Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole
Abstract
1. Introduction
2. Results
2.1. Tetraniliprole Relative Toxicity and Resistance Development
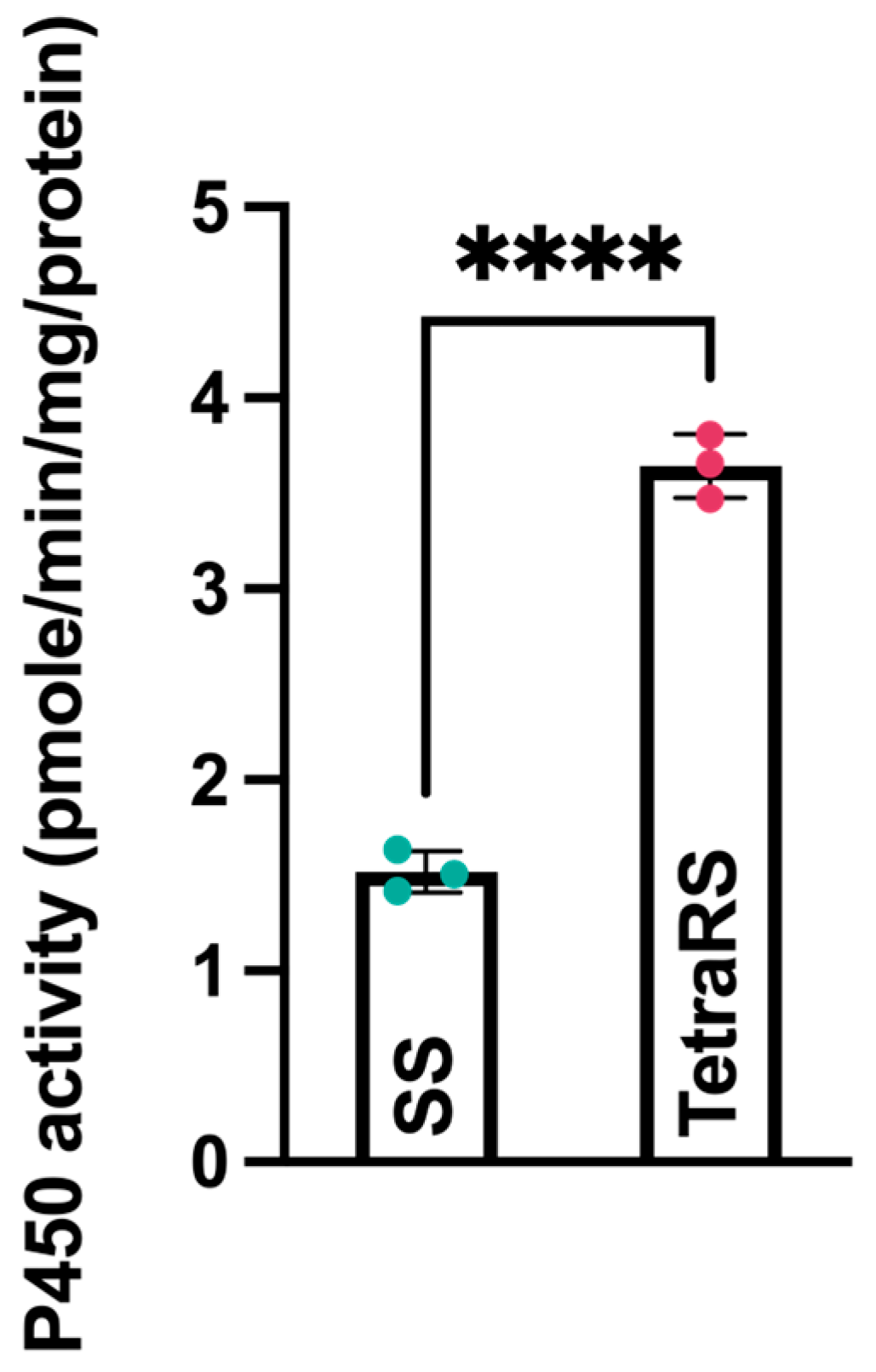
2.2. Effects of Tetraniliprole Resistance on Life-History Traits and P450 Enzyme Activity
2.3. Transcriptome Data Annotation
| Samples | Reads | Bases | GC (%) | N (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| SS-1 | 23,470,720 | 7,024,623,252 | 46.75 | 0.04 | 98.24 | 95.34 |
| SS-2 | 24,722,615 | 7,400,591,111 | 45.92 | 0.04 | 98.07 | 94.84 |
| SS-3 | 26,083,946 | 7,806,153,973 | 46.58 | 0.04 | 98.25 | 95.35 |
| TetraRS-1 | 23,478,474 | 7,027,641,397 | 46.28 | 0.04 | 98.13 | 94.99 |
| TetraRS-2 | 25,200,234 | 7,540,917,370 | 46.7 | 0.04 | 98.2 | 95.24 |
| TetraRS-3 | 23,393,925 | 6,999,448,021 | 47.11 | 0.04 | 98.14 | 95.06 |
2.4. Differentially Expressed Genes in TetraRS and SS Tuta Absoluta
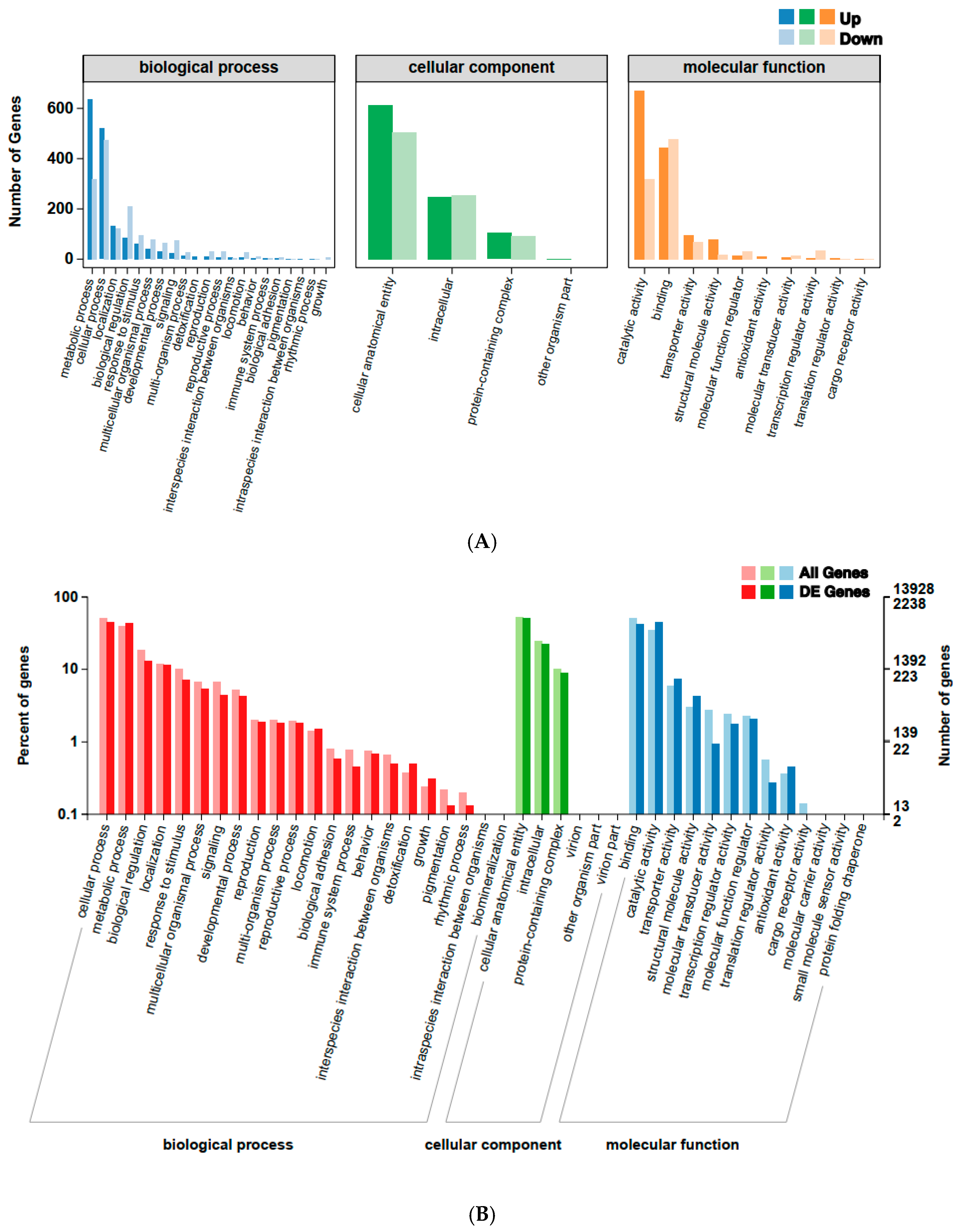
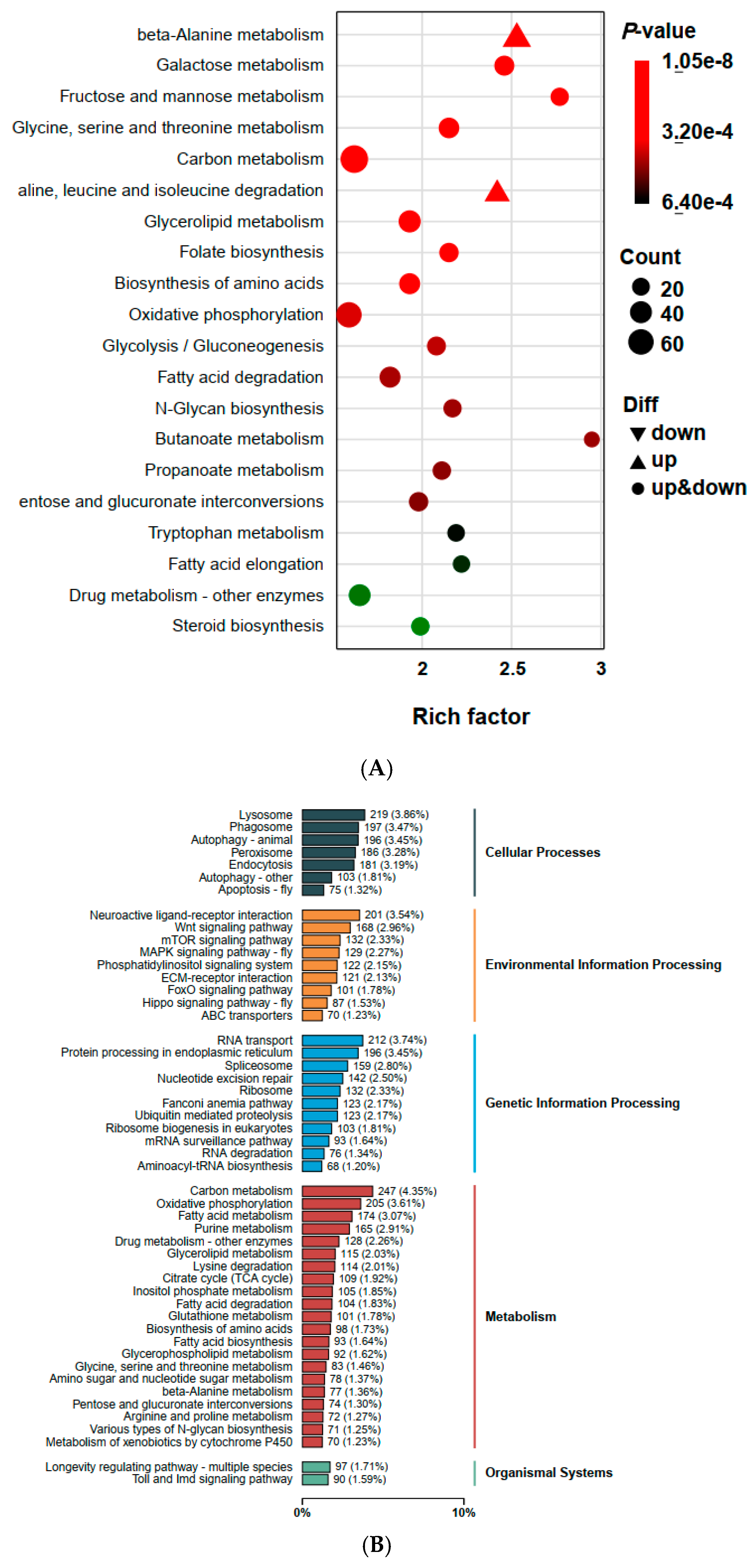
2.5. Functional Annotation of DEGs
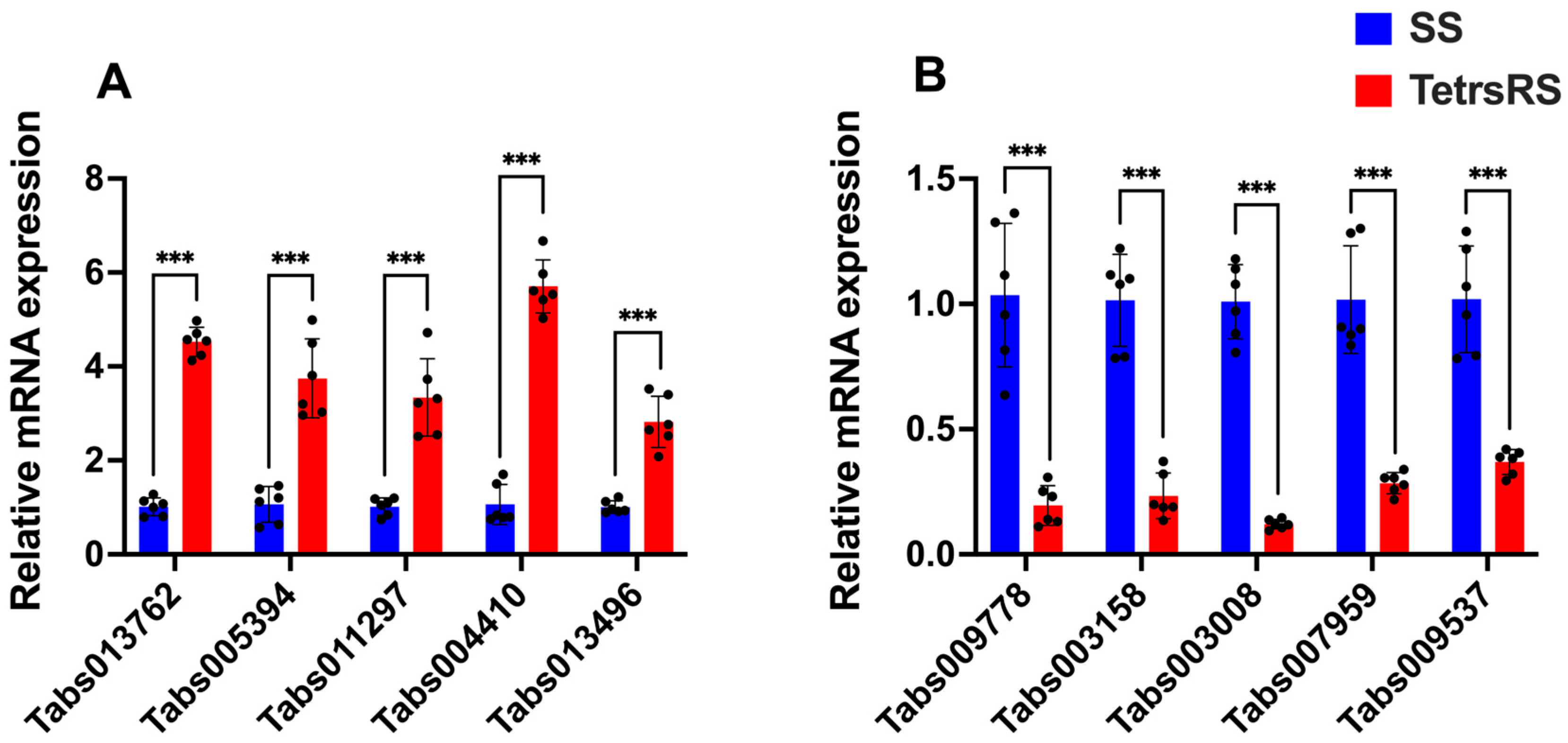
2.6. Expression Levels of Candidate Genes
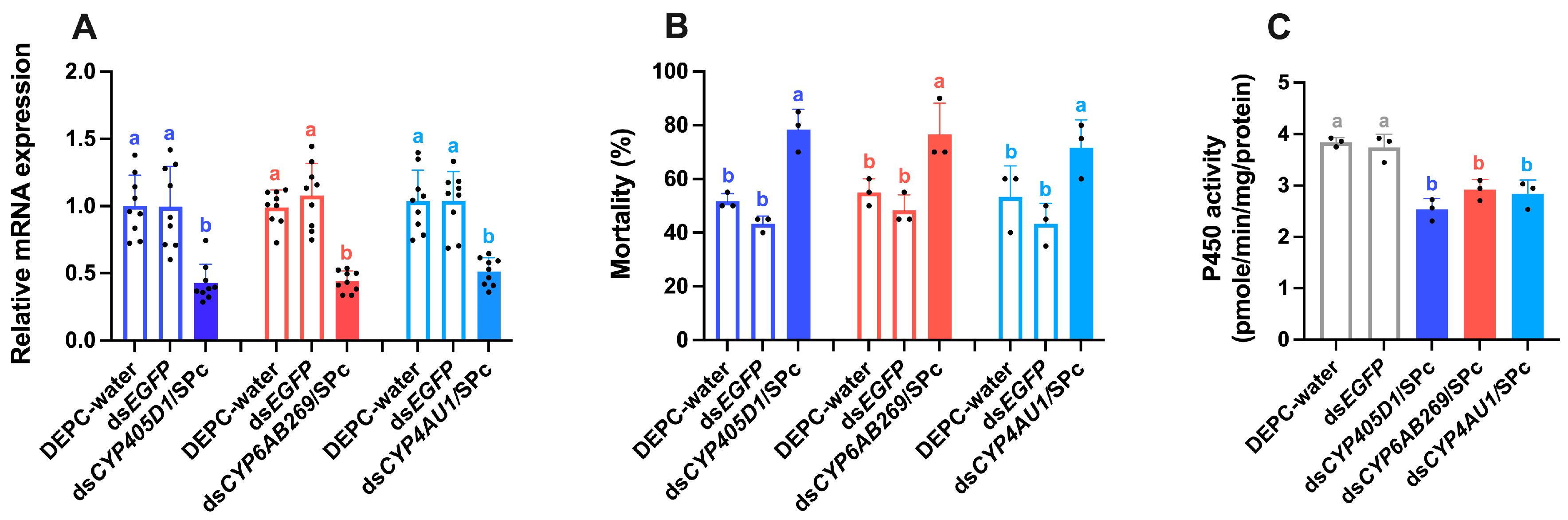
2.7. Nanocarrier-Mediated Silencing of P450 Genes in Resistant Tuta absoluta
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Bioassays
4.3. Resistance Selection
4.4. Fitness Comparisons
4.5. P450 Enzyme Activity
4.6. RNA Extraction and Transcriptome Sequencing
4.7. RT-qPCR of Targeted Genes
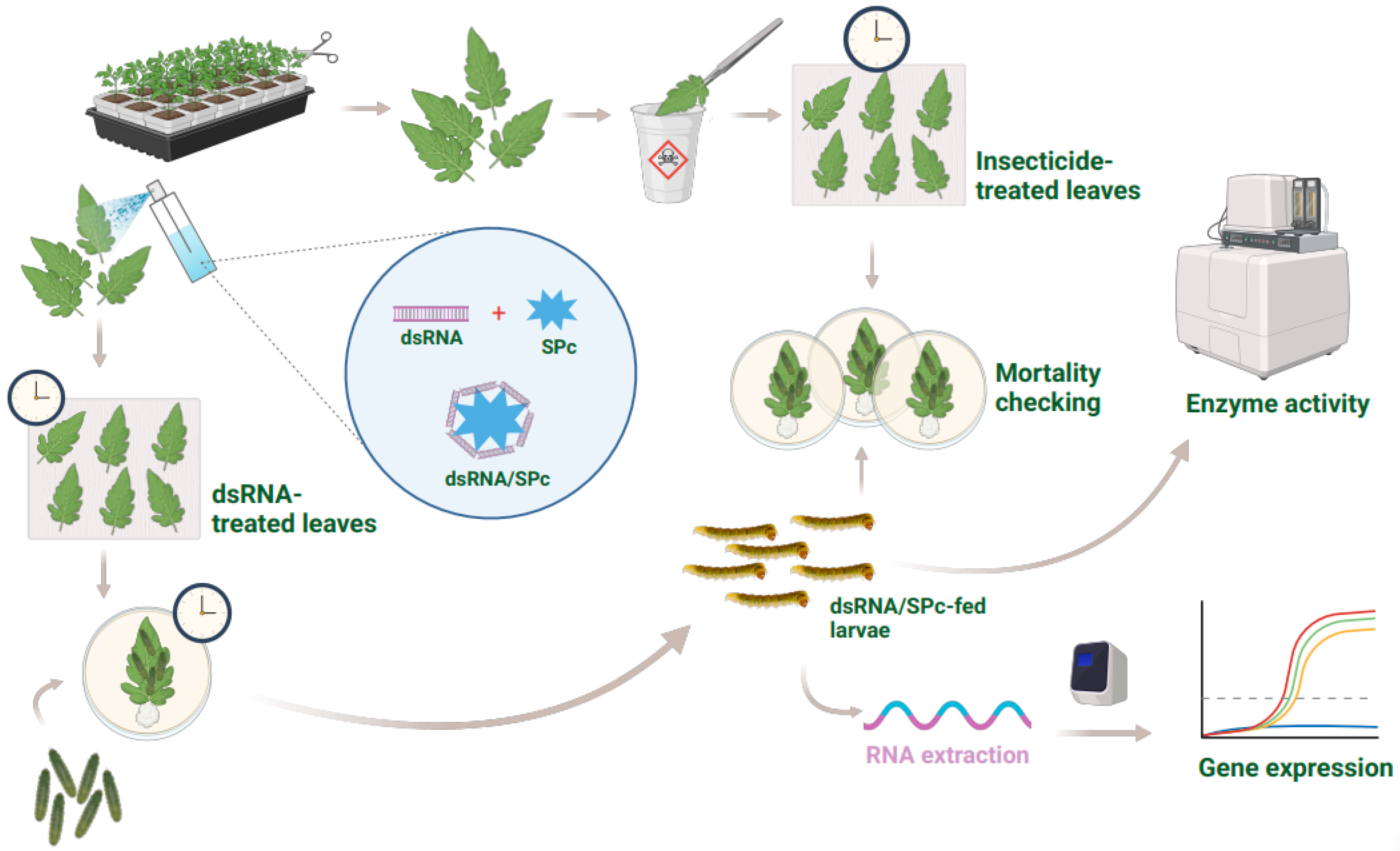
4.8. Preparation of Double-Stranded RNA (dsRNA) and dsRNA/SPc Nanoparticle Complex
4.9. Nanocarrier-Mediated RNA Interference (RNAi) and Toxicity Bioassays
4.10. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agathokleous, E.; Barceló, D.; Aschner, M.; Azevedo, R.A.; Bhattacharya, P.; Costantini, D.; Cutler, G.C.; De Marco, A.; Docea, A.O.; Dórea, J.G. Rethinking subthreshold effects in regulatory chemical risk assessments. Environ. Sci. Technol. 2022, 56, 11095–11099. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Desneux, N.; Han, P.; Mansour, R.; Arnó, J.; Brévault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.; Karimi, J.; Konan, K.A.J. Integrated pest management of Tuta absoluta: Practical implementations across different world regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Wang, M.-H.; Ismoilov, K.; Liu, W.-X.; Bai, M.; Bai, X.-S.; Chen, B.; Chen, H.; Chen, H.-s.; Dong, Y.-C.; Fang, K. Tuta absoluta management in China: Progress and prospects. Entomol. Gen. 2024, 44, 269–278. [Google Scholar] [CrossRef]
- Guedes, R.; Roditakis, E.; Campos, M.; Haddi, K.; Bielza, P.; Siqueira, H.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Ma, D.; Yang, S.; Jiang, J.; Zhu, J.; Li, B.; Mu, W.; Dou, D.; Liu, F. Toxicity, residue and risk assessment of tetraniliprole in soil-earthworm microcosms. Ecotoxicol. Environ. Saf. 2021, 213, 112061. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, B.; Qu, C.; Gong, J.; Li, W.; Luo, C.; Wang, R. Resistance monitoring for six insecticides in vegetable field-collected populations of Spodoptera litura from China. Horticulturae 2022, 8, 255. [Google Scholar] [CrossRef]
- Suganthi, A.; Krishnamoorthy, S.; Sathiah, N.; Rabindra, R.; Muthukrishnan, N.; Jeyarani, S.; Karthik, P.; Selvi, C.; Kumar, G.A.; Srinivasan, T. Bioefficacy, persistent toxicity, and persistence of translocated residues of seed treatment insecticides in maize against fall armyworm, Spodoptera frugiperda (JE Smith, 1797). Crop Prot. 2022, 154, 105892. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.W.; Kang, D.H.; Kim, H.K.; Kim, G.H. Evaluation of the insecticidal toxicity of various pesticides for Atractomorpha lata (Orthoptera: Pyrgomorphidae) control. Entomol. Res. 2023, 53, 209–218. [Google Scholar] [CrossRef]
- Scott, I.M.; Vickruck, J.; Hann, S.; Krolikowski, S.; MacKinley, P.; Stokes-Rees, J.; Hatten, G.; Moffat, C. Regional differences in susceptibility to spinosyn insecticides registered for Colorado potato beetle management in Canada. Pestic. Biochem. Physiol. 2023, 193, 105459. [Google Scholar] [CrossRef]
- Xu, T.; Hu, F.; Yue, R.; Hu, B.; Bi, S.; Tong, Y.; Xu, L. Risk Assessment and Fitness Cost of Tetraniliprole Resistance in Ostrinia furnacalis (Lepidoptera: Crambidae). Agronomy 2025, 15, 531. [Google Scholar] [CrossRef]
- Mbuji, A.L.; Xue, Z.; Guo, M.; Li, M.; Lv, S.; Zhang, L. Resistance and fitness costs of the Helicoverpa armigera after selection with the tetraniliprole newly developed diamide insecticide. Crop Prot. 2024, 179, 106622. [Google Scholar] [CrossRef]
- Qu, C.; Yao, J.; Huang, J.; Che, W.; Fang, Y.; Luo, C.; Wang, R. Tetraniliprole resistance in field-collected populations of Tuta absoluta (Lepidoptera: Gelechiidae) from China: Baseline susceptibility, cross-resistance, inheritance, and biochemical mechanism. Pestic. Biochem. Physiol. 2024, 203, 106019. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-M.; Zhao, Y.-X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.-F.; Wu, S.-F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.T.; Ling, Y.; Wang, L.; Ni, H.; Guo, D.; Dong, B.B.; Huang, Q.; Long, L.P.; Zhang, S. Insecticide resistance monitoring of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) and its mechanism to chlorantraniliprole. Pest Manag. Sci. 2023, 79, 3290–3299. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Liu, C.; Hu, W.K.; Liu, J.W.; Zheng, L.J.; Gao, M.Y.; Guo, F.R.; Qiao, S.T.; Liu, J.L. Risk assessment, fitness cost, cross-resistance, and mechanism of tetraniliprole resistance in the rice stem borer, Chilo suppressalis. Insect Sci. 2024, 31, 835–846. [Google Scholar] [CrossRef]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; del Rosario Martínez-Aguirre, M.; Bielza, P.; Morou, E. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806. [Google Scholar] [CrossRef] [PubMed]
- Burand, J.P.; Hunter, W.B. RNAi: Future in insect management. J. Invertebr. Pathol. 2013, 112, S68–S74. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Zhang, J. Strategies to improve the efficiency of RNAi-mediated crop protection for pest control. Entomol. Gen. 2023, 43, 5–19. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.-Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2023, 43, 21–30. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A facile-synthesized star polycation constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef]
- Chao, Z.; Ma, Z.; Zhang, Y.; Yan, S.; Shen, J. Establishment of star polycation-based RNA interference system in all developmental stages of fall armyworm Spodoptera frugiperda. Entomol. Gen. 2023, 43, 127–137. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Bi, S.; Tang, Y.; Zhang, G.; Yan, S.; Wan, F.; Lü, Z.; Liu, W. A promising approach to an environmentally friendly pest management solution: Nanocarrier-delivered dsRNA towards controlling the destructive invasive pest Tuta absoluta. Environ. Sci. Nano 2023, 10, 1003–1015. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Wang, Y.-S.; GAO, Y.-H.; Liu, W.-X.; Zhang, R.; Fu, W.-J.; Xian, X.-Q.; Jun, W.; Kuang, M.; Wan, F.-H. First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef]
- Gul, H.; Gadratagi, B.G.; Güncan, A.; Tyagi, S.; Ullah, F.; Desneux, N.; Liu, X. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023, 14, 1238111. [Google Scholar] [CrossRef]
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19. [Google Scholar] [CrossRef]
- Ingham, V.; Wagstaff, S.; Ranson, H. Transcriptomic meta-signatures identified in Anopheles gambiae populations reveal previously undetected insecticide resistance mechanisms. Nat. Commun. 2018, 9, 5282. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Mutz, K.-O.; Heilkenbrinker, A.; Lönne, M.; Walter, J.-G.; Stahl, F. Transcriptome analysis using next-generation sequencing. Curr. Opin. Biotechnol. 2013, 24, 22–30. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Sun, Y.; Tian, X.; Zhu, S.; Wang, Y.; Gao, H.; Shi, C.; Zhu, X. Transcriptome-Based Identification and Characterization of Genes Associated with Resistance to Beta-Cypermethrin in Rhopalosiphum padi (Hemiptera: Aphididae). Agriculture 2023, 13, 235. [Google Scholar] [CrossRef]
- Pu, J.; Chung, H. New and emerging mechanisms of insecticide resistance. Curr. Opin. Insect Sci. 2024, 63, 101184. [Google Scholar] [CrossRef]
- Contreras, H.; Bradley, T. Metabolic rate controls respiratory pattern in insects. J. Exp. Biol. 2009, 212, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kodrík, D.; Bednářová, A.; Zemanová, M.; Krishnan, N. Hormonal regulation of response to oxidative stress in insects—An update. Int. J. Mol. Sci. 2015, 16, 25788–25816. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, F.; Zhu, J.; Fu, D.; Wang, Z.; Xiao, R. Combined PacBio Iso-Seq and Illumina RNA-Seq Analysis of the Tuta absoluta (Meyrick) Transcriptome and Cytochrome P450 Genes. Insects 2023, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Li, W.; Yang, W.; Shi, Y.; Yang, X.; Liu, S.; Liao, X.; Shi, L. Comprehensive analysis of the overexpressed cytochrome P450-based insecticide resistance mechanism in Spodoptera litura. J. Hazard. Mater. 2024, 461, 132605. [Google Scholar] [CrossRef]
- Li, W.-T.; Lin, J.-Y.; Liu, J.-J.; Hafeez, M.; Deng, S.-W.; Chen, H.-Y.; Ren, R.-J.; Rana, M.S.; Wang, R.-L. Molecular insights into the functional analysis of P450 CYP321A7 gene in the involvement of detoxification of lambda-cyhalothrin in Spodoptera frugiperda. Pestic. Biochem. Physiol. 2024, 203, 106009. [Google Scholar] [CrossRef]
- Stavrakaki, M.; Ilias, A.; Ioannidis, P.; Vontas, J.; Roditakis, E. Investigating mechanisms associated with emamectin benzoate resistance in the tomato borer Tuta absoluta. J. Pest Sci. 2022, 95, 1163–1177. [Google Scholar] [CrossRef]
- Huang ShuiJin, H.S.; Qin WenJing, Q.W.; Chen Qiong, C.Q. Cloning and mRNA expression levels of cytochrome P450 genes CYP4M14 and CYP4S9 in the common cutworm Spodoptera litura (Fabricius). Sci. Agric. Sin. 2010, 43, 3115–3124. [Google Scholar]
- Baek, J.H.; Clark, J.M.; Lee, S.H. Cross-strain comparison of cypermethrin-induced cytochrome P450 transcription under different induction conditions in diamondback moth. Pestic. Biochem. Physiol. 2010, 96, 43–50. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Hafeez, M.; Desneux, N.; Song, D. Silencing of Cytochrome P450 genes CYP6CY14 and CYP6DC1 in Aphis gossypii by RNA interference enhances susceptibility to clothianidin. Entomol. Gen. 2023, 43, 669–678. [Google Scholar] [CrossRef]
- Xiao, Q.; Deng, L.; Elzaki, M.E.A.; Zhu, L.; Xu, Y.; Han, X.; Wang, C.; Han, Z.; Wu, M. The inducible CYP4C71 can metabolize imidacloprid in Laodelphax striatellus (Hemiptera: Delphacidae). J. Econ. Entomol. 2020, 113, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Generations | LC50 (95% CI) a mg/L | Slope ± SE b | χ2 c | p-Value | RR d |
|---|---|---|---|---|---|
| F0 | 0.03 (0.02–0.03) | 2.37 ± 0.22 | 7.03 | 0.99 | - |
| F1 | 0.03 (0.02–0.03) | 2.22 ± 0.19 | 5.35 | 0.99 | 1.03 |
| F2 | 0.04 (0.04–0.05) | 2.05 ± 0.17 | 5.26 | 0.99 | 1.55 |
| F3 | 0.08 (0.06–0.09) | 1.80 ± 0.15 | 7.41 | 0.99 | 2.65 |
| F4 | 0.15 (0.12–0.19) | 1.53 ± 0.14 | 8.18 | 0.98 | 5.20 |
| F5 | 0.25 (0.21–0.31) | 1.94 ± 0.16 | 10.85 | 0.93 | 8.79 |
| F6 | 0.36 (0.31–0.43) | 2.14 ± 0.17 | 17.80 | 0.54 | 12.48 |
| F7 | 0.48 (0.40–0.56) | 2.20 ± 0.17 | 5.28 | 0.99 | 16.44 |
| F8 | 0.60 (0.49–0.74) | 1.70 ± 0.13 | 14.19 | 0.77 | 20.80 |
| Primer Name | Forward Sequence | Reverse Sequence |
|---|---|---|
| Tabs013762 | TGCACGAGTTTCCAGGAGAT | TCTTCCCGTGTCTCTTCCAC |
| Tabs005394 | GATCGTCCTAGCGCTTTGTG | GGCAGAGGTTTGTCGTGTTT |
| Tabs004410 | TTCAACATGCACAGTACCGC | GGCTGTTTTCGGGAAGGAAG |
| Tabs007959 | GCAGTCAAGGAAAGGCTAGC | TGTCGGTCACCTGTGTTTCT |
| Tabs009537 | AGGACCAATCTGCCGAAAGA | AGTACAGCTGAAGGAACGCT |
| Tabs011636 | ACTCTTACTCCGTGGCTGAC | CAGCGGTGTATTCGACCTTG |
| Tabs008791 | AATCAGATCGACGCAGCAAC | CGGGTACAAGGCCGTAATTG |
| Tabs012471 | GGATGGAGACTCGTTCGACT | GTACTTTCAACCGGCGGATC |
| Tabs011297 | TTTCAAATCCTTCGGCCGTG | CCAGGATCGCTAGGGTTCTC |
| Tabs013496 | GACAAAAGCGCGGGTAGTAG | CCACGGGTAGTCACATCCTT |
| EF1α | GAAGCCTGGTATGGTTGTCGT | GGGTGGGTTGTTCTTTGTG |
| RPL28 | TCAGACGTGCTGAACACACA | GCCAGTCTTGGACAACCATT |
| dsEGFP | TAATACGACTCACTATAGGGAAGTTCAGCGTGTCCGGCGAGG | TAATACGACTCACTATAGGGCACCTTGATGCCGTTCTTCTGC |
| dsCYP405D1 | taatacgactcactatagggGATACCGACCGTTCCAAAGA | taatacgactcactatagggAGAAAATTGTGGTTCGGTGC |
| dsCYP6AB269 | taatacgactcactatagggACGAAAACTCCGCTTTCAGA | taatacgactcactatagggGCAAGCTGGTGTAACGTGAA |
| dsCYP4AU1 | taatacgactcactatagggCATTGACTCCATCACCATCG | taatacgactcactatagggATCAGGGTTGAACAAGTCCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, F.; Ullah, Z.; Gul, H.; Li, X.; Pan, Y.; Zhang, H.; Zhang, Z.; Huang, J.; Emmanouil, R.; Guedes, R.N.C.; et al. Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole. Int. J. Mol. Sci. 2025, 26, 5180. https://doi.org/10.3390/ijms26115180
Ullah F, Ullah Z, Gul H, Li X, Pan Y, Zhang H, Zhang Z, Huang J, Emmanouil R, Guedes RNC, et al. Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole. International Journal of Molecular Sciences. 2025; 26(11):5180. https://doi.org/10.3390/ijms26115180
Chicago/Turabian StyleUllah, Farman, Zeeshan Ullah, Hina Gul, Xiaowei Li, Yuhan Pan, Haixia Zhang, Zhijun Zhang, Jun Huang, Roditakis Emmanouil, Raul Narciso C. Guedes, and et al. 2025. "Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole" International Journal of Molecular Sciences 26, no. 11: 5180. https://doi.org/10.3390/ijms26115180
APA StyleUllah, F., Ullah, Z., Gul, H., Li, X., Pan, Y., Zhang, H., Zhang, Z., Huang, J., Emmanouil, R., Guedes, R. N. C., Desneux, N., & Lu, Y. (2025). Proactive Resistance Management Studies Highlight the Role of Cytochrome P450 Genes in the Resistance of Tuta absoluta Against Tetraniliprole. International Journal of Molecular Sciences, 26(11), 5180. https://doi.org/10.3390/ijms26115180










