Divergent Immune Pathways in Coronary Artery Disease and Aortic Stenosis: The Role of Chronic Inflammation and Senescence
Abstract
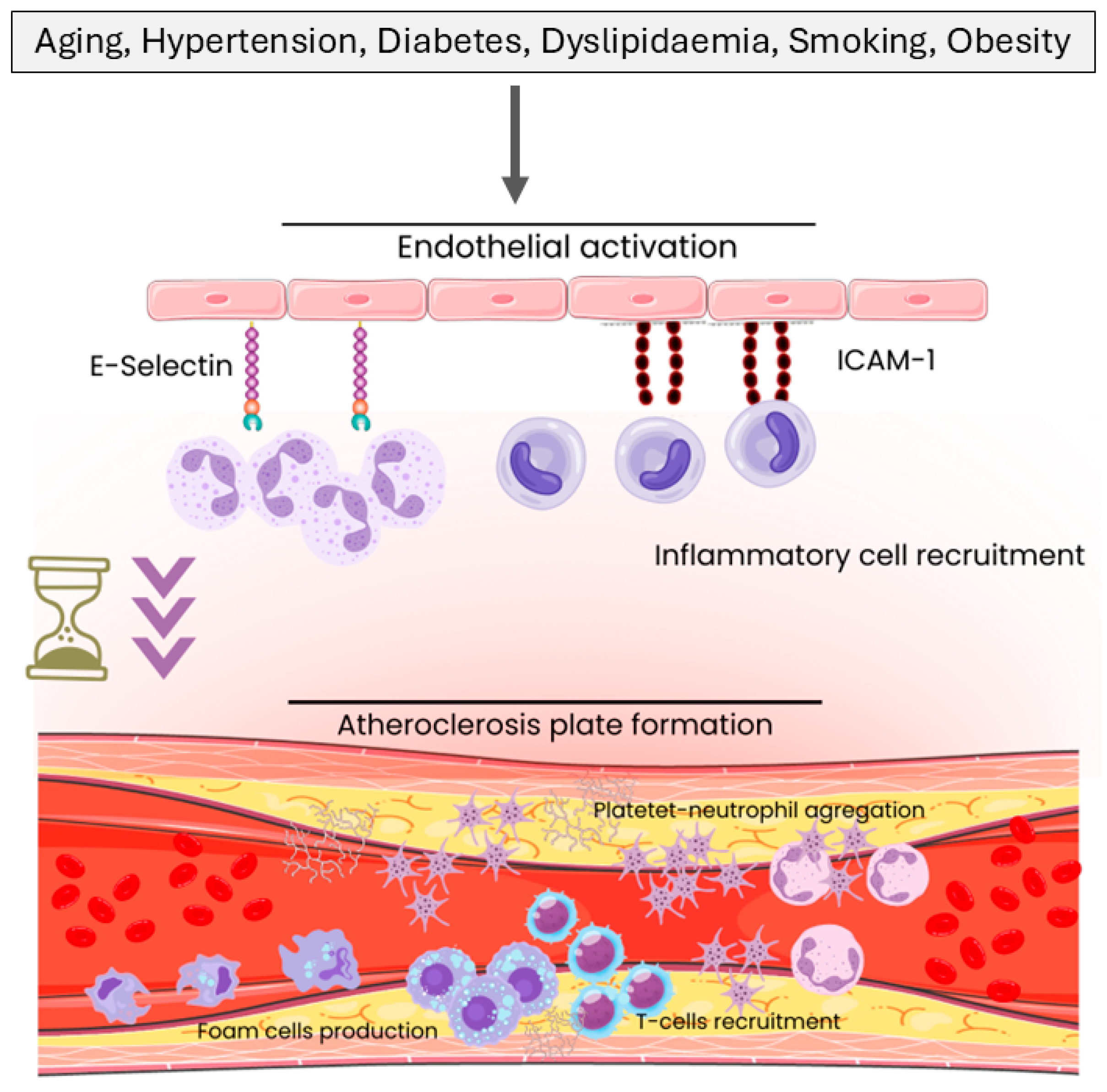
1. Introduction
2. Results
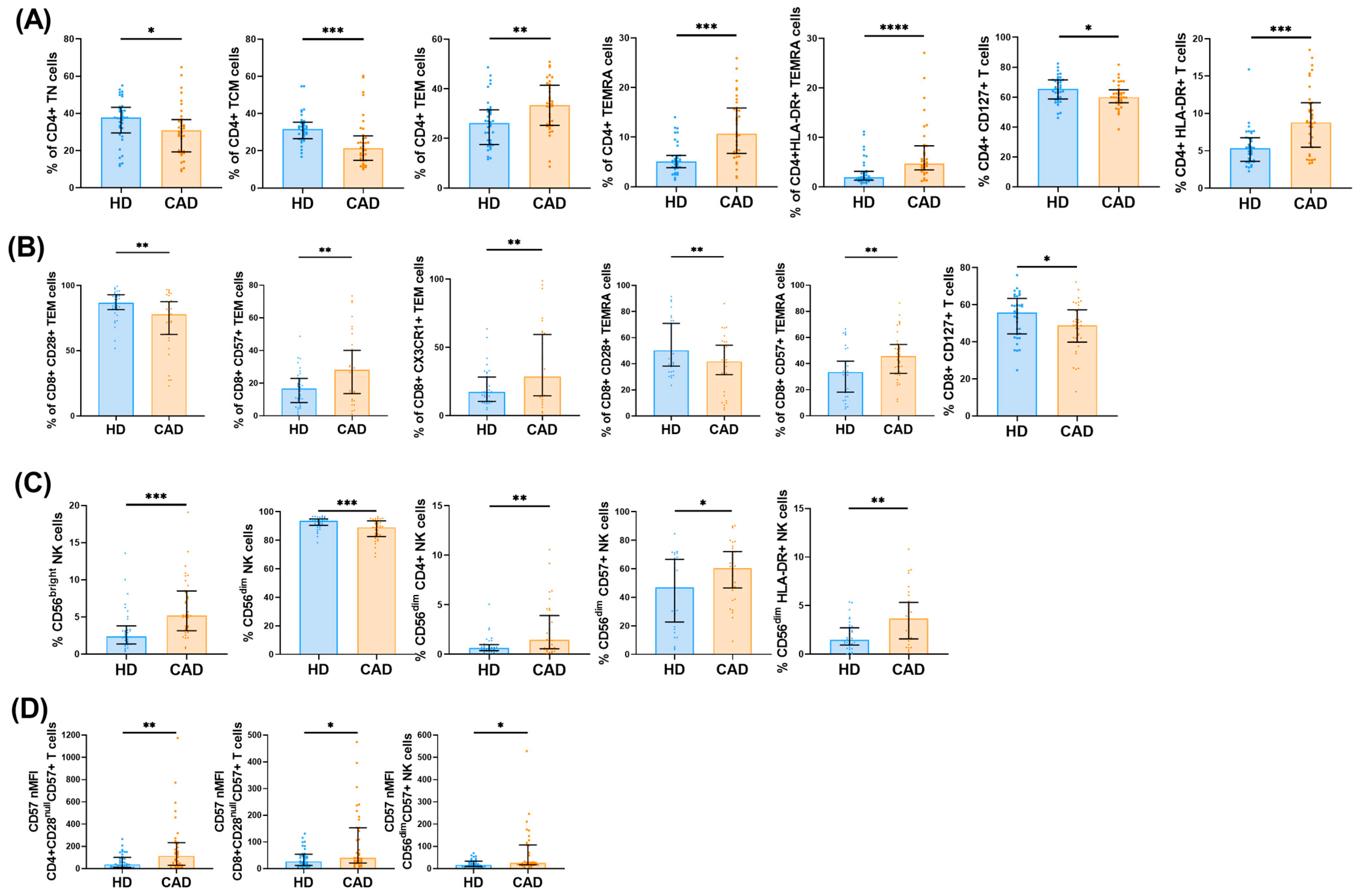
2.1. Peripheral Immune Cell Profiles in CAD Patients
2.2. Peripheral Immune Cell Profiles in iCAD and ASCAD Patients
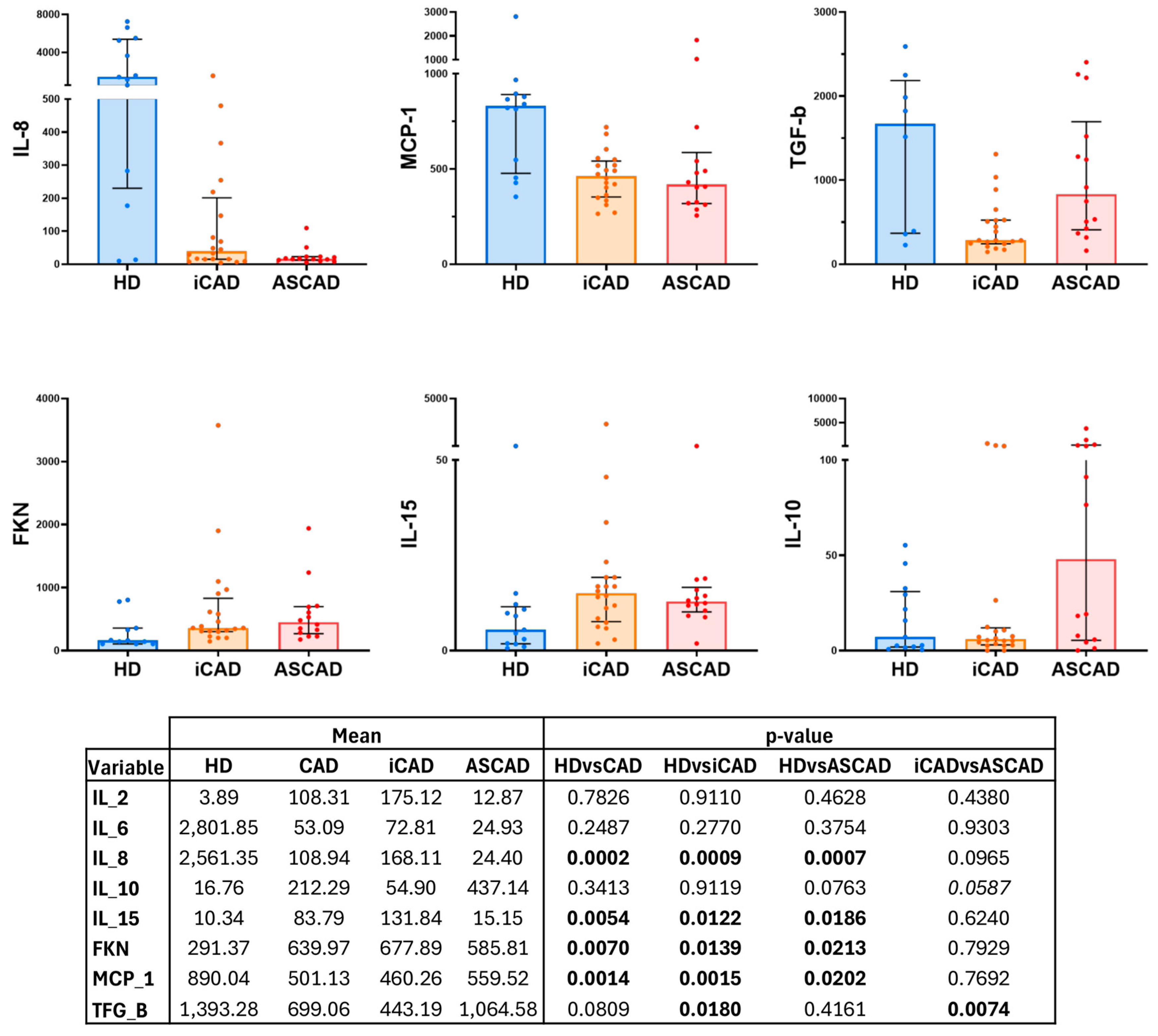
2.3. Inflammatory Status in Patients with CAD
2.4. Predictive Model
3. Discussion
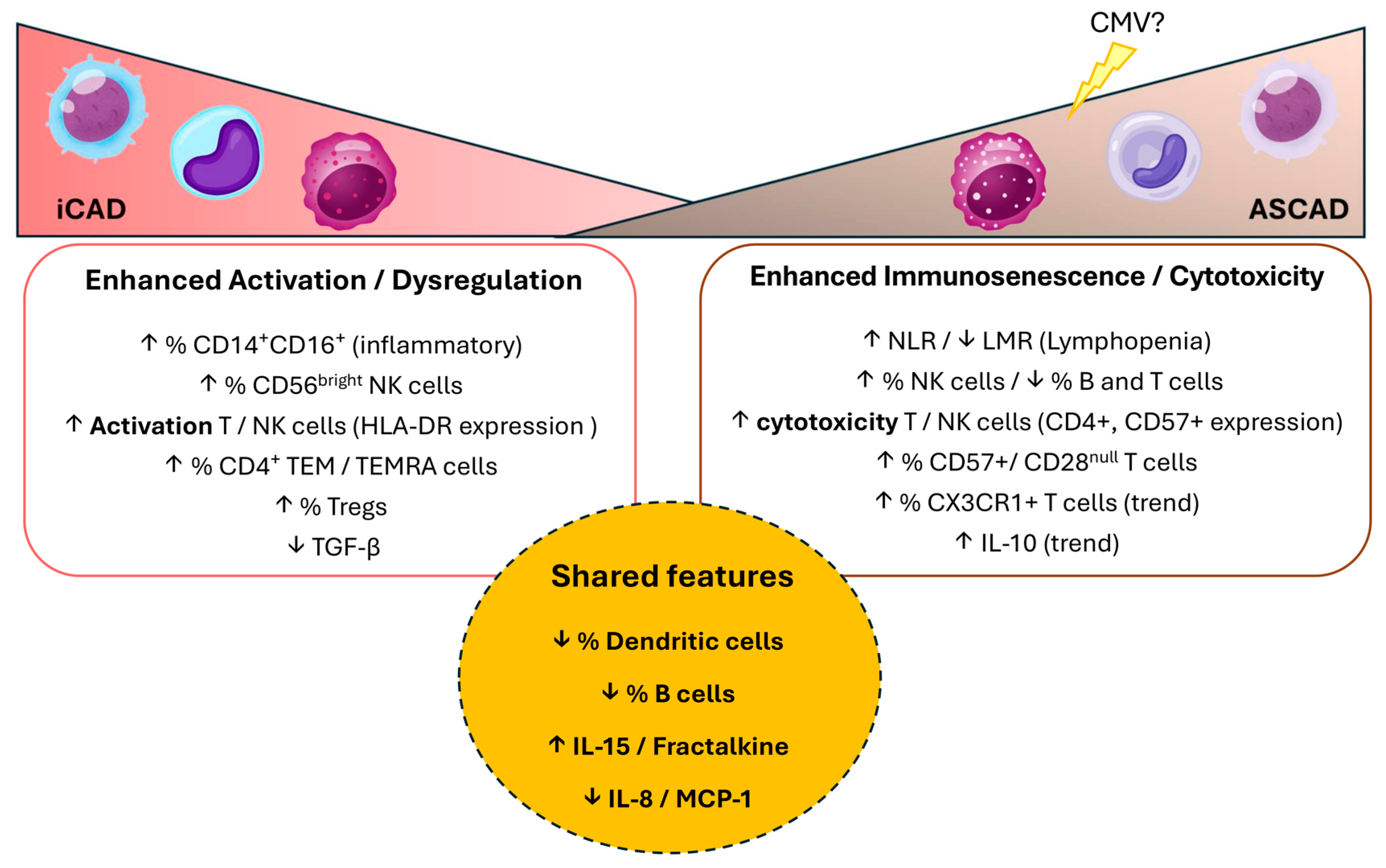
3.1. General Immune Dysregulation in CAD
3.2. Distinct Immune Features of iCAD: A Predominantly Activated Immune State
3.3. Distinct Immune Features of ASCAD: A Predominantly Immunosenescent and Cytotoxic Profile
3.4. Immunosenescence as a Key Driver of ASCAD Pathophysiology
3.5. Potential Cytometry Panel for Disease Distinction
4. Materials and Methods
4.1. Study Design and Participants
4.2. Ethics Approval
4.3. Sample Collection, Processing, and Data Acquisition
4.4. Processing Data, Modelling Process, and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | coronary artery disease |
| HDs | healthy donors |
| iCAD | isolated coronary artery disease |
| ASCAD | aortic stenosis and coronary artery disease |
| TEM | effector memory T cells |
| TEMRA | terminally differentiated effector memory T cells |
| DC | dendritic cells |
| LMR | lymphocyte-to-monocyte ratio |
| NLR | neutrophil-to-lymphocyte ratio |
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.-X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- Broeders, W.; Bekkering, S.; El Messaoudi, S.; Joosten, L.A.B.; van Royen, N.; Riksen, N.P. Innate immune cells in the pathophysiology of calcific aortic valve disease: Lessons to be learned from atherosclerotic cardiovascular disease? Basic. Res. Cardiol. 2022, 117, 28. [Google Scholar] [CrossRef]
- Ghamar Talepoor, A.; Doroudchi, M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Front. Immunol. 2022, 13, 945016. [Google Scholar] [CrossRef]
- Vallejo, A.N.; Brandes, J.C.; Weyand, C.M.; Goronzy, J.J. Modulation of CD28 expression: Distinct regulatory pathways during activation and replicative senescence. J. Immunol. 1999, 162, 6572–6579. [Google Scholar] [CrossRef]
- Formentini, M.; Navas, A.; Hassouneh, F.; Lopez-Sejas, N.; Alonso, C.; Tarazona, R.; Solana, R.; Pera, A. Impact of Cytomegalovirus and Age on T-Cell Subsets Defined by CD161, CD300a, and/or CD57 Expression in Healthy Andalusians. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1946–1953. [Google Scholar] [CrossRef]
- Alvarez-Heredia, P.; Reina-Alfonso, I.; Dominguez-Del-Castillo, J.J.; Gutierrez-Gonzalez, C.; Hassouneh, F.; Batista-Duharte, A.; Perez, A.B.; Tarazona, R.; Solana, R.; Pera, A. Accelerated T-Cell Immunosenescence in Cytomegalovirus-Seropositive Individuals After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2023, 228, 576–585. [Google Scholar] [CrossRef]
- Broadley, I.; Pera, A.; Morrow, G.; Davies, K.A.; Kern, F. Expansions of Cytotoxic CD4(+)CD28(-) T Cells Drive Excess Cardiovascular Mortality in Rheumatoid Arthritis and Other Chronic Inflammatory Conditions and Are Triggered by CMV Infection. Front. Immunol. 2017, 8, 195. [Google Scholar] [CrossRef]
- Namekawa, T.; Snyder, M.R.; Yen, J.H.; Goehring, B.E.; Leibson, P.J.; Weyand, C.M.; Goronzy, J.J. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J. Immunol. 2000, 165, 1138–1145. [Google Scholar] [CrossRef]
- Teo, F.H.; de Oliveira, R.T.; Mamoni, R.L.; Ferreira, M.C.; Nadruz, W., Jr.; Coelho, O.R.; Fernandes Jde, L.; Blotta, M.H. Characterization of CD4+CD28null T cells in patients with coronary artery disease and individuals with risk factors for atherosclerosis. Cell. Immunol. 2013, 281, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Araguas, E.T.; Baboonian, C.; Kaski, J.C. CD4+ CD28 null T cells in coronary artery disease: When helpers become killers. Cardiovasc. Res. 2009, 81, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bergström, I.; Backteman, K.; Lundberg, A.; Ernerudh, J.; Jonasson, L. Persistent accumulation of interferon-γ-producing CD8+CD56+ T cells in blood from patients with coronary artery disease. Atherosclerosis 2012, 224, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Moro-García, M.A.; López Iglesias, F.; Avanzas, P.; Echeverría, A.; López-Larrea, C.; Morís de la Tassa, C.; Alonso-Arias, R. Disease complexity in acute coronary syndrome is related to the patient’s immunological status. Int. J. Cardiol. 2015, 189, 115–123. [Google Scholar] [CrossRef]
- Paradis, J.M.; Fried, J.; Nazif, T.; Kirtane, A.; Harjai, K.; Khalique, O.; Grubb, K.; George, I.; Hahn, R.; Williams, M.; et al. Aortic stenosis and coronary artery disease: What do we know? What don’t we know? A comprehensive review of the literature with proposed treatment algorithms. Eur. Heart J. 2014, 35, 2069–2082. [Google Scholar] [CrossRef]
- Pujari, S.H.; Agasthi, P. Aortic Stenosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Di Gioia, G.; Bartunek, J.; Tesorio, T.; Vukcevic, V.; Aleksandric, S.; Dobric, M.; Franco, D.; Barbato, E.; Banovic, M. Pathophysiology, Diagnosis, and Treatment of Patients with Concomitant Severe Aortic Stenosis and Coronary Artery Disease: A Closer Look to the Unresolved Perplexity. J. Clin. Med. 2021, 10, 1617. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Singh, M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep. 2015, 7, 08. [Google Scholar] [CrossRef]
- Kott, K.A.; Chan, A.S.; Vernon, S.T.; Hansen, T.; Kim, T.; de Dreu, M.; Gunasegaran, B.; Murphy, A.J.; Patrick, E.; Psaltis, P.J.; et al. Mass cytometry analysis reveals altered immune profiles in patients with coronary artery disease. Clin. Transl. Immunol. 2023, 12, e1462. [Google Scholar] [CrossRef]
- Iqneibi, S.; Saigusa, R.; Khan, A.; Oliaeimotlagh, M.; Armstrong Suthahar, S.S.; Kumar, S.; Alimadadi, A.; Durant, C.P.; Ghosheh, Y.; McNamara, C.A.; et al. Single cell transcriptomics reveals recent CD8T cell receptor signaling in patients with coronary artery disease. Front. Immunol. 2023, 14, 1239148. [Google Scholar] [CrossRef]
- Bullenkamp, J.; Mengoni, V.; Kaur, S.; Chhetri, I.; Dimou, P.; Astroulakis, Z.M.J.; Kaski, J.C.; Dumitriu, I.E. Interleukin-7 and interleukin-15 drive CD4+CD28null T lymphocyte expansion and function in patients with acute coronary syndrome. Cardiovasc. Res. 2021, 117, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Weber, C.; Forlow, S.B.; Sperandio, M.; Thatte, J.; Mack, M.; Jung, S.; Littman, D.R.; Ley, K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J. Clin. Investig. 2001, 108, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Matsui, K.; Murakami, Y.; Shimada, K. Monocyte chemoattractant protein-1 and coronary artery disease. Clin. Cardiol. 2002, 25, 143–147. [Google Scholar] [CrossRef]
- Romuk, E.; Skrzep-Poloczek, B.; Wojciechowska, C.; Tomasik, A.; Birkner, E.; Wodniecki, J.; Gabrylewicz, B.; Ochala, A.; Tendera, M. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur. J. Clin. Investig. 2002, 32, 657–661. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Peters, R.J.; Hack, C.E.; Day, N.E.; Luben, R.; Bingham, S.A.; Wareham, N.J.; Reitsma, P.H.; Khaw, K.T. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1503–1508. [Google Scholar] [CrossRef]
- Robinson, T.O.; Schluns, K.S. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol. Lett. 2017, 190, 159–168. [Google Scholar] [CrossRef]
- Loh, S.X.; Ekinci, Y.; Spray, L.; Jeyalan, V.; Olin, T.; Richardson, G.; Austin, D.; Alkhalil, M.; Spyridopoulos, I. Fractalkine Signalling (CX(3)CL1/CX(3)CR1 Axis) as an Emerging Target in Coronary Artery Disease. J. Clin. Med. 2023, 12, 4821. [Google Scholar] [CrossRef]
- Hafiane, A.; Pisaturo, A.; Favari, E.; Bortnick, A.E. Atherosclerosis, calcific aortic valve disease and mitral annular calcification: Same or different? Int. J. Cardiol. 2025, 420, 132741. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, G.; Li, Y.; Yu, B.; Guo, Z.; Chen, X.; Liu, T.; Li, G. Prognosis impact of multiple novel lymphocyte-based inflammatory indices in patients with initially diagnosed coronary artery disease. Immun. Inflamm. Dis. 2024, 12, e1340. [Google Scholar] [CrossRef]
- Winchester, R.; Wiesendanger, M.; O’Brien, W.; Zhang, H.Z.; Maurer, M.S.; Gillam, L.D.; Schwartz, A.; Marboe, C.; Stewart, A.S. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J. Immunol. 2011, 187, 1006–1014. [Google Scholar] [CrossRef]
- Álvarez-Heredia, P.; Domínguez-del-Castillo, J.J.; Reina-Alfonso, I.; Gutiérrez-González, C.; Hassouneh, F.; Batista-Duharte, A.; Trujillo-Aguilera, A.; López-Romero, R.; Muñoz, I.; Solana, R.; et al. A Straightforward Cytometry-Based Protocol for the Comprehensive Analysis of the Inflammatory Valve Infiltrate in Aortic Stenosis. Int. J. Mol. Sci. 2023, 24, 2194. [Google Scholar] [CrossRef] [PubMed]
- Jonasson, L.; Tompa, A.; Wikby, A. Expansion of peripheral CD8+ T cells in patients with coronary artery disease: Relation to cytomegalovirus infection. J. Intern. Med. 2003, 254, 472–478. [Google Scholar] [CrossRef] [PubMed]
- García-Torre, A.; Bueno-García, E.; Moro-García, M.A.; López-Martínez, R.; Rioseras, B.; Díaz-Molina, B.; Lambert, J.L.; Alonso-Arias, R. IL-10 indirectly modulates functional activity of CD4(+)CD28(null) T-lymphocytes through LFA-3 and HLA class II inhibition. Immunology 2024, 173, 296–309. [Google Scholar] [CrossRef]
- Pera, A.; Caserta, S.; Albanese, F.; Blowers, P.; Morrow, G.; Terrazzini, N.; Smith, H.E.; Rajkumar, C.; Reus, B.; Msonda, J.R.; et al. CD28(null) pro-atherogenic CD4 T-cells explain the link between CMV infection and an increased risk of cardiovascular death. Theranostics 2018, 8, 4509–4519. [Google Scholar] [CrossRef]
- Pera, A.; Broadley, I.; Davies, K.A.; Kern, F. Cytomegalovirus as a Driver of Excess Cardiovascular Mortality in Rheumatoid Arthritis: A Red Herring or a Smoking Gun? Circ. Res. 2017, 120, 274–277. [Google Scholar] [CrossRef]
- Chow, F.S.; Jusko, W.J. Immunosuppressive interactions among calcium channel antagonists and selected corticosteroids and macrolides using human whole blood lymphocytes. Drug Metab. Pharmacokinet. 2004, 19, 413–421. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef]
- Felkle, D.; Jarczynski, M.; Kaleta, K.; Zieba, K.; Nazimek, K. The immunomodulatory effects of antihypertensive therapy: A review. Biomed. Pharmacother. 2022, 153, 113287. [Google Scholar] [CrossRef]



| Absolute Numbers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean | p-Value | |||||||
| HD | CAD | iCAD | ASCAD | HD vs. CAD | HD vs. iCAD | HD vs. ASCAD | iCAD vs. ASCAD | |
| LEUKOCYTES | 7.1061 | 8.0683 | 7.8380 | 8.3563 | 0.0561 | 0.2141 | 0.0703 | 0.4875 |
| NEUTROPHILS | 4.1519 | 5.1683 | 4.6815 | 5.7769 | 0.0250 | 0.3514 | 0.0069 | 0.0620 |
| BASOPHILS | 0.0696 | 0.0468 | 0.0464 | 0.0474 | 0.0016 | 0.0043 | 0.0251 | 0.9164 |
| MONOCITES | 0.4403 | 0.5139 | 0.5050 | 0.5250 | 0.1424 | 0.1928 | 0.1493 | 0.5772 |
| LYMPHOCYTES | 2.1608 | 1.8561 | 1.9975 | 1.6794 | 0.0474 | 0.3129 | 0.0209 | 0.1387 |
| NLR | 1.9925 | 3.2962 | 2.4635 | 4.3370 | 0.0006 | 0.0957 | 0.0000 | 0.0215 |
| LMR | 5.3645 | 3.8324 | 4.2943 | 3.2551 | 0.0026 | 0.1401 | 0.0003 | 0.0457 |
| CD4:CD8 Ratio | 2.9422 | 2.4564 | 2.3919 | 2.5369 | 0.3668 | 0.4399 | 0.4979 | 0.7410 |
| DCs | 0.0643 | 0.0370 | 0.0348 | 0.0400 | 0.0000 | 0.0000 | 0.0005 | 0.1794 |
| mDCs | 0.0497 | 0.0259 | 0.0261 | 0.0256 | 0.0000 | 0.0040 | 0.6263 | 0.0277 |
| pDCs | 0.0085 | 0.0060 | 0.0067 | 0.0050 | 0.0067 | 0.0001 | 0.0003 | 0.0253 |
| dn DCs | 0.0047 | 0.0045 | 0.0019 | 0.0081 | 0.0977 | 0.0982 | 0.0040 | 0.1438 |
| CD14+CD16lo | 0.3231 | 0.2770 | 0.2086 | 0.3626 | 0.0295 | 0.0100 | 0.2069 | 0.1317 |
| CD14+CD16+ | 0.0495 | 0.0566 | 0.0525 | 0.0618 | 0.5406 | 0.8828 | 0.3922 | 0.1491 |
| CD14loCD16++ | 0.0088 | 0.0054 | 0.0041 | 0.0071 | 0.0021 | 0.0010 | 0.1071 | 0.6258 |
| B cells | 0.3657 | 0.1578 | 0.1613 | 0.1534 | 0.0000 | 0.0000 | 0.0000 | 0.7413 |
| NK cells | 0.4136 | 0.2610 | 0.2173 | 0.3157 | 0.0001 | 0.0000 | 0.0201 | 0.0389 |
| T cells | 1.9152 | 1.2096 | 1.2688 | 1.1355 | 0.0000 | 0.0000 | 0.0001 | 0.4565 |
| CD4 | 1.2864 | 0.7546 | 0.8371 | 0.6515 | 0.0000 | 0.0000 | 0.0000 | 0.0957 |
| CD8 | 0.5472 | 0.3981 | 0.3755 | 0.4263 | 0.0004 | 0.0057 | 0.0026 | 0.0827 |
| CD4hiCD8lo | 0.0349 | 0.0136 | 0.0122 | 0.0152 | 0.1652 | 0.0370 | 0.9765 | 0.0949 |
| DP | 0.0131 | 0.0068 | 0.0070 | 0.0065 | 0.0018 | 0.0215 | 0.0048 | 0.3049 |
| DN | 0.0682 | 0.0501 | 0.0492 | 0.0512 | 0.0188 | 0.1065 | 0.0246 | 0.7176 |
| DN TCRγδ | 0.0567 | 0.0397 | 0.0403 | 0.0390 | 0.0361 | 0.1985 | 0.0289 | 0.4984 |
| DN TCRab | 0.0116 | 0.0104 | 0.0089 | 0.0122 | 0.0902 | 0.0890 | 0.3202 | 0.9624 |
| T reg | 0.0829 | 0.0540 | 0.0659 | 0.0390 | 0.0001 | 0.0256 | 0.0000 | 0.0039 |
| Percentages | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean | p-Value | |||||||
| HD | CAD | iCAD | ASCAD | HD vs. CAD | HD vs. iCAD | HD vs. ASCAD | iCAD vs. ASCAD | |
| NEUTROPHILS | 57.6645 | 63.1828 | 59.2507 | 68.0978 | 0.0208 | 0.5958 | 0.0008 | 0.0188 |
| BASOPHILS | 0.8164 | 0.6069 | 0.6027 | 0.6122 | 0.0121 | 0.0646 | 0.0238 | 0.4402 |
| MONOCITES | 6.3300 | 6.5400 | 6.4982 | 6.5922 | 0.6704 | 0.8337 | 0.6224 | 0.8789 |
| LYMPHOCYTES | 30.9218 | 23.8535 | 26.2981 | 20.7977 | 0.0001 | 0.0310 | 0.0001 | 0.0949 |
| DCs | 0.7534 | 0.4980 | 0.4545 | 0.5561 | 0.0000 | 0.0000 | 0.0062 | 0.0743 |
| mDCs | 0.5821 | 0.3412 | 0.3388 | 0.3443 | 0.0000 | 0.0104 | 0.7348 | 0.1222 |
| pDCs | 0.0997 | 0.0793 | 0.0892 | 0.0662 | 0.0643 | 0.0003 | 0.0008 | 0.0143 |
| dn DCs | 0.0551 | 0.0691 | 0.0245 | 0.1285 | 0.1268 | 0.3629 | 0.0262 | 0.1470 |
| CD14+CD16lo | 79.8951 | 76.7538 | 72.6970 | 81.8248 | 0.5256 | 0.0526 | 0.4035 | 0.0010 |
| CD14+CD16+ | 13.0839 | 18.9086 | 24.3082 | 12.1591 | 0.0736 | 0.0004 | 0.2717 | 0.0179 |
| CD14loCD16++ | 2.2437 | 1.6752 | 1.7062 | 1.6364 | 0.0242 | 0.0596 | 0.0280 | 0.2114 |
| B cells | 4.4097 | 2.0638 | 2.2182 | 1.8708 | 0.0000 | 0.0000 | 0.0000 | 0.5819 |
| NK cells | 12.3130 | 16.0074 | 13.6464 | 18.9587 | 0.0774 | 0.4093 | 0.0312 | 0.1783 |
| T cells | 70.2394 | 68.1008 | 70.4790 | 65.1281 | 0.4838 | 0.8952 | 0.1828 | 0.2358 |
| CD4 | 67.3019 | 63.7722 | 65.3295 | 61.8256 | 0.3974 | 0.4891 | 0.4493 | 0.9124 |
| CD8 | 28.2339 | 31.3919 | 29.8570 | 33.3106 | 0.4174 | 0.5126 | 0.6062 | 0.8381 |
| CD4hiCD8lo | 2.7153 | 2.0528 | 1.6825 | 2.5156 | 0.5468 | 0.3382 | 0.0292 | 0.0071 |
| DP | 0.6736 | 0.5498 | 4.2526 | 0.5349 | 0.2408 | 0.9932 | 0.2330 | 0.7652 |
| DN | 3.7772 | 4.2866 | 80.3963 | 4.3290 | 0.7076 | 0.6903 | 0.4979 | 0.7176 |
| DN TCRγδ | 78.8747 | 79.1023 | 19.6037 | 77.4848 | 0.4165 | 0.6903 | 0.3399 | 0.4984 |
| DN TCRab | 21.1253 | 20.8977 | 0.5618 | 22.5152 | 0.4165 | 0.4603 | 0.3399 | 0.4984 |
| T reg | 6.5278 | 7.2093 | 8.1446 | 6.0401 | 0.1584 | 0.0057 | 0.4828 | 0.0149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-del-Castillo, J.J.; Álvarez-Heredia, P.; Reina-Alfonso, I.; Vallejo-Bermúdez, M.-I.; López-Romero, R.; Moreno-Moreno, J.A.; Bilbao-Carrasco, L.; Moya-Gonzalez, J.; Muñoz-Calero, M.; Tarazona, R.; et al. Divergent Immune Pathways in Coronary Artery Disease and Aortic Stenosis: The Role of Chronic Inflammation and Senescence. Int. J. Mol. Sci. 2025, 26, 5248. https://doi.org/10.3390/ijms26115248
Domínguez-del-Castillo JJ, Álvarez-Heredia P, Reina-Alfonso I, Vallejo-Bermúdez M-I, López-Romero R, Moreno-Moreno JA, Bilbao-Carrasco L, Moya-Gonzalez J, Muñoz-Calero M, Tarazona R, et al. Divergent Immune Pathways in Coronary Artery Disease and Aortic Stenosis: The Role of Chronic Inflammation and Senescence. International Journal of Molecular Sciences. 2025; 26(11):5248. https://doi.org/10.3390/ijms26115248
Chicago/Turabian StyleDomínguez-del-Castillo, José Joaquín, Pablo Álvarez-Heredia, Irene Reina-Alfonso, Maria-Isabel Vallejo-Bermúdez, Rosalía López-Romero, Jose Antonio Moreno-Moreno, Lucía Bilbao-Carrasco, Javier Moya-Gonzalez, María Muñoz-Calero, Raquel Tarazona, and et al. 2025. "Divergent Immune Pathways in Coronary Artery Disease and Aortic Stenosis: The Role of Chronic Inflammation and Senescence" International Journal of Molecular Sciences 26, no. 11: 5248. https://doi.org/10.3390/ijms26115248
APA StyleDomínguez-del-Castillo, J. J., Álvarez-Heredia, P., Reina-Alfonso, I., Vallejo-Bermúdez, M.-I., López-Romero, R., Moreno-Moreno, J. A., Bilbao-Carrasco, L., Moya-Gonzalez, J., Muñoz-Calero, M., Tarazona, R., Solana, R., Batista-Duharte, A., Muñoz, I., & Pera, A. (2025). Divergent Immune Pathways in Coronary Artery Disease and Aortic Stenosis: The Role of Chronic Inflammation and Senescence. International Journal of Molecular Sciences, 26(11), 5248. https://doi.org/10.3390/ijms26115248











