Synergy of Tetracyclines and Potassium Azeloyl Diglycinate (Azeloglycine) in Hydrogels: Evaluation of Stability, Antimicrobial Activity, and Physicochemical Properties
Abstract
1. Introduction
2. Results
2.1. pH Values of Hydrogel Formulations
2.2. Stability Evaluation of Antibiotics Based on HPLC Chromatography
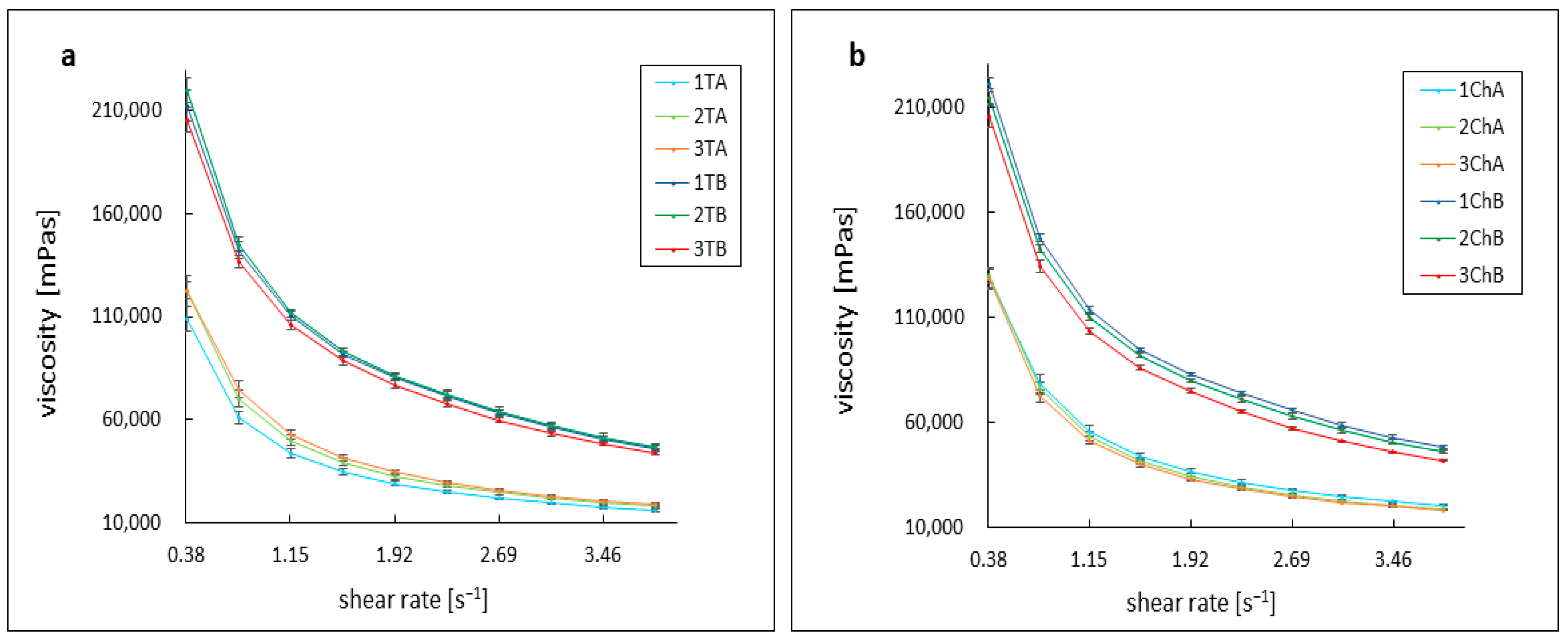
2.3. Assessment of Rheological Parameters
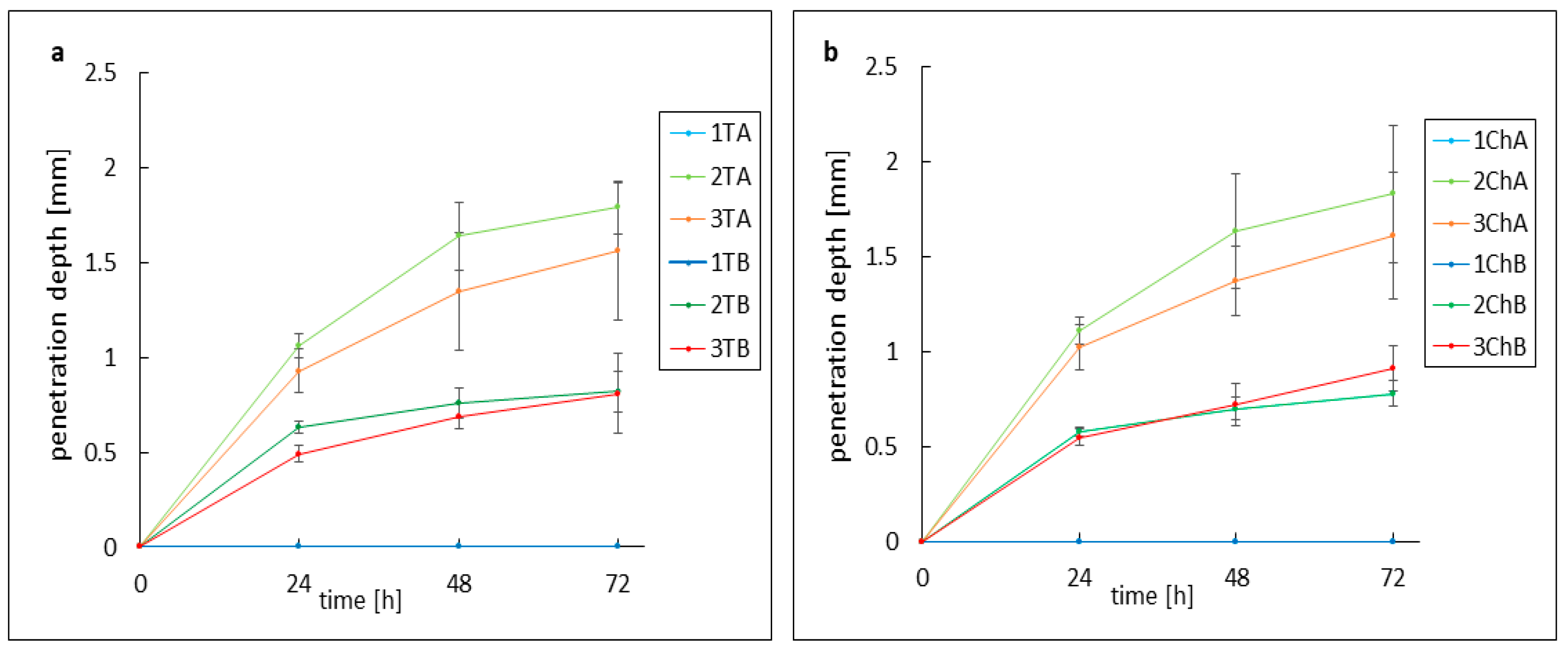
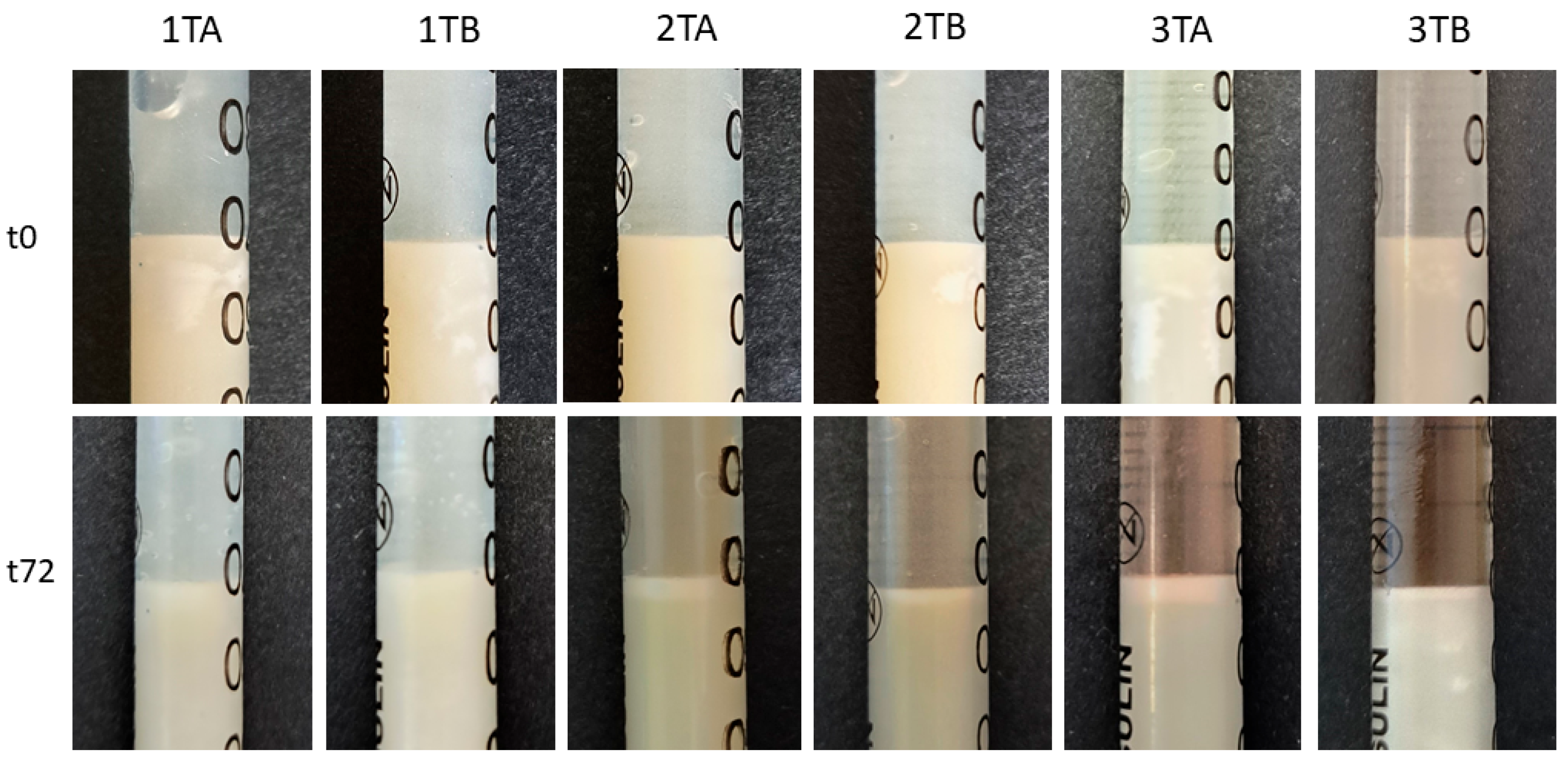
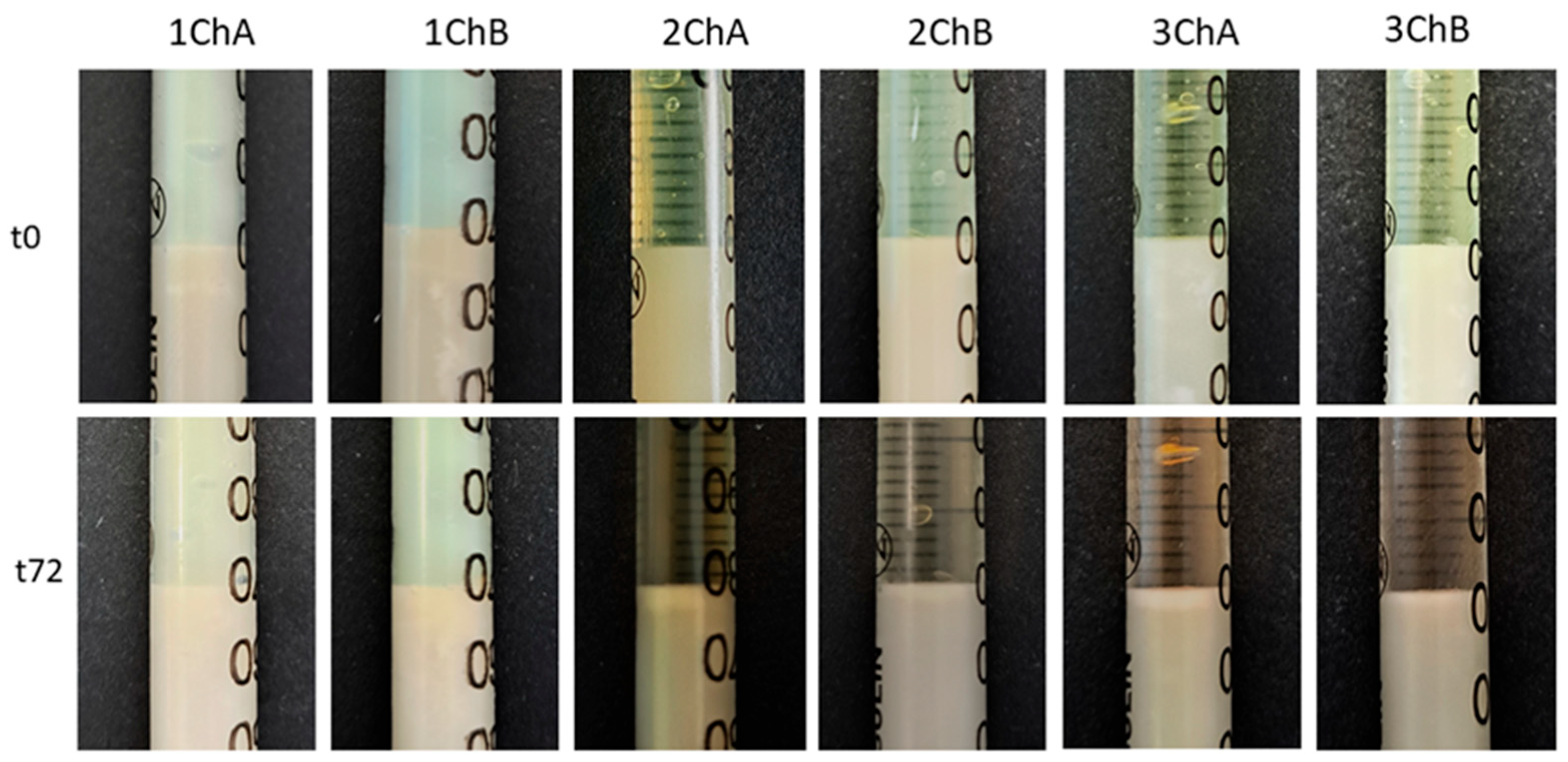
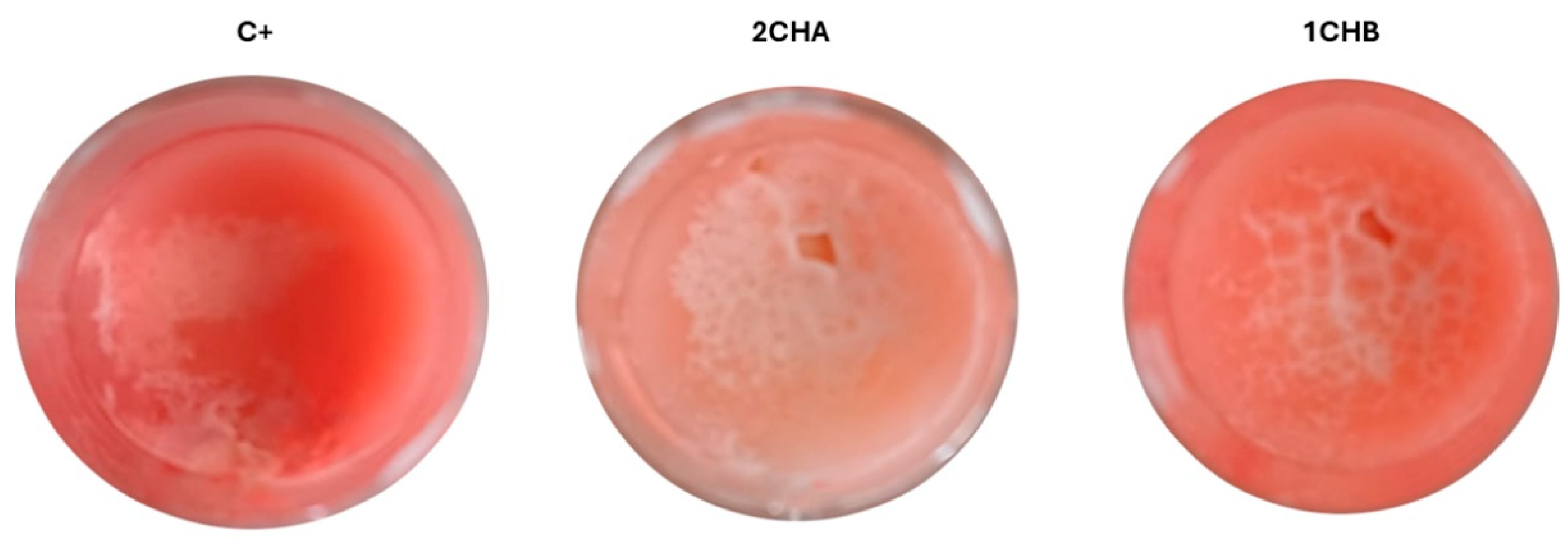
2.4. Evaluation of the Activity of Preparations Against Model Skin Sebum
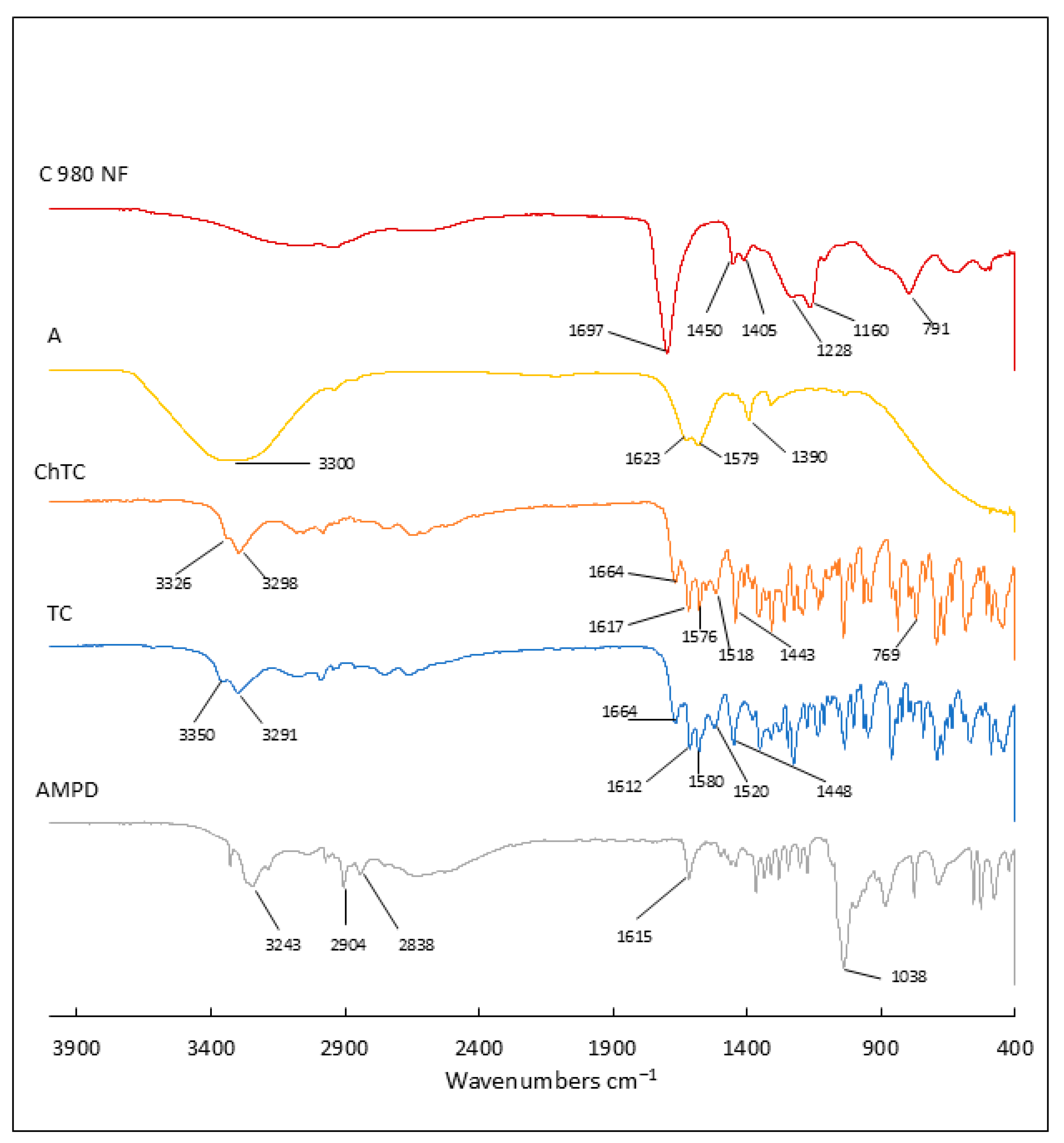
2.5. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy Analysis
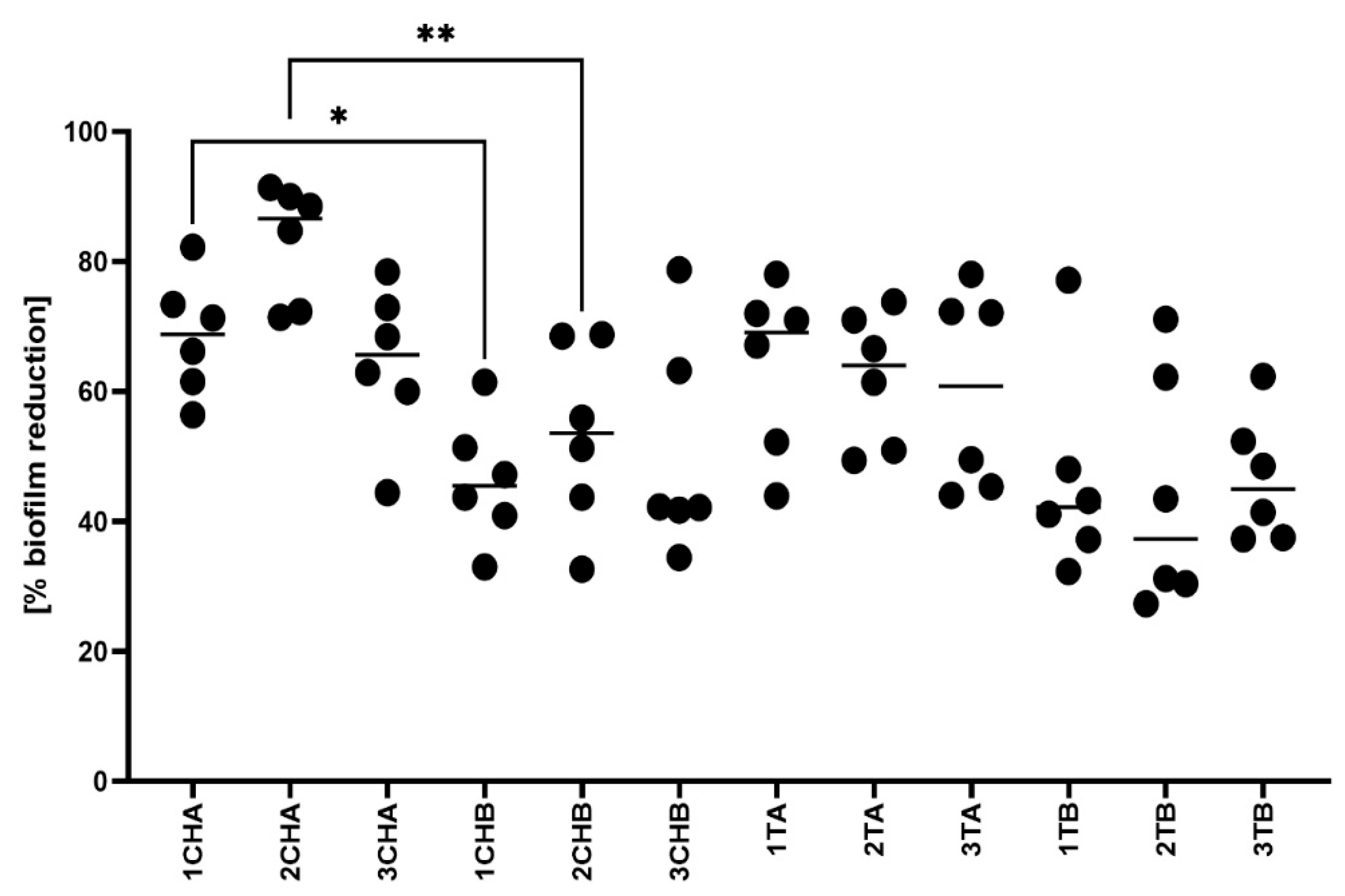
2.6. Microbiological Activity of the Formulations
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Hydrophilic Formulations
4.3. Measurement of pH Value
4.4. Antibiotic Stability Analysis by HPLC
4.4.1. Preparation of Hydrogel Samples
4.4.2. HPLC Analysis
4.5. Determination of the Viscosity Curve of the Preparations
4.6. Artificial Skin Sebum Preparation
4.7. Evaluation of Hydrogels’ Activity Against Artificial Sebum Components
4.8. ATR–FTIR Measurements of Freeze-Dried Formulations
4.9. Assessment of Staphylococcal Growth Inhibition Zone After Exposure on Hydrogels Using Modified Disk Diffusion Method
4.10. Assessment of Staphylococcal Biofilm Reduction in the Artificial Sebum
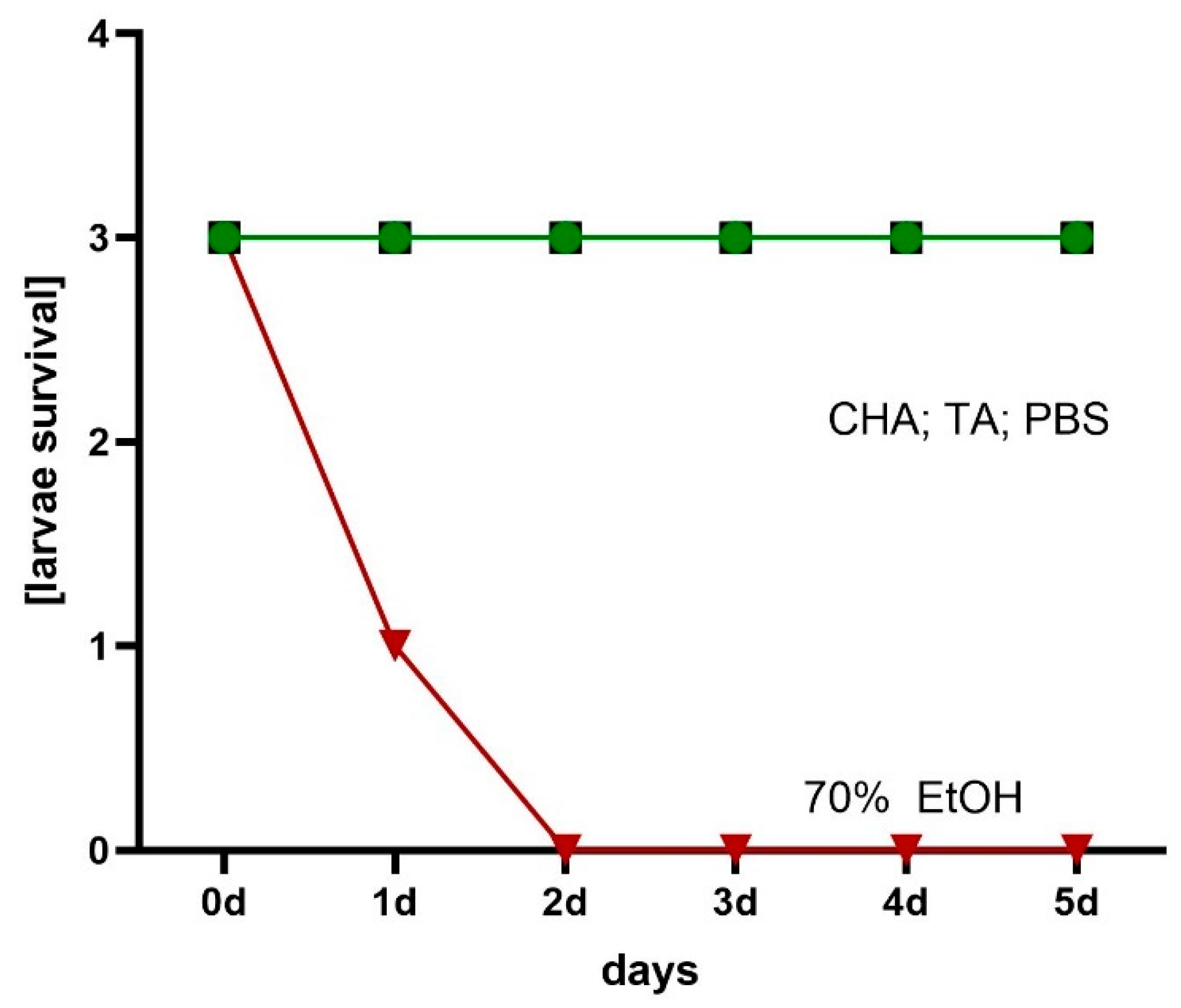
4.11. Assessment of Hydrogels’ Local Cytotoxicity Towards Galleria mellonella Larvae
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPD | 2-amino-2-methyl-1,3-propandiol |
| ChTC | chlortetracycline hydrochloride |
| ChTA | formulation with chlortetracycline and azeloglycine |
| ChTB | formulation with chlortetracycline and without azeloglycine |
| FTIR | Fourier transform infrared spectroscopy |
| HPLC | high-performance liquid chromatography |
| TC | tetracycline hydrochloride |
| TA | formulation with tetracycline and azeloglycine |
| TB | formulation with tetracycline and without azeloglycine |
References
- Dréno, B. Recent Data on Epidemiology of Acne. Ann. Dermatol. Venereol. 2010, 137, 3–5. [Google Scholar] [CrossRef]
- Fried, R.G.; Wechsler, A. Psychological Problems in the Acne Patient. Dermatol. Ther. 2006, 19, 237–240. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Stein Gold, L.F.; Tan, J.K.L.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Farrar, M.D.; Ingham, E. Acne: Inflammation. Clin. Dermatol. 2004, 22, 380–384. [Google Scholar] [CrossRef]
- Schwartz Robert, A. Al-Mutairi Nawaf Topical Antibiotics in Dermatology An Update. Gulf J. Dermatol. Venereol. 2010, 17, 1–19. [Google Scholar]
- Drake, L.; Reyes-Hadsall, S.; Barbieri, J.S.; Mostaghimi, A. New Developments in Topical Acne Therapy. Am. J. Clin. Dermatol. 2022, 23, 125–136. [Google Scholar] [CrossRef]
- Katsambas, A.; Papakonstantinou, A. Acne: Systemic Treatment. Clin. Dermatol. 2004, 22, 412–418. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef]
- Tsankov, N.; Broshtilova, V.; Kazandjieva, J. Tetracyclines in Dermatology. Disease-a-Month 2004, 50, 332–344. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.S. Tetracyclines Revisited: Tetracyclines in the Field of Dermatology. Dermatology 2024, 240, 844–858. [Google Scholar] [CrossRef]
- Dawson, C.R.; Daghfous, T.; Messadi, M.; Hoshiwara, I.; Vastine, D.W.; Yoneda, C.; Schacter, J. Severe Endemic Trachoma in Tunisia II. A Controlled Therapy Trial of Topically Applied Chlortetracycline and Erythromycin. Arch. Ophthalmol. 1974, 92, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Burton, J. A Placebo-Controlled Study to Evaluate the Efficacy of Topical Tetracycline and Oral Tetracycline in the Treatment of Mild to Moderate Acne. J. Int. Med. Res. 1990, 18, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Triviño, F.J.; Pérez-López, I.; Ruíz-Villaverde, R. Doxycycline, an Antibiotic or an Anti-Inflammatory Agent? The Most Common Uses in Dermatology. Actas Dermosifiliogr. 2020, 111, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. An Overview of Topical Antibiotics for Acne Treatment. Dermatology 1998, 196, 130–134. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic Properties and Their Clinical Implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Sieber, M.A.; Hegel, J.K.E. Azelaic Acid: Properties and Mode of Action. Skin. Pharmacol. Physiol. 2013, 27, 9–17. [Google Scholar] [CrossRef]
- Webster, G. Combination Azelaic Acid Therapy for Acne Vulgaris. J. Am. Acad. Dermatol. 2000, 43, 47–50. [Google Scholar] [CrossRef]
- Sadeghzadeh-Bazargan, A.; Behrangi, E.; Najar Nobari, N.; Ghassemi, M.; Roohaninasab, M.; Goodarzi, A. Systematic Review of Clinical Studies Assessing the Needling for Treatment of Melasma: Focusing on Efficacy, Safety, and Recurrence Rate. J. Cosmet. Dermatol. 2022, 21, 1857–1873. [Google Scholar] [CrossRef]
- Maramaldi, G.; Esposito, M.A. Potassium Azeloyl Diglycinate: A Multifunctional Skin Lightener. Cosmet. Toilet. 2008, 117, 43–72. [Google Scholar]
- Rohmani, S.; Dinda, K.E.; Ainurofiq, A. Formulation and Evaluation of the Cream Made from Potassium Azeloyl Diglycinate as an Anti-Aging. J. Phys. Conf. Ser. 2021, 1912, 1–12. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Musiał, W. The Influence of Increasing Concentrations of AMPD on the Efficacy of Its Penetration into a Model Skin Sebum Layer. Pharmaceutics 2020, 12, 1228. [Google Scholar] [CrossRef] [PubMed]
- Musial, W.; Kubis, A. Preliminary Evaluation of Interactions between Selected Alcoholamines and Model Skin Sebum Components. Chem. Pharm. Bull. 2006, 54, 1076–1081. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Złocińska, A.; Musiał, W. Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride. Pharmaceutics 2021, 13, 1381. [Google Scholar] [CrossRef]
- Wróblewska, M.; Słyż, J.; Winnicka, K. Rheological and Textural Properties of Hydrogels, Containing Sulfur as a Model Drug, Made Using Different Polymers Types. Polim. Polym. 2019, 64, 208–215. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological Characterization of Carbopol ® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Shafiei, M.; Balhoff, M.; Hayman, N.W. Chemical and Microstructural Controls on Viscoplasticity in Carbopol Hydrogel. Polymer 2018, 139, 44–51. [Google Scholar] [CrossRef]
- Curran, S.J.; Hayes, R.E.; Afacan, A.; Williams, M.C.; Tanguy, P.A. Properties of Carbopol Solutions as Models for Yield-Stress Fluids. J. Food Sci. Food Eng. Phys. Prop. 2002, 67, 176–180. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Pączek, K.; Weselak, A.; Musiał, W. Effect of Hydrogel Substrate Components on the Stability of Tetracycline Hydrochloride and Swelling Activity against Model Skin Sebum. Int. J. Mol. Sci. 2023, 24, 2678. [Google Scholar] [CrossRef]
- Marchetti, M.; Perini, A.; Zanella, M.; Benetti, F.; Donelli, D. Study on the Fate of the Carbopol® Polymer in the Use of Hand Sanitizer Gels: An Experimental Model to Monitor Its Physical State from Product Manufacturing up to the Final Hand Rinse. Microplastics 2024, 3, 390–404. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; Van Bronswijk, W. In Situ FTIR-ATR Examination of Poly(Acrylic Acid) Adsorbed onto Hematite at Low PH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Fourier Transform Infrared Spectroscopy for the Analysis of Neutralizer-Carbomer and Surfactant-Carbomer Interactions in Aqueous, Hydroalcoholic, and Anhydrous Gel Formulations. Am. Assoc. Pharm. Sci. J. 2004, 6, 1–7. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C.; Nanda, U.N. Qualitative Analysis of Environmentally Responsive Biodegradable Smart Carbopol Polymer. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 8–13. [Google Scholar]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C.; Naik, S.; Nanda, U.N. FTIR and Raman Spectroscopy as a Tool for Analyzing Sustained Release Hydrogel of Ciprofloxacin/Carbopol Polymer. IJPSR 2011, 2, 268–277. [Google Scholar] [CrossRef]
- Taleb, M.A.; Hussien, A.M.; Al-Fiky, A.F.; El-Sayed, H. Preparation of Fatty Acid-Amino Diol Condensate and Its Utilization as a Durable Nonionic Softener for PAN Fabrics. Heliyon 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Kobryń, J.; Demski, P.; Raszewski, B.; Zięba, T.; Musiał, W. Effect of Co-Solvents, Modified Starch and Physical Parameters on the Solubility and Release Rate of Cryptotanshinone from Alcohologels. Molecules 2024, 29, 5877. [Google Scholar] [CrossRef]
- Hou, H.; Dai, Z.; Liu, X.; Yao, Y.; Liao, Q.; Yu, C.; Li, D. Reutilization of the Expired Tetracycline for Lithium Ion Battery Anode. Sci. Total Environ. 2018, 630, 495–501. [Google Scholar] [CrossRef]
- Mikulski, C.M.; Fleming, J.; Fleming, D.; Karayannis, N.M. Chelates of Tetracycline with First Row Transition Metal Perchlorates. Inorganica Chim. Acta. 1988, 144, 9–16. [Google Scholar] [CrossRef]
- Apiwongngam, J.; Limwikrant, W.; Jintapattanakit, A.; Jaturanpinyo, M. Enhanced Supersaturation of Chlortetracycline Hydrochloride by Amorphous Solid Dispersion. J. Drug Deliv. Sci. Technol. 2018, 47, 417–426. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Dong, Y. hua Oxidative Degradation Kinetics and Products of Chlortetracycline by Manganese Dioxide. J. Hazard. Mater. 2011, 193, 128–138. [Google Scholar] [CrossRef]
- Caminati, G.; Focardi, C.; Gabrielli, G.; Gambinossi, F.; Mecheri, B.; Nocentini, M.; Puggelli, M. Spectroscopic Investigation of Tetracycline Interaction with Phospholipid Langmuir-Blodgett Films. Mater. Sci. Eng. C 2002, 22, 301–305. [Google Scholar] [CrossRef]
- Li, Z.; Kolb, V.M.K.; Jiang, W.T.; Hong, H. FTIR and XRD Investigations of Tetracycline Intercalation in Smectites. Clays Clay Min. 2010, 58, 462–474. [Google Scholar] [CrossRef]
- Chang, P.H.; Jean, J.S.; Jiang, W.T.; Li, Z. Mechanism of Tetracycline Sorption on Rectorite. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 94–99. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ragadhita, R.; Fiandini, M. Interpretation of Fourier Transform Infrared Spectra (FTIR): A Practical Approach in the Polymer/Plastic Thermal Decomposition. Indones. J. Sci. Technol. 2023, 8, 113–126. [Google Scholar] [CrossRef]
- Nattkemper, A.; Schleiden, T.; Migliavacca, J.M.; Melin, T. Monitoring Crystallization Kinetics of Azelaic Acid by in Situ FTIR Spectroscopy in Three-Phase Systems. Chem. Eng. Technol. 2003, 26, 881–889. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, A.K.; Singh, V.B.; Rai, S.B. Vibrational Spectrum of Glycine Molecule. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2741–2746. [Google Scholar] [CrossRef]
- Santoro, M.; Marchetti, P.; Rossi, F.; Perale, G.; Castiglione, F.; Mele, A.; Masi, M. Smart Approach to Evaluate Drug Diffusivity in Injectable Agar-Carbomer Hydrogels for Drug Delivery. J. Phys. Chem. B 2011, 115, 2503–2510. [Google Scholar] [CrossRef]
- Tan, J.K.L.; Bhate, K. A Global Perspective on the Epidemiology of Acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef]
- Juhl, C.R.; Bergholdt, H.K.M.; Miller, I.M.; Jemec, G.B.E.; Kanters, J.K.; Ellervik, C. Dairy Intake and Acne Vulgaris: A Systematic Review and Meta-Analysis of 78,529 Children, Adolescents, and Young Adults. Nutrients 2018, 10, 1049. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Teng, Y.; Li, S.; Tang, H.; Tao, X.; Fan, Y.; Huang, Y. Medical Applications of Hydrogels in Skin Infections: A Review. Infect. Drug Resist. 2023, 16, 391–401. [Google Scholar] [CrossRef]
- Pavlačková, J.; Pecháčková, H.; Egner, P.; Mokrejš, P.; Gál, R.; Janalíková, M. The Effect of Cosmetic Treatment and Gel Laser Therapy on the Improvement of Comedogenic Skin Type. Gels 2023, 9, 370. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Roberts, M.C. Tetracycline Therapy: Update. Clin. Infect. Dis. 2003, 36, 462–467. [Google Scholar] [CrossRef]
- Solomons, B. Aureomycin: Its Topical Use In Some Skin Diseases. Br. Med. J. 1951, 2, 525–527. [Google Scholar] [CrossRef]
- Darougar, S.; Viswalingam, N.; El-Sheikh, H.; Hunter, P.A.; Yearsley, P. A Double-Blind Comparison of Topical Therapy of Chlamydial Ocular Infection (TRIC Infection) with Rifampicin or Chlortetracycline. Br. J. Ophthalmol. 1981, 65, 549–552. [Google Scholar] [CrossRef]
- Esposito, S.; Bassetti, M.; Concia, E.; De Simone, G.; De Rosa, F.G.; Grossi, P.; Novelli, A.; Menichetti, F.; Petrosillo, N.; Tinelli, M.; et al. Diagnosis and Management of Skin and Soft-Tissue Infections (SSTI). A Literature Review and Consensus Statement: An Update. J. Chemother. 2017, 29, 197–214. [Google Scholar] [CrossRef]
- Karadag, A.S.; Aslan Kayıran, M.; Wu, C.Y.; Chen, W.; Parish, L.C. Antibiotic Resistance in Acne: Changes, Consequences and Concerns. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 73–78. [Google Scholar] [CrossRef]
- Fernandez-Obregon, A.C.; Rohrback, J.; Reichel, M.A.; Willis, C. Current Use of Anti-Infectives in Dermatology. Expert Rev. Anti. Infect. Ther. 2005, 3, 557–591. [Google Scholar] [CrossRef]
- Ochsendorf, F. Minocycline in Acne Vulgaris Benefits and Risks. Am. J. Clin. Dermatol. 2010, 11, 327–341. [Google Scholar] [CrossRef]
- Le, C.-Y.; Ye, Y.-J.; Xu, J.; Li, L.; Feng, X.-Q.; Chen, N.-P.; Zhu, B.-Q.; Ding, Z.-S.; Qian, C.-D. Hinokitiol Selectively Enhances the Antibacterial Activity of Tetracyclines against Staphylococcus Aureus. Microbiol. Spectr. 2023, 11, e0320522. [Google Scholar] [CrossRef]
- Luhaibi, D.K.; Ali, H.H.M.; Al-Ani, I.; Shalan, N.; Al-Akayleh, F.; Al-Remawi, M.; Nasereddin, J.; Qinna, N.A.; Al-Adham, I.; Khanfar, M. The Formulation and Evaluation of Deep Eutectic Vehicles for the Topical Delivery of Azelaic Acid for Acne Treatment. Molecules 2023, 28, 6927. [Google Scholar] [CrossRef]
- Berardesca, E.; Iorizzo, M.; Abril, E.; Guglielmini, G.; Caserini, M.; Palmieri, R.; Piérard, G.E. Clinical and Instrumental Assessment of the Effects of a New Product Based on Hydroxypropyl Chitosan and Potassium Azeloyl Diglycinate in the Management of Rosacea. J. Cosmet. Dermatol. 2012, 11, 37–41. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Szczepaniak, G. Anti-Acne Preparations Containing Tetracycline, Azelaic Acid and Azeloglycine: Optimization of Stability and Physicochemical Properties. Polim. Med. 2024, 54, 155–159. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of Metronidazole, Tetracycline HCl and Famotidine Alone and in Combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef]
- Pena, A.; Carmona, A.; Barbosa, A.; Lino, C.M.; Silveira, M.I.; Castillo, B. Determination of Tetracycline and Its Major Degradation Products by Liquid Chromatography with Fluorescence Detection. J. Pharm. Biomed. Anal. 1998, 18, 839–845. [Google Scholar] [CrossRef]
- Cervini, P.; MacHado, L.C.M.; Ferreira, A.P.G.; Ambrozini, B.; Cavalheiro, É.T.G. Thermal Decomposition of Tetracycline and Chlortetracycline. J. Anal. Appl. Pyrolysis 2016, 118, 317–324. [Google Scholar] [CrossRef]
- Gajda, A.; Posyniak, A. Tetracyclines and Their Epimers in Animal Tissues by High-Performance Liquid Chromatography. Bull. Vet. Inst. Pulawy 2009, 53, 263–267. [Google Scholar]
- Jia, A.; Xiao, Y.; Hu, J.; Asami, M.; Kunikane, S. Simultaneous Determination of Tetracyclines and Their Degradation Products in Environmental Waters by Liquid Chromatography-Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of Tetracyclines and Tetracycline Degradation Products to Environmentally Relevant Bacteria, Including Selected Tetracycline-Resistant Bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Keßler, D.N.; Fokuhl, V.K.; Petri, M.S.; Spielmeyer, A. Abiotic Transformation Products of Tetracycline and Chlortetracycline in Salt Solutions and Manure. Chemosphere 2019, 224, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Palilis, L.P.; Lino, C.M.; Silveira, M.I.; Calokerinos, A.C. Determination of Tetracycline and Its Major Degradation Products by Chemiluminescence. Anal. Chim. Acta. 2000, 405, 51–56. [Google Scholar] [CrossRef]
- Hoener, B.-A.; Sokoloski, T.D.; Mitscher, L.A.; Malspeis, L. Kinetics of Dehydration of Epitetracycline in Solution. J. Pharm. Sci. 1974, 63, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G. Determination of Anhydrotetracycline and 4-Epianhydrotetracycline in a Tetracycline Mixture. J. Pharm. Sci. 1964, 53, 1551–1552. [Google Scholar] [CrossRef]
- Hasan, T.; Allen, M.; Cooperman, B.S. Anhydrotetracycline Is a Major Product of Tetracycline Photolysis. J. Org. Chem. 1985, 50, 1755–1757. [Google Scholar] [CrossRef]
- Davies, A.K.; McKellar, J.F.; Phillips, G.O.; Reid, A.G. Photochemical Oxidation of Tetracycline in Aqueous Solution. J. Chem. Soc. Perkin Trans. 1979, 2, 369–375. [Google Scholar] [CrossRef]
- Oka, H.; Ikai, Y.; Kawamura, N.; Yamada, M.; Harada, K.; Ito, S.; Suzuki, M. Photodecomposition Products of Tetracycline in Aqueous Solution. J. Agric. Food Chem. 1989, 37, 226–231. [Google Scholar] [CrossRef]
- Kennedy, D.G.; McCracken, R.J.; Carey, M.P.; Blanchflower, W.J.; Hewitt, S.A. Iso- and Epi-Iso-Chlortetracycline Are the Principal Metabolites of Chlortetracycline in the Hen’s Egg. J. Chromatogr. A 1998, 812, 327–337. [Google Scholar] [CrossRef]
- Gómez-Pacheco, C.V.; Sánchez-Polo, M.; Rivera-Utrilla, J.; López-Peñalver, J.J. Tetracycline Degradation in Aqueous Phase by Ultraviolet Radiation. Chem. Eng. J. 2012, 187, 89–95. [Google Scholar] [CrossRef]
- López-Peñalver, J.J.; Sánchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of Tetracyclines in Aqueous Solution by Using UV and UV/H2O2 Oxidation Processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Drexel, R.E.; Olack, G.; Jones, C.; Santim, R.; Morrison, H.; Chmurny, G. Lumitetracycline: A Novel New Tetracycline Photoproduct. J. Org. Chem. 1990, 55, 2471–2478. [Google Scholar] [CrossRef]
- Hasan, T.; Kochevar, I.E.; McAuliffe, D.J.; Cooperman, B.S.; Abdulah, D. Mechanism of Tetracycline Phototoxicity. J. Investig. Dermatol. 1984, 83, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal Surface Lipids. Derm. Endocrinol. 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Schiller, A.; Müller, B.; Spencer, N.D.; Derler, S. Fabrication, Characterisation and Tribological Investigation of Artificial Skin Surface Lipid Films. Tribol. Lett. 2009, 34, 81–93. [Google Scholar] [CrossRef]
- Smith, K.R.; Thiboutot, D.M. Skin Lipids. Sebaceous Gland Lipids: Friend or Foe? J. Lipid. Res. 2008, 49, 271–281. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of Materials in Artificial Skin Surface Film Liquids. Toxicol. Vitr. 2006, 20, 1265–1283. [Google Scholar] [CrossRef]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The Mechanism of Skin Lipids Influencing Skin Status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Harvey, C.J.; Wertz, P.W. Formulation and Stability of a Novel Artificial Sebum under Conditions of Storage and Use. Int. J. Cosmet. Sci. 2010, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Musial, W.; Kubis, A. Preliminary Assessment of Alginic Acid as a Factor Buffering Triethanolamine Interacting with Artificial Skin Sebum. Eur. J. Pharm. Biopharm. 2003, 55, 237–240. [Google Scholar] [CrossRef] [PubMed]






| Formulation | pH Value ± SD |
|---|---|
| 1TA | 6.41 ± 0.01 |
| 1TB | 6.63 ± 0.05 |
| 1ChA | 6.62 ± 0.02 |
| 1ChB | 6.72 ± 0.01 |
| 2TA | 7.20 ± 0.01 |
| 2TB | 7.45 ± 0.01 |
| 2ChA | 7.21 ± 0.01 |
| 2ChB | 7.35 ± 0.01 |
| 3TA | 8.19 ± 0.01 |
| 3TB | 8.29 ± 0.01 |
| 3ChA | 8.17 ± 0.02 |
| 3ChB | 8.24 ± 0.02 |
| Formulation | TC [g] | ChTC [g] | Azeloglycine [g] | AMPD [g] | Carbopol 980 NF [g] | Water [g] |
|---|---|---|---|---|---|---|
| 1TA | 0.2 | 0.0 | 2.0 | 0.7 | 0.7 | 96.4 |
| 2TA | 0.2 | 0.0 | 2.0 | 0.9 | 0.7 | 96.2 |
| 3TA | 0.2 | 0.0 | 2.0 | 1.1 | 0.7 | 96.0 |
| 1TB | 0.2 | 0.0 | 0.0 | 0.7 | 0.7 | 98.4 |
| 2TB | 0.2 | 0.0 | 0.0 | 0.9 | 0.7 | 98.2 |
| 3TB | 0.2 | 0.0 | 0.0 | 1.1 | 0.7 | 98.0 |
| 1ChA | 0.0 | 0.2 | 2.0 | 0.7 | 0.7 | 96.4 |
| 2ChA | 0.0 | 0.2 | 2.0 | 0.9 | 0.7 | 96.2 |
| 3ChA | 0.0 | 0.2 | 2.0 | 1.1 | 0.7 | 96.0 |
| 1ChB | 0.0 | 0.2 | 0.0 | 0.7 | 0.7 | 98.4 |
| 2ChB | 0.0 | 0.2 | 0.0 | 0.9 | 0.7 | 98.2 |
| 3ChB | 0.0 | 0.2 | 0.0 | 1.1 | 0.7 | 98.0 |
| 1.0A | 0.0 | 0.0 | 2.0 | 0.7 | 0.7 | 96.6 |
| 2.0A | 0.0 | 0.0 | 2.0 | 0.9 | 0.7 | 96.4 |
| 3.0A | 0.0 | 0.0 | 2.0 | 1.1 | 0.7 | 96.2 |
| 1.0B | 0.0 | 0.0 | 0.0 | 0.7 | 0.7 | 98.6 |
| 2.0B | 0.0 | 0.0 | 0.0 | 0.9 | 0.7 | 98.4 |
| 3.0B | 0.0 | 0.0 | 0.0 | 1.1 | 0.7 | 98.2 |
| Components | Artificial Skin Sebum |
|---|---|
| Cholesterol | 4% |
| Squalene | 12% |
| Stearic acid as a free fatty acid | 24% |
| Lanolin as wax | 26% |
| Pork lard as triglyceride | 34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzębska, A.; Junka, A.; Musiał, W. Synergy of Tetracyclines and Potassium Azeloyl Diglycinate (Azeloglycine) in Hydrogels: Evaluation of Stability, Antimicrobial Activity, and Physicochemical Properties. Int. J. Mol. Sci. 2025, 26, 5239. https://doi.org/10.3390/ijms26115239
Kostrzębska A, Junka A, Musiał W. Synergy of Tetracyclines and Potassium Azeloyl Diglycinate (Azeloglycine) in Hydrogels: Evaluation of Stability, Antimicrobial Activity, and Physicochemical Properties. International Journal of Molecular Sciences. 2025; 26(11):5239. https://doi.org/10.3390/ijms26115239
Chicago/Turabian StyleKostrzębska, Agnieszka, Adam Junka, and Witold Musiał. 2025. "Synergy of Tetracyclines and Potassium Azeloyl Diglycinate (Azeloglycine) in Hydrogels: Evaluation of Stability, Antimicrobial Activity, and Physicochemical Properties" International Journal of Molecular Sciences 26, no. 11: 5239. https://doi.org/10.3390/ijms26115239
APA StyleKostrzębska, A., Junka, A., & Musiał, W. (2025). Synergy of Tetracyclines and Potassium Azeloyl Diglycinate (Azeloglycine) in Hydrogels: Evaluation of Stability, Antimicrobial Activity, and Physicochemical Properties. International Journal of Molecular Sciences, 26(11), 5239. https://doi.org/10.3390/ijms26115239







