Abstract
Myosin-binding protein C (MyBP-C) comprises a family of myofilament proteins that maintain sarcomeric structure and regulate actomyosin crossbridge cycling. Pathogenic variants in MYBPC1, the gene encoding the slow skeletal isoform (sMyBP-C), lead to a dominant congenital myopathy, termed Myotrem, characterized by muscle weakness, hypotonia, and a distinctive tremor of myogenic origin, in the absence of neuropathy. However, the molecular mechanism(s) of myogenic tremorgenesis is largely unknown. One potential mechanism is aberrant myofilament stretch activation, which is defined as a delayed increase in force after a rapid stretch. We utilized the Myotrem murine model harboring the pathogenic MYBPC1 E248K variant to test the hypothesis that stretch activation is augmented in permeabilized Myotrem E248K soleus fibers. We found that stretch activation was significantly increased in E248K soleus muscle fibers. Interestingly, once submaximally Ca2+ activated, a subpopulation of slow-twitch E248K fibers exhibited spontaneous pulsatile sarcomere oscillations. This pulsing behavior generated a sinusoidal waveform pattern in sarcomere length, which often persisted on a timescale of minutes. These results align with sMyBP-C as key regulator of the synchronous activation of myofilaments by dampening both spontaneous oscillatory activity and stretch-dependent activation. We propose that the presence of sMyBP-C-E248K disrupts this regulation, thereby driving pathogenic myogenic tremors.
1. Introduction
Pathogenic variants in MYBPC1, the gene encoding the slow skeletal isoform of Myosin Binding Protein-C (sMyBP-C), are linked to a newly recognized form of congenital myopathy termed Myotrem (OMIM #618524). Myotrem is characterized by congenital hypotonia, early onset muscle weakness, skeletal deformities, and, most characteristically, tremor of myogenic origin [1]. The myogenic tremor is largely postural, accentuated with action, and is present mainly in the upper and lower extremities as well as the tongue and facial muscles [1]. sMyBP-C is ubiquitously expressed in skeletal muscle in both slow- and fast-twitch fibers, although in different amounts and isoform combinations [2]. Localized to the C-zone of the sarcomeric A-band [3], sMyBP-C is constitutively bound to the thick filament at its COOH-terminal end [4], while its NH2-terminus interacts dynamically with both myosin [5] and actin [6,7]. sMyBP-C plays a role in the assembly and maintenance of sarcomeric organization [8] and critically regulates actomyosin crossbridge cycling. Given that sMyBP-C expression is restricted to skeletal muscle and that Myotrem patients exhibit no neuropathy, the tremor is believed to originate in the muscle itself starting at the level of the sarcomere [1]. However, the molecular mechanisms of myogenic tremorgenesis remain unknown.
Stretch activation is a property of striated muscle whereby rapid mechanical stretch of an isolated muscle fiber leads to a delayed increase in force [9,10]. This stretch-activated response occurs in three phases. In phase 1, mechanical stretch strains actomyosin cross-bridges, generating a rapid increase in tension. In phase 2, tension rapidly decreases as strained cross-bridges detach. Finally, in phase 3, there is a delayed increase in muscle tension, known as stretch activation.
Stretch activation is often studied in insect flight muscles, where it is more pronounced and facilitates asynchronous muscle contraction with membrane depolarization [11]. However, stretch activation is also a property of vertebrae striated muscles and contributes to physiological function [9,12,13,14,15]. In cardiac myocytes, which rhythmically contract with each heartbeat, stretch activation is thought to contribute to length-dependent activation and the Frank–Starling mechanism [16]. Although the mechanisms of stretch activation are not fully understood, alterations in the levels or phosphorylation status of MyBP-C have been shown to regulate stretch activation in cardiac myocytes [13] and skeletal muscle fibers [15]. It seems plausible that aberrant stretch activation may be a potential mechanism contributing to myogenic tremor. Thus, we tested the hypothesis that stretch activation is augmented in permeabilized soleus muscle fibers from MYBPC1 E248K knock-in (KI) Myotrem mice.
2. Results
2.1. Contractile Properties of Soleus Muscle Fibers
Permeabilized fibers from wild type (WT) and MYBPC1 E248K knock-in (KI) soleus muscles were first evaluated for their morphometric characteristics and mechanical performance in relaxing and maximal Ca2+ activating solutions (Table 1). Fiber preparation length, width, resting sarcomere length, and passive tension were comparable between groups. However, maximal Ca2+ activated force was significantly reduced in E248K KI soleus fibers (Table 1), an observation consistent with similar measurements in permeabilized E248K KI extensor digitorum longus (EDL) fibers [17]. Moreover, the rate constant of force development after a slack–re-stretch maneuver was comparable between WT (pCa 4.5 ktr = 10.5 ± 1.1 s−1) and E248K KI (pCa 4.5 ktr = 10.3 ± 1.1 s−1) soleus fibers during maximal Ca2+ activation.

Table 1.
Cellular Characteristics of WT vs MYBPC1 E248K KI Permeabilized Soleus Skeletal Muscle Fibers.
2.2. Stretch Activation Properties of Soleus Muscle Fibers
We next investigated whether stretch activation properties were augmented in E248K KI soleus fibers. Fibers were subjected to rapid step–stretches that yielded a 1–4% change in sarcomere length during submaximal Ca2+ activations, and the resulting triphasic stretch-activated response was recorded (Figure 1A–C). Stretches that failed to induce delayed force development (Figure 1B) were assigned a Pto value of zero and were included in the analysis. Stretch activation, quantified by Pto/Po, was significantly greater in E248K KI soleus fibers compared to WT (WT Pto/Po = 0.111 ± 0.028; E248K KI Pto/Po = 0.240 ± 0.017, **** p < 0.0001; Figure 1D). Interestingly, all E248K KI fibers displayed stretch activation (i.e., delayed force transient after rapid stretch), whereas only ~50% of WT soleus fibers exhibited stretch activation, which was a major contributor to group differences.
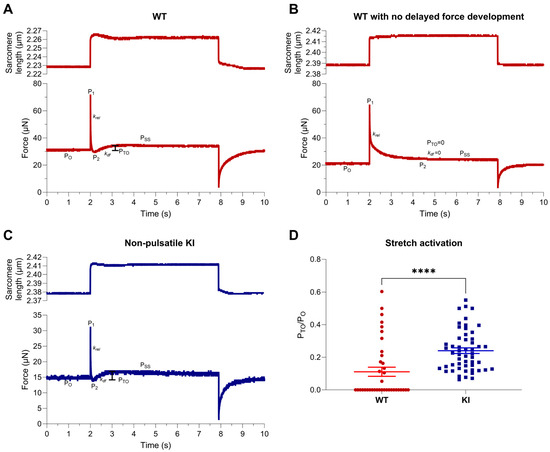
Figure 1.
Stretch activation responses of permeabilized WT and E248K KI soleus fibers. (A–C). Representative sarcomere length and force traces during a stretch activation maneuver of a 1% sarcomere length stretch of permeabilized WT (A,B) and E248K KI (C) soleus fibers. Stretch activation is quantified as the delayed transient force overshoot (PTO) after sarcomere length stretch. On each trace, properties of the stretch-activated response are indicated, which include pre-stretch tension (Po), an initial force spike that coincides with stretch (P1), minimum of quick force decay (P2), rapid force decay rate (krel), delayed force re-development rate (kdf), and delayed force overshoot or stretch activation (PTO), before resolving to a new stretch-activated steady state tension (PSS). Fibers that did not exhibit delayed force redevelopment (B) had PTO and kdf values of zero. (D) Stretch activation was significantly increased in E248K KI soleus muscle fibers, as indexed by delayed force overshoot after stretch (PTO/PO). Values are calculated from n = 38 stretch maneuvers of n′ = 8 fibers from n″ = 5 WT mice and n = 53 stretch maneuvers of n′ = 11 fibers from n″ = 5 E248K KI mice. Data are expressed as mean ± SEM and statistical significance was determined by Mann–Whitney test; WT PTO/Po = 0.111 ± 0.028; E248K KI PTO/Po = 0.240 ± 0.017, **** p < 0.0001.
Further evaluation of stretch activation parameters indicated that step–stretch length (Figure 2A) and pre-stretch steady state tension (Figure 2B) were similar between WT and E248K KI fibers. However, post-stretch, E248K KI fibers exhibited significantly reduced maximum force peak (P1/PO; Figure 2C) and minimum force decay (P2/PO; Figure 2D) tension, faster rapid force decay (krel, Figure 2E) and slower delayed force development (kdf, Figure 2F) kinetics, while stretch-induced steady state tension (PSS/PO, Figure 2G) remained comparable to WT. Taken together, the observed differences in stretch activation parameters implicate altered interactions between mutant sMyBP-C and the sarcomeric thick and thin filaments. For instance, the difference between P2 and PTO is primarily a function of the rate of phase 2 decay (i.e., rapid force decay, krel) compared to the rate of phase 3 increase (i.e., delayed force development, kdf). Thus, the significantly reduced P2/PO values in the E248K KI fibers in phase 2 indicate faster detachment of strained cross-bridges in the presence of similar or slightly lower rates of cross-bridge force development (kdf) in phase 3. Moreover, the lower P1/PO values in the E248K KI fibers are also consistent with faster cross-bridge attachment rates but may also arise from reduced stiffness per bridge.
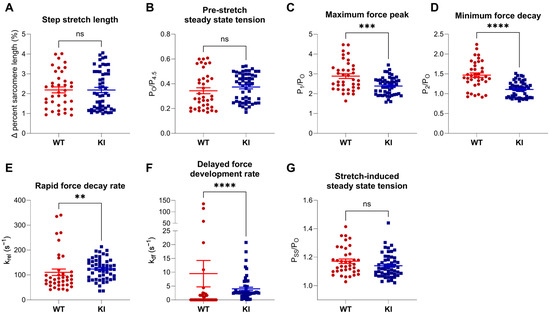
Figure 2.
Stretch activation properties of permeabilized WT and E248K KI soleus fibers. Stretch activation properties of soleus fibers undergoing stretch maneuvers during submaximal Ca2+ activation (pCa 6.2–6.5). (A) Fibers underwent similar step stretches ranging from 1–4% sarcomere length (p = 0.94). (B) Pre-stretch steady state tension was similar between WT and E248K KI fibers, (p = 0.17). During the stretch maneuver, the calculated maximum force peak (C; *** p = 0.0004), minimum tension of force decay (D; **** p < 0.0001), rapid force decay rate (E; ** p = 0.0046), and delayed forced development rates (F; **** p < 0.0001) were all depressed in E248K KI fibers compared to WT. After the stretch, the resolved stretched-induced steady state tension remained similar between WT and E248K KI fibers (G; p = 0.10). Values are calculated from n = 38 stretch maneuvers of n′ = 8 fibers from n″ = 5 WT mice and n = 53 stretch maneuvers of n′ = 11 fibers from n″ = 8 KI mice. Data are expressed as mean ± SEM and statistical significance was determined by Welch’s t-test (C), or Mann–Whitney test if normality failed (A,B,D,E); ns: not significant.
2.3. Pulsatile Activity of a Subpopulation of E248K KI Soleus Muscle Fibers
Permeabilized fibers from WT and E248K KI soleus muscles were subsequently subjected to mechanical measurements in submaximal Ca2+ conditions. All eight WT fibers and eight of 11 E248K KI fibers remained at constant sarcomere length during sub-maximal Ca2+ activation, consistent with prototypic behavior (Figure 1A–C and Supplemental Video S1). However, a subpopulation, three of 11, of E248K KI fibers exhibited pulsatile spontaneous oscillatory contraction (SPOC)-like behavior during sub-maximal Ca2+ activation (Figure 3 and Supplemental Videos S2–S4). This unsolicited pulsatile SPOC-like activity consisted of rhythmic oscillations in sarcomere length that lasted for minutes and persisted during subsequent small rapid mechanical stretches (Figure 3A). Sarcomere length traces of these pulsatile E248K KI fibers revealed a unique sinusoidal pattern with temporally equivalent lengthening and shortening phases, reaching a consistent amplitude with each period. Despite this internal consistency, amplitude and frequency values varied amongst the three pulsatile E248K KI fibers (Figure 3B). Pulse amplitude and frequency were also altered within the same fiber in a pCa-dependent manner, where lower Ca2+ concentrations reduced pulse amplitude and frequency (Figure 3B). Pulsatile behavior was not observed in pCa 9.0 relaxing solution, where sarcomere lengths of relaxed fibers remained constant.
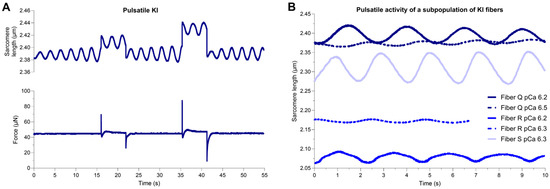
Figure 3.
Pulsatile SPOC-like activity in permeabilized E248K KI soleus fibers. (A,B) A sub-population of E248K KI permeabilized soleus fibers exhibited pulsatile sarcomere length oscillations during submaximal Ca2+ activation. The pulses oscillated rhythmically at ~0.5 Hz and persisted throughout the stretch activation protocol. Pulsatile fibers were not observed in the WT group under any tested condition. (A) Representative sarcomere length and force traces of a pulsatile E248K KI fiber undergoing two stretch maneuvers at pCa 6.2. Data are shown over a 55 s period with a sampling rate of 250 Hz and 1000 Hz for sarcomere length and force, respectively. (B) Sarcomere length traces of E248K KI fibers that exhibit unsolicited pulsatile activity in submaximal Ca2+ solutions (pCa 6.2–6.5). Navy, periwinkle, and bright blue represent traces from different (n = 3) pulsatile soleus fibers from three E248K KI mice. Data shown are over a 10 s period of steady-state equilibrium with a sampling rate of 250 Hz.
2.4. Fiber-Type Specific Stretch Activation Properties
Given the spontaneous pulsatile activity observed in a subset of E248K KI soleus fibers, we aimed to explore fiber characteristics that potentially contribute to pulsatile behavior. Soleus fibers underwent immunoblot analysis to determine fiber-type by probing for the slow isoform of myosin heavy chain (MHC) and sMyBP-C, while SYPRO Ruby stain was used to evaluate total protein levels in the tested fibers (Figure 4A). Interestingly, all three pulsatile E248K KI fibers were identified as slow type-I based on their positive expression of slow-MHC, a finding corroborated by our mechanical measurements, where all pulsatile E248K KI fibers exhibited a slower tension re-development rate constant (ktr) in maximal Ca2+ activating conditions of less than 7 s−1 (Table 1). Notably, one of six non-pulsatile E248K KI fibers was also type-I, suggesting that while a slow fiber type-I background may be necessary for E248K to elicit pulsatile behavior, it is not solely sufficient.
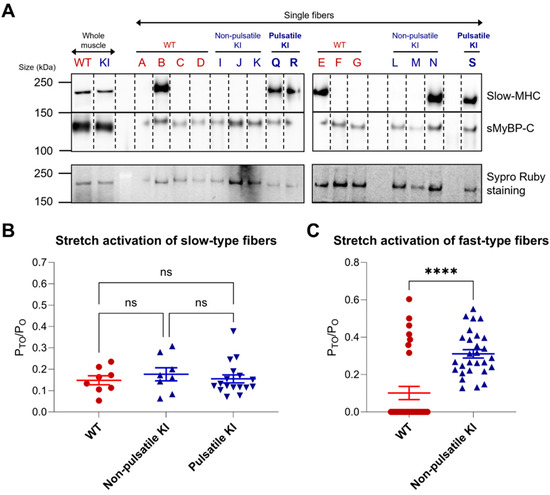
Figure 4.
Fiber typing and stretch activation properties of WT and E248K KI soleus fibers. (A) Single fiber SDS-gel electrophoresis and Western blotting were performed on WT, non-pulsatile E248K KI, and pulsatile E248K KI fibers after mechanical measurements. Lysates from whole soleus muscles from WT and E2348K KI mice were included as positive controls, and SYPRO Ruby was used to visualize total protein levels. Blots were probed for slow myosin heavy chain (MHC) and sMyBP-C. Slow-MHC was positively identified in 2/7 (29%) WT, 1/6 (17%) non-pulsatile E248K KI, and 3/3 (100%) pulsatile E248K KI fibers by Western blotting. (B,C) Post-hoc analysis of stretch activation properties were stratified according to fiber type and pulsatile activity. Fiber type was defined by mechanical properties with slow fibers exhibiting a ktr value less than 7 s−1 and fast fibers displaying a ktr values greater than or equal to 7 s−1. (B) Stretch activation (PTO/PO) was comparable among WT, non-pulsatile E248K KI, and pulsatile E248K KI type-I slow fibers. Data are expressed as mean ± SEM. Values are calculated from n = 8 stretch maneuvers of n′ = 2 WT & n′ = 2 non-pulsatile E248K KI fibers, and n = 17 stretch maneuvers of n’ = 3 pulsatile E248K KI type-I slow fibers. Statistical significance was determined by Kruskal–Wallis one-way ANOVA; ns: not significant. (C) While none of the E248K KI fast type-II fibers exhibited pulsatile activity, they developed enhanced stretch activation when compared to their WT counterparts. Values are calculated from n = 30 stretch maneuvers of n′ = 6 WT and n = 28 stretch maneuvers of n′ = 6 E248K KI fast type-II fibers. Statistical significance was determined by Mann–Whitney test; **** p < 0.0001.
Considering the enhanced stretch activation observed in the E248K KI soleus fibers compared to WT (Figure 1D), we proceeded to assess the stretch activation characteristics relative to fiber type. Fiber type was classified based on mechanical properties, with a ktr value less than 7 s−1 indicating slow fibers, and a ktr value greater than or equal to 7 s−1 indicating fast fibers. No difference in stretch activation (Pto/Po) was observed among WT, non-pulsatile E248K KI, and pulsatile E248K KI slow type-I fibers (Figure 4B). In contrast, E248K KI fast type-II fibers exhibited significantly enhanced stretch activation compared to their WT counterparts (Figure 4C). Interestingly, all WT fibers that lacked delayed force development were of the fast type. Thus, although stretch activation does not appear to contribute to the pulsatile activity of slow type-I E248K KI fibers per se, the elevated stretch activation observed in fast type-II E248K KI fibers may augment the muscle’s overall response to stretch, potentially contributing to the propagation of the pulsatile behavior present in a subgroup of slow type-I Myotrem fibers.
3. Discussion
Dominant missense variants of MYBPC1, the gene encoding sMyBP-C, are linked to a new myopathy termed Myotrem (OMIM #618524), associated with tremor [1]. Given the absence of neuropathy in individuals with Myotrem [1], and the restricted expression of MYBPC1 in skeletal muscle, this characteristic tremor is believed to originate within the muscle itself [1,17]. Despite this understanding, the mechanism responsible for myogenic tremor has remained unclear. To study Myotrem, we generated a KI mouse model carrying the dominant MYBPC1 E248K variant [17]. Consistent with the pathological manifestations of Myotrem seen in humans, heterozygous E248K KI mice present with skeletal muscle weakness, manifesting as reduced force production and suppressed contractility kinetics, as well as early-onset tremor [17]. Considering that neuromuscular diseases often display sex-dimorphic presentation [18], our studies exclusively focused on males. Nonetheless, since MYBPC1 E248K Myotrem mice exhibit no evidence of sex dimorphism at young ages [17], similar results might be expected of female mice as well.
We first postulated that augmented stretch activation, which is defined as a delayed increase in force after a rapid stretch, may contribute to myogenic tremorgenesis. We therefore tested the hypothesis that stretch activation is increased in permeabilized E248K KI soleus muscle fibers. We report enhanced stretch activation in fast type-II E248K KI soleus muscle fibers when compared to their WT counterparts. Although the exact mechanism(s) of stretch activation remains unclear, alterations in the expression or phosphorylation profile of MyBP-C influence stretch activation in both cardiac and skeletal muscles [13,15] (Figure 5A). Our prior work has shown that the E248K variant significantly increases myosin binding [1]. We therefore postulate that upon stretch, this enhanced myosin binding may provide structural support, facilitating myosin displacement towards actin, thereby promoting crossbridge recruitment via mechanotransduction (Figure 5B).
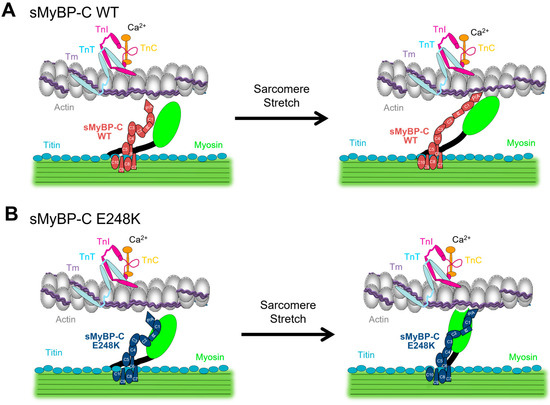
Figure 5.
Proposed model of enhanced stretch activation in the presence of the MYBPC1 E248K Myotrem variant. (A) In submaximal Ca2+ conditions, stretch may allow for lateral displacement of thick and thin filaments, promoting crossbridge formation as part of the stretch activation response, which is (at least partially) regulated by sMyBP-C. (B) Introduction of the MYBPC1 E248K variant, enhances binding of sMyBP-C to myosin. This potentially allows sMyBP-C to provide structural support, thereby upon stretch, myosin is more likely to engage in crossbridge formation leading to enhanced stretch activation.
Unexpectedly, we also identified a subset of slow type-I E248K KI fibers that exhibit rhythmic, pulsatile sarcomere oscillations during submaximal Ca2+ activation. We postulate that these pulsatile slow type-I E248K KI fibers may serve as initiators of the tremor, with the autonomous oscillatory signal being propagated and amplified via enhanced stretch activation in neighboring fast type-II fibers, ultimately producing a recordable tremor. The intrinsic pulsatile SPOC-like phenotype we uncovered in a subpopulation of E248K KI slow type-I fibers is independent of neurological input or stimulation through canonical excitation–contraction coupling. Notably, SPOC is typically characterized by a saw-tooth pattern of rapid lengthening and slow shortening cycles [12], which contrasts with the highly regular sinusoidal pattern of contraction and persistence exhibited by pulsing E248K KI slow type-I fibers. A case of highly synchronous SPOC-like activity has been reported in permeabilized rabbit psoas fibers under isotonic conditions [19], which mirrors the regularity of the pulsatile activity observed in E248K KI slow type-I fibers. However, this synchronous SPOC-like activity persisted on a timescale of seconds, whereas the pulsatile behavior we describe in E248K KI slow type-I fibers spans a timescale of minutes.
Pulsatile activity in E248K KI slow type-I fibers was dormant in relaxing solution, present in submaximal Ca2+ solutions, and typically absent at maximal Ca2+ concentration. Consequently, Ca2+ is required for pulsing, but pulsatile behavior is mostly distinct from maximal Ca2+ activated contraction. This finding aligns with Myotrem tremor on a physiological level, as tremor is mainly absent at rest but induced by posture, action and intention [1,20], which largely occur at submaximal activation [21]. Pulse frequency and amplitude also varied within pulsatile fibers in a pCa-dependent manner, as well as among pulsatile fibers. This supports the hypothesis of multiple tremor initiators within the body’s musculature, consistent with dual-channel electromyography (EMG) recordings in Myotrem patients revealing temporally asynchronous tremors between the two legs and tremor of incongruent frequency between the two arms [1].
Recent work has postulated that cardiac MyBP-C may regulate SPOC generation, as removal of its NH2-terminal region in cardiac myocytes leads to an increase in SPOC activity [22], prompting a newly proposed role of MyBP-C as a SPOC “wave breaker” [12]. Harris argued that removal of the NH2-terminus of cardiac MyBP-C, the regulatory center of the protein, accentuates SPOC generation by accelerating the detachment of strained cross-bridges [12,22], an idea consistent with our findings in E248K KI soleus fibers, which demonstrate reduced minimum force decay (P2/PO) during stretch activation. Relatedly, the expression levels and phosphorylation profile of the cardiac and slow skeletal MyBP-C proteins were found to modulate stretch activation responses in permeabilized cardiac and skeletal muscle fibers [13,15,23]. Thus, it is conceivable that pathogenic variants in MYBPC1 may give rise to a SPOC-like phenotype.
Importantly, while all pulsatile E248K KI fibers are slow type-I, not all slow type-I E248K KI fibers display SPOC-like activity, indicating that a slow type-I fiber is likely necessary but not sufficient to elicit pulsatile activity. Moreover, while stretch activation among WT, E248K KI non-pulsatile, and E248K KI pulsatile slow type-I fibers is comparable, E248K KI fast type-II fibers display significantly enhanced stretch activation relative to their WT counterparts, with only ~50% of WT fast type-II fibers (versus ~100% of E248K KI fast type-II fibers) demonstrating the capacity to generate such a response. We therefore posit that pulsatile and non-pulsatile E248K KI fibers may serve distinct roles in myogenic tremorgenesis, yet likely work in concert to predispose dyssynchronous muscle fiber activity. When partially activated in submaximal Ca2+ conditions induced by posture or intention, a subpopulation of slow type-I Myotrem fibers exhibits spontaneous pulsatile activity. This mechanical perturbation may be transmitted to adjacent fast type-II fibers displaying enhanced stretch activation. As these fast type-II fibers develop force and contract in response to stretch, they may propagate the pulsatile activity initiated by the slow type-I fibers, resulting in the generation of recordable tremor.
While this is an intriguing notion, there are several unresolved questions, including the identification of the intrinsic characteristics of the potentially distinct fiber subsets. The molecular complexity of the sMyBP-C subfamily, encompassing >14 splice variants in humans expressed in varying combinations, ratios and amounts across individual muscles, fibers, and likely sarcomeres [2], that may undergo phosphorylation at both constitutive and variant-specific sites [24], likely play a key role in defining the disparate fiber subpopulations within a muscle. Moreover, the EMG patient studies describe preservation in tremor frequency upon loading, suggesting some degree of central nervous system (CNS) involvement in the observable tremor [1]. The muscle spindle and Golgi tendon organ serve as sensory receptors in skeletal muscle, which, upon stretch or compaction due to force generation, send an afferent signal to the CNS [25,26]. Thus, it is plausible that in pathological conditions, such as Myotrem, pulsatile activity of a fiber subset coupled with enhanced stretch activation of adjacent fibers may affect proprioception, resulting in propagation of a measurable tremor on the organ level via sensory receptors. Further studies ranging from quantitative modeling of myofilament regulation to measurements of contractile activity within a single fiber and contractile behavior between adjacent muscle fibers, will be necessary to fully decipher the etiologies of myogenic tremorgenesis.
Currently, there are no effective treatments for patients diagnosed with Myotrem. β-blockers, commonly prescribed to treat heart rhythm disturbances and essential tremor, and primidone, typically used to control epileptic seizures, have proven ineffective in treating Myotrem tremor. Further, other treatments used to mitigate neurogenic tremor, such as Levodopa for Parkinson’s disease [27], are unlikely to alleviate myogenic tremor, which occurs in the absence of neuropathy. Our studies highlight a potential mechanism underlying myogenic tremor, offering valuable insights for designing targeted therapies for myogenic tremorgenesis.
4. Materials and Methods
4.1. Study Approval
All animal work, including housing, husbandry, animal care, and monitoring, were conducted under protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. The generation of the Myotrem C57BL/6J murine model expressing the murine Mybpc1 E249K substitution corresponding to the human E248K variant was previously described [17]. KI Myotrem mice are referred to as the MYBPC1 E248K KI murine model to avoid confusion. Studies use healthy two-month-old WT and heterozygous E248K KI male mice that have not undergone any previous procedures. To maximize consistency, this study focuses on measurements from male mice, as many neuromuscular disorders are known to affect males and females differently [18]. Sample sizes were determined from power analysis and information from previous studies [15].
4.2. Solutions
Relaxing solution consisted of 1 mM dithiothreitol (DTT), 100 mM KCl, 10 mM imidazole, 2.0 mM EGTA, 4.0 mM ATP, and 1 mM free (5 mM total) MgCl2, supplemented with Pefabloc protease inhibitor (Supelco, MilliporeSigma, Burlington, MA, USA). Basal Ca2+ activating solution contained 7.00 mM EGTA, 20 mM imidazole, and 14.50 mM PCr. Minimal Ca2+ activating solution (pCa 9.0) was supplemented with 5.42 mM MgCl2, 72.37 mM KCl, 0.016 mM CaCl2, and 4.7 mM ATP, ionic strength 180 mM. Maximal Ca2+ activating solution (pCa 4.5) was supplemented with 5.26 mM MgCl2, 60.25 mM KCl, 7.01 mM CaCl2, and 4.81 mM ATP, ionic strength 180 mM. Submaximal activating solutions of intermediate pCa values ranging between 5.9 and 6.5 were made by combining the appropriate ratios of minimal and maximal Ca2+ solutions.
4.3. Fiber Preparation and Experimental Setup
Fiber preparation, experimental setup, stretch-activated force measurements, and data analysis occurred as described previously [15] by a blinded experimenter. In short, mice were euthanized via asphyxiation using carbon dioxide followed by cervical dislocation. Soleus muscle was isolated and secured at each tendon to a toothpick to preserve sarcomere length. The tissue was incubated in a 1:1 relaxing solution:glycerol at −20 °C to allow for chemical skinning as previously reported [15,17,28,29,30,31,32]. As in the aforementioned studies, no detergents were applied to these fibers bundles; this yields the possibility of internal organellar calcium stores [33]. On the day of experimentation, single fibers were dissected, and the ends of the fiber were secured to stainless steel troughs with 3-0 monofilament sutures and subsequently mounted to a capacitance gauge force transducer (model 403, sensitivity of 20 mV/mg, resonant frequency of 600 Hz; Aurora Scientific, St. Aurora, ON, Canada) and a length control fitted to a DC torque motor (model 308c; Aurora Scientific). The apparatus was mounted over an Olympus IX70 inverted microscope (with a ×40 objective (Olympus UWD 40, 0.55 N.A., Olympus, Center Valley, PA, USA) on a pneumatic vibration isolation table. Morphological measurements were made while the fiber preparation was relaxed. Sarcomere length was monitored continuously using the IonOptix SarcLen video system and fast Fourier transform analysis of a ~220 × 30 μm region of interest and all measurements were performed at 15 ± 1 °C. A total of eight fibers from five WT mice and 11 fibers from five KI mice were harvested for experimentation (Table 1).
4.4. Slack–Re-Stretch Protocol
Slack–re-stretch experiments were performed as described previously [15,34] to measure crossbridge kinetics in maximal and submaximal Ca2+ activating conditions (pCa 4.5). In brief, permeabilized fibers were placed in maximal Ca2+ activating solution (pCa 4.5) at optimal length and steady state was allowed to develop. Subsequently, the fiber was slackened, rapidly re-stretched to mechanically detach cross-bridges to a value slightly greater than optimal length for ~2 ms and returned to optimal muscle length allowing for crossbridge formation and force redevelopment. The resulting force curve was fit to a single exponential equation:
where the force, F, at any time x, is related to the residual tension immediately after the slack–re-stretch maneuver (Fres), the maximal isometric force (Fmax), and the rate constant of force development, (ktr). For each fiber, the slack–re-stretch maneuver was performed at the onset and conclusion of the mechanical protocols and the reported ktr value is the average of these two repeats.
4.5. Step–Stretch Protocol and Data Analysis
Following the initial slack–re-stretch protocol, the fiber was transferred to submaximal Ca2+ activating solution and subjected to a step–stretch protocol to investigate stretch activation properties. During each submaximal Ca2+ activation, the fiber was allowed to reach steady-state tension (PO) and then up to five separate, fixed voltage (0.05, 0.10, 0.20, 0.30, 0.40 V, motor calibration 235 µm/V) fiber length stretches were applied, which yielded sarcomere length stretches ranging from ~0.5 to 8% sarcomere length. For analysis, group data was pooled for Ca2+ activated forces between 20 and 60% maximal and sarcomere length stretches of 1 to 4% since we previously did not observe independent variable-dependence of stretch activation parameters over these ranges [15]. The rapid fiber stretches elicited changes in force that exhibited the prototypical triphasic stretch-activated response: immediate sharp increase in force (P1) in phase 1; subsequent decrease in force (P2) in phase 2, that occurs with a kinetic constant of krel; and stretch activation in phase 3, which is the slow delayed development in force (PTO) following stretch that occurs at a time constant of kdf. The force trace during this phase was fit to a single exponential rise to maximum equation:
where A is the amplitude of the exponential phase and F is the force at x seconds of phase 3. The fiber then reached a new steady state tension. This stretch was held for about 6 sec before the fiber was shortened to its original sarcomere length (Figure 1A–C). The fiber was allowed to reach steady-state tension again at this original length before undergoing subsequent step stretches, and the stretch-activated response of each stretch was recorded. For each fiber, this protocol was repeated in two different submaximal Ca2+ solutions: one that elicited ~50% of the fiber’s maximal Ca2+ activated force (pCa where PO/PpCa4.5 = 50%), and another that elicited ~25% of the fiber’s maximal Ca2+ activated force (pCa where PO/PpCa4.5 = 25%). Stretch activation tension measurements, i.e., phase 1 maximum force (P1), phase 2 minimum force (P2), stretch-activated force development (PTO), and stretch-induced steady state force (PSS) were normalized to the initial steady state force (PO).
4.6. Single Fiber Immunoblot Analysis
Following mechanical measurements, single permeabilized fibers were incubated in relaxing solution with sodium dodecyl sulfate (SDS). Individual fibers were fractionated by SDS-PAGE using a 4–12% tris-acetate gel (Invitrogen, Waltham, MA, USA) and transferred to nitrocellulose membrane. Lysates from whole soleus WT and KI muscles were included as positive controls and total protein was visualized using SYPRO Ruby protein staining (Thermo Scientific, Waltham, MA, USA). The blot was subsequently cut into strips and probed with antibodies against the slow isoform of myosin heavy chain (mouse monoclonal, Sigma-Aldrich, M8421, 1:1000, Millipore Sigma, St. Louis, MO, USA) and sMyBP-C (rabbit polyclonal, Sigma-Aldrich, SAB3501005, 1:1000, Millipore Sigma, St. Louis, MO, USA) at 4 °C overnight. After washing, the strips were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, Danvers, MA, USA), followed by ECL substrate (Thermo Scientific, Waltham, MA, USA). Poorly preserved fibers were excluded from analysis and thus reported immunoblot data reflects seven of eight WT fibers, six of eight quiescent KI fibers, and three of three pulsing KI fibers.
4.7. Statistical Analysis
Statistical tests, sample sizes (n), and p-values are provided in figure legends. Shapiro–Wilk’s test was used to assess normality, and an F test was used to compare variances. Comparisons between two normal datasets of similar variance were performed using Student’s 2-tailed t-test, whereas comparisons of datasets that failed the normality or variance tests were performed with Mann–Whitney test and Welch’s t-test, respectively. Comparisons between three non-normal datasets were performed using Kruskal–Wallis test. Statistical analysis was performed using SigmaPlot (version 14; Palo Alto, CA, USA).and GraphPad Prism (version 10.4.2; San Diego, CA, USA). Values are expressed as mean ± SEM; ns: not significant; * p < 0.05; ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26115252/s1.
Author Contributions
Conceptualization, J.M.M., L.M.H., C.W.W., K.S.M. and A.K.-K.; methodology, L.M.H. and K.S.M.; validation, L.M.H. and K.S.M.; formal analysis, J.M.M., L.M.H., S.C. and K.S.M.; investigation, L.M.H. and S.C.; resources, K.S.M. and A.K.-K.; writing—original draft preparation, J.M.M. and K.S.M.; writing—review and editing, J.M.M., L.M.H., S.C., C.W.W., K.S.M. and A.K.-K.; visualization, J.M.M., L.M.H., S.C. and K.S.M.; supervision, K.S.M. and A.K.-K.; project administration, K.S.M. and A.K.-K.; funding acquisition, K.S.M. and A.K.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant numbers R01-AR076373 (A.K.-K.) and T32-AR007592 (J.M.M.), and the National Heart, Lung, and Blood Institute grant number R01-HL148785 (K.S.M.).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore (protocol code 1122011 approved 13 January 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Source data, including raw data values and original blot images, and Supplemental Videos, can be accessed at DOI: 10.6084/m9.figshare.28801487.
Acknowledgments
The graphical abstract was prepared with BioRender under the agreement number UO285HU29U.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CNS | Central nervous system |
| EDL | Extensor digitorum longus |
| kdf | Delayed force development rate |
| KI | Knock-In |
| krel | Rapid force decay rate |
| MHC | Myosin Heavy Chain |
| MyBP-C | Myosin binding protein-C |
| PO | Pre-stretch steady state tension |
| PSS | Stretch-induced steady state tension |
| PTO | Stretch activation |
| P1 | Maximum force peak |
| P2 | Minimum force decay |
| sMyBP-C | Slow skeletal myosin binding protein-C |
| SPOC | Spontaneous oscillatory contraction |
| WT | Wild type |
References
- Stavusis, J.; Lace, B.; Schafer, J.; Geist, J.; Inashkina, I.; Kidere, D.; Pajusalu, S.; Wright, N.T.; Saak, A.; Weinhold, M.; et al. Novel Mutations in MYBPC1 Are Associated with Myogenic Tremor and Mild Myopathy. Ann. Neurol. 2019, 86, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.A.; Kontrogianni-Konstantopoulos, A. Myosin Binding Protein-C Slow: A Multifaceted Family of Proteins with a Complex Expression Profile in Fast and Slow Twitch Skeletal Muscles. Front. Physiol. 2013, 4, 391. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.; Offer, G. The Location of C-Protein in Rabbit Skeletal Muscle. Proc. R. Soc. Lond. B Biol. Sci. 1976, 192, 451–461. [Google Scholar] [CrossRef]
- Okagaki, T.; Weber, F.E.; Fischman, D.A.; Vaughan, K.T.; Mikawa, T.; Reinach, F.C. The Major Myosin-Binding Domain of Skeletal Muscle MyBP-C (C Protein) Resides in the COOH-Terminal, Immunoglobulin C2 Motif. J. Cell Biol. 1993, 123, 619–626. [Google Scholar] [CrossRef]
- Gruen, M.; Prinz, H.; Gautel, M. cAPK-Phosphorylation Controls the Interaction of the Regulatory Domain of Cardiac Myosin Binding Protein C with Myosin-S2 in an on-off Fashion. FEBS Lett. 1999, 453, 254–259. [Google Scholar] [CrossRef]
- Moos, C. Fluorescence Microscope Study of the Binding of Added C Protein to Skeletal Muscle Myofibrils. J. Cell Biol. 1981, 90, 25–31. [Google Scholar] [CrossRef]
- Kulikovskaya, I.; McClellan, G.; Flavigny, J.; Carrier, L.; Winegrad, S. Effect of MyBP-C Binding to Actin on Contractility in Heart Muscle. J. Gen. Physiol. 2003, 122, 761–774. [Google Scholar] [CrossRef]
- Geist, J.; Ward, C.W.; Kontrogianni-Konstantopoulos, A. Structure before Function: Myosin Binding Protein-C Slow Is a Structural Protein with Regulatory Properties. FASEB J. 2018, 32, fj201800624R. [Google Scholar] [CrossRef]
- Campbell, K.B.; Chandra, M. Functions of Stretch Activation in Heart Muscle. J. Gen. Physiol. 2006, 127, 89–94. [Google Scholar] [CrossRef]
- Linari, M.; Reedy, M.K.; Reedy, M.C.; Lombardi, V.; Piazzesi, G. Ca-Activation and Stretch-Activation in Insect Flight Muscle. Biophys. J. 2004, 87, 1101–1111. [Google Scholar] [CrossRef]
- Josephson, R.K.; Malamud, J.G.; Stokes, D.R. Asynchronous Muscle: A Primer. J. Exp. Biol. 2000, 203, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.P. Making waves: A Proposed New Role for Myosin-Binding Protein C in Regulating Oscillatory Contractions in Vertebrate Striated Muscle. J. Gen. Physiol. 2021, 153, e202012729. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, J.E.; Dunning, S.B.; Moss, R.L. Ablation of Cardiac Myosin-Binding Protein-C Accelerates Stretch Activation in Murine Skinned Myocardium. Circ. Res. 2006, 98, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Straight, C.R.; Bell, K.M.; Slosberg, J.N.; Miller, M.S.; Swank, D.M. A Myosin-Based Mechanism for Stretch Activation and Its Possible Role Revealed by Varying Phosphate Concentration in Fast and Slow Mouse Skeletal Muscle Fibers. Am. J. Physiol. Cell Physiol. 2019, 317, C1143–C1152. [Google Scholar] [CrossRef]
- Robinett, J.C.; Hanft, L.M.; Biesiadecki, B.; McDonald, K.S. Molecular Regulation of Stretch Activation. Am. J. Physiol. Cell Physiol. 2022, 323, C1728–C1739. [Google Scholar] [CrossRef]
- Stelzer, J.E.; Moss, R.L. Contributions of Stretch Activation to Length-Dependent Contraction in Murine Myocardium. J. Gen. Physiol. 2006, 128, 461–471. [Google Scholar] [CrossRef]
- Geist Hauserman, J.; Stavusis, J.; Joca, H.C.; Robinett, J.C.; Hanft, L.; Vandermeulen, J.; Zhao, R.; Stains, J.P.; Konstantopoulos, K.; McDonald, K.S.; et al. Sarcomeric Deficits Underlie MYBPC1-Associated Myopathy with Myogenic Tremor. JCI Insight 2021, 6, e147612. [Google Scholar] [CrossRef]
- Vinciguerra, C.; Iacono, S.; Bevilacqua, L.; Landolfi, A.; Piscosquito, G.; Ginanneschi, F.; Schiro, G.; Di Stefano, V.; Brighina, F.; Barone, P.; et al. Sex Differences in Neuromuscular Disorders. Mech. Ageing Dev. 2023, 211, 111793. [Google Scholar] [CrossRef]
- Yasuda, K.; Shindo, Y.; Ishiwata, S. Synchronous Behavior of Spontaneous Oscillations of Sarcomeres in Skeletal Myofibrils under Isotonic Conditions. Biophys. J. 1996, 70, 1823–1829. [Google Scholar] [CrossRef]
- Schaefer, J.; Saak, A.; Bonnemann, C.G.; Jackson, S. Myogenic Tremor—A Novel Tremor Entity. Curr. Opin. Neurol. 2021, 34, 706–713. [Google Scholar] [CrossRef]
- Semmler, J.G.; Kornatz, K.W.; Dinenno, D.V.; Zhou, S.; Enoka, R.M. Motor Unit Synchronisation Is Enhanced during Slow Lengthening Contractions of a Hand Muscle. J. Physiol. 2002, 545, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Napierski, N.C.; Granger, K.; Langlais, P.R.; Moran, H.R.; Strom, J.; Touma, K.; Harris, S.P. A Novel “Cut and Paste” Method for in situ Replacement of cMyBP-C Reveals a New Role for cMyBP-C in the Regulation of Contractile Oscillations. Circ. Res. 2020, 126, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, J.E.; Patel, J.R.; Moss, R.L. Protein Kinase A-Mediated Acceleration of the Stretch Activation Response in Murine Skinned Myocardium Is Eliminated by Ablation of cMyBP-C. Circ. Res. 2006, 99, 884–890. [Google Scholar] [CrossRef]
- Ackermann, M.A.; Kontrogianni-Konstantopoulos, A. Myosin Binding Protein-C Slow Is a Novel Substrate for Protein Kinase A (PKA) and C (PKC) in Skeletal Muscle. J. Proteome Res. 2011, 10, 4547–4555. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Kistemaker, D.A.; Van Soest, A.J.; Wong, J.D.; Kurtzer, I.; Gribble, P.L. Control of Position and Movement Is Simplified by Combined Muscle Spindle and Golgi Tendon Organ Feedback. J. Neurophysiol. 2013, 109, 1126–1139. [Google Scholar] [CrossRef]
- Koller, W.C.; Rueda, M.G. Mechanism of Action of Dopaminergic Agents in Parkinson’s Disease. Neurology 1998, 50, S11–S14, discussion S44–S48. [Google Scholar] [CrossRef]
- McDonald, K.S.; Fitts, R.H. Effect of Hindlimb Unweighting on Single Soleus Fiber Maximal Shortening Velocity and ATPase Activity. J. Appl. Physiol. 1993, 74, 2949–2957. [Google Scholar] [CrossRef]
- McDonald, K.S.; Fitts, R.H. Effect of Hindlimb Unloading on Rat Soleus Fiber Force, Stiffness, and Calcium Sensitivity. J. Appl. Physiol. 1995, 79, 1796–1802. [Google Scholar] [CrossRef]
- McDonald, K.S.; Wolff, M.R.; Moss, R.L. Sarcomere Length Dependence of the Rate of Tension Redevelopment and Submaximal Tension in Rat and Rabbit Skinned Skeletal Muscle Fibers. J. Physiol. 1997, 501, 607–621. [Google Scholar] [CrossRef]
- McDonald, K.S. Ca2+ Dependence of Loaded Shortening in Rat Skinned Cardiac Myocytes and Skeletal Muscle Fibers. J. Physiol. 2000, 525, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Hanft, L.M.; McDonald, K.S. Length Dependence of Force Generation Exhibit Similarities between Rat Cardiac Myocytes and Skeletal Muscle Fibres. J. Physiol. 2010, 588, 2891–2903. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B. An Indirect Proof of Stretch-Induced Ca++ Release from the Sarcoplasmic Reticulum in Glycerinated Skeletal and Heart Muscle Preparations (Author’s Transl). Basic. Res. Cardiol. 1979, 74, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Hinken, A.C.; McDonald, K.S. Inorganic Phosphate Speeds Loaded Shortening in Rat Skinned Cardiac Myocytes. Am. J. Physiol. Cell Physiol. 2004, 287, C500–C507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).