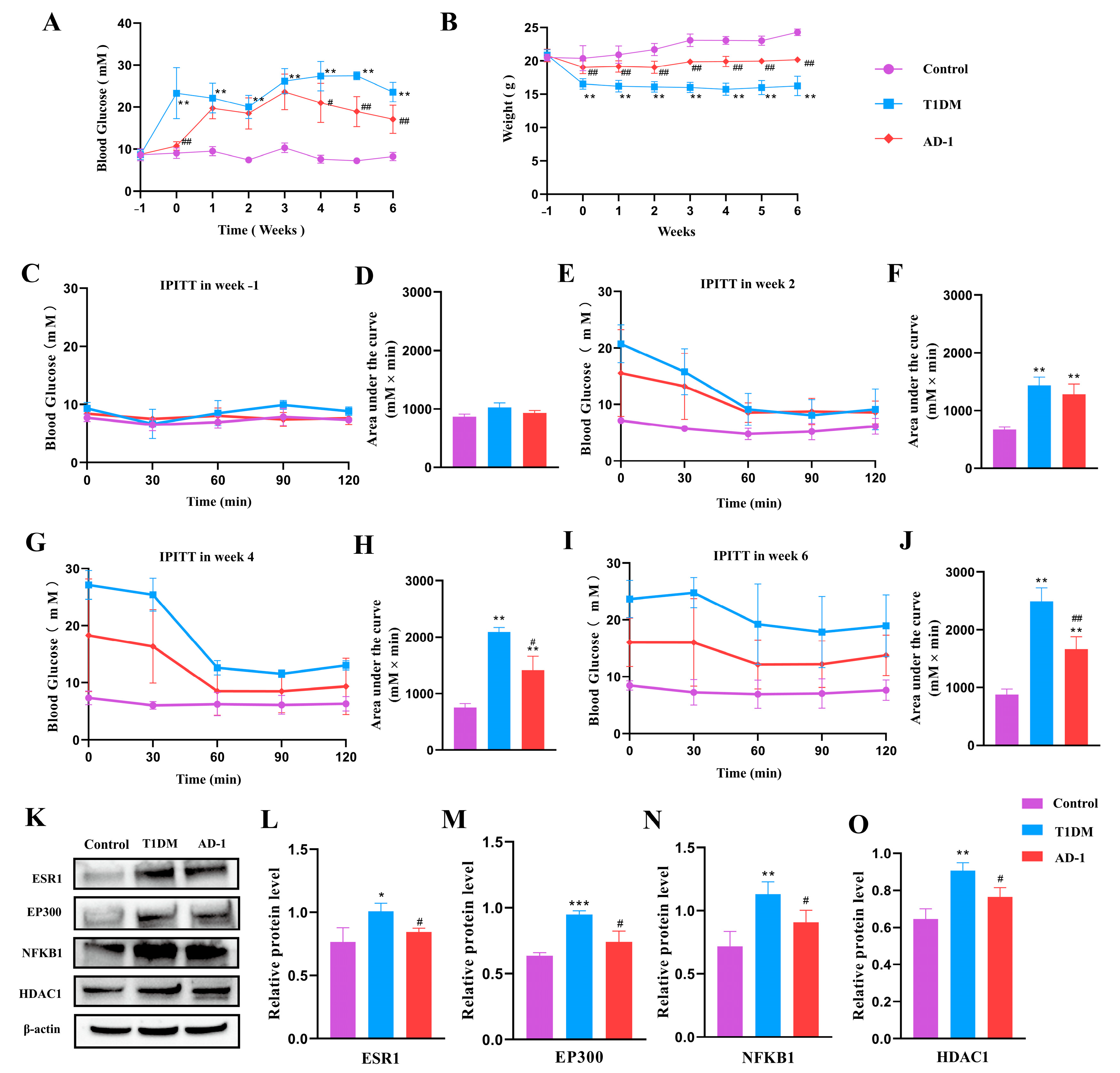
2.6. Effect of Ginsenoside Derivative AD-1 on STZ-Induced T1DM Mice and Verification of Prediction Target Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiment
4.3. Screening of Ginsenoside Compound Composition
4.4. Collection and Collation of Disease Targets
4.5. Acquisition of Key Targets
4.6. Construction of PPI Network and Screening of Core Targets
4.7. GO and KEGG
4.8. Verification of Molecular Docking
4.9. MD Simulation
4.10. Fasting Glucose and IPITT
4.11. Western Blot Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregg, E.W.; Buckley, J.; Ali, M.K.; Davies, J.; Flood, D.; Mehta, R.; Griffiths, B.; Lim, L.L.; Manne-Goehler, J.; Pearson-Stuttard, J.; et al. Improving health outcomes of people with diabetes: Target setting for the WHO Global Diabetes Compact. Lancet 2023, 401, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023) [Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien. Klin. Wochenschr. 2023, 135 (Suppl. S1), 7–17. (In German) [Google Scholar] [CrossRef] [PubMed]
- Gora, I.M.; Ciechanowska, A.; Ladyzynski, P. NLRP3 Inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells 2021, 10, 314. [Google Scholar] [CrossRef]
- Wu, R.; Mumtaz, M.; Maxwell, A.J.; Isaacs, S.R.; Laiho, J.E.; Rawlinson, W.D.; Hyöty, H.; Craig, M.E.; Kim, K.W. Respiratory infections and type 1 diabetes: Potential roles in pathogenesis. Rev. Med. Virol. 2023, 33, e2429. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Cai, Y.; Yang, Y.; Yuan, J.; Chen, Q. Inhibition of the pyroptosis-associated inflammasome pathway: The important potential mechanism of ginsenosides in ameliorating diabetes and its complications. Eur. J. Med. Chem. 2023, 253, 115336. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, L.; Liu, G.; Wang, H. Research progress of active ingredients of scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 2022, 148, 112690. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.S. Pharmacological effects of ginseng: Multiple constituents and multiple actions on humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, M.; Zhang, Z.; Song, Z.; Xu, J.; Zhang, M.; Gong, M. Overview of panax ginseng and its active ingredients protective mechanism on cardiovascular diseases. J. Ethnopharmacol. 2024, 334, 118506. [Google Scholar] [CrossRef]
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in saponin diversity of panax ginseng. Molecules 2020, 25, 3452. [Google Scholar] [CrossRef]
- You, D.; Hillerman, S.; Locke, G.; Chaudhry, C.; Stromko, C.; Murtaza, A.; Fan, Y.; Koenitzer, J.; Chen, Y.; Briceno, S.; et al. Enhanced antitumor immunity by a novel small molecule HPK1 inhibitor. J. Immunother. Cancer 2021, 9, e001402. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liu, Q.P.; An, P.; Jia, M.; Luan, X.; Tang, J.Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2022, 95, 153883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; He, D.; Zhang, H.; Xie, J.; Zhang, S.; Tian, X.; Zeng, H.; Qin, Y.; Huang, L. Ginsenoside Rb1 protects against diabetes-associated metabolic disorders in Kkay mice by reshaping gut microbiota and fecal metabolic profiles. J. Ethnopharmacol. 2023, 303, 115997. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Zhang, X.; Tong, H.; Sun, X.; Sun, G. Ginsenoside Rb1 protects against diabetic cardiomyopathy by regulating the adipocytokine pathway. J. Inflamm. Res. 2022, 15, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhang, X.; Zhou, Q.; Xu, Y.; Sun, L.; Wen, Q.; Wang, W.; Chen, Q. Antioxidant and anti-inflammatory properties of ginsenoside Rg1 for hyperglycemia in type 2 diabetes mellitus: Systematic reviews and meta-analyses of animal studies. Front. Pharmacol. 2023, 14, 1179705. [Google Scholar] [CrossRef]
- Dong, X.; Kong, L.; Huang, L.; Su, Y.; Li, X.; Yang, L.; Ji, P.; Li, W.; Li, W. Ginsenoside Rg1 treatment protects against cognitive dysfunction via inhibiting PLC-CN-NFAT1 signaling in T2DM mice. J. Ginseng Res. 2023, 47, 458–468. [Google Scholar] [CrossRef]
- Moon, J.H.; Oh, C.M.; Kim, H. Serotonin in the regulation of systemic energy metabolism. J. Diabetes Investig. 2022, 13, 1639–1645. [Google Scholar] [CrossRef]
- Rial-Pensado, E.; Canaple, L.; Guyot, R.; Clemmensen, C.; Wiersema, J.; Wu, S.; Richard, S.; Boelen, A.; Müller, T.D.; López, M.; et al. Neuronal blockade of thyroid hormone signaling increases sensitivity to diet-Induced obesity in adult male mice. Endocrinology 2023, 164, bqad034. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S.; et al. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodríguez, R.A.; Trejo-Solís, C.; Cabrera-Cano, A.; Gómez-Manzo, S.; Dávila-Borja, V.M. Hypoxia as a modulator of inflammation and immune response in cancer. Cancers 2022, 14, 2291. [Google Scholar] [CrossRef]
- Kim, K.E.; Lee, J.; Shin, H.J.; Jeong, E.A.; Jang, H.M.; Ahn, Y.J.; An, H.S.; Lee, J.Y.; Shin, M.C.; Kim, S.K.; et al. Lipocalin-2 activates hepatic stellate cells and promotes nonalcoholic steatohepatitis in high-fat diet-fed Ob/Ob mice. Hepatology 2023, 77, 888–901. [Google Scholar] [CrossRef]
- Pan, P.; Wen, Z.; Ma, F.; Lei, Z.; Pan, C.; Fei, Q.; Tian, E.; Wang, Y.; Zhu, Q.; Li, H.; et al. Bisphenol S stimulates Leydig cell proliferation but inhibits differentiation in pubertal male rats through multiple mechanisms. Environ. Toxicol. 2023, 38, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fan, X.; Zuo, L.; Guo, Q.; Su, X.; Xi, G.; Zhang, Z.; Zhang, J.; Zheng, G. Estrogen receptor 1 gene polymorphisms are associated with metabolic syndrome in postmenopausal women in China. BMC Endocr. Disord. 2018, 18, 65. [Google Scholar] [CrossRef]
- Ereqat, S.; Cauchi, S.; Eweidat, K.; Elqadi, M.; Nasereddin, A. Estrogen receptor 1 gene polymorphisms (PvuII and XbaI) are associated with type 2 diabetes in palestinian women. PeerJ 2019, 7, e7164. [Google Scholar] [CrossRef]
- Gregorio, K.C.R.; Laurindo, C.P.; Machado, U.F. Estrogen and glycemic homeostasis: The fundamental role of nuclear estrogen receptors ESR1/ESR2 in glucose transporter GLUT4 regulation. Cells 2021, 10, 99. [Google Scholar] [CrossRef]
- Wild, K.T.; Nomakuchi, T.T.; Sheppard, S.E.; Leavens, K.F.; De León, D.D.; Zackai, E.H. Hyperinsulinism in an individual with an EP300 variant of rubinstein-taybi syndrome. Am. J. Med. Genet. A 2021, 185, 1251–1255. [Google Scholar] [CrossRef]
- Zhang, E.; Mohammed Al-Amily, I.; Mohammed, S.; Luan, C.; Asplund, O.; Ahmed, M.; Ye, Y.; Ben-Hail, D.; Soni, A.; Vishnu, N.; et al. Preserving insulin secretion in diabetes by inhibiting VDAC1 overexpression and surface translocation in β Cells. Cell Metab. 2019, 29, 64–77. e6. [Google Scholar] [CrossRef]
- Di Pietrantonio, N.; Di Tomo, P.; Mandatori, D.; Formoso, G.; Pandolfi, A. Diabetes and its cardiovascular complications: Potential role of the acetyltransferase p300. Cells 2023, 12, 431. [Google Scholar] [CrossRef]
- Errafii, K.; Boujraf, S.; Chikri, M. Transcriptomic analysis from normal glucose tolerance to T2D of obese individuals using bioinformatic tools. Int. J. Mol. Sci. 2023, 24, 6337. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.; Boshra, M.S.; Agwa, S.H.A.; Hakeem, M.S.A.; Meteini, M.S.E.; Matboli, M. Identification of a multi-messenger RNA signature as type 2 diabetes mellitus candidate genes involved in crosstalk between inflammation and insulin resistance. Biomolecules 2022, 12, 1230. [Google Scholar] [CrossRef]
- Wróblewski, A.; Strycharz, J.; Oszajca, K.; Czarny, P.; Świderska, E.; Matyjas, T.; Zieleniak, A.; Rucińska, M.; Pomorski, L.; Drzewoski, J.; et al. Dysregulation of inflammation, oxidative stress, and glucose metabolism-related genes and miRNAs in visceral adipose tissue of women with type 2 diabetes mellitus. Med. Sci. Monit. 2023, 29, e939299. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Zohouri, A. Carvedilol prevents pancreatic β-cell damage and the development of type 1 diabetes in mice by the inhibition of proinflammatory cytokines, NF-κB, COX-2, iNOS and oxidative stress. Cytokine 2021, 138, 155394. [Google Scholar] [CrossRef]
- Domingo-Relloso, A.; Gribble, M.O.; Riffo-Campos, A.L.; Haack, K.; Cole, S.A.; Tellez-Plaza, M.; Umans, J.G.; Fretts, A.M.; Zhang, Y.; Fallin, M.D.; et al. Epigenetics of type 2 diabetes and diabetes-related outcomes in the strong heart study. Clin. Epigenet. 2022, 14, 177. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Fu, H.; Kang, F.; Song, J.; Xu, M.; Ning, G.; Wang, J.; Wang, W.; Wang, Q. Mettl3-mediated m6A methylation controls pancreatic bipotent progenitor fate and islet formation. Diabetes 2024, 73, 237–249. [Google Scholar] [CrossRef]
- Darden, C.M.; Vasu, S.; Mattke, J.; Liu, Y.; Rhodes, C.J.; Naziruddin, B.; Lawrence, M.C. Calcineurin/NFATc2 and PI3K/AKT signaling maintains β-cell identity and function during metabolic and inflammatory stress. iScience 2022, 25, 104125. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Ielciu, I.; Mitre, A.O.; Filip, G.A.; Oniga, I.; Vlase, L.; Benedec, D.; Gheldiu, A.M.; Toma, V.A.; Mihart, B.; et al. Targeting oxidative stress reduction and inhibition of HDAC1, MECP2, and NF-kB pathways in rats with experimentally induced hyperglycemia by administration of Thymus marshallianus willd. Extracts. Front. Pharmacol. 2020, 11, 581470. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, Y.; Liu, X.; Zhou, L.; Zhang, C.; Shao, L. Histone deacetylase inhibition by MS-275 potentiates glucose-stimulated insulin secretion without affecting glucose oxidation. Life Sci. 2020, 257, 118073. [Google Scholar] [CrossRef]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. 1997, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Bauer-Mehren, A.; Rautschka, M.; Sanz, F.; Furlong, L.I. DisGeNET: A Cytoscape plugin to visualize, integrate, search and analyze gene-disease networks. Bioinformatics 2010, 26, 2924–2926. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Fang, X.; Lee, Y.H.; Jang, J.H.; Kim, S.J.; Kim, S.H.; Kim, D.H.; Na, H.K.; Kim, K.O.; Baek, J.H.; Surh, Y.J. ARD1 stabilizes NRF2 through direct interaction and promotes colon cancer progression. Life Sci. 2023, 313, 121217. [Google Scholar] [CrossRef]

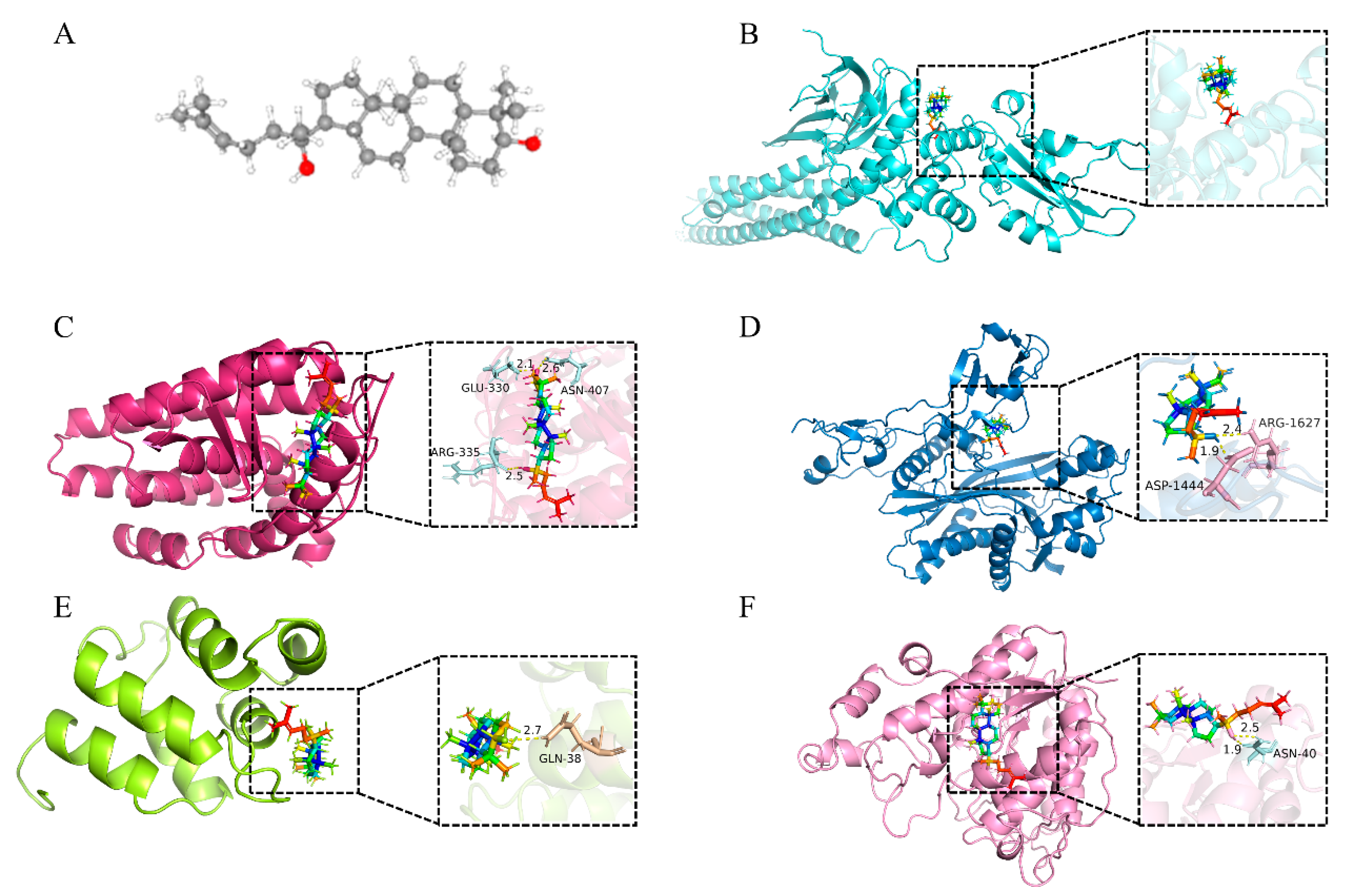

| No. | Sybol | Uniprot ID | Protein Names | Degree | PDB | Docking Score (kcal/mol) |
|---|---|---|---|---|---|---|
| 1 | STAT3 | P40763 | Signal transducer and activator of transcription 3 | 49 | 6NJS | −9.00 |
| 2 | ESR1 | P03372 | Estrogen receptor | 45 | 6V8T | −10.25 |
| 3 | EP300 | Q09472 | Histone acetyltransferase p300 | 39 | 8GZC | −9.19 |
| 4 | NFKB1 | P19838 | Nuclear factor NF-kappa-B p105 subunit | 36 | 2DBF | −8.88 |
| 5 | HDAC1 | Q13547 | Histone deacetylase 1 | 36 | 6Z2J | −9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Li, F.; Xue, D.; Shi, X.; Fang, X.; Li, J.; Fu, Y.; Zhao, Y.; Jin, D. Mechanism of Ginsenosides in the Treatment of Diabetes Mellitus Based on Network Pharmacology and Molecular Docking. Int. J. Mol. Sci. 2025, 26, 5300. https://doi.org/10.3390/ijms26115300
Huang S, Li F, Xue D, Shi X, Fang X, Li J, Fu Y, Zhao Y, Jin D. Mechanism of Ginsenosides in the Treatment of Diabetes Mellitus Based on Network Pharmacology and Molecular Docking. International Journal of Molecular Sciences. 2025; 26(11):5300. https://doi.org/10.3390/ijms26115300
Chicago/Turabian StyleHuang, Shengnan, Fangfang Li, Dedi Xue, Xinyuan Shi, Xizhu Fang, Jiawei Li, Yuan Fu, Yuqing Zhao, and Dan Jin. 2025. "Mechanism of Ginsenosides in the Treatment of Diabetes Mellitus Based on Network Pharmacology and Molecular Docking" International Journal of Molecular Sciences 26, no. 11: 5300. https://doi.org/10.3390/ijms26115300
APA StyleHuang, S., Li, F., Xue, D., Shi, X., Fang, X., Li, J., Fu, Y., Zhao, Y., & Jin, D. (2025). Mechanism of Ginsenosides in the Treatment of Diabetes Mellitus Based on Network Pharmacology and Molecular Docking. International Journal of Molecular Sciences, 26(11), 5300. https://doi.org/10.3390/ijms26115300





