The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy
Abstract
1. Senescence Versus Aging
2. Biological Versus Chronological Age
3. Evidence That the Neural Retina Ages
4. The Effect of Senescence Is Dependent on the Stage of DR Pathogenesis
5. DM Exacerbates Processes That Promote the Senescence of Endothelial Cells Within Retinal Vessels, Thereby Accelerating the Senescence of the Retinal Vasculature
6. Approaches to Detect Senescence Within Retinal Vessels and Cultured Cells
| Disease State | Experimental System | Technique | Readouts | References |
|---|---|---|---|---|
| DR | Retinal Explant | ELISA | ↑ HMGB1 ↑ IL-1β | [30] |
| mRECs | Flow Cytometry | Cells positive for CellEvent Senescence Green Flow Cytometry Assay | ||
| Immunocytochemistry | ↑ β-galactosidase activity | |||
| Cell Proliferation | Edu assay | |||
| HRECs | Immunocytochemistry | ↑ β-galactosidase activity | ||
| Cell Proliferation | Edu assay | |||
| Murine RV | PCR | ↑ p16 ↑ p21 ↑ Igfbp3 ↑ p53 | ||
| Freshly Isolated Murine RV | SA-β-Gal Assay | ↑ β-galactosidase activity | ||
| DR and neovascular retinal diseases | HMRECs | PCR | ↑ Cdkn2a (Ink4a) ↑ Cdkn1a | [36] |
| OIR P17 Retina | PCR | Concomitant peaks of senescence (↑ Cdkn2a (Ink4a), Cdkn1a, Serpine1, Vegfa) and maximal pathological angiogenesis | ||
| SA-β-Gal Assay | Maximal stained cells in inner retina where new vessels form | |||
| Western Blot | p16INK4a PAI1 (product of Serpine1 gene) | |||
| DropSeq | GSVA showed ↑ senescence transcripts in clusters of endothelial cells, pericytes, astrocytes, Müller glia | |||
| 10X Single-Cell RNA Seq | Supervised clustering for senescence showed enrichment in endothelial cells | |||
| DR | HRECs | SA-β-Gal Assay | ↑ β-galactosidase activity | [37] |
| Western Blot | ↑ p21 | |||
| Flow Cytometry | G0/G1cell cycle arrest | |||
| DR | HMRECs | PCR | ↑ p53 ↑ p16 ↑ p21 ↑ IL-1β ↑ IGFBP7 | [38] |
| Western Blot | ↑ p53 ↑ IL-1β ↑ IGFBP7 ↑ ICAM-1 ↑ MCP-1 ↑ MMP9 | |||
| SA-β-Gal Assay | ↑ β-galactosidase activity | |||
| Immunofluorescence | ↑ p53 | |||
| db/db mouse retina | Single-Cell RNA Seq | Enrichment of senescence and p53 pathways in KEGG pathway analysis for endothelial cluster | ||
| p53 enrichment only in endothelial cluster | ||||
| Prolonged DM and DR | HMRECs | CASY Cell counter | ↓ growth | [39] |
| ↑ cell diameter | ||||
| ↑ cell area | ||||
| ↓ population doublings | ||||
| ↓ Hayflick limit | ||||
| SA-β-Gal Assay | ↑ β-galactosidase activity | |||
| Western Blot | ↓ pRb ↓ HMGB2 ↓ Sirt1 ↑ p53 p21, p16 (although inconsistent change) | |||
| db/db mouse retina | Histology | Costaining of β-galactosidase activity and Isolectin B4 | ||
| Akimba mouse | Single-Cell RNA Seq | Enrichment of SASP and senescence genes in ECs | ||
| Retinopathy | OIR P17 Retina | Single-Cell RNA Seq | Enrichment of senescence and SASP in endothelial cell cluster | [16] |
| GSVA indicating enrichment of senescence transcripts | ||||
| Confocal | Colocalization of Isolectin B4 and promyelocytic leukemia (PML) protein for senescent endothelial cells | |||
| Colocalization NG2 and promyelocytic leukemia protein for pericytes | ||||
| RAS colocalization with Isolectin B4+ endothelial cells in preretinal NV | ||||
| Colocalization of Isolectin B4 and high pERK1/2 | ||||
| OIR P19 Retina | SA-β-Gal Assay | β-galactosidase activity | ||
| PCR | ↑ p21 ↑ Serpine1 ↑ IL-1β ↑ p53 ↑ Vegfa ↑ Tnfα ↑ Tgfβ | |||
| NA | HRECs | SA-β-Gal Assay | ↑ β-galactosidase activity | [40] |
| PCR | ↑ p53 | |||
| Population doubling time | ↑ | |||
| DCF Assay | ↑ intracellular oxidative stress | |||
| NA | HRECs | SA-β-Gal Assay | ↑ β-galactosidase activity | [41] |
| PCR | ↑ CDKN1A (p21 CIP1) ↑ IL6 | |||
| Chronic DM and implication in DR | HRECs | SA-β-Gal Assay | ↑ β-galactosidase activity | [42] |
| TRAP PCR Assay | ↓ Telomerase activity | |||
| PCR | ↑ p53 ↑ p21 ↓ SIRT1 (regulator of senescence) | |||
| Western Blot | ↑ p53 ↑ p21 ↓ SIRT1 (regulator of senescence) | |||
| NA | HRECs | TRAP PCR Assay | ↓ Telomerase activity | [43] |
| PCR | ↑ PAI-1 ↑ TERF2 ↓ TERT ↓ SIRT1 | |||
| SA-β-Gal Assay | ↑ β-galactosidase activity | |||
| Western Blot | ↑ PAI-1 ↓ SIRT1 (regulator of senescence) | |||
| DR | HMRECs | Western Blot | ↑ p21 ↓ SIRT3 (regulator of senescence) | [44] |
| SA-β-Gal Assay | ↑ β-galactosidase activity | |||
| DME | HMRECs | Ionizing radiation | Actin filament organization showing impaired monolayer formation | [17] |
| Alamar Blue Assay | ↓ | |||
| Standard microscopy | ↓ cell density | |||
| SA-β-Gal Assay | ↑ β-galactosidase activity | |||
| Fluorescence microscopy | ↑ PML bodies | |||
| Western Blot | ↑ p16 ↑ p21 ↑ p53 | |||
| PCR | ↑ CDKN1A ↑ CDKN2A/INK4a ↑ SERPINE1 ↑ IL6 ↑ IL8 ↑ TNF ↑ERN1 | |||
| Immunoblot | ↑ γH2AX ↑ PAI-1 (SERPINE1) | |||
| Human Retina | Immunofluorescence | ↑ p16INK4A | ||
| Colocalization of PAI-1 and COL4-positive ECs | ||||
| STZ Retina | Single-Cell RNA Seq | Enrichment of senescence signature in ECs | ||
| NA | HRECs | SA-β-Gal Assay | ↑ β-galactosidase activity | [45] |
| Western Blot | ↑ p16Ink4a ↑ p21Waf1 ↓ SIRT1 (regulator of senescence) |
7. The Role of Mitochondria in Senescence
8. The Role of Mitochondria in Cell Fate Decisions
9. Genes That Initiate and Commit Cells to Senescence
10. Resilience to DR (RDR)
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Oubaha, M.; Miloudi, K.; Dejda, A.; Guber, V.; Mawambo, G.; Germain, M.A.; Bourdel, G.; Popovic, N.; Rezende, F.A.; Kaufman, R.J.; et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci. Transl. Med. 2016, 8, 362ra144. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.H.; Minaker, S.A.; Lahaie Luna, G.; Bapat, P.; Farahvash, A.; Garg, A.; Bhambra, N.; Muni, R.H. Changes in aqueous and vitreous inflammatory cytokine levels in nonproliferative diabetic retinopathy: Systematic review and meta-analysis. Can. J. Ophthalmol. 2025, 60, e100–e116. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Y.; Zhang, J.; Wang, W.; Hu, W.; He, M.; Zhu, Z. Retinal age gap as a predictive biomarker for future risk of clinically significant diabetic retinopathy. Acta Diabetol. 2024, 61, 373–380. [Google Scholar] [CrossRef]
- Abreu-Gonzalez, R.; Rodríguez-Martín, J.; Quezada-Peralta, G.; Rodrigo-Bello, J.; Gil-Hernández, M.; Bermúdez-Pérez, C.; Donate-López, J. Retinal age as a predictive biomarker of the diabetic retinopathy grade. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2023, 98, 265–269. [Google Scholar] [CrossRef]
- Campello, L.; Singh, N.; Advani, J.; Mondal, A.K.; Corso-Diaz, X.; Swaroop, A. Aging of the Retina: Molecular and Metabolic Turbulences and Potential Interventions. Annu. Rev. Vis. Sci. 2021, 7, 633–664. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, D.; Guankai, P.; Tan, Z.; Shang, X.; Hu, W.; Liao, H.; Zhang, X.; Huang, Y.; Yu, H.; et al. Retinal age gap as a predictive biomarker for mortality risk. Br. J. Ophthalmol. 2023, 107, 547–554. [Google Scholar] [CrossRef]
- Zhu, J.D.; Tarachand, S.P.; Abdulwahab, Q.; Samuel, M.A. Structure, Function, and Molecular Landscapes of the Aging Retina. Annu. Rev. Vis. Sci. 2023, 9, 177–199. [Google Scholar] [CrossRef]
- Ferdous, S.; Liao, K.L.; Gefke, I.D.; Summers, V.R.; Wu, W.; Donaldson, K.J.; Kim, Y.-K.; Sellers, J.T.; Dixon, J.A.; Shelton, D.A.; et al. Age-Related Retinal Changes in Wild-Type C57BL/6J Mice Between 2 and 32 Months. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Persidina, O.; Balasubramanian, G.; Nguyen, T.; Pradeep, A.; Hetling, J.R.; McAnany, J.J. Effects of normal aging on the mouse retina assessed by full-field flash and flicker electroretinography. Sci. Rep. 2023, 13, 8860. [Google Scholar]
- Xu, Q.; Rydz, C.; Nguyen Huu, V.A.; Rocha, L.; Palomino La Torre, C.; Lee, I.; Cho, W.; Jabari, M.; Donello, J.; Lyon, D.C.; et al. Stress induced aging in mouse eye. Aging Cell 2022, 21, e13737. [Google Scholar] [CrossRef]
- Campello, L.; Brooks, M.J.; Fadl, B.R.; Choi, H.S.; Pal, S.; Swaroop, A. Transcriptional Heterogeneity and Differential Response of Rod Photoreceptor Pathway Uncovered by Single-Cell RNA Sequencing of the Aging Mouse Retina. Aging Cell 2025, 24, e70001. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.L.; Fang, Y.F.; Sun, J.X.; Dou, G.R. Senescent endothelial cells: A potential target for diabetic retinopathy. Angiogenesis 2024, 27, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef]
- Crespo-Garcia, S.; Fournier, F.; Diaz-Marin, R.; Klier, S.; Ragusa, D.; Masaki, L.; Cagnone, G.; Blot, G.; Hafiane, I.; Dejda, A.; et al. Therapeutic targeting of cellular senescence in diabetic macular edema: Preclinical and phase 1 trial results. Nat. Med. 2024, 30, 443–454. [Google Scholar] [CrossRef]
- Klier, S.; Dananberg, J.; Masaki, L.; Bhisitkul, R.B.; Khanani, A.M.; Maturi, R.; Salehi-Had, H.; Mallinckrodt, C.H.; Rathmell, J.M.; Ghosh, A.; et al. Safety and Efficacy of Senolytic UBX1325 in Diabetic Macular Edema. NEJM Évid. 2025, 4, EVIDoa2400009. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- di Fagagna, F.D.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial Dysfunction Accounts for the Stochastic Heterogeneity in Telomere-Dependent Senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; Van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, R.; Lacelle, C.; Wang, E. Senescent fibroblasts resist apoptosis by downregulating caspase-3. Mech. Ageing Dev. 2004, 125, 777–783. [Google Scholar] [CrossRef]
- Harder, J.M.; Ding, Q.; Fernandes, K.A.; Cherry, J.D.; Gan, L.; Libby, R.T. BCL2L1 (BCL-X) promotes survival of adult and developing retinal ganglion cells. Mol. Cell Neurosci. 2012, 51, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nickells, R.W.; Semaan, S.J.; Schlamp, C.L. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog. Brain Res. 2008, 173, 423–435. [Google Scholar]
- Serikbaeva, A.; Li, Y.; Ma, S.; Yi, D.; Kazlauskas, A. Resilience to diabetic retinopathy. Prog. Retin. Eye Res. 2024, 101, 101271. [Google Scholar] [CrossRef]
- Liu, H.; Ghosh, S.; Vaidya, T.; Bammidi, S.; Huang, C.; Shang, P.; Nair, A.P.; Chowdhury, O.; Stepicheva, N.A.; Strizhakova, A.; et al. Activated cGAS/STING signaling elicits endothelial cell senescence in early diabetic retinopathy. JCI Insight 2023, 8, e168945. [Google Scholar]
- Li, Y.; Zhu, L.; Cai, M.-X.; Wang, Z.-L.; Zhuang, M.; Tan, C.-Y.; Xie, T.-H.; Yao, Y.; Wei, T.-T. TGR5 supresses cGAS/STING pathway by inhibiting GRP75-mediated endoplasmic reticulum-mitochondrial coupling in diabetic retinopathy. Cell Death Dis. 2023, 14, 583. [Google Scholar] [CrossRef]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.I.; et al. p16(Ink4a) and senescence-associated beta-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging 2017, 9, 1867–1884. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Burd, C.E.; Sorrentino, J.A.; Clark, K.S.; Darr, D.B.; Krishnamurthy, J.; Deal, A.M.; Bardeesy, N.; Castrillon, D.H.; Beach, D.H.; Sharpless, N.E. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013, 152, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef]
- Crespo-Garcia, S.; Tsuruda, P.R.; Dejda, A.; Ryan, R.D.; Fournier, F.; Chaney, S.Y.; Pilon, F.; Dogan, T.; Cagnone, G.; Patel, P.; et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 2021, 33, 818–832.e7. [Google Scholar] [CrossRef]
- Li, S.; Sun, D.; Chen, S.; Zhang, S.; Gu, Q.; Shen, Y.; Xu, L.; Xu, X.; Wei, F.; Wang, N. UCP2-SIRT3 Signaling Relieved Hyperglycemia-Induced Oxidative Stress and Senescence in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 14. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, M.; Xu, R.; Fu, L.; Xue, M.; Xu, C.; Tang, C.; Fang, T.; Liu, X.; Sun, B.; et al. p53 accelerates endothelial cell senescence in diabetic retinopathy by enhancing FoxO3a ubiquitylation and degradation via UBE2L6. Exp. Gerontol. 2024, 188, 112391. [Google Scholar] [CrossRef]
- Bertelli, P.M.; Pedrini, E.; Hughes, D.; McDonnell, S.; Pathak, V.; Peixoto, E.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. Long term high glucose exposure induces premature senescence in retinal endothelial cells. Front. Physiol. 2022, 13, 929118. [Google Scholar] [CrossRef]
- Gericke, A.; Suminska-Jasinska, K.; Breborowicz, A. Sulodexide reduces glucose induced senescence in human retinal endothelial cells. Sci. Rep. 2021, 11, 11532. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Robinson, R.; Greenway, G.; Glass, J.; Budkin, S.; Sharma, S. Blockade of interleukin-6 trans-signaling prevents mitochondrial dysfunction and cellular senescence in retinal endothelial cells. Exp. Eye Res. 2023, 237, 109721. [Google Scholar] [CrossRef]
- Hou, L.; Du, J.; Dong, Y.; Wang, M.; Wang, L.; Zhao, J. Liraglutide prevents cellular senescence in human retinal endothelial cells (HRECs) mediated by SIRT1: An implication in diabetes retinopathy. Hum. Cell 2024, 37, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Mi, Y.; Ren, K.; Wang, S.; Li, M.; Yang, D. The inhibitory effects of Dulaglutide on cellular senescence against high glucose in human retinal endothelial cells. Hum. Cell 2022, 35, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, S.; Chen, S.; Zhang, S.; Gu, Q.; Shen, Y.; Wei, F.; Wang, N. TRIM25 inhibition attenuates inflammation, senescence, and oxidative stress in microvascular endothelial cells induced by hyperglycemia. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 81–91. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Jadeja, R.N.; Warren, M.; Powell, F.L.; Raju, R.; Gutsaeva, D.; Khurana, S.; Martin, P.M.; Bartoli, M. MicroRNA-34a (miR-34a) Mediates Retinal Endothelial Cell Premature Senescence through Mitochondrial Dysfunction and Loss of Antioxidant Activities. Antioxidants 2019, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondria in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Preclinical Evaluation of Oral Urolithin-A for the Treatment of Acute Campylobacteriosis in Campylobacter jejuni Infected Microbiota-Depleted IL-10(-/-) Mice. Pathogens 2020, 10, 7. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef]
- Skeie, J.M.; Nishimura, D.Y.; Wang, C.L.; Schmidt, G.A.; Aldrich, B.T.; Greiner, M.A. Mitophagy: An Emerging Target in Ocular Pathology. Investig. Ophthalmol. Vis. Sci. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zou, H. Research Progress on Mitochondrial Dysfunction in Diabetic Retinopathy. Antioxidants 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Alka, K.; Kumar, J.; Kowluru, R.A. Impaired mitochondrial dynamics and removal of the damaged mitochondria in diabetic retinopathy. Front. Endocrinol. 2023, 14, 1160155. [Google Scholar] [CrossRef] [PubMed]
- Guduric-Fuchs, J.; Pedrini, E.; Bertelli, P.M.; McDonnell, S.; Pathak, V.; McLoughlin, K.; O’Neill, C.L.; Stitt, A.W.; Medina, R.J. A new gene signature for endothelial senescence identifies self-RNA sensing by retinoic acid-inducible gene I as a molecular facilitator of vascular aging. Aging Cell 2024, 23, e14240. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Marzoog, B.A. Autophagy Behavior in Endothelial Cell Regeneration. Curr. Aging Sci. 2024, 17, 58–67. [Google Scholar] [CrossRef]
- Song, P.; Zou, M.H. Redox regulation of endothelial cell fate. Cell Mol. Life Sci. 2014, 71, 3219–3239. [Google Scholar] [CrossRef]
- Mameli, E.; Martello, A.; Caporali, A. Autophagy at the interface of endothelial cell homeostasis and vascular disease. FEBS J. 2022, 289, 2976–2991. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Bourdeau, V.; Rowell, M.-C.; Amirimehr, D.; Guillon, J.; Kalegari, P.; Barua, A.; Trinh, V.Q.-H.; Azzi, F.; Turcotte, S.; et al. A senescence restriction point acting on chromatin integrates oncogenic signals. Cell Rep. 2024, 43, 114044. [Google Scholar] [CrossRef]
- Martínez-Zamudio, R.I.; Roux, P.-F.; de Freitas, J.A.N.; Robinson, L.; Doré, G.; Sun, B.; Belenki, D.; Milanovic, M.; Herbig, U.; Schmitt, C.A.; et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat. Cell Biol. 2020, 22, 842–855. [Google Scholar] [CrossRef]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S.; et al. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef]
- Mallette, F.A.; Gaumont-Leclerc, M.F.; Huot, G.; Ferbeyre, G. Myc down-regulation as a mechanism to activate the Rb pathway in STAT5A-induced senescence. J. Biol. Chem. 2007, 282, 34938–34944. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Zebedee, Z.; Huot, T.J.; Stinson, J.A.; Sugimoto, M.; Ohashi, Y.; Sharrocks, A.D.; Peters, G.; Hara, E. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 2001, 409, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Wang, J.-J.; Lin, J.-G.; Ye, W.-L.; Zou, J.-M.; Liang, L.-Y.; Yang, P.-L.; Qiu, W.-L.; Li, Y.-Y.; Yang, S.-J.; et al. Cytosolic DNA initiates a vicious circle of aging-related endothelial inflammation and mitochondrial dysfunction via STING: The inhibitory effect of Cilostazol. Acta Pharmacol. Sin. 2024, 45, 1879–1897. [Google Scholar] [CrossRef]
- Baris, A.; Fraile-Bethencourt, E.; Eubanks, J.; Khou, S.; Anand, S. Thymidine phosphorylase facilitates retinoic acid inducible gene-I induced endothelial dysfunction. Cell Death Dis. 2023, 14, 294. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Yu, M.G.; Gordin, D.; Fu, J.; Park, K.; Li, Q.; King, G.L. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr. Rev. 2024, 45, 227–252. [Google Scholar] [PubMed]
- Serikbaeva, A.; Li, Y.; Ganesh, B.; Zelkha, R.; Kazlauskas, A. Hyperglycemia Promotes Mitophagy and Thereby Mitigates Hyperglycemia-Induced Damage. Am. J. Pathol. 2022, 192, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, S.; Kazlauskas, A. Manifestation of Pathology in Animal Models of Diabetic Retinopathy Is Delayed from the Onset of Diabetes. Int. J. Mol. Sci. 2024, 25, 1610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Baccouche, B.; Del-Risco, N.; Park, J.; Song, A.; McAnany, J.J.; Kazlauskas, A. The Slow Progression of Diabetic Retinopathy Is Associated with Transient Protection of Retinal Vessels from Death. Int. J. Mol. Sci. 2023, 24, 10869. [Google Scholar] [CrossRef]
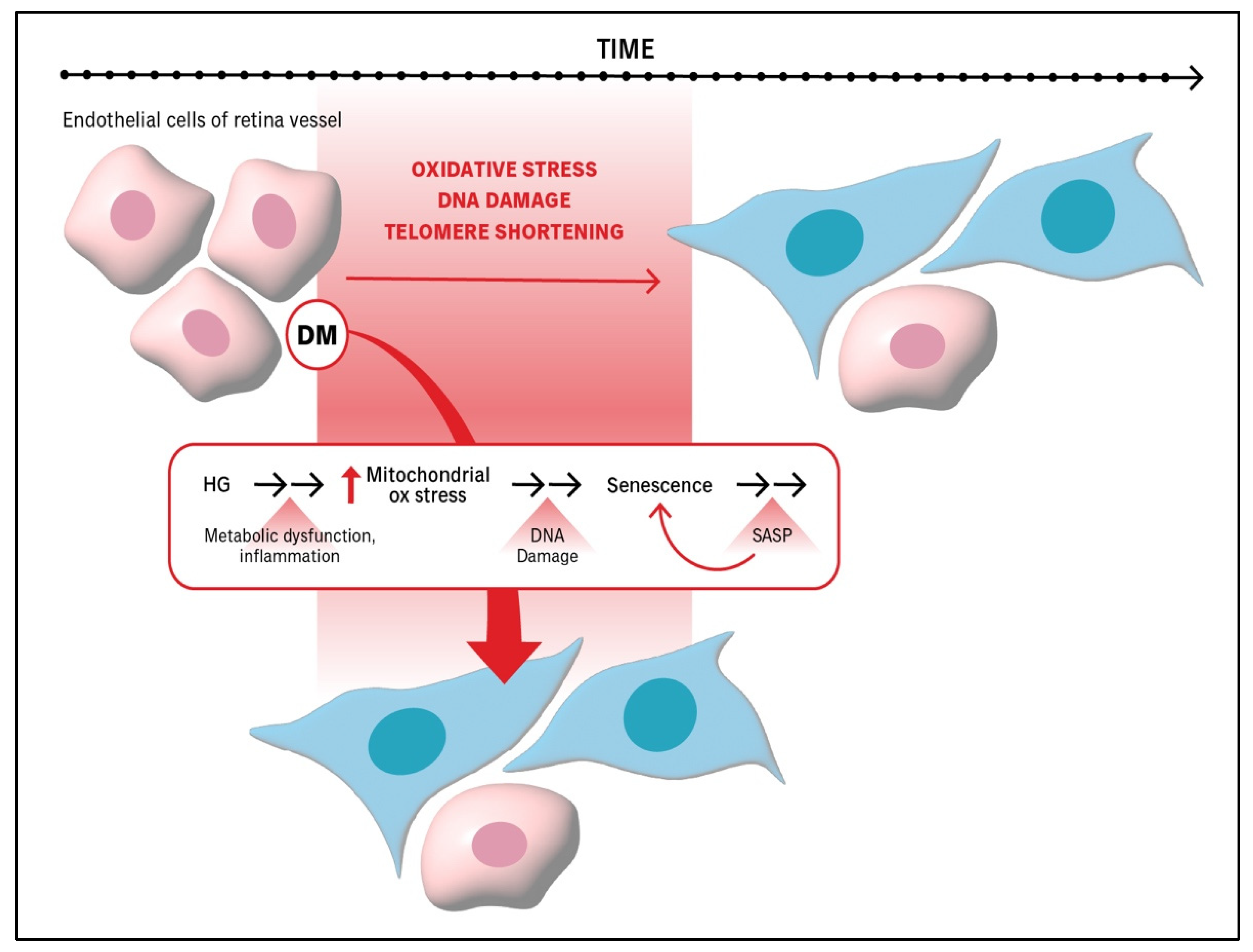
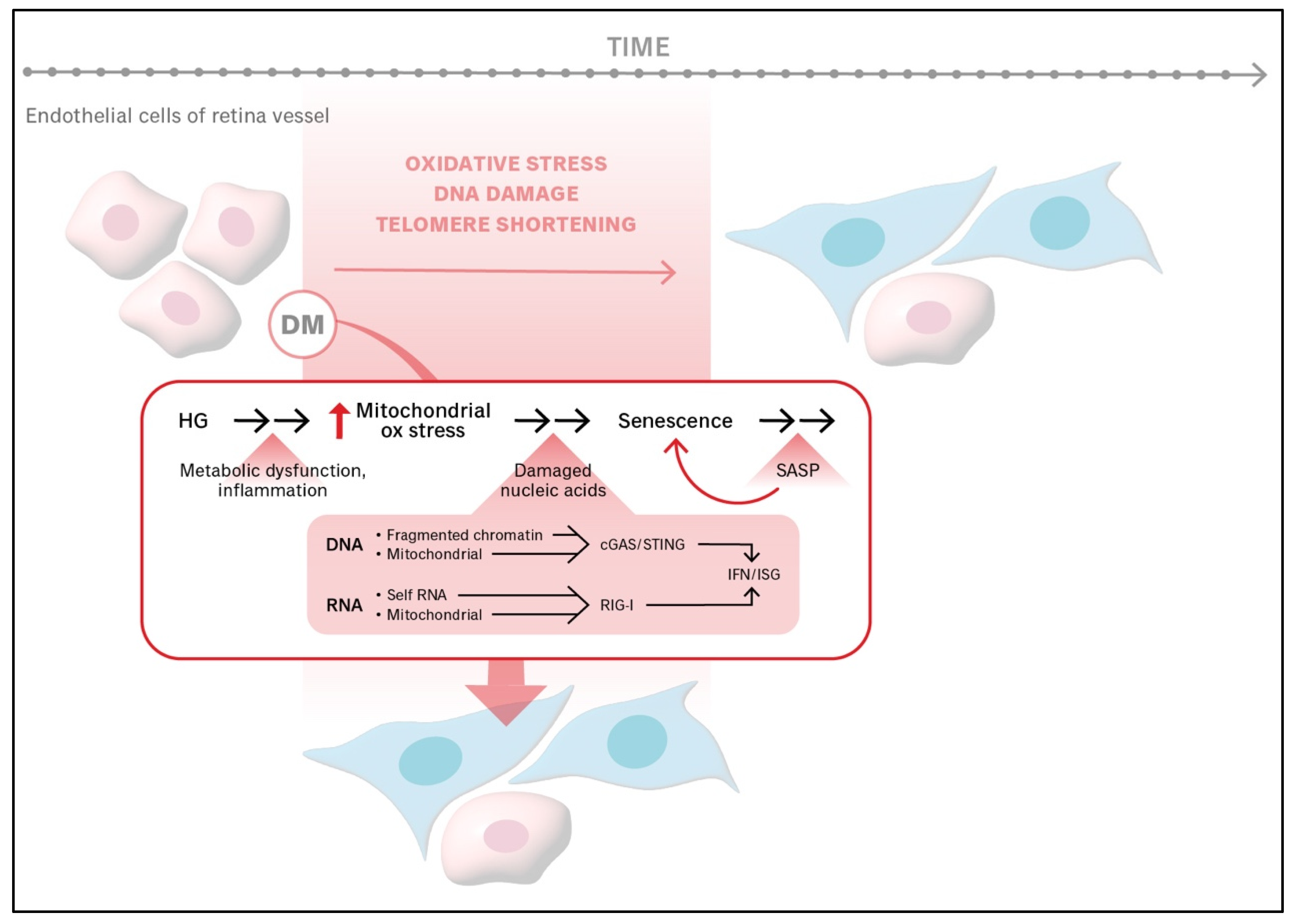

| Stage of DR Pathogenesis | Impact of Senescence |
|---|---|
| Deterioration of RDR | Unknown |
| Vascular dysfunction that does not compromise vision | Exacerbates pathology by promoting SASP-mediated inflammation |
| Vision-compromising vascular dysfunction | Exacerbates or mitigates vascular dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, M.; Haider, S.; Chang, H.Z.Y.; Kazlauskas, A. The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy. Int. J. Mol. Sci. 2025, 26, 5211. https://doi.org/10.3390/ijms26115211
Gandhi M, Haider S, Chang HZY, Kazlauskas A. The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy. International Journal of Molecular Sciences. 2025; 26(11):5211. https://doi.org/10.3390/ijms26115211
Chicago/Turabian StyleGandhi, Manav, Shahzaib Haider, Helena Zin Ying Chang, and Andrius Kazlauskas. 2025. "The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy" International Journal of Molecular Sciences 26, no. 11: 5211. https://doi.org/10.3390/ijms26115211
APA StyleGandhi, M., Haider, S., Chang, H. Z. Y., & Kazlauskas, A. (2025). The Role of Endothelial Senescence in the Pathogenesis of Diabetic Retinopathy. International Journal of Molecular Sciences, 26(11), 5211. https://doi.org/10.3390/ijms26115211







