The Trivalent Recombinant Chimeric Proteins Containing Immunodominant Fragments of Toxoplasma gondii SAG1 and SAG2 Antigens in Their Core—A Good Diagnostic Tool for Detecting IgG Antibodies in Human Serum Samples
Abstract
1. Introduction
2. Results
2.1. Plasmids Construction, Expression, and Purification of Recombinant Chimeric Proteins
2.2. Immunoreactivity of Human Anti-T. gondii Antibodies in the ELISA Test
3. Discussion
4. Materials and Methods
4.1. Parasite Culture and Preparation of the Toxoplasma Lysate Antigen (TLA)
4.2. T. gondii RNA Isolation and cDNA Synthesis
4.3. Construction of the Recombinant Plasmids
4.4. Production and Purification of Recombinant Proteins
4.5. Human Serum Samples
4.6. IgG ELISA—Human Serum Samples
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Almeria, S.; Dubey, J.P. Foodborne Transmission of Toxoplasma gondii Infection in the Last Decade. An Overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Attias, M.; Teixeira, D.E.; Benchimol, M.; Vommaro, R.C.; Crepaldi, P.H.; De Souza, W. The Life-Cycle of Toxoplasma gondii Reviewed Using Animations. Parasit. Vectors 2020, 13, 588. [Google Scholar] [CrossRef]
- Lafferty, K.D. Sea Otter Health: Challenging a Pet Hypothesis. Int. J. Parasitol. Parasites Wildl. 2015, 4, 291. [Google Scholar] [CrossRef]
- Verma, S.K.; Knowles, S.; Cerqueira-Cézar, C.K.; Kwok, O.C.; Jiang, T.; Su, C.; Dubey, J.P. An Update on Toxoplasma gondii Infections in Northern Sea Otters (Enhydra Lutris Kenyoni) from Washington State, USA. Vet. Parasitol. 2018, 258, 133–137. [Google Scholar] [CrossRef]
- Jones, J.L.; Dubey, J.P. Foodborne Toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii Prevalence in Farm Animals in the United States. Int. J. Parasitol. 2013, 43, 107–113. [Google Scholar] [CrossRef]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii Infection in Humans and Animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of Human Toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef]
- Miyagaki, M.; Zong, Y.; Yang, M.; Zhang, J.; Zou, Y.; Ohno-Matsui, K.; Kamoi, K. Ocular Toxoplasmosis: Advances in Toxoplasma gondii Biology, Clinical Manifestations, Diagnostics, and Therapy. Pathogens 2024, 13, 898. [Google Scholar] [CrossRef]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: An Underestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef]
- Gatkowska, J.; Dzitko, K.; Ferra, B.T.; Holec-Gąsior, L.; Kawka, M.; Dziadek, B. The Impact of the Antigenic Composition of Chimeric Proteins on Their Immunoprotective Activity against Chronic Toxoplasmosis in Mice. Vaccines 2019, 7, 154. [Google Scholar] [CrossRef]
- Lappalainen, M.; Hedman, K. Serodiagnosis of Toxoplasmosis. The Impact of Measurement of IgG Avidity. Ann. Ist. Super. Sanita 2004, 40, 81–88. [Google Scholar] [PubMed]
- Laboudi, M.; Sadak, A. Serodiagnosis of Toxoplasmosis: The Effect of Measurement of IgG Avidity in Pregnant Women in Rabat in Morocco. Acta Trop. 2017, 172, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Pfrepper, K.I.; Enders, G.; Gohl, M.; Krczal, D.; Hlobil, H.; Wassenberg, D.; Soutschek, E. Seroreactivity to and Avidity for Recombinant Antigens in Toxoplasmosis. Clin. Diagn. Lab. Immunol. 2005, 12, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, D.; Joss, A.W.L.; Pennington, T.H.; Ho-Yen, D.O. Do IgA, IgE, and IgG Avidity Tests Have Any Value in the Diagnosis of Toxoplasma Infection in Pregnancy? J. Clin. Pathol. 1998, 51, 312–315. [Google Scholar] [CrossRef]
- Bessieres, M.H.; Roques, C.; Berrebi, A.; Barre, V.; Cazaux, M.; Seguela, J.P. IgA Antibody Response during Acquired and Congenital Toxoplasmosis. J. Clin. Pathol. 1992, 45, 605–608. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.D.; Huang, S.Y.; Zhu, X.Q. Diagnosis of Toxoplasmosis and Typing of Toxoplasma gondii. Parasit. Vectors 2015, 8, 292. [Google Scholar] [CrossRef]
- Holec-Gąsior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H. Moving towards Improved Vaccines for Toxoplasma gondii. Expert. Opin. Biol. Ther. 2018, 18, 273–280. [Google Scholar] [CrossRef]
- Sam-Yellowe, T.Y. Rhoptry Organelles of the Apicomplexa: Their Role in Host Cell Invasion and Intracellular Survival. Parasitol. Today 1996, 12, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, V.B.; Giddings, O.K.; Sibley, L.D. Secretion of Micronemal Proteins Is Associated with Toxoplasma Invasion of Host Cells. Cell. Microbiol. 1999, 1, 225–235. [Google Scholar] [CrossRef]
- Lekutis, C.; Ferguson, D.J.P.; Boothroyd, J.C. Toxoplasma gondii: Identification of a Developmentally Regulated Family of Genes Related to SAG2. Exp. Parasitol. 2000, 96, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lekutis, C.; Ferguson, D.J.P.; Grigg, M.E.; Camps, M.; Boothroyd, J.C. Surface Antigens of Toxoplasma gondii: Variations on a Theme. Int. J. Parasitol. 2001, 31, 1285–1292. [Google Scholar] [CrossRef]
- Joiner, K.A.; Roos, D.S. Secretory Traffic in the Eukaryotic Parasite Toxoplasma gondii. J. Cell Biol. 2002, 157, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Mercier, C.; Adjogble, K.D.Z.; Däubener, W.; Delauw, M.-F.-C. Dense Granules: Are They Key Organelles to Help Understand the Parasitophorous Vacuole of All Apicomplexa Parasites? Int. J. Parasitol. 2005, 35, 829–849. [Google Scholar] [CrossRef]
- Mercier, C.; Dubremetz, J.F.; Rauscher, B.; Lecordier, L.; Sibley, L.D.; Cesbron-Delauw, M.F. Biogenesis of Nanotubular Network in Toxoplasma Parasitophorous Vacuole Induced by Parasite Proteins. Mol. Biol. Cell 2002, 13, 2397–2409. [Google Scholar] [CrossRef]
- Manger, I.D.; Hehl, A.B.; Boothroyd, J.C. The Surface of Toxoplasma Tachyzoites Is Dominated by a Family of Glycosylphosphatidylinositol-Anchored Antigens Related to SAG1. Infect. Immun. 1998, 66, 2237–2244. [Google Scholar] [CrossRef]
- Jung, C.; Lee, C.Y.F.; Grigg, M.E. The SRS Superfamily of Toxoplasma Surface Proteins. Int. J. Parasitol. 2004, 34, 285–296. [Google Scholar] [CrossRef]
- Nagel, S.D.; Boothroyd, J.C. The Major Surface Antigen, P30, of Toxoplasma gondii Is Anchored by a Glycolipid. J. Biol. Chem. 1989, 264, 5569–5574. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Sibley, L.D. Sequential Protein Secretion Front Three Distinct Organelles of Toxoplasma gondii Accompanies Invasion of Human Fibroblasts. Eur. J. Cell Biol. 1997, 73, 114–123. [Google Scholar] [PubMed]
- Bradley, P.J.; Sibley, L.D. Rhoptries: An Arsenal of Secreted Virulence Factors. Curr. Opin. Microbiol. 2007, 10, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, F.C.; Zhou, C.X.; Zhu, X.Q. Research Advances in Interactions Related to Toxoplasma gondii Microneme Proteins. Exp. Parasitol. 2017, 176, 89–98. [Google Scholar] [CrossRef]
- Cortez, E.; Stumbo, A.C.; Saldanha-Gama, R.; Villela, C.G.; Barja-Fidalgo, C.; Rodrigues, C.A.; das Graças Henriques, M.; Benchimol, M.; Barbosa, H.S.; Porto, L.C.; et al. Immunolocalization of an Osteopontin-like Protein in Dense Granules of Toxoplasma gondii Tachyzoites and Its Association with the Parasitophorous Vacuole. Micron 2008, 39, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Katris, N.J.; Ke, H.; McFadden, G.I.; van Dooren, G.G.; Waller, R.F. Calcium Negatively Regulates Secretion from Dense Granules in Toxoplasma gondii. Cell. Microbiol. 2019, 21, e13011. [Google Scholar] [CrossRef]
- Ferra, B.T.; Chyb, M.; Sołowińska, K.; Holec-Gąsior, L.; Skwarecka, M.; Baranowicz, K.; Gatkowska, J. The Development of Toxoplasma gondii Recombinant Trivalent Chimeric Proteins as an Alternative to Toxoplasma Lysate Antigen (TLA) in Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Immunoglobulin G (IgG) in Small Ruminants. Int. J. Mol. Sci. 2024, 25, 4384. [Google Scholar] [CrossRef]
- Kijlstra, A.; Jongert, E. Control of the Risk of Human Toxoplasmosis Transmitted by Meat. Int. J. Parasitol. 2008, 38, 1359–1370. [Google Scholar] [CrossRef]
- Kijlstra, A.; Jongert, E. Toxoplasma-Safe Meat: Close to Reality? Trends Parasitol. 2009, 25, 18–22. [Google Scholar] [CrossRef]
- Boelaert, F.; Stoicescu, A.; Amore, G.; Messens, W.; Hempen, M.; Rizzi, V.; Antoniou, S.E.; Baldinelli, F.; Dorbek-Kolin, E.; Van der Stede, Y.; et al. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Planas, N.; Llobat, L. Toxoplasma gondii in Foods: Prevalence, Control, and Safety. Foods 2022, 11, 2542. [Google Scholar] [CrossRef]
- Flegr, J.; Prandota, J.; Sovičková, M.; Israili, Z.H. Toxoplasmosis—A Global Threat. Correlation of Latent Toxoplasmosis with Specific Disease Burden in a Set of 88 Countries. PLoS ONE 2014, 9, e90203. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.; Holec-Gąsior, L.; Graźlewska, W. Toxoplasma gondii Recombinant Antigens in the Serodiagnosis of Toxoplasmosis in Domestic and Farm Animals. Animals 2020, 10, 1245. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.L.; Thiruvengadam, G.; Lee, W.W.; Fong, M.Y. Immunogenic Characterization of the Chimeric Surface Antigen 1 and 2 (SAG1/2) of Toxoplasma gondii Expressed in the Yeast Pichia Pastoris. Parasitol. Res. 2011, 109, 871–878. [Google Scholar] [CrossRef]
- Beghetto, E.; Spadoni, A.; Bruno, L.; Buffolano, W.; Gargano, N. Chimeric Antigens of Toxoplasma gondii: Toward Standardization of Toxoplasmosis Serodiagnosis Using Recombinant Products. J. Clin. Microbiol. 2006, 44, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jiang, M.; Wang, Y.; Qu, L.; Gong, R.; Si, J. Evaluation of a Recombinant Multiepitope Peptide for Serodiagnosis of Toxoplasma gondii Infection. Clin. Vaccine Immunol. 2012, 19, 338–342. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, M.; Qu, L.; Sun, L.; Wang, Y.; Gong, L.; Gong, R.; Si, J. Toxoplasma gondii: Enzyme-Linked Immunosorbent Assay Based on a Recombinant Multi-Epitope Peptide for Distinguishing Recent from Past Infection in Human Sera. Exp. Parasitol. 2013, 133, 95–100. [Google Scholar] [CrossRef]
- Drapała, D.; Holec-Gąsior, L.; Kur, J. New Recombinant Chimeric Antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the Serodiagnosis of Human Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D.; Lautenbach, D.; Kur, J. A New MIC1-MAG1 Recombinant Chimeric Antigen Can Be Used Instead of the Toxoplasma gondii Lysate Antigen in Serodiagnosis of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 57–63. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Ferra, B.; Drapala, D. MIC1-MAG1-SAG1 Chimeric Protein, a Most Effective Antigen for Detection of Human Toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A New Toxoplasma gondii Chimeric Antigen Containing Fragments of SAG2, GRA1, and ROP1 Proteins—Impact of Immunodominant Sequences Size on Its Diagnostic Usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef][Green Version]
- Ferra, B.T.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The First Study on the Usefulness of Recombinant Tetravalent Chimeric Proteins Containing Fragments of SAG2, GRA1, ROP1 and AMA1 Antigens in the Detection of Specific Anti-Toxoplasma gondii Antibodies in Mouse and Human Sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef] [PubMed]
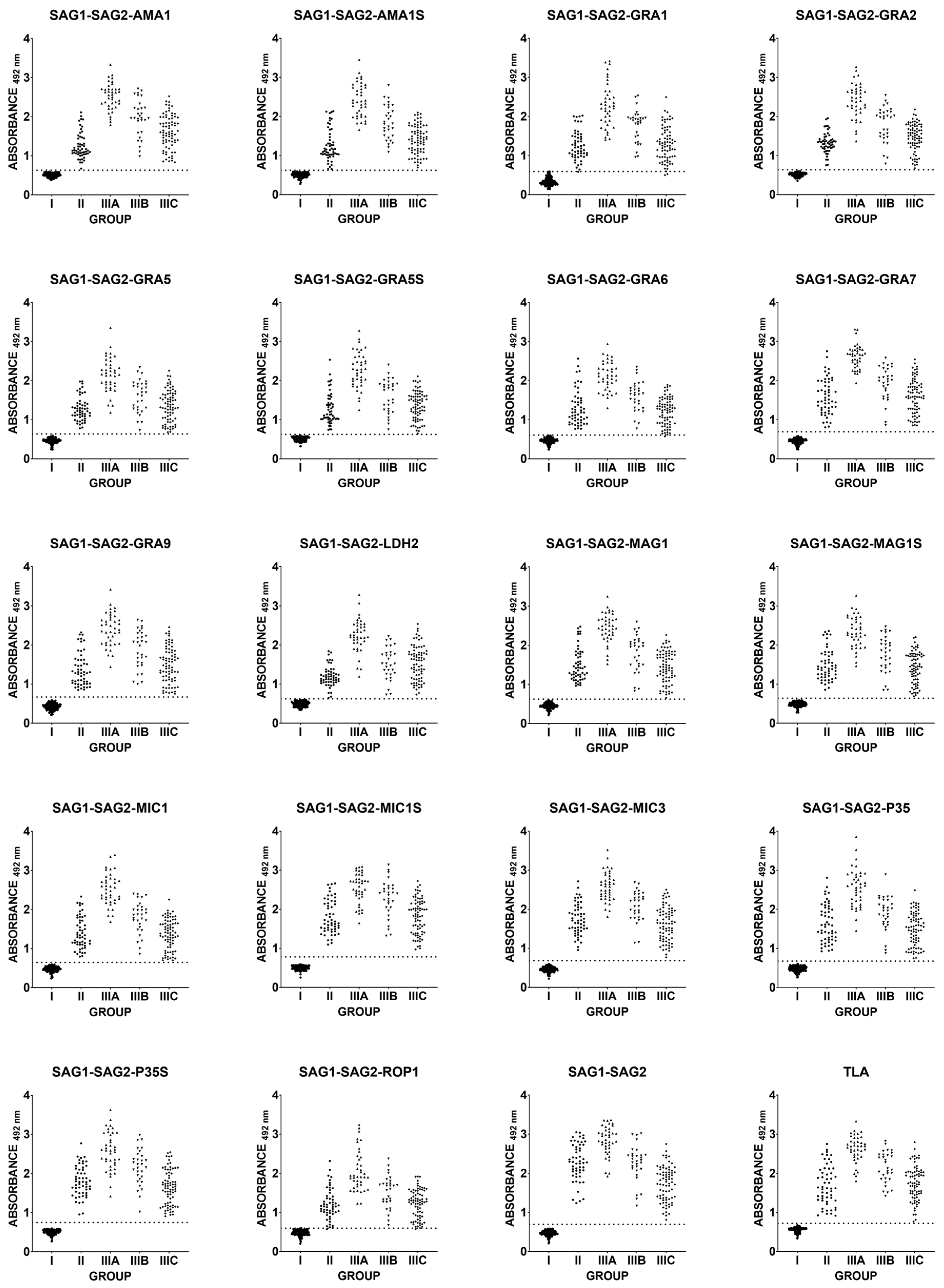
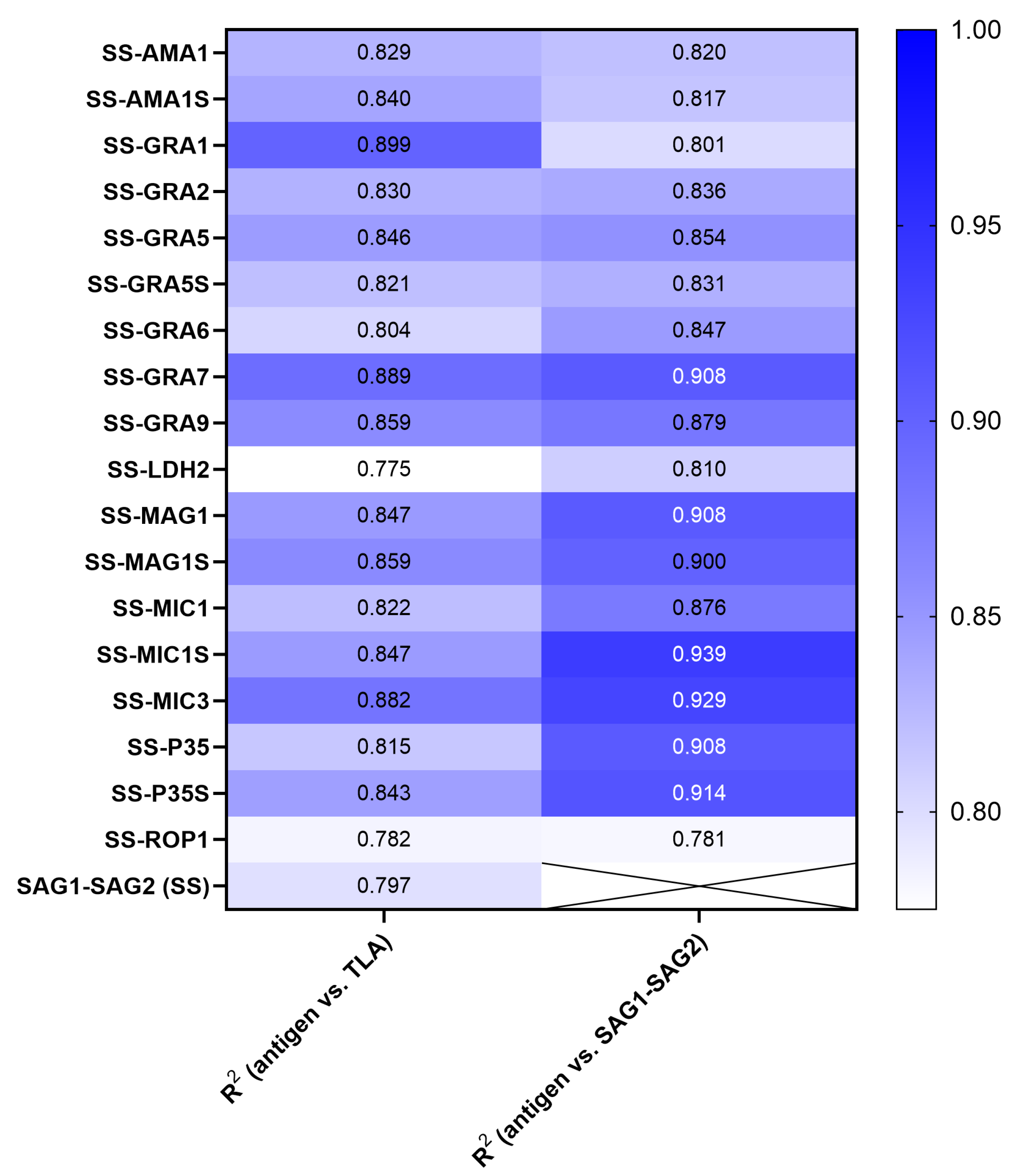
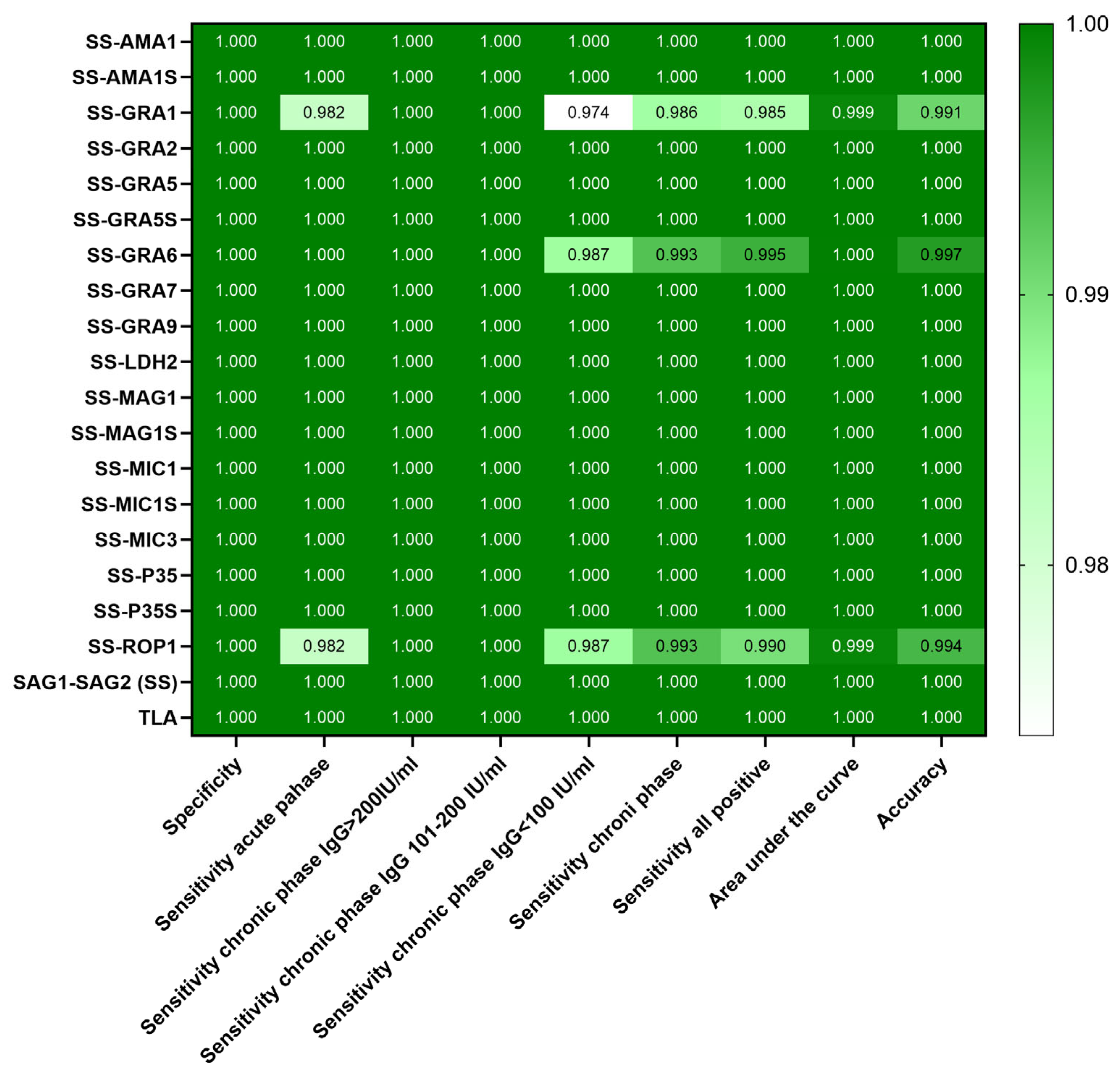
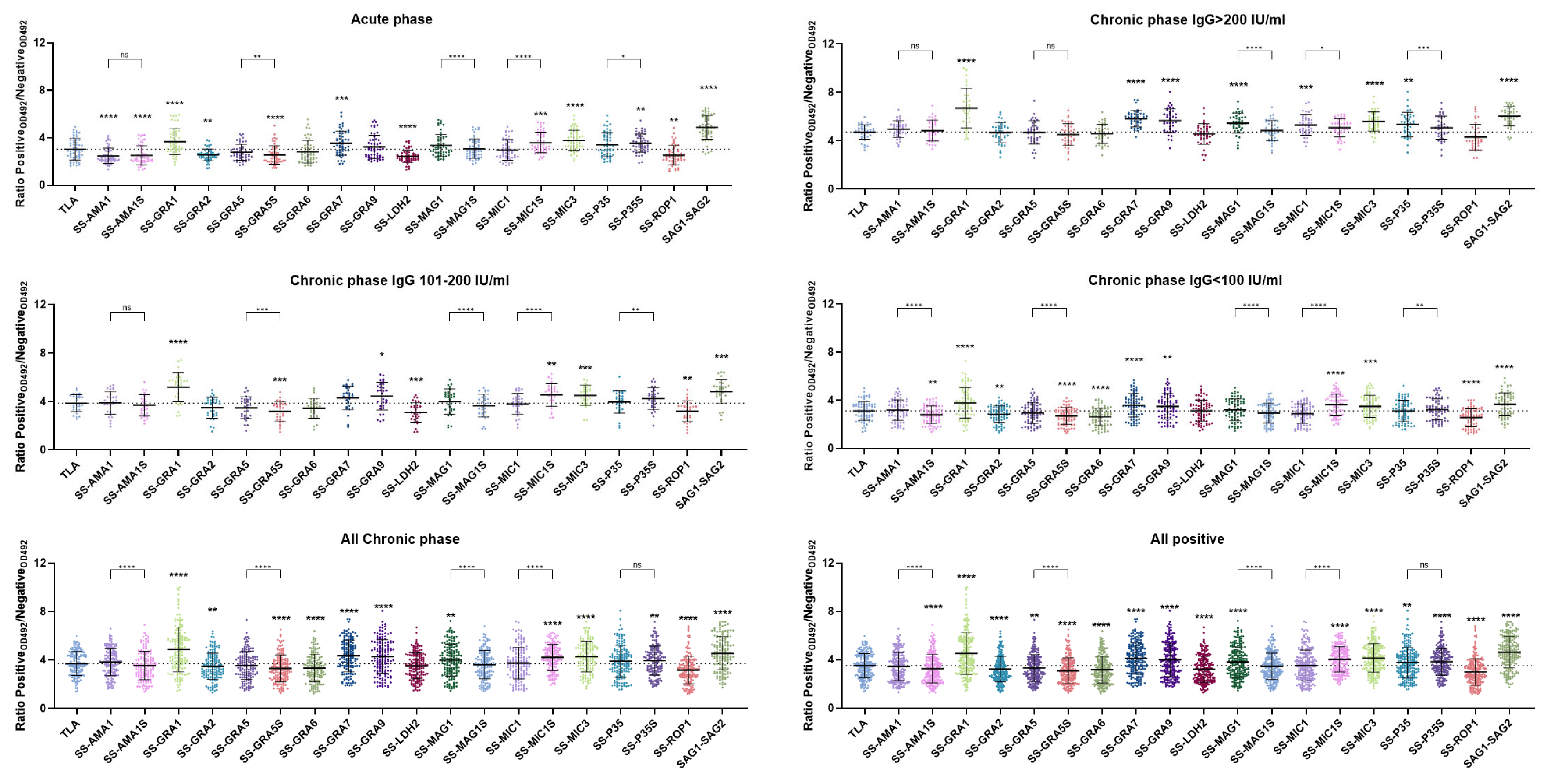
| Recombinant Chimeric Protein | vs. | r (95% CI) | r2 | p Value |
|---|---|---|---|---|
| SAG1-SAG2-AMA1 | TLA SAG1-SAG2 | 0.9102 (0.8900–0.9269) 0.9055 (0.8843–0.9230) | 0.8285 0.8200 | <0.0001 <0.0001 |
| SAG1-SAG2-AMA1S | TLA SAG1-SAG2 | 0.9163 (0.8973–0.9319) 0.9038 (0.8822–0.9217) | 0.8396 0.8169 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA1 | TLA SAG1-SAG2 | 0.9481 (0.9361–0.9579) 0.8948 (0.8713–0.9142) | 0.8989 0.8007 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA2 | TLA SAG1-SAG2 | 0.9112 (0.8911–0.9277) 0.9142 (0.8948–0.9302) | 0.8302 0.8358 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA5 | TLA SAG1-SAG2 | 0.9198 (0.9016–0.9348) 0.9241 (0.9068–0.9383) | 0.8460 0.8540 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA5S | TLA SAG1-SAG2 | 0.9058 (0.8846–0.9233) 0.9117 (0.8918–0.9281) | 0.8205 0.8312 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA6 | TLA SAG1-SAG2 | 0.8967 (0.8735–0.9157) 0.9202 (0.9021–0.9351) | 0.8040 0.8467 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA7 | TLA SAG1-SAG2 | 0.9428 (0.9296–0.9536) 0.9529 (0.9419–0.9618) | 0.8889 0.9079 | <0.0001 <0.0001 |
| SAG1-SAG2-GRA9 | TLA SAG1-SAG2 | 0.9267 (0.9100–0.9404) 0.9373 (0.9229–0.9490) | 0.8587 0.8785 | <0.0001 <0.0001 |
| SAG1-SAG2-LDH2 | TLA SAG1-SAG2 | 0.8804 (0.8539–0.9023) 0.8998 (0.8773–0.9183) | 0.7751 0.8096 | <0.0001 <0.0001 |
| SAG1-SAG2-MAG1 | TLA SAG1-SAG2 | 0.9202 (0.9021–0.9351) 0.9531 (0.9422–0.9619) | 0.8469 0.9083 | <0.0001 <0.0001 |
| SAG1-SAG2-MAG1S | TLA SAG1-SAG2 | 0.9270 (0.9103–0.9407) 0.9485 (0.9366–0.9582) | 0.8593 0.8997 | <0.0001 <0.0001 |
| SAG1-SAG2-MIC1 | TLA SAG1-SAG2 | 0.9065 (0.8854–0.9238) 0.9358 (0.9211–0.9479) | 0.8217 0.8757 | <0.0001 <0.0001 |
| SAG1-SAG2-MIC1S | TLA SAG1-SAG2 | 0.9201 (0.9019–0.9350) 0.9689 (0.9617–0.9748) | 0.8466 0.9388 | <0.0001 <0.0001 |
| SAG1-SAG2-MIC3 | TLA SAG1-SAG2 | 0.9391 (0.9251–0.9505) 0.9641 (0.9557–0.9709) | 0.8819 0.9294 | <0.0001 <0.0001 |
| SAG1-SAG2-P35 | TLA SAG1-SAG2 | 0.9026 (0.8807–0.9206) 0.9530 (0.9421–0.9619) | 0.8147 0.9083 | <0.0001 <0.0001 |
| SAG1-SAG2-P35S | TLA SAG1-SAG2 | 0.9184 (0.8998–0.9336) 0.9561 (0.9459–0.9644) | 0.8434 0.9141 | <0.0001 <0.0001 |
| SAG1-SAG2-ROP1 | TLA SAG1-SAG2 | 0.8845 (0.8588–0.9057) 0.8837 (0.8578–0.9050) | 0.7823 0.7808 | <0.0001 <0.0001 |
| SAG1-SAG2 | TLA | 0.8928 (0.8688–0.9125) | 0.7970 | <0.0001 |
| Recombinant Chimeric Protein | Calculated Cut-Off | ROC Cut-Off | Sensitivity [%] (95% CI) | Specificity [%] (95% CI) | Area Under the Curve (95% CI) | p Value |
|---|---|---|---|---|---|---|
| SAG1-SAG2-AMA1 | 0.6083 | 0.6320 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-AMA1S | 0.6169 | 0.6233 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-GRA1 | 0.5445 | 0.5918 | 98.5 (95.68–99.59) | 100 (97.29–100) | 0.9992 (0.9981–1.000) | <0.0001 |
| SAG1-SAG2-GRA2 | 0.6113 | 0.6363 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-GRA5 | 0.5958 | 0.6350 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-GRA5S | 0.6149 | 0.6240 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-GRA6 | 0.6015 | 0.6075 | 99.5 (97.22–99.97) | 100 (97.29–100) | 0.9999 (0.9995–1.000) | <0.0001 |
| SAG1-SAG2-GRA7 | 0.5948 | 0.6908 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-GRA9 | 0.5898 | 0.6695 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-LDH2 | 0.6134 | 0.6233 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-MAG1 | 0.5911 | 0.6233 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-MAG1S | 0.6112 | 0.6380 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-MIC1 | 0.6080 | 0.6433 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-MIC1S | 0.6089 | 0.7798 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-MIC3 | 0.5967 | 0.6813 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-P35 | 0.6144 | 0.6738 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-P35S | 0.6246 | 0.7523 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| SAG1-SAG2-ROP1 | 0.6184 | 0.5985 | 99 (96.43–99.82) | 100 (97.29–100) | 0.9993 (0.9982–1.000) | <0.0001 |
| SAG1-SAG2 | 0.6064 | 0.6995 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| TLA | 0.6804 | 0.7245 | 100 (98.12–100) | 100 (97.29–100) | 1.000 (1.000–1.000) | <0.0001 |
| Group of Serum Samples | Phase of T. gondii Infection | Number of Serum Samples | IgM | IgG | IgG Avidity | |
|---|---|---|---|---|---|---|
| I | uninfected | 138 | − | − | not tested | |
| II | suspected acute | 54 | + | + | low | |
| III | IIIA | chronic | 40 | +/− | >200 IU/mL | high |
| IIIB | 30 | − | 101–200 IU/mL | |||
| IIIC | 76 | − | ≤100 IU/mL | |||
| IIIA-C | 146 | +/− | + | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferra, B.T.; Chyb, M.; Skwarecka, M.; Gatkowska, J. The Trivalent Recombinant Chimeric Proteins Containing Immunodominant Fragments of Toxoplasma gondii SAG1 and SAG2 Antigens in Their Core—A Good Diagnostic Tool for Detecting IgG Antibodies in Human Serum Samples. Int. J. Mol. Sci. 2025, 26, 5621. https://doi.org/10.3390/ijms26125621
Ferra BT, Chyb M, Skwarecka M, Gatkowska J. The Trivalent Recombinant Chimeric Proteins Containing Immunodominant Fragments of Toxoplasma gondii SAG1 and SAG2 Antigens in Their Core—A Good Diagnostic Tool for Detecting IgG Antibodies in Human Serum Samples. International Journal of Molecular Sciences. 2025; 26(12):5621. https://doi.org/10.3390/ijms26125621
Chicago/Turabian StyleFerra, Bartłomiej Tomasz, Maciej Chyb, Marta Skwarecka, and Justyna Gatkowska. 2025. "The Trivalent Recombinant Chimeric Proteins Containing Immunodominant Fragments of Toxoplasma gondii SAG1 and SAG2 Antigens in Their Core—A Good Diagnostic Tool for Detecting IgG Antibodies in Human Serum Samples" International Journal of Molecular Sciences 26, no. 12: 5621. https://doi.org/10.3390/ijms26125621
APA StyleFerra, B. T., Chyb, M., Skwarecka, M., & Gatkowska, J. (2025). The Trivalent Recombinant Chimeric Proteins Containing Immunodominant Fragments of Toxoplasma gondii SAG1 and SAG2 Antigens in Their Core—A Good Diagnostic Tool for Detecting IgG Antibodies in Human Serum Samples. International Journal of Molecular Sciences, 26(12), 5621. https://doi.org/10.3390/ijms26125621







