Dual Modulation of Autophagy and Apoptosis as Anticancer Mechanism of Action of Khaya grandiofoliola in Colon Carcinoma Cells
Abstract
1. Introduction
2. Results
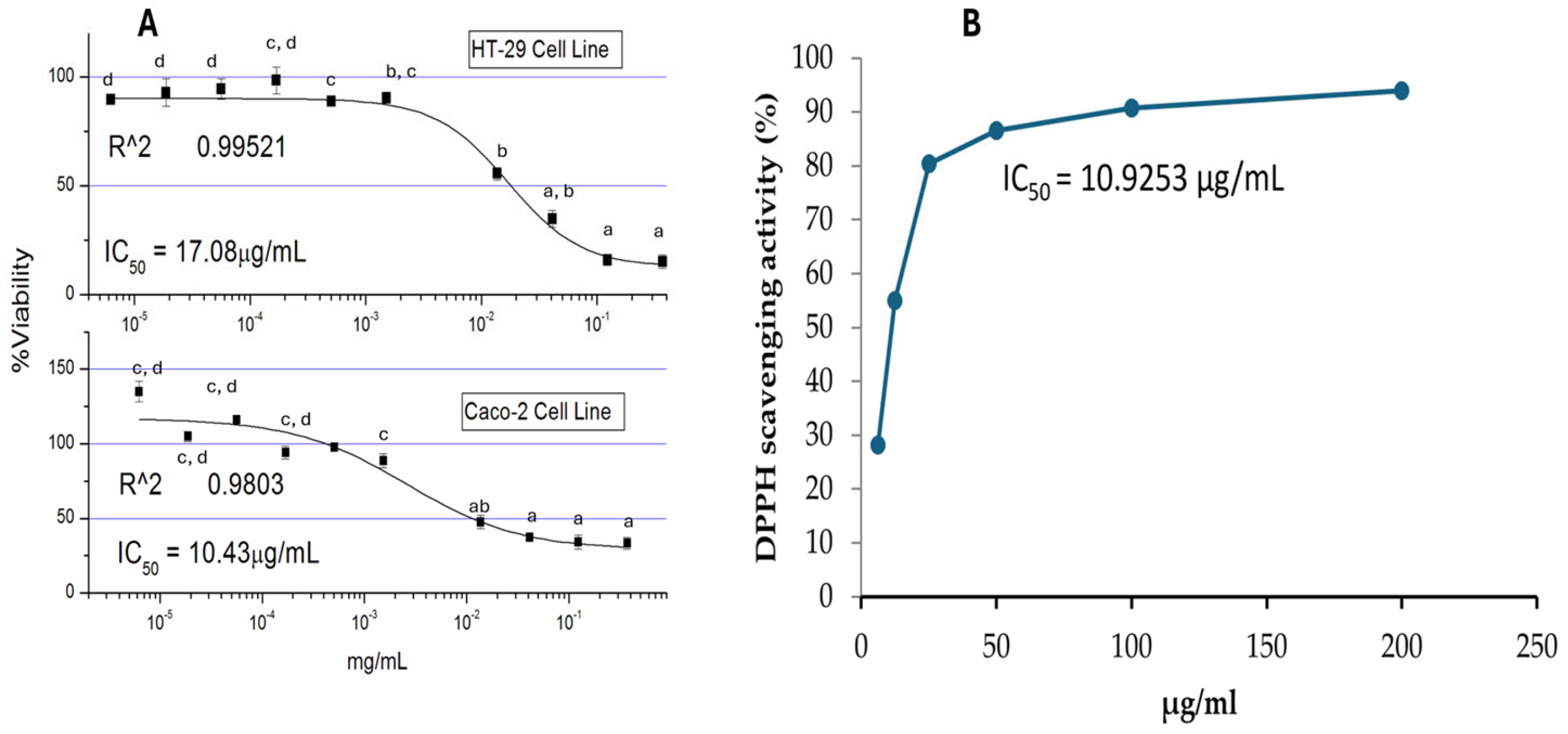
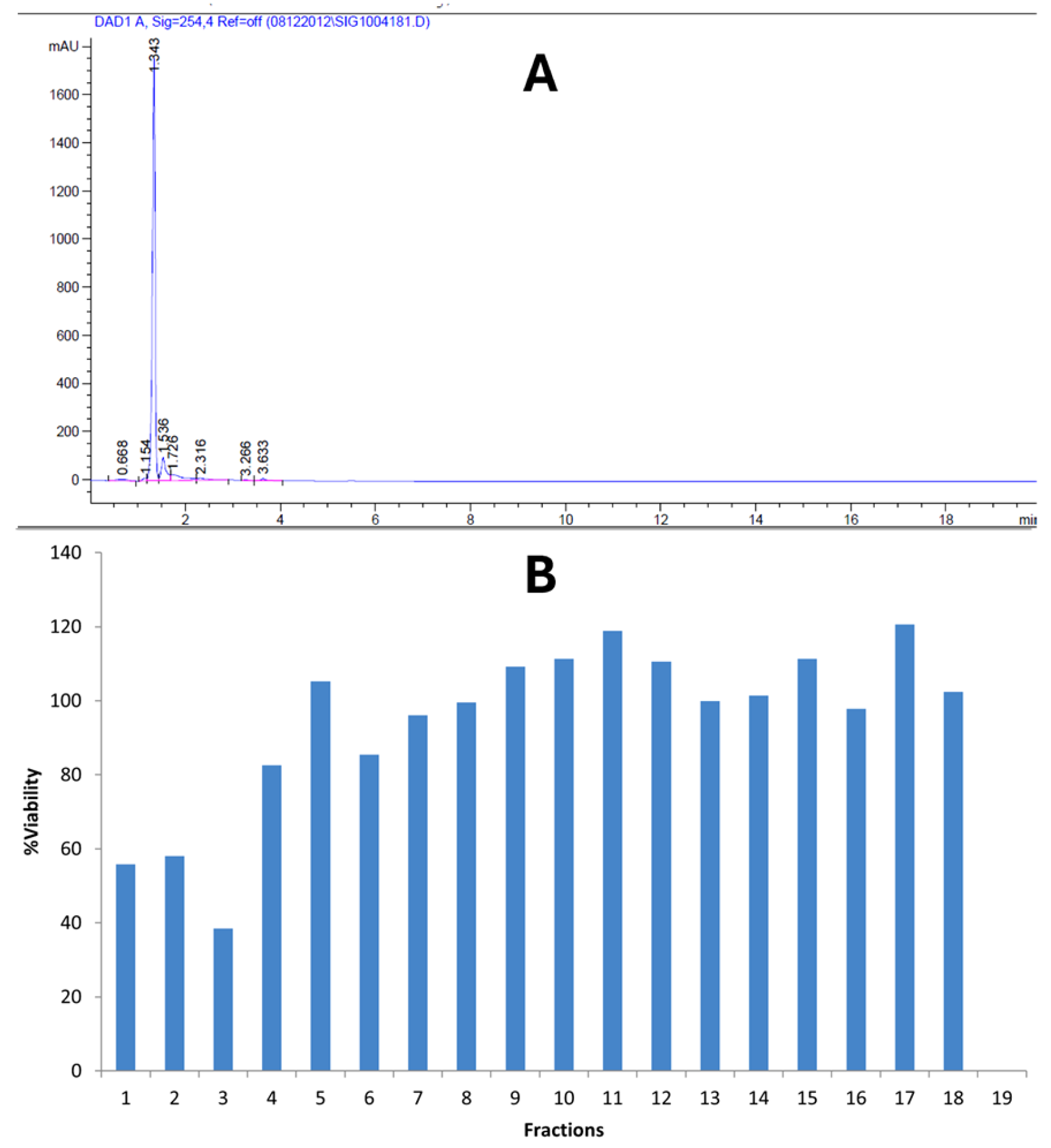
2.1. Bioactivity and Antioxidant Potentials of Kh
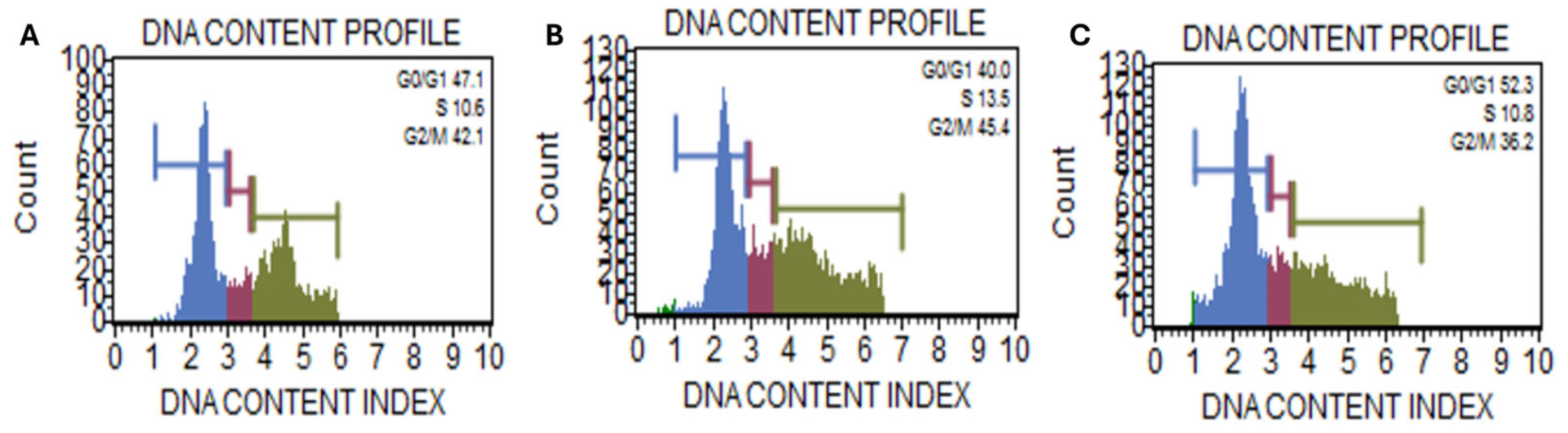
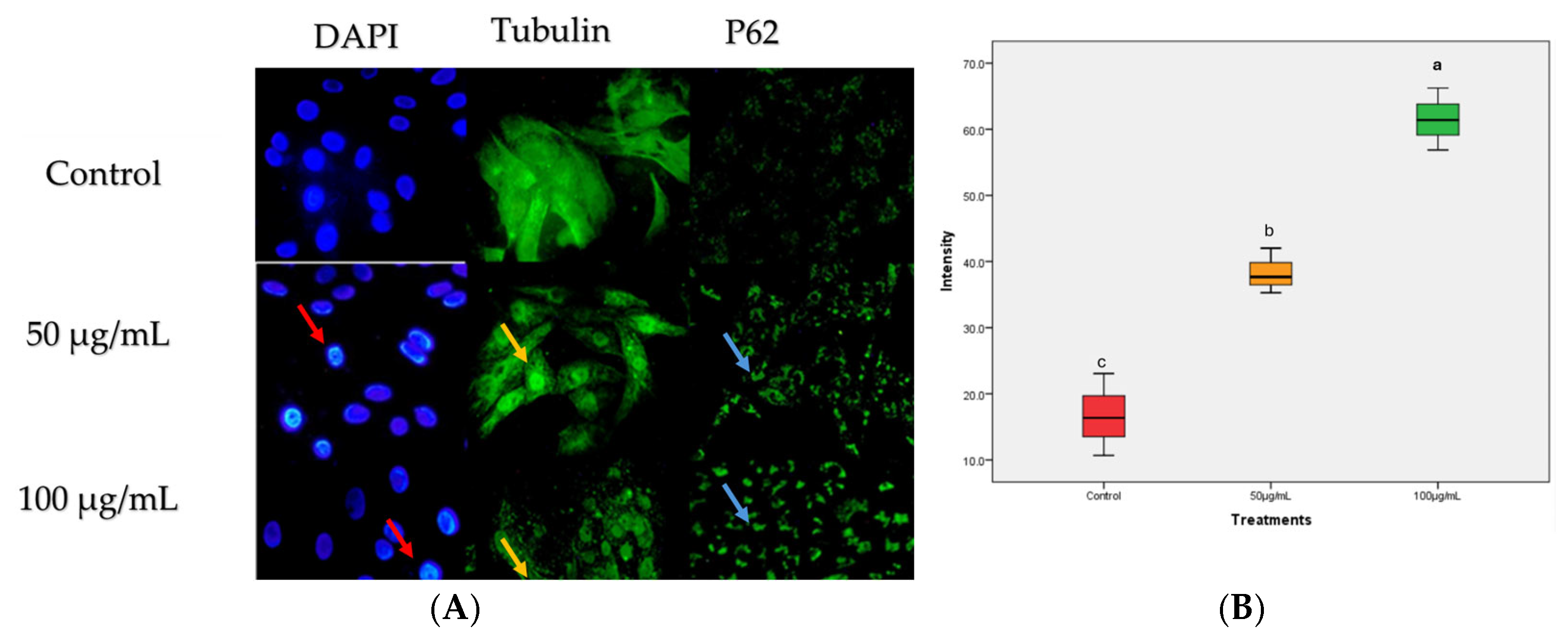
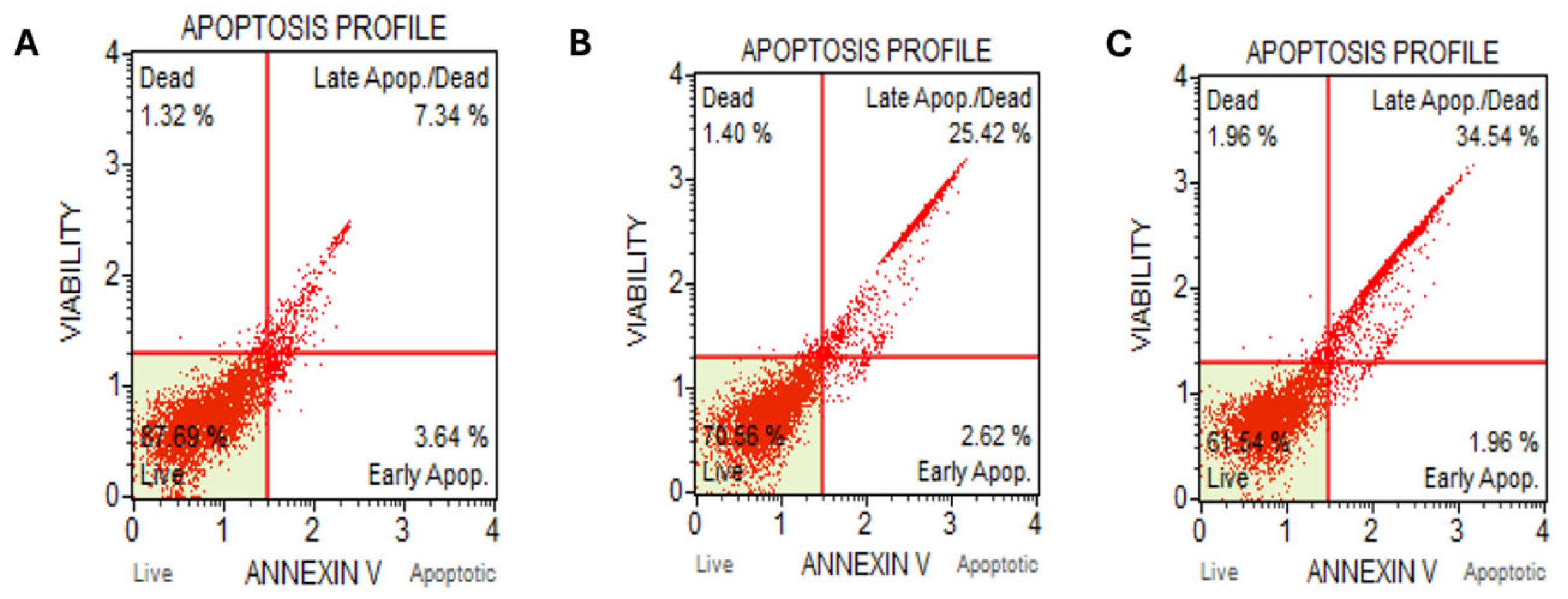
2.2. Kh Induced Dual Apoptotic and Autophagic Flux Degradation Inhibition in Caco-2 Cells
2.3. Kh Phytochemical Profile
3. Discussion
4. Materials and Methods
4.1. Plant Extraction
4.2. DPPH Assay
4.3. Bioactivity Test
4.4. Cellular Phenotyping Assay
4.5. Cell Cycle Fraction Analysis
4.6. Annexin V FITC Assay
4.7. Phytochemical Analysis
4.7.1. HPLC Separation
4.7.2. GCMS Identification
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Kh | Khaya grandiofoliola |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HPLC | High-performance liquid chromatography |
| GCMS | Gas chromatography mass spectrometry |
References
- Anifowose, S.O.; Alqahtani, W.S.; Al-Dahmash, B.A.; Sasse, F.; Jalouli, M.; Aboul-Soud, M.A.; Badjah-Hadj-Ahmed, A.Y.; Elnakady, Y.A. Efforts in bioprospecting research: A survey of novel anticancer phytochemicals reported in the last decade. Molecules 2022, 27, 8307. [Google Scholar] [CrossRef] [PubMed]
- Mukaila, Y.O.; Ajao, A.A.-n.; Moteetee, A.N. Khaya grandifoliola C. DC. (Meliaceae: Sapindales): Ethnobotany, phytochemistry, pharmacological properties, and toxicology. J. Ethnopharmacol. 2021, 278, 114253. [Google Scholar] [CrossRef] [PubMed]
- Elbana, R.M.; Taie, H.A.; Moustafa, A.M.Y.; Marzouk, M. LC-MS/MS analyses of Khaya grandifoliola and in vitro Antioxidant Activity and Cytotoxicity of Khaya senegalensis and Khaya grandifoliola against Ehrlich Ascites Carcinoma Cells. Egypt. J. Chem. 2024, 67, 509–531. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr. Sci. 2001, 80, 1387–1396. [Google Scholar]
- Jiang, Y.-H.; Jiang, T.-J.; Lv, X.-F.; Yu, X.-F.; Chi, W.-Q. Antimicrobial mexicanolide limonoids from the seeds of Khaya senegalensis. J. Asian Nat. Prod. Res. 2022, 24, 634–640. [Google Scholar] [CrossRef]
- Kouam, A.F.; Njayou, F.N.; Yuan, F.; Oladejo, B.O.; Mkounga, P.; Song, F.; Moundipa, P.F. Protective mechanisms of limonoids from Khaya grandifoliola against cisplatin-toxicity in L-02 hepatocytes: Targeting JNK activation and nuclear translocation of Nrf2. Methods 2019, 25, 28. [Google Scholar]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Chien, J.-H.; Chang, K.-F.; Lee, S.-C.; Lee, C.-J.; Chen, Y.-T.; Lai, H.-C.; Lu, Y.-C.; Tsai, N.-M. Cedrol restricts the growth of colorectal cancer in vitro and in vivo by inducing cell cycle arrest and caspase-dependent apoptotic cell death. Int. J. Med. Sci. 2022, 19, 1953. [Google Scholar] [CrossRef]
- Guy-Armand, G.N.; Cedric, Y.; Christelle Nadia, N.A.; Azizi, M.A.; Aboubakar Sidiki, N.N.; Jemimah Sandra, T.N.; Alex Kevin, T.D.; Payne, V.K. Antiplasmodial, antioxidant and cytotoxicity activity of ethanol and aqueous extracts of Khaya grandifoliola stem bark. J. Trop. Med. 2023, 2023, 8062453. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A.; Ito, Y.; Ahmed, S.; Radwan, N.; Ahmed, H.S.; Eid, N. Targeting autophagy with natural products as a potential therapeutic approach for cancer. Int. J. Mol. Sci. 2021, 22, 9807. [Google Scholar] [CrossRef]
- Li, D.; Geng, D.; Wang, M. Advances in natural products modulating autophagy influenced by cellular stress conditions and their anticancer roles in the treatment of ovarian cancer. FASEB J. 2024, 38, e70075. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Qian, T.; He, L.; Kim, J.-S.; Elmore, S.P.; Cascio, W.E.; Brenner, D.A. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid. Redox Signal. 2002, 4, 769–781. [Google Scholar] [CrossRef]
- Butler, D.; Bahr, B.A. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid. Redox Signal. 2006, 8, 185–196. [Google Scholar] [CrossRef]
- Rambold, A.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef]
- Gordy, C.; He, Y.-W. The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell 2012, 3, 17–27. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, Y.; Xing, D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011, 278, 403–413. [Google Scholar] [CrossRef]
- Harris, M.; Thompson, C. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000, 7, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-D.; Qin, Z.-H. Beclin 1, Bcl-2 and autophagy. Autophagy Biol. Dis. Basic Sci. 2019, 1206, 109–126. [Google Scholar]
- Palabiyik, A.A. The role of Bcl-2 in controlling the transition between autophagy and apoptosis. Mol. Med. Rep. 2025, 32, 172. [Google Scholar] [CrossRef]
- Schmidt, T.J. Structure-activity relationships of sesquiterpene lactones. Stud. Nat. Prod. Chem. 2006, 33, 309–392. [Google Scholar]
- Yong, Z.; Zibao, H.; Zhi, Z.; Ning, M.; Ruiqi, W.; Mimi, C.; Xiaowen, H.; Lin, D.; Zhixuan, X.; Qiang, L. Nootkatone, a sesquiterpene ketone from Alpiniae oxyphyllae fructus, ameliorates metabolic-associated fatty liver by regulating AMPK and MAPK signaling. Front. Pharmacol. 2022, 13, 909280. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Khaw-On, P. Terpinen-4-ol induces autophagic and apoptotic cell death in human leukemic HL-60 cells. Asian Pac. J. Cancer Prev. 2013, 14, 7537–7542. [Google Scholar] [CrossRef]
- Bocca, C.; Gabriel, L.; Bozzo, F.; Miglietta, A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004, 147, 79–86. [Google Scholar] [CrossRef]
- Ncube, E.N.; Steenkamp, L.; Dubery, I.A. Ambrafuran (AmbroxTM) synthesis from natural plant product precursors. Molecules 2020, 25, 3851. [Google Scholar] [CrossRef]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef]
- Chang, K.-F.; Huang, X.-F.; Chang, J.T.; Huang, Y.-C.; Lo, W.-S.; Hsiao, C.-Y.; Tsai, N.-M. Cedrol, a sesquiterpene alcohol, enhances the anticancer efficacy of temozolomide in attenuating drug resistance via regulation of the DNA damage response and MGMT expression. J. Nat. Prod. 2020, 83, 3021–3029. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Salih, A.M.; Alattas, N.M.; Alsubaie, Q.D.; Anifowose, S.O. Bidah Pomegranate Landrace: Chemical Composition, Antioxidant, Antibacterial, and Anticancer Activity. Life 2025, 15, 489. [Google Scholar] [CrossRef]

| Peak | Rt (min) | Compound Name | Chemical Class | Area (%) | MF | Mw |
|---|---|---|---|---|---|---|
| 1 | 8.94 | 7-epi-cis-Sesquisabinene hydrate | Sesquiterpene alcohol | 2.5593 | C15H26O | 222 |
| 2 | 10.973 | Z,Z-2,5-Pentadecadien-1-ol | Long-chain alcohol | 1.356 | C15H28O | 224 |
| 3 | 11.751 | 13-nor-Eremophil-1(10)-en-11-one | Sesquiterpene ketone | 4.2434 | C14H22O | 206 |
| 4 | 12.873 | 2,5,5,8a-Tetramethyl-3,5,6,7,8,8a-hexahydro-2H-naphthalen-1-one | Sesquiterpene ketone | 3.1604 | C14H22O | 206 |
| 5 | 13.007 | Edulan II | Furan derivative | 1.6017 | C13H20O | 192 |
| 6 | 13.431 | 2-Butenal, 2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | Monoterpene derivative | 3.081 | C14H22O | 206 |
| 7 | 13.656 | Ethanone, 1-(5,6,7,8-tetrahydro-2,8,8-trimethyl-4H-cyclohepta[b]furan-5-yl)- | Sesquiterpene ketone | 1.6636 | C14H20O2 | 220 |
| 8 | 15.724 | 2-Propenoic acid, 3(2,4-dimethoxyphenyl)-, (E)- | Phenylpropanoid derivative | 8.2974 | C11H12O4 | 208 |
| 9 | 16.612 | Cedrol | Sesquiterpene alcohol | 2.126 | C15H26O | 222 |
| 10 | 16.812 | 2-Acetoxy-1,1,10-trimethyl-6,9-epidioxydecalin | Peroxide-containing sesquiterpenoid | 2.0345 | C15H24O4 | 268 |
| 11 | 17.099 | Cedrane, 8-propoxy- | Sesquiterpene ether | 8.6444 | C18H32O | 264 |
| 12 | 17.237 | Z, E-2,13-Octadecadien-1-ol | Long-chain alcohol | 2.2318 | C18H34O | 266 |
| 13 | 18.479 | (7a-Isopropenyl-4,5-dimethyloctahydroinden-4-yl) methanol | Sesquiterpene alcohol | 20.5561 | C15H26O | 222 |
| 14 | 18.985 | Patchouli alcohol | Sesquiterpene alcohol | 5.6829 | C15H26O | 222 |
| 15 | 19.605 | 1,2-Longidione | Sesquiterpene diketone | 2.1346 | C15H22O2 | 234 |
| 16 | 19.902 | 3a,9-Dimethyldodecahydrocyclohepta[d]inden-3-one | Polycyclic ketone | 5.6674 | C16H26O | 234 |
| 17 | 22.909 | Ambrox | Diterpene alcohol | 3.4322 | C16H28O | 236 |
| 18 | 23.049 | 1-(3-Hydroxypropyl) -5,5,8a-trimethyldecahydronaphthalen-2-ol | Sesquiterpene alcohol | 3.189 | C16H30O2 | 254 |
| 19 | 23.839 | 4,7,10-Hexadecatrienoic acid, methyl ester | Fatty acid methyl ester | 2.0859 | C17H28O2 | 264 |
| 20 | 24.375 | 5,8,11,14-Eicosatetraenoic acid, methyl ester, (all-Z)- | Fatty acid methyl ester | 2.1711 | C21H34O2 | 318 |
| 21 | 24.982 | 7-Acetyl-6-ethyl-1,1,4,4-tetramethyltetralin | Tetralin derivative | 9.167 | C18H26O | 258 |
| 22 | 27.823 | Ethylene brassylate | Macrocyclic musk | 4.9144 | C15H26O4 | 270 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anifowose, S.O.; Oladejo, M.K.; Salih, A.M.; Almutairi, L.M.; Almansour, M.I.; Al-Dahmash, B.; Al Mosallam, M.S.; Alanazi, I.O.; Rady, A. Dual Modulation of Autophagy and Apoptosis as Anticancer Mechanism of Action of Khaya grandiofoliola in Colon Carcinoma Cells. Int. J. Mol. Sci. 2025, 26, 5247. https://doi.org/10.3390/ijms26115247
Anifowose SO, Oladejo MK, Salih AM, Almutairi LM, Almansour MI, Al-Dahmash B, Al Mosallam MS, Alanazi IO, Rady A. Dual Modulation of Autophagy and Apoptosis as Anticancer Mechanism of Action of Khaya grandiofoliola in Colon Carcinoma Cells. International Journal of Molecular Sciences. 2025; 26(11):5247. https://doi.org/10.3390/ijms26115247
Chicago/Turabian StyleAnifowose, Saheed O., Musa K. Oladejo, Abdalrhaman M. Salih, Layali M. Almutairi, Mansour I. Almansour, Badr Al-Dahmash, Mobarak S. Al Mosallam, Ibrahim O. Alanazi, and Ahmed Rady. 2025. "Dual Modulation of Autophagy and Apoptosis as Anticancer Mechanism of Action of Khaya grandiofoliola in Colon Carcinoma Cells" International Journal of Molecular Sciences 26, no. 11: 5247. https://doi.org/10.3390/ijms26115247
APA StyleAnifowose, S. O., Oladejo, M. K., Salih, A. M., Almutairi, L. M., Almansour, M. I., Al-Dahmash, B., Al Mosallam, M. S., Alanazi, I. O., & Rady, A. (2025). Dual Modulation of Autophagy and Apoptosis as Anticancer Mechanism of Action of Khaya grandiofoliola in Colon Carcinoma Cells. International Journal of Molecular Sciences, 26(11), 5247. https://doi.org/10.3390/ijms26115247







