Leveraging the Polymorphism of the Merozoite Surface Protein 2 (MSP2) to Engineer Molecular Tools for Predicting Malaria Episodes in a Community
Abstract
1. Introduction
2. Results
2.1. Study Characteristics
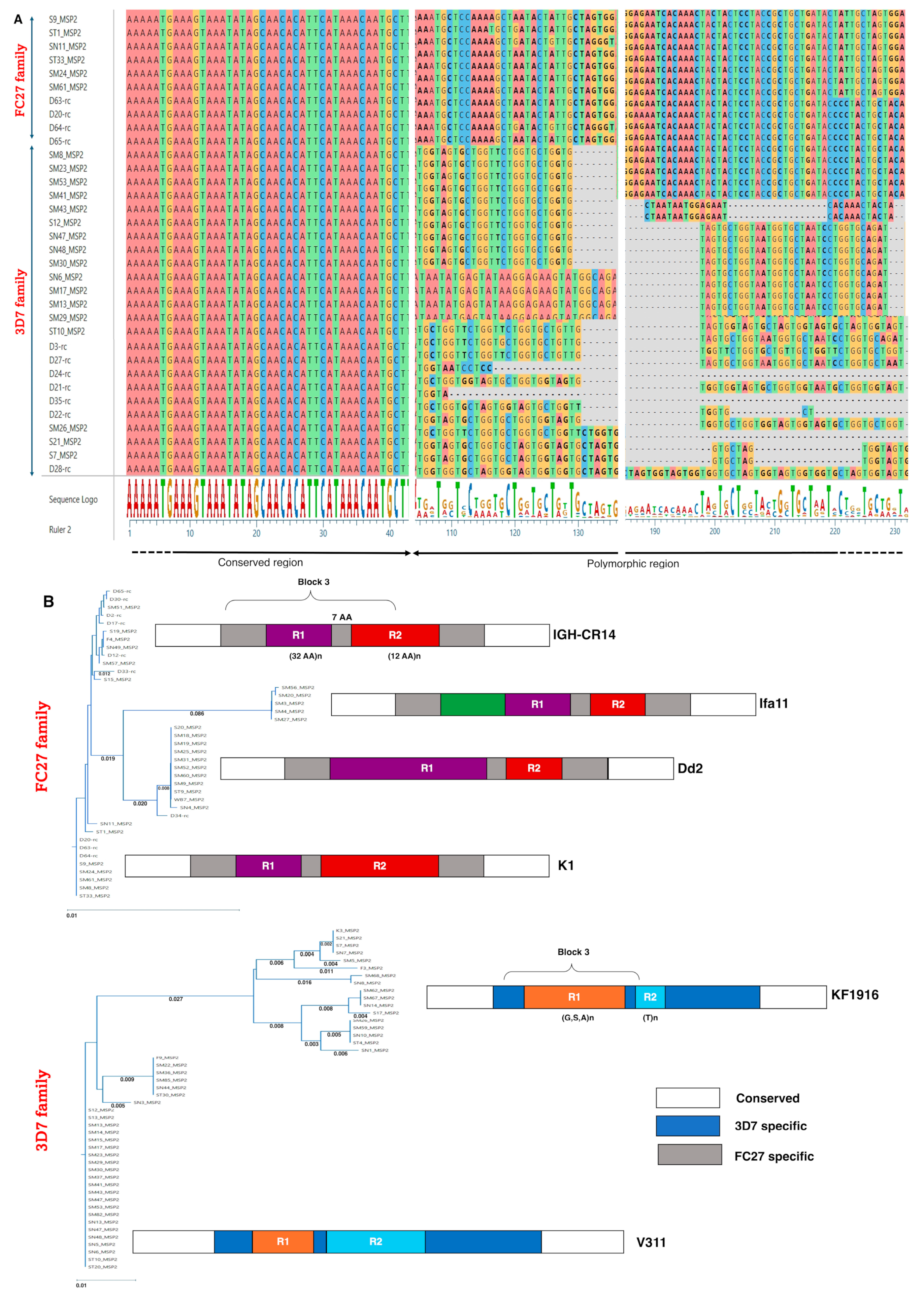
2.2. The Polymorphism of the Pfmsp2 Gene from KFHR Clinical Isolates Revealed 3D7 Strains Predominant over FC27
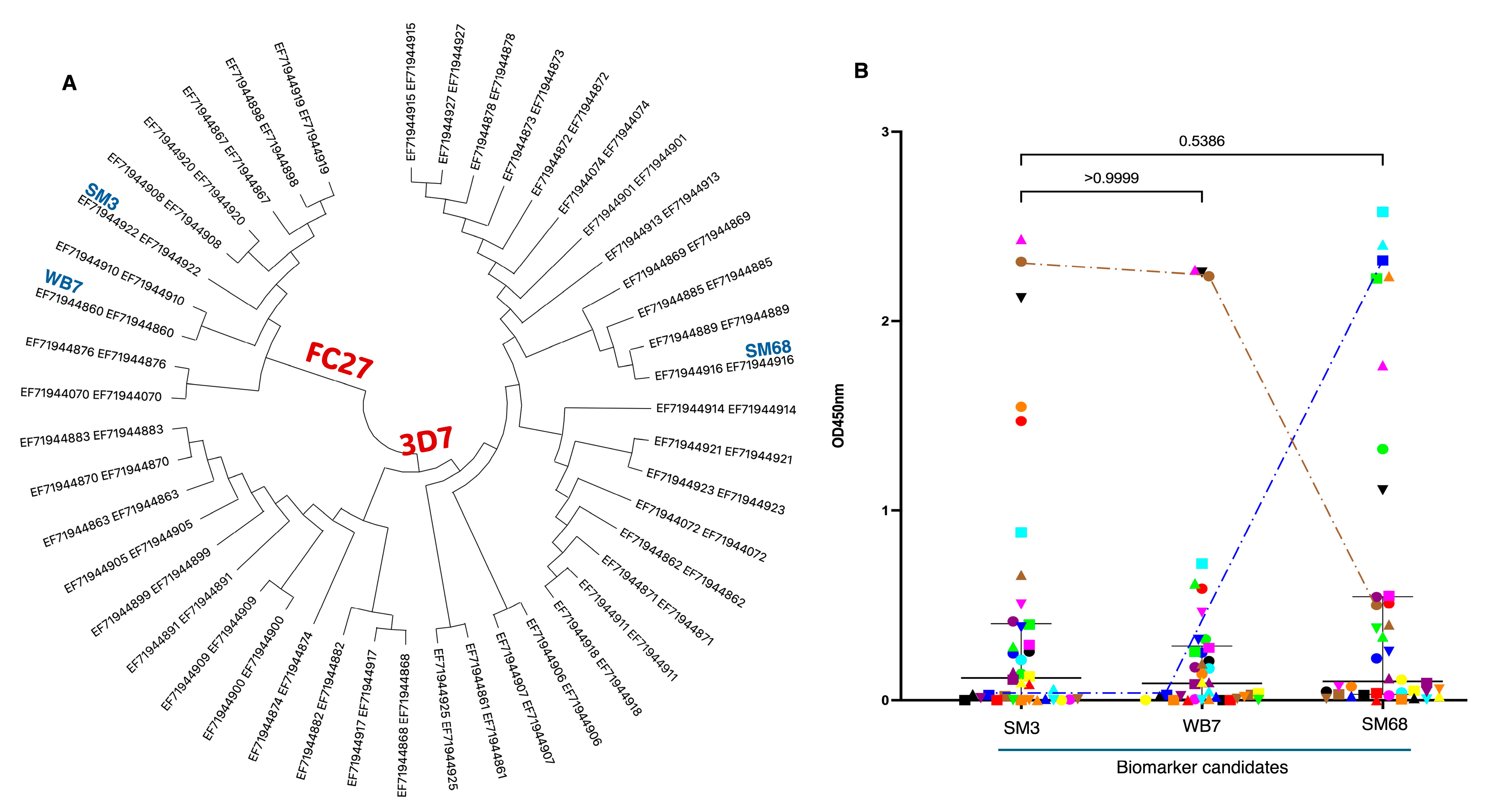
2.3. Cloning, Expression, and Characterization of MSP2 Selected Antigens: SM3, WB7, and SM68
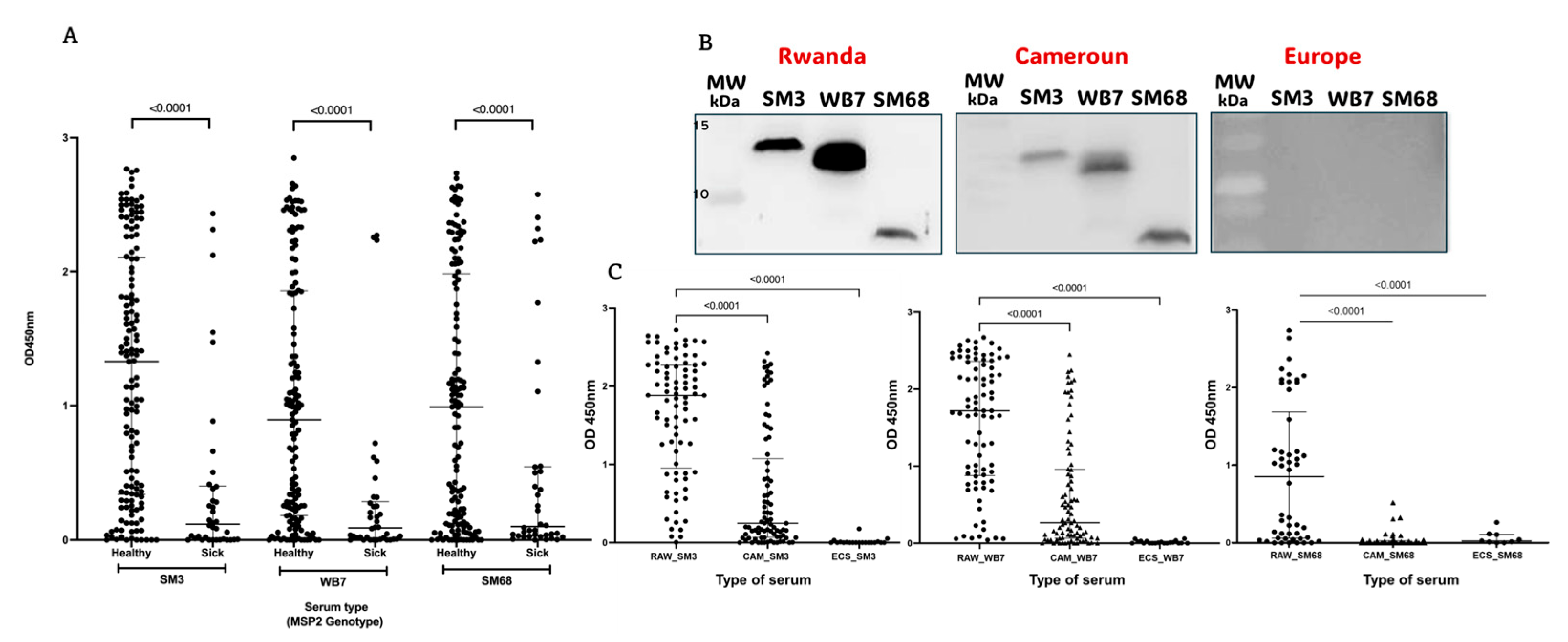
2.4. Serum Samples from Malaria Patients Respond Differentially to the Candidate Biomarkers SM3, WB7, and SM68
2.5. The Candidate Biomarkers SM3, WB7, and SM68 Effectively Discriminate Sera from Inhabitants of Geographically Distinct Regions
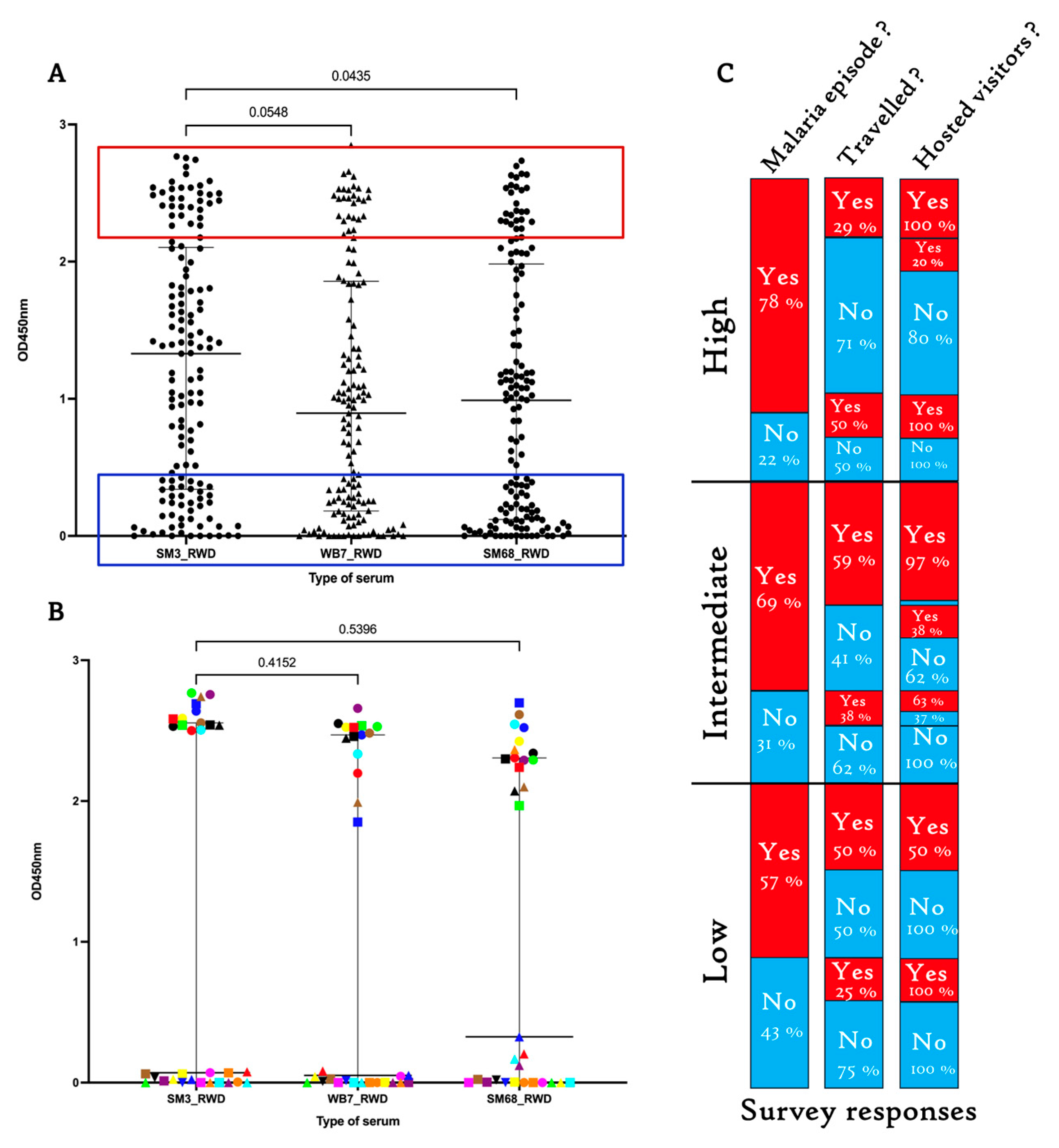
2.6. Sera from Healthy Rwandans Living in Bugesera Exhibited Similar Response Patterns to the SM3, WB7, and SM68 Candidate Biomarkers
2.7. The High Humoral Responses to the Three Candidate Biomarkers Are Predominantly Enriched in the IgG3 Antibody Subclass
3. Discussion
3.1. Allelic Variation and Malaria Episode Prediction
3.2. Distribution of Plasmodium falciparum MSP2 Allelic Families and Biomarker Engineering
3.3. Differential Serum Responses to Candidate Biomarkers
3.4. MSP2-Mediated Genetic Diversity and Infection Risk
3.5. IgG Subclass Responses and Malaria Immunity
4. Materials and Methods
4.1. Ethical Consideration
4.2. Study Site and Population
4.3. Parasite DNA Extraction
4.4. Plasmodium falciparum Genotyping
4.5. DNA Sequencing
4.6. Cloning of msp2 Polymorphic Gene Regions, Expression, and Purification of Antigens
4.7. Western Blotting
4.8. Serological Assessment of the Expressed Antigens
4.9. Qualitative Analysis of P. falciparum Infection
4.10. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSP | Merozoite surface protein |
| AMA | Apical merozoite antigen |
| IgG | Immunoglobulin G |
| ELISA | Enzyme Linked ImmunoSorbent Assay |
| OD | Optical Density |
| PCR | Polymerase Chain Reaction |
| BSA | Bovine Serum Albumin |
| MOI | multiplicity of infection |
| CEO | Chief Executive Officer |
| ACT | Artemisinin-based Combination Therapy |
| SPC | Société des Provenderies du Cameroun |
| ARES | Académie de Recherche et de l’Enseignement Supérieur |
| KFHR | King Faisal Hospital Rwanda |
References
- World Health Organization. World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 15 December 2024).
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.E.; Schultz, L.; Buckee, C.O.; Recker, M. Daily changes in Plasmodium falciparum populations during malaria infection in humans. PLoS Pathog. 2013, 9, e1003392. [Google Scholar] [CrossRef]
- Smith, J.D.; Rowe, J.A.; Higgins, M.K.; Lavstsen, T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 2013, 15, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Abukari, Z.; Okonu, R.; Nyarko, S.B.; Lo, A.C.; Dieng, C.C.; Salifu, S.P.; Gyan, B.A.; Lo, E.; Amoah, L.E. The Diversity, Multiplicity of Infection and Population Structure of P. falciparum Parasites Circulating in Asymptomatic Carriers Living in High and Low Malaria Transmission Settings of Ghana. Genes 2019, 10, 434. [Google Scholar] [CrossRef]
- Andrade, B.B.; Barral-Netto, M. Biomarkers for susceptibility to infection and disease severity in human malaria. Mem. Inst. Oswaldo Cruz 2011, 106, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Djoufounna, J.; Mayi, M.P.A.; Bamou, R.; Foyet, J.V.; Tabue, R.; Lontsi-Demano, M.; Achu-Fosah, D.; Antonio-Nkondjio, C.; Tchuinkam, T. High prevalence of asymptomatic Plasmodium falciparum malaria in Makenene, a locality in the forest-savannah transition zone, Centre Region of Cameroon. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100104. [Google Scholar] [CrossRef]
- Ndong, I.C.; Okyere, D.; Enos, J.Y.; Mensah, B.A.; Nyarko, A.; Abuaku, B.; Amambua-Ngwa, A.; Merle, C.S.C.; Koram, K.A.; Ahorlu, C.S. Prevalence of asymptomatic malaria parasitaemia following mass testing and treatment in Pakro sub-district of Ghana. BMC Public Health 2019, 19, 1622. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Raghavendra, K.; Bhatt, R.M.; Swain, D.K.; Valecha, N.; Kleinschmidt, I. Burden of asymptomatic malaria among a tribal population in a forested village of central India: A hidden challenge for malaria control in India. Public Health 2017, 147, 92–97. [Google Scholar] [CrossRef]
- Sumbele, I.U.N.; Teh, R.N.; Nkeudem, G.A.; Sandie, S.M.; Moyeh, M.N.; Shey, R.A.; Shintouo, C.M.; Ghogomu, S.M.; Batiha, G.E.S.; Alkazmi, L.; et al. Asymptomatic and sub-microscopic Plasmodium falciparum infection in children in the Mount Cameroon area: A cross-sectional study on altitudinal influence, haematological parameters and risk factors. Malar. J. 2021, 20, 382. [Google Scholar] [CrossRef]
- Nguitragool, W.; Mueller, I.; Kumpitak, C.; Saeseu, T.; Bantuchai, S.; Yorsaeng, R.; Yimsamran, S.; Maneeboonyang, W.; Sa-Angchai, P.; Chaimungkun, W.; et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasites Vectors 2017, 10, 512. [Google Scholar] [CrossRef]
- Somé, A.F.; Bazié, T.; Zongo, I.; Yerbanga, R.S.; Nikiéma, F.; Neya, C.; Taho, L.K.; Ouédraogo, J.B. Plasmodium falciparum msp1 and msp2 genetic diversity and allele frequencies in parasites isolated from symptomatic malaria patients in Bobo-Dioulasso, Burkina Faso. Parasites Vectors 2018, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Apinjoh, T.O.; Tata, R.B.; Anchang-Kimbi, J.K.; Chi, H.F.; Fon, E.M.; Mugri, R.N.; Tangoh, D.A.; Nyingchu, R.V.; Ghogomu, S.M.; Nkuo-Akenji, T.; et al. Plasmodium falciparum merozoite surface protein 1 block 2 gene polymorphism in field isolates along the slope of mount Cameroon: A cross—Sectional study. BMC Infect. Dis. 2015, 15, 309. [Google Scholar] [CrossRef] [PubMed]
- Balam, S.; Olugbile, S.; Servis, C.; Diakité, M.; D’Alessandro, A.; Frank, G.; Moret, R.; Nebie, I.; Tanner, M.; Felger, I.; et al. Plasmodium falciparum merozoite surface protein 2: Epitope mapping and fine specificity of human antibody response against non-polymorphic domains. Malar. J. 2014, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, R.A.; de Koning-Ward, T.F.; Burt, R.A.; Bockarie, M.; Reeder, J.C.; Cowman, A.F.; Crabb, B.S. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 2001, 193, 1403–1412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Genton, B.; Betuela, I.; Felger, I.; Al-Yaman, F.; Anders, R.F.; Saul, A.; Rare, L.; Baisor, M.; Lorry, K.; Brown, G.V.; et al. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 2002, 185, 820–827. [Google Scholar] [CrossRef]
- Felger, I.; Tavul, L.; Kabintik, S.; Marshall, V.; Genton, B.; Alpers, M.; Beck, H.P. Plasmodium falciparum: Extensive Polymorphism in Merozoite Surface Antigen 2 Alleles in an Area with Endemic Malaria in Papua New Guinea. Exp. Parasitol. 1994, 79, 106–116. [Google Scholar] [CrossRef]
- Mohammed, H.; Kassa, M.; Mekete, K.; Assefa, A.; Taye, G.; Commons, R.J. Genetic diversity of the msp-1, msp-2, and glurp genes of Plasmodium falciparum isolates in Northwest Ethiopia. Malar. J. 2018, 17, 386. [Google Scholar] [CrossRef]
- Shintouo, C.M.; Shey, R.A.; Nebangwa, D.N.; Esoh, K.K.; Nongley, N.F.; Nguve, J.E.; Giron, P.; Mutesa, L.; Vanhamme, L.; Souopgui, J.; et al. In Silico Design and Validation of OvMANE1, a Chimeric Antigen for Human Onchocerciasis Diagnosis. Pathogens 2020, 9, 495. [Google Scholar] [CrossRef]
- Ferreira, M.U.; Da Silva Nunes, M.; Wunderlich, G. Antigenic diversity and immune evasion by malaria parasites. Clin. Vaccine Immunol. 2004, 11, 987–995. [Google Scholar] [CrossRef]
- Simpson, S.V.; Nundu, S.S.; Arima, H.; Kaneko, O.; Mita, T.; Culleton, R.; Yamamoto, T. The diversity of Plasmodium falciparum isolates from asymptomatic and symptomatic school-age children in Kinshasa Province, Democratic Republic of Congo. Malar. J. 2023, 22, 102. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.; Israr, M.; Shah, M.; Shams, S.; Khan, W.; Shah, M.; Siraj, M.; Akbar, K.; Naz, T.; et al. Genomic miscellany and allelic frequencies of Plasmodium falciparum msp-1, msp-2 and glurp in parasite isolates. PLoS ONE 2022, 17, e0264654. [Google Scholar] [CrossRef] [PubMed]
- Mehlin, C.; Boni, E.; Buckner, F.S.; Engel, L.; Feist, T.; Gelb, M.H.; Haji, L.; Kim, D.; Liu, C.; Mueller, N.; et al. Heterologous expression of proteins from Plasmodium falciparum: Results from 1000 genes. Mol. Biochem. Parasitol. 2006, 148, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Letcher, B.; Maciuca, S.; Iqbal, Z. Role for gene conversion in the evolution of cell-surface antigens of the malaria parasite Plasmodium falciparum. PLoS Biol. 2024, 22, e3002507. [Google Scholar] [CrossRef] [PubMed]
- Mbugi, E.V.; Hartog, G.d.; Veenemans, J.; Chilongola, J.O.; Verhoef, H.; Savelkoul, H.F.J. Nutrient Deficiencies and Potential Alteration in Plasma Levels of Naturally Acquired Malaria-Specific Antibody Responses in Tanzanian Children. Front. Nutr. 2022, 9, 872710. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Vidal, M.; Jiménez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Díez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef]
- Froger, A.; Hall, J.E. Transformation of plasmid DNA into E. coli using the heat shock method. J. Vis. Exp. 2007, 6, 253. [Google Scholar] [CrossRef]
- Arezumand, R.; Mahdian , R.; Behdani, M.; Khanahmad, H.; Langari, J.; Namvarasl, N.; Hassanzadeh-Ghasabeh, R.; Zeinali, S. Recombinant expression and purification of human placental growth factor 1 and specific camel heavy chain polyclonal antibody preparation. Saudi J. Biol. Sci. 2014, 21, 35–39. [Google Scholar] [CrossRef]


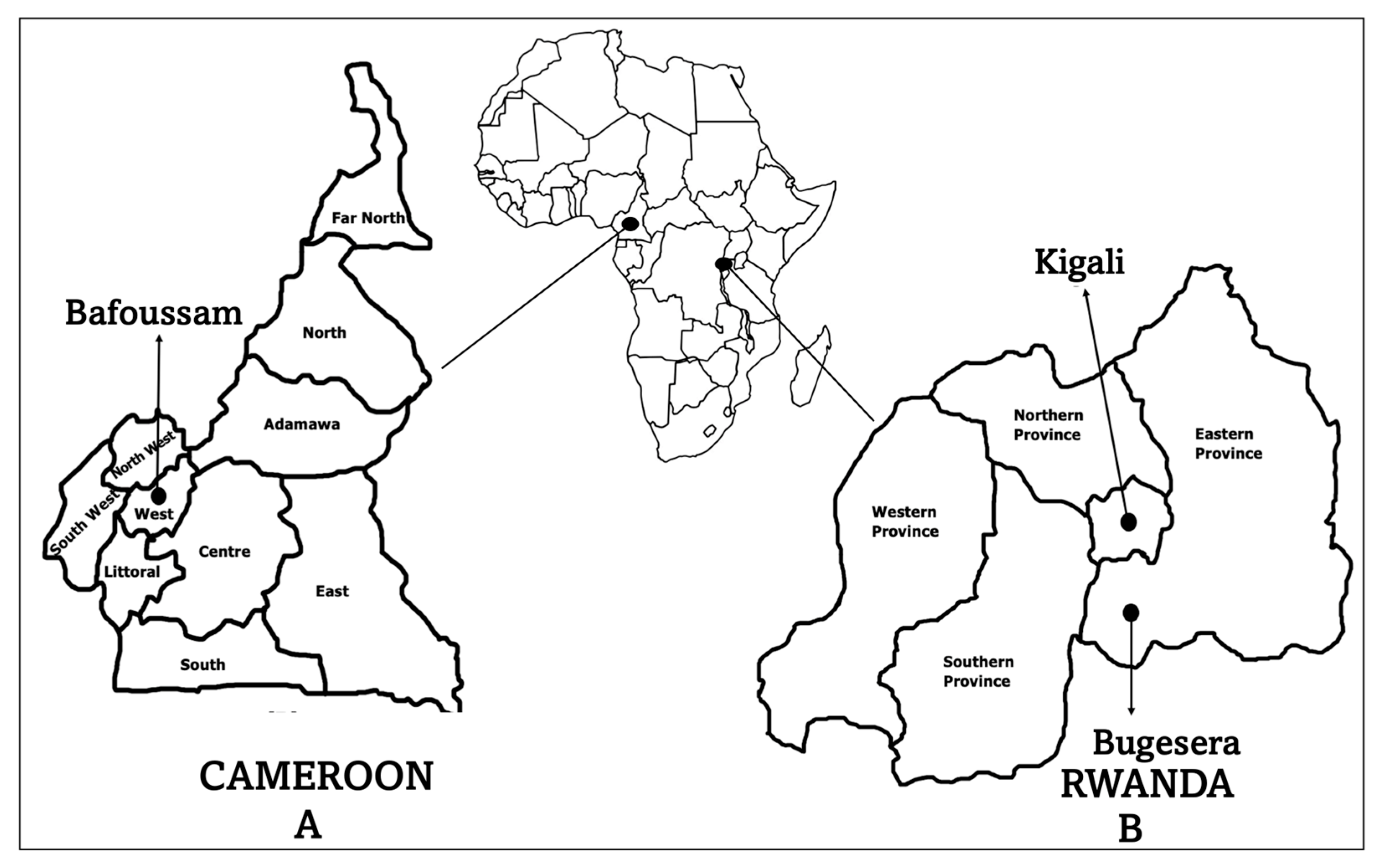
| Characteristics | Kigali, RWD (n = 75) | Bugesera, RWD (n = 145) | Bafoussam, CMR (n = 120) |
|---|---|---|---|
| Clinical status | Symptomatic | Healthy | Healthy |
| Gender, n (%) | |||
| Female | 35 (46.7) | 61 (42.1) | 30 (25.0) |
| Male | 40 (53.3) | 84 (57.9) | 90 (75.0) |
| Age in years, n (%) | |||
| 1–17 (children) | 18 (24.0) | 2 (1.4) | 0 (0.0) |
| 18–49 (adults) | 48 (64.0) | 127 (87.6) | 86 (71.7) |
| ≥50 (elderly) | 9 (12.0) | 16 (11.0) | 34 (28.3) |
| Oligonucleotide | Sequence (5′-3′) |
|---|---|
| MSP2-Forward | ATGAAGGTAATTAAAACATTGTCTATTATA |
| MSP2-Reverse | ATATGGCAAAAGATAAAACAAGTGTTGCTG |
| 3D7-Forward | AGTTGAAACATATGAGTATAAGGAGAAGTATG |
| 3D7-Reverse | AATATCTCGAGGGTACTGGTAGATGCTTCTGCATCAT |
| FC27-Forward | AGTTGAAACATATGAGTATAAGGAGAAGTATGGC |
| FC27-Reverse | AATATCTCGAGTGCATTGCCAGAACTTGAACTTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalimba, E.M.; Noukimi, S.F.; Mbonimpa, J.-B.; Shintouo, C.M.; Ouali, R.; Diallo, M.T.; Vicario, A.; Vandecasteele, S.; Nchang, A.S.; Shinyuy, L.M.; et al. Leveraging the Polymorphism of the Merozoite Surface Protein 2 (MSP2) to Engineer Molecular Tools for Predicting Malaria Episodes in a Community. Int. J. Mol. Sci. 2025, 26, 5277. https://doi.org/10.3390/ijms26115277
Kalimba EM, Noukimi SF, Mbonimpa J-B, Shintouo CM, Ouali R, Diallo MT, Vicario A, Vandecasteele S, Nchang AS, Shinyuy LM, et al. Leveraging the Polymorphism of the Merozoite Surface Protein 2 (MSP2) to Engineer Molecular Tools for Predicting Malaria Episodes in a Community. International Journal of Molecular Sciences. 2025; 26(11):5277. https://doi.org/10.3390/ijms26115277
Chicago/Turabian StyleKalimba, Edgar Mutebwa, Sandra Fankem Noukimi, Jean-Bosco Mbonimpa, Cabirou Mounchili Shintouo, Radouane Ouali, Mariama Telly Diallo, Antoine Vicario, Samuel Vandecasteele, Abenwie Suh Nchang, Lahngong Methodius Shinyuy, and et al. 2025. "Leveraging the Polymorphism of the Merozoite Surface Protein 2 (MSP2) to Engineer Molecular Tools for Predicting Malaria Episodes in a Community" International Journal of Molecular Sciences 26, no. 11: 5277. https://doi.org/10.3390/ijms26115277
APA StyleKalimba, E. M., Noukimi, S. F., Mbonimpa, J.-B., Shintouo, C. M., Ouali, R., Diallo, M. T., Vicario, A., Vandecasteele, S., Nchang, A. S., Shinyuy, L. M., Efeti, M. T., Nsengiyumva Ishimwe, A. N., Biryuwenze, A. B., Habimana, A. M., Mugisha, L. d. M. F. N., Ayadi, S., Shey, R. A., Njemini, R., Ghogomu, S. M., & Souopgui, J. (2025). Leveraging the Polymorphism of the Merozoite Surface Protein 2 (MSP2) to Engineer Molecular Tools for Predicting Malaria Episodes in a Community. International Journal of Molecular Sciences, 26(11), 5277. https://doi.org/10.3390/ijms26115277











