Long-Term miRNA Changes Predicting Resiliency Factors of Post-Traumatic Stress Disorder in a Large Military Cohort—Millennium Cohort Study
Abstract
1. Introduction
2. Results
2.1. Five Trajectories of PTSD Symptoms
2.2. miRNA Associated with Changes in PTSD Symptoms
2.3. Comparative Analysis of miRNA Expression Patterns Across PTSD Trajectories
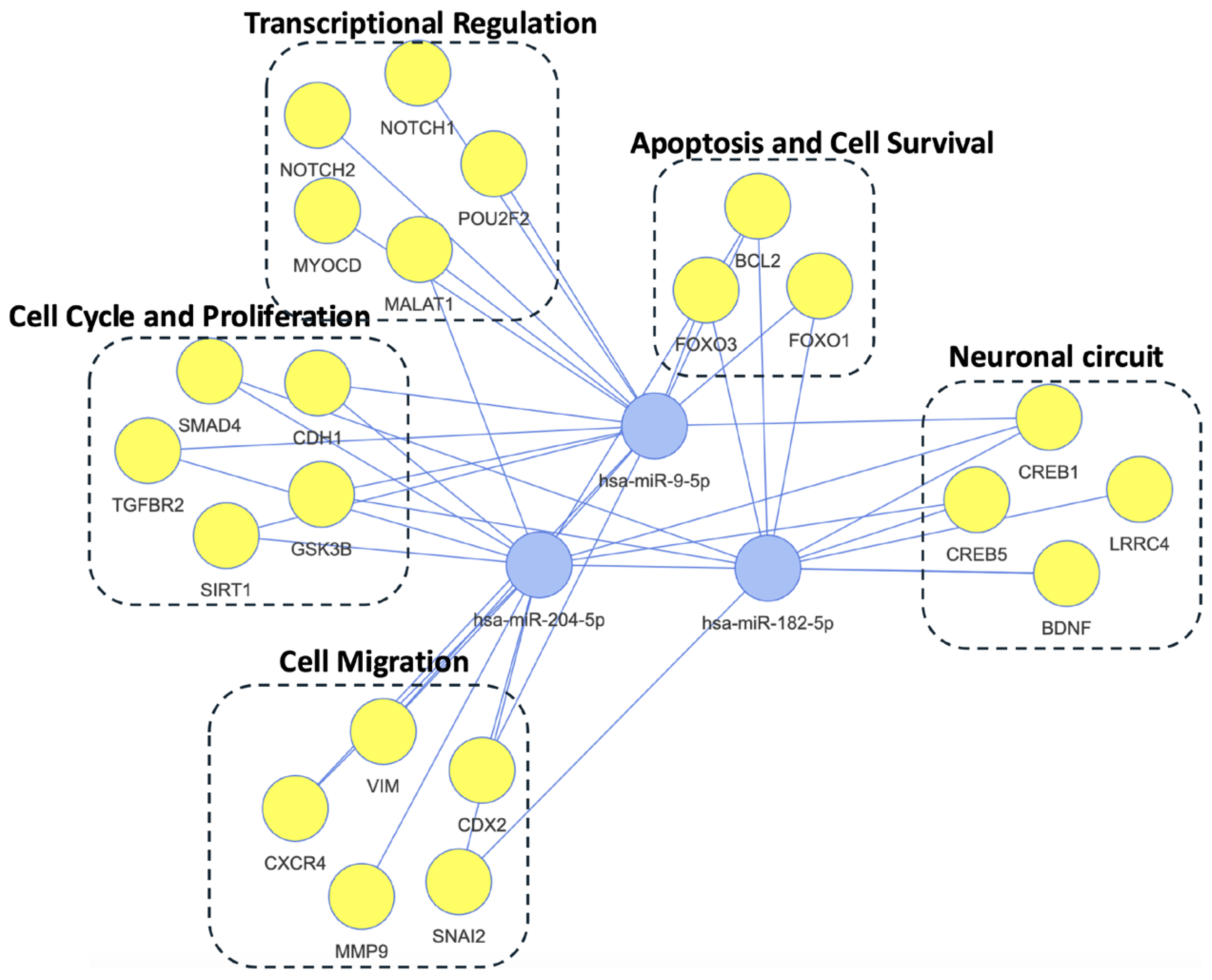
2.3.1. Adaptive vs. Resilient Trajectories
2.3.2. New-Onset vs. Resilient Trajectories
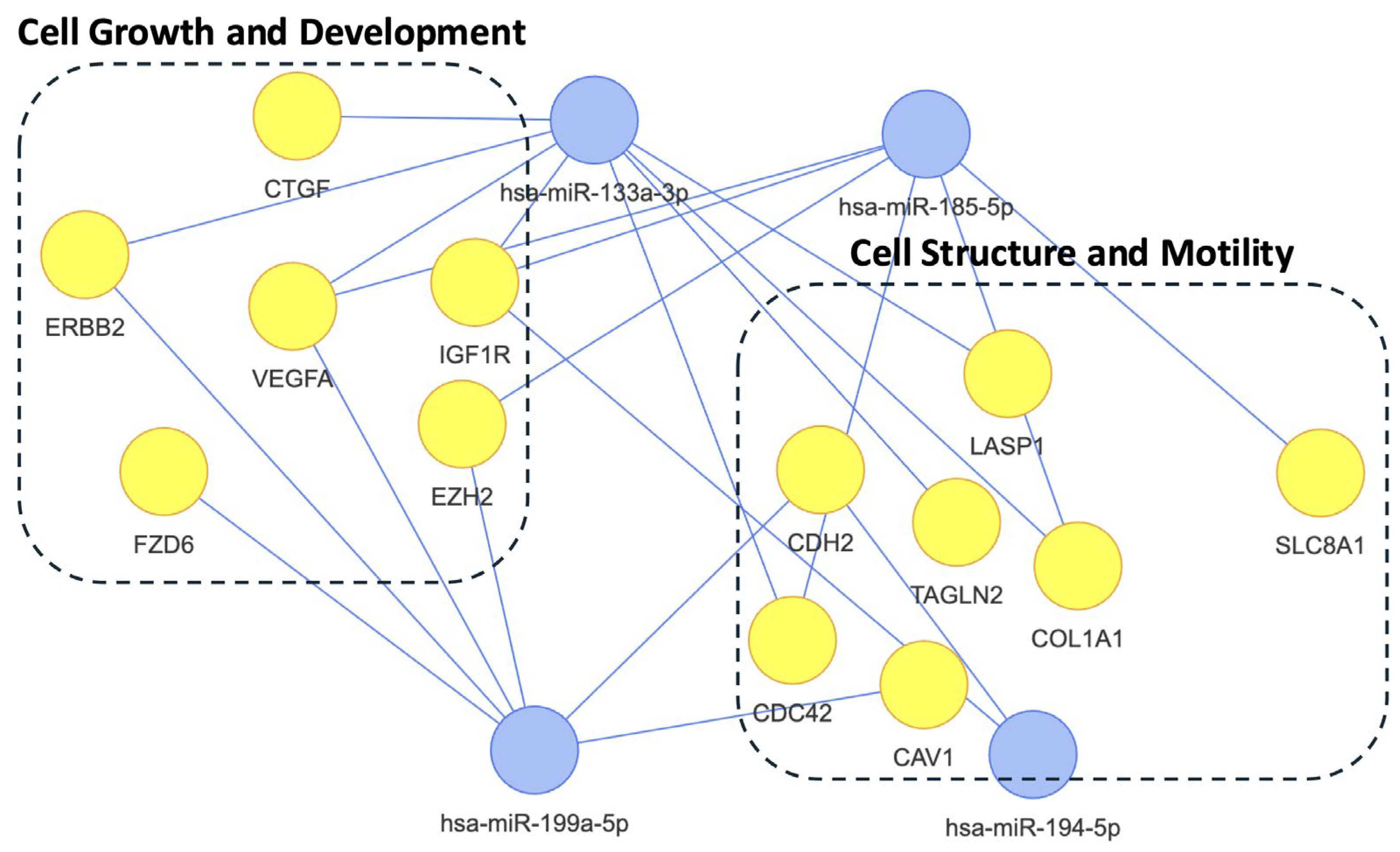
2.4. Differential Analysis Between PTSD Positive and Negative
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. miRNA Sequencing
4.3. Statistical Analysis
4.3.1. Cluster Analysis
4.3.2. Differential Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP Response Element-Binding Protein |
| DoDSR | Department of Defense Serum Repository |
| FDR | False discovery rate |
| LGMM | Latent growth mixture model |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PCL | PTSD Checklist |
| TGF-alpha | Transforming Growth Factor-alpha |
| PTSD | Post-traumatic stress disorder |
References
- Yehuda, R.; Hoge, C.W.; McFarlane, A.C.; Vermetten, E.; Lanius, R.A.; Nievergelt, C.M.; Hobfoll, S.E.; Koenen, K.C.; Neylan, T.C.; Hyman, S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Primers 2015, 1, 15057. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A.; Liberzon, I.; Marmar, C. Post-traumatic stress disorder. N. Eng. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Hoge, C.W.; Castro, C.A.; Messer, S.C.; McGurk, D.; Cotting, D.I.; Koffman, R.L. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N. Eng. J. Med. 2004, 351, 13–22. [Google Scholar] [CrossRef]
- Dean, K.R.; Hammamieh, R.; Mellon, S.H.; Abu-Amara, D.; Flory, J.D.; Guffanti, G.; Wang, K.; Daigle, B.J., Jr.; Gautam, A.; Lee, I. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol. Psychiatry 2020, 25, 3337–3349. [Google Scholar] [CrossRef]
- Maurel, O.M.; Torrisi, S.A.; Barbagallo, C.; Purrello, M.; Salomone, S.; Drago, F.; Ragusa, M.; Leggio, G.M. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p is associated with modulation of BDNF and FKBP5 in brain areas of PTSD-related susceptible and resilient mice. Int. J. Mol. Sci. 2021, 22, 5157. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, X.; Du, M.; Wu, C.; Fu, J.; Tan, W.; Wu, B.; Zhang, J.; Liao, Z. Recent advances in the role of miRNAs in post-traumatic stress disorder and traumatic brain injury. Mol. Psychiatry 2023, 28, 2630–2644. [Google Scholar] [CrossRef]
- Huang, G.; Iqbal, J.; Shen, D.; Xue, Y.-X.; Yang, M.; Jia, X. MicroRNA expression profiles of stress susceptibility and resilience in the prelimbic and infralimbic cortex of rats after single prolonged stress. Front. Psychiatry 2023, 14, 1247714. [Google Scholar] [CrossRef]
- Ji, L.-L.; Ye, Y.; Nie, P.-Y.; Peng, J.-B.; Fu, C.-H.; Wang, Z.-Y.; Tong, L. Dysregulation of miR-142 results in anxiety-like behaviors following single prolonged stress. Behav. Brain Res. 2019, 365, 157–163. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Tong, L.; Chen, Y.; Fu, C.-H.; Peng, J.-B.; Ji, L.-L. MiR-153 downregulation alleviates PTSD-like behaviors and reduces cell apoptosis by upregulating the Sigma-1 receptor in the hippocampus of rats exposed to single-prolonged stress. Exp. Neurol. 2022, 352, 114034. [Google Scholar] [CrossRef]
- Lee, M.Y.; Baxter, D.; Scherler, K.; Kim, T.-K.; Wu, X.; Abu-Amara, D.; Flory, J.; Yehuda, R.; Marmar, C.; Jett, M. Distinct profiles of cell-free microRNAs in plasma of veterans with post-traumatic stress disorder. J. Clin. Med. 2019, 8, 963. [Google Scholar] [CrossRef]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef] [PubMed]
- Schultebraucks, K.; Qian, M.; Abu-Amara, D.; Dean, K.; Laska, E.; Siegel, C.; Gautam, A.; Guffanti, G.; Hammamieh, R.; Misganaw, B. Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: A machine-learning approach for analyzing multivariate predictors. Mol. Psychiatry 2021, 26, 5011–5022. [Google Scholar] [CrossRef] [PubMed]
- Belding, J.N.; Castañeda, S.F.; Jacobson, I.G.; LeardMann, C.A.; Porter, B.; Powell, T.M.; Kolaja, C.A.; Seelig, A.D.; Matsuno, R.K.; Carey, F.R. The Millennium Cohort Study: The first 20 years of research dedicated to understanding the long-term health of US Service Members and Veterans. Ann. Epidemiol. 2022, 67, 61–72. [Google Scholar] [CrossRef]
- Chesbrough, K.B.; Ryan, M.A.; Amoroso, P.; Boyko, E.J.; Gackstetter, G.D.; Hooper, T.I.; Riddle, J.R.; Gray, G.C.; Group, M.C.S. The Millennium Cohort Study: A 21-year prospective cohort study of 140,000 military personnel. Mil. Med. 2002, 167, 483–488. [Google Scholar] [CrossRef]
- Conrad, D.; Wilker, S.; Schneider, A.; Karabatsiakis, A.; Pfeiffer, A.; Kolassa, S.; Freytag, V.; Vukojevic, V.; Vogler, C.; Milnik, A. Integrated genetic, epigenetic, and gene set enrichment analyses identify NOTCH as a potential mediator for PTSD risk after trauma: Results from two independent African cohorts. Psychophysiology 2020, 57, e13288. [Google Scholar] [CrossRef]
- Katrinli, S.; Oliveira, N.C.; Felger, J.C.; Michopoulos, V.; Smith, A.K. The role of the immune system in posttraumatic stress disorder. Transl. Psychiatry 2022, 12, 313. [Google Scholar] [CrossRef]
- Hung, Y.-Y.; Huang, Y.-L.; Chang, C.; Kang, H.-Y. Deficiency in androgen receptor aggravates the depressive-like behaviors in chronic mild stress model of depression. Cells 2019, 8, 1021. [Google Scholar] [CrossRef]
- Guan, W.; Wu, X.Y.; Jin, X.; Sheng, X.M.; Fan, Y. miR-204-5p Plays a Critical Role in the Pathogenesis of Depression and Anti-depression Action of Venlafaxine in the Hippocampus of Mice. Curr. Med. Chem. 2024, 31, 3412–3425. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Sheng, X.M.; Jin, X.; Guan, W. MiR-182-5p: A Novel Biomarker in the Treatment of Depression in CSDS-Induced Mice. Int. J. Neuropsychopharmacol. 2024, 27, pyad064. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; van den Heuvel, L.; Bucker, J.; Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241928. [Google Scholar] [CrossRef]
- Yang, R.; Xu, C.; Bierer, L.M.; Flory, J.D.; Gautam, A.; Bader, H.N.; Lehrner, A.; Makotkine, I.; Desarnaud, F.; Miller, S.A. Longitudinal genome-wide methylation study of PTSD treatment using prolonged exposure and hydrocortisone. Transl. Psychiatry 2021, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gautam, A.; Getnet, D.; Daigle, B.J.; Miller, S.; Misganaw, B.; Dean, K.R.; Kumar, R.; Muhie, S.; Wang, K. Epigenetic biotypes of post-traumatic stress disorder in war-zone exposed veteran and active duty males. Mol. Psychiatry 2021, 26, 4300–4314. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; Guerreiro-Costa, L.N.F.; Lins-Silva, D.; Guimaraes, D.F.; Souza, L.S.; Leal, G.C.; Caliman-Fontes, A.T.; Beanes, G.; Costa, R.D.S.; Quarantini, L.C. MicroRNAs as diagnostic biomarkers and predictors of antidepressant response in major depressive disorder: A systematic review. Cureus 2024, 16, e56910. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Tian, X.; Luo, Y.; Zhong, J.; Zhang, H.; Li, H.; Cen, B.; Jiang, T.; Sun, X. microRNA-9-5p alleviates blood-brain barrier damage and neuroinflammation after traumatic brain injury. J. Neurochem. 2020, 153, 710–726. [Google Scholar] [CrossRef]
- Belzeaux, R.; Bergon, A.; Jeanjean, V.; Loriod, B.; Formisano-Tréziny, C.; Verrier, L.; Loundou, A.; Baumstarck-Barrau, K.; Boyer, L.; Gall, V. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl. Psychiatry 2012, 2, e185. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S. Micro RNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Qu, S.; Yang, L.; Liu, Z. MicroRNA-194 reduces inflammatory response and human dermal microvascular endothelial cells permeability through suppression of TGF-β/SMAD pathway by inhibiting THBS1 in chronic idiopathic urticaria. J. Cell. Biochem. 2020, 121, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, S.F.; Belding, J.N.; Kolaja, C.A.; LeardMann, C.A.; Jacobson, I.G.; Rivera, A.C.; Carey, F.R.; Boparai, S.; Walstrom, J.L.; Sheppard, B.D. Cohort Profile Update: The US Millennium Cohort Study—Evaluating the impact of military experiences on service members and veteran health. Int. J. Epidemiol. 2023, 52, e222–e231. [Google Scholar] [CrossRef]
- McDonald, J.S.; Milosevic, D.; Reddi, H.V.; Grebe, S.K.; Algeciras-Schimnich, A. Analysis of circulating microRNA: Preanalytical and analytical challenges. Clin. Chem. 2011, 57, 833–840. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer: Berlin/Heidelberg, Germany, 2005; pp. 397–420. [Google Scholar]
- Law, C.W.; Alhamdoosh, M.; Su, S.; Dong, X.; Tian, L.; Smyth, G.K.; Ritchie, M.E. RNA-seq analysis is easy as 1-2-3 with limma, Glimma and edgeR. F1000Research 2016, 5, ISCB Comm J-1408. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-D.; Lin, F.-M.; Wu, W.-Y.; Liang, C.; Huang, W.-C.; Chan, W.-L.; Tsai, W.-T.; Chen, G.-Z.; Lee, C.-J.; Chiu, C.-M. miRTarBase: A database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2011, 39 (Suppl. 1), D163–D169. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef]
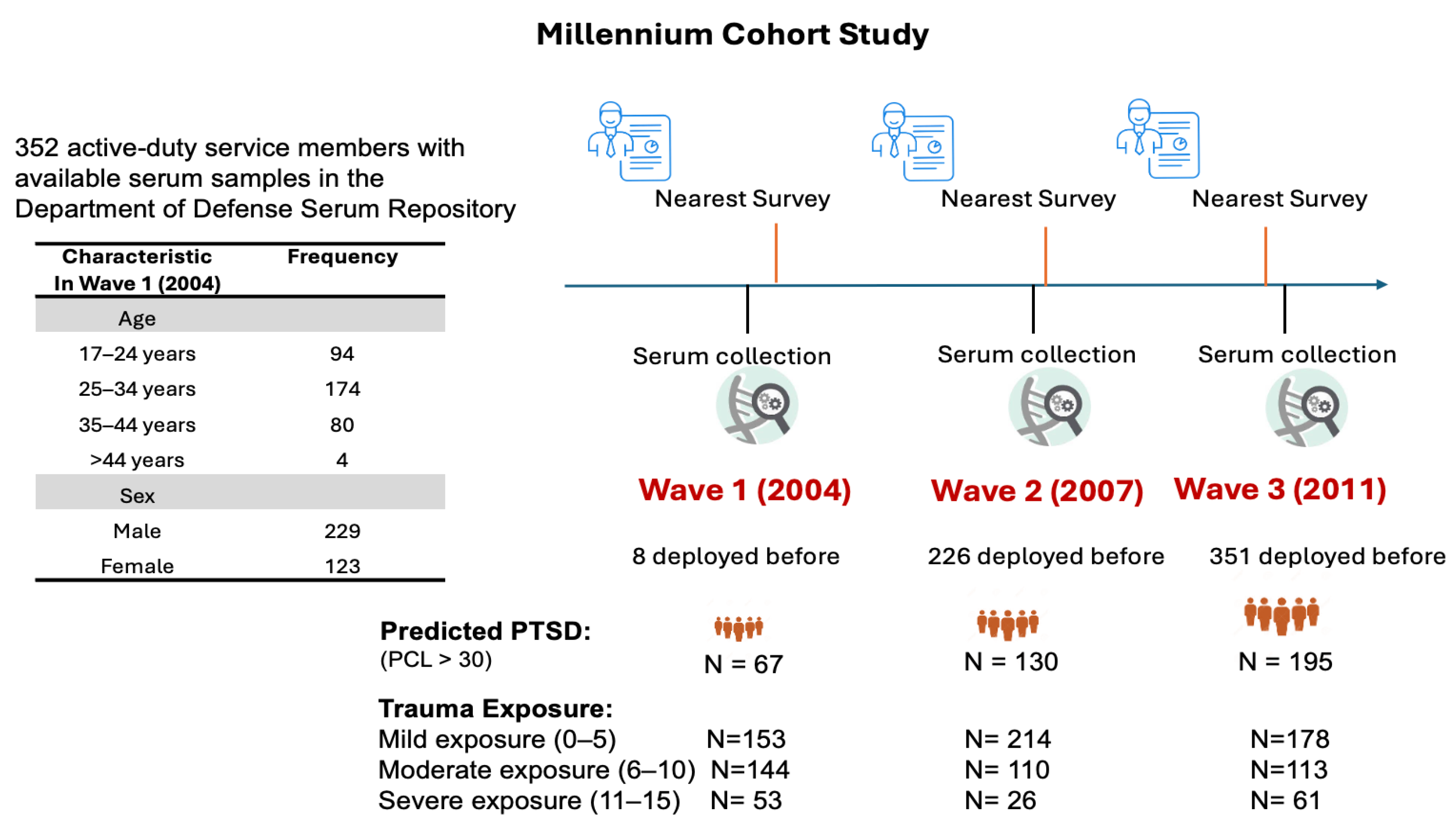
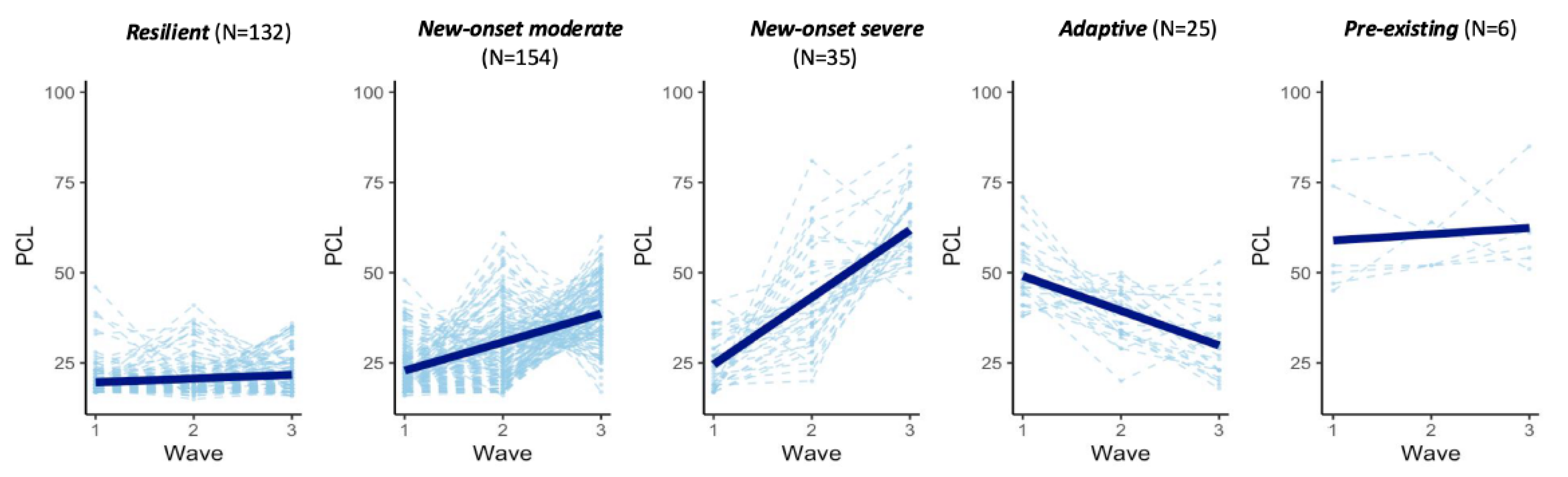

| Wave 1 | Wave 2 | Wave 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Control | PTSD | p-Value | Control | PTSD | p-Value | Control | PTSD | p-Value |
| n | 283 | 67 | 220 | 130 | 157 | 195 | |||
| Sex = female (%) | 97 (34.3%) | 25 (37.3%) | 0.744 | 78 (35.5%) | 45 (34.6%) | 0.966 | 61 (38.9%) | 62 (31.8%) | 0.205 |
| Trauma exposure (mean (SD)) | 6.92 (2.48) | 7.70 (2.82) | 0.04 | 6.86 (2.39) | 7.41 (2.79) | 0.006 | 6.33 (2.29) | 7.46 (2.88) | 0.006 |
| Age category (%) | 0.471 | 0.072 | 0.445 | ||||||
| 70 (24.7%) | 22 (32.8%) | 14 (6.4%) | 19 (14.6%) | 0 (0.0%) | 0 (0.0%) | |||
| 146 (51.6%) | 28 (41.8%) | 112 (50.9%) | 56 (43.1%) | 64 (40.8%) | 74 (37.9%) | |||
| 64 (22.6%) | 16 (23.9%) | 75 (34.1%) | 43 (33.1%) | 74 (47.1%) | 88 (45.1%) | |||
| 3 (1.1%) | 1 (1.5%) | 19 (8.6%) | 12 (9.2%) | 19 (12.1%) | 33 (16.9%) | |||
| PHQ (mean (SD)) | 31.74 (10.31) | 48.90 (15.50) | <0.001 | 33.57 (11.28) | 48.76 (14.85) | <0.001 | 21.91 (17.60) | 35.32 (26.62) | <0.001 |
| Cigarette pack/week (mean (SD)) | 1.84 (0.77) | 1.97 (0.87) | 0.248 | 1.81 (0.77) | 1.98 (0.81) | 0.069 | 1.88 (0.90) | 2.07 (0.91) | 0.076 |
| Sleep (mean (SD)) | 6.43 (1.33) | 5.93 (1.65) | 0.020 | 6.22 (1.10) | 5.85 (1.59) | 0.016 | 6.39 (1.36) | 5.84 (1.51) | <0.001 |
| PCL (mean (SD)) | 20.25 (3.49) | 43.22 (10.87) | <0.001 | 20.95 (3.96) | 41.52 (10.02) | <0.001 | 21.61 (4.30) | 45.25 (11.53) | <0.001 |
| PTSD vs. Control | Female vs. Male | ||||||
|---|---|---|---|---|---|---|---|
| miRNA | logFC | logCPM | p-Value | FDR | logFC | p-Value | FDR |
| hsa-miR-182-5p | 0.4341 | 10.9460 | 6.33 × 10−7 | 0.0002 | −0.4488 | 1.42 × 10−7 | 3.90 × 10−6 |
| hsa-miR-204-5p | 0.5628 | 7.0284 | 1.30 × 10−6 | 0.0002 | −0.6623 | 2.19 × 10−8 | 8.75 × 10−7 |
| hsa-miR-9-5p | 0.5634 | 6.1838 | 2.37 × 10−5 | 0.0022 | −0.6993 | 1.07 × 10−7 | 3.28 × 10−6 |
| PTSD vs. Control | Female vs. Male | ||||||
|---|---|---|---|---|---|---|---|
| miRNA | logFC | logCPM | p-Value | FDR | logFC | p-Value | FDR |
| hsa-miR-185-5p | −0.4523 | 6.4460 | 9.02 × 10−5 | 4.13 × 10−3 | 0.2834 | 0.016 | 0.0819 |
| hsa-miR-194-5p | −0.5232 | 6.5525 | 2.34 × 10−7 | 4.81 × 10−5 | 0.4883 | 2.16 × 10−6 | 0.0001 |
| hsa-miR-199a-5p | −0.5414 | 6.6457 | 3.50 × 10−7 | 4.81 × 10−5 | −0.1039 | 0.3332 | 0.4724 |
| hsa-miR-133a-3p | −0.9689 | 9.2582 | 1.98 × 10−6 | 1.81 × 10−4 | −0.1321 | 0.5322 | 0.6623 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Kannan, S.; Gautam, A.; Powell, T.M.; LeardMann, C.A.; Hoke, A.V.; Dimitrov, G.I.; Jett, M.; Donoho, C.J.; Rull, R.P.; et al. Long-Term miRNA Changes Predicting Resiliency Factors of Post-Traumatic Stress Disorder in a Large Military Cohort—Millennium Cohort Study. Int. J. Mol. Sci. 2025, 26, 5195. https://doi.org/10.3390/ijms26115195
Yang R, Kannan S, Gautam A, Powell TM, LeardMann CA, Hoke AV, Dimitrov GI, Jett M, Donoho CJ, Rull RP, et al. Long-Term miRNA Changes Predicting Resiliency Factors of Post-Traumatic Stress Disorder in a Large Military Cohort—Millennium Cohort Study. International Journal of Molecular Sciences. 2025; 26(11):5195. https://doi.org/10.3390/ijms26115195
Chicago/Turabian StyleYang, Ruoting, Swapna Kannan, Aarti Gautam, Teresa M. Powell, Cynthia A. LeardMann, Allison V. Hoke, George I. Dimitrov, Marti Jett, Carrie J. Donoho, Rudolph P. Rull, and et al. 2025. "Long-Term miRNA Changes Predicting Resiliency Factors of Post-Traumatic Stress Disorder in a Large Military Cohort—Millennium Cohort Study" International Journal of Molecular Sciences 26, no. 11: 5195. https://doi.org/10.3390/ijms26115195
APA StyleYang, R., Kannan, S., Gautam, A., Powell, T. M., LeardMann, C. A., Hoke, A. V., Dimitrov, G. I., Jett, M., Donoho, C. J., Rull, R. P., & Hammamieh, R. (2025). Long-Term miRNA Changes Predicting Resiliency Factors of Post-Traumatic Stress Disorder in a Large Military Cohort—Millennium Cohort Study. International Journal of Molecular Sciences, 26(11), 5195. https://doi.org/10.3390/ijms26115195






