Synergistic Effects of Amitraz and Dinotefuran on Honey Bee Health: Impacts on Survival, Gene Expression, and Hypopharyngeal Gland Morphology
Abstract
1. Introduction
2. Results
2.1. Toxicity Assessment of Amitraz and Dinotefuran in Honey Bees
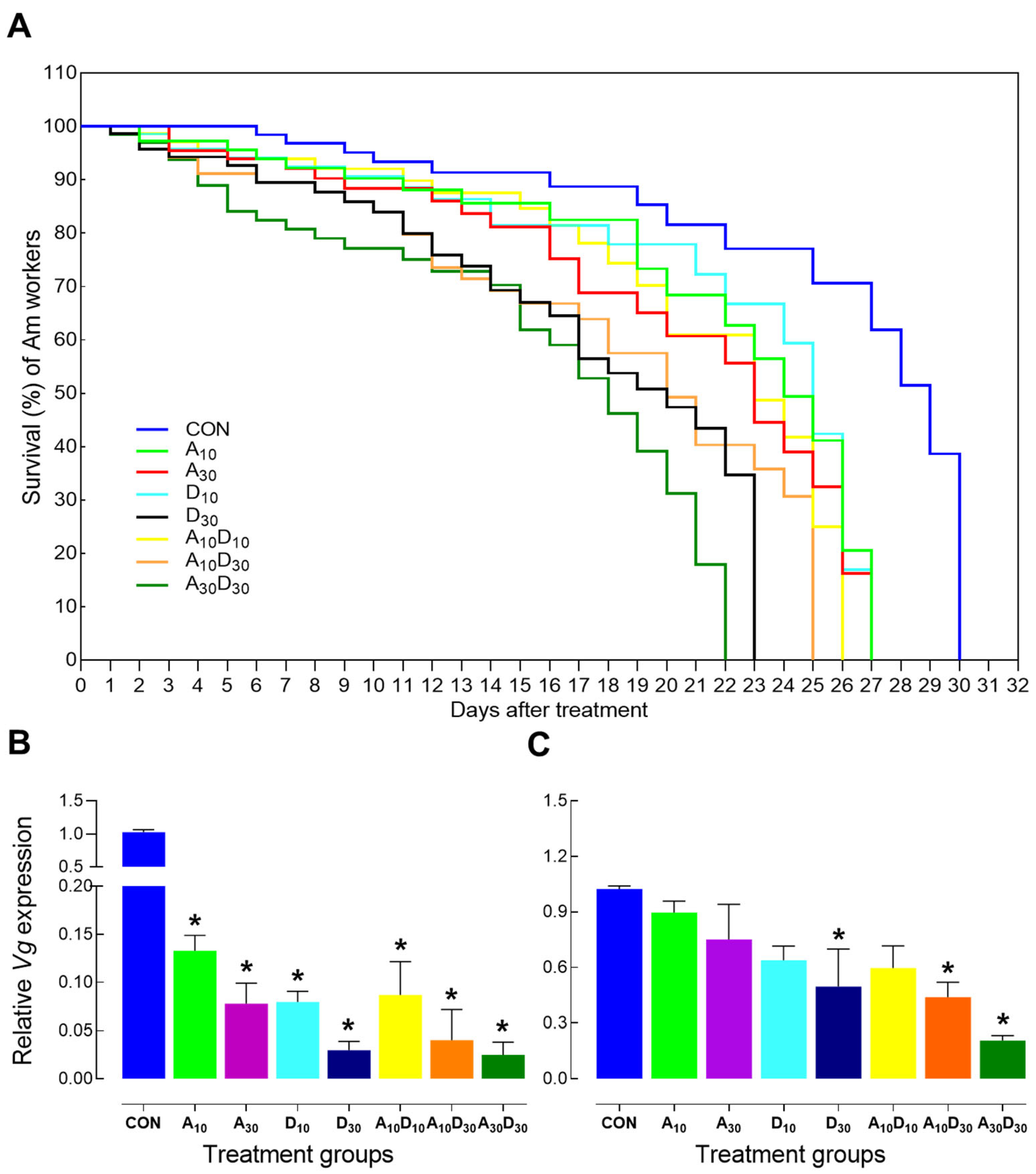
2.2. Effect of Amitraz, Dinotefuran, and Their Mixture on Honey Bee Survival
2.3. Effects of Amitraz, Dinotefuran, and Their Mixture on Stress-Related Gene Expression
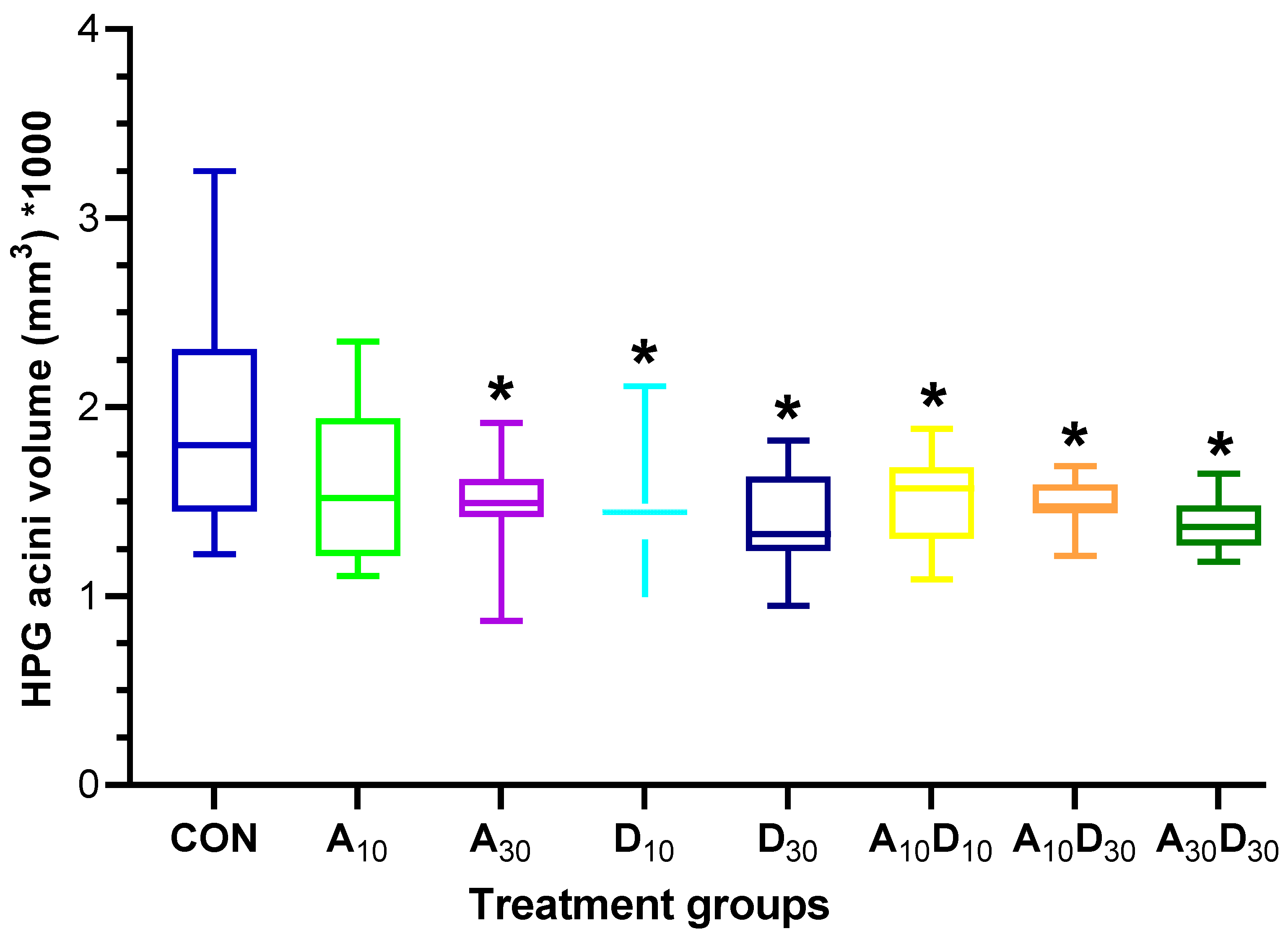
2.4. Effects of Amitraz, Diotefuran, and Their Mixture on Honey Bee Hypopharyngeal Glands Morphology
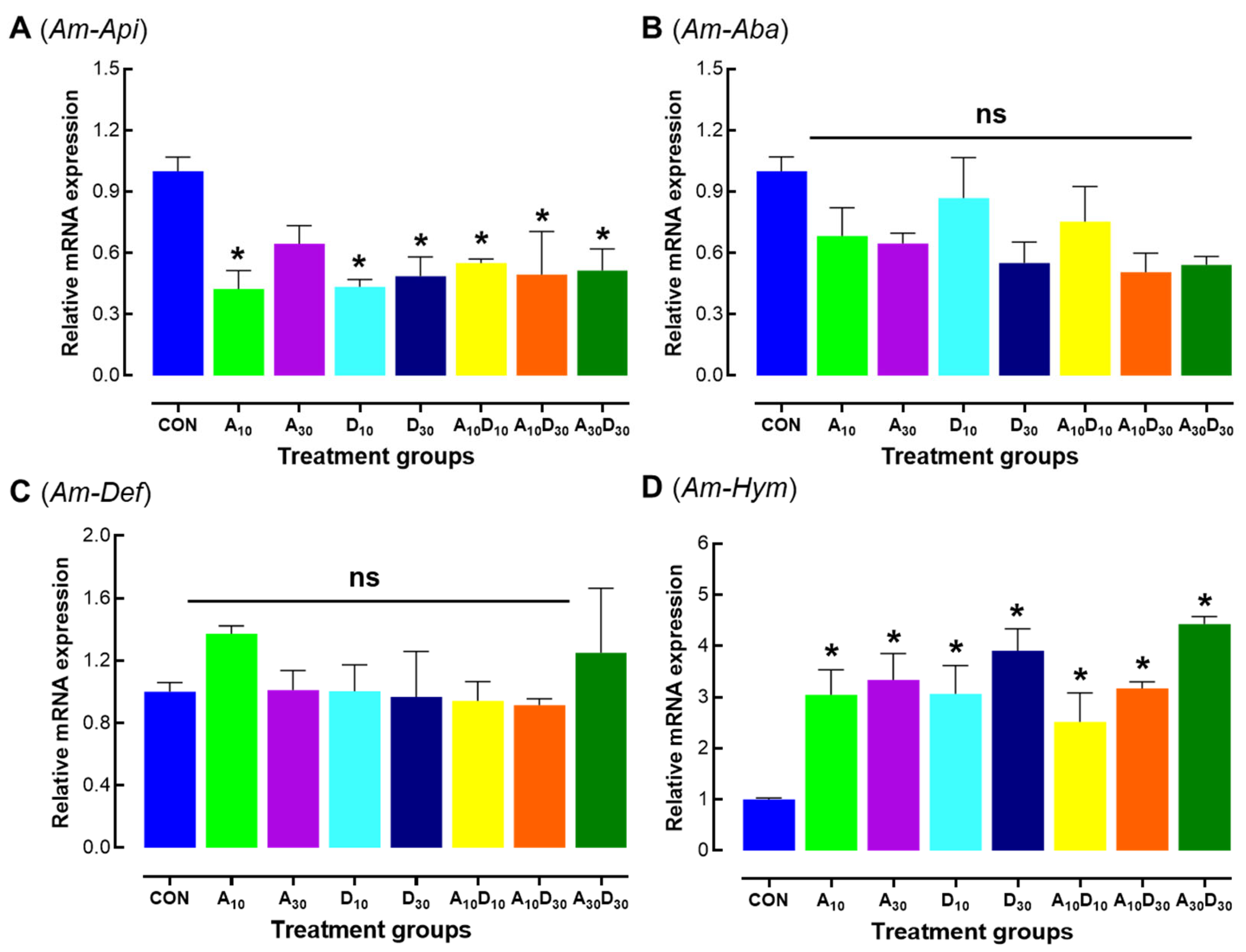
2.5. Effects of Amitraz, Dinotefuran, and Their Mixture on Immune-Related Gene Expression
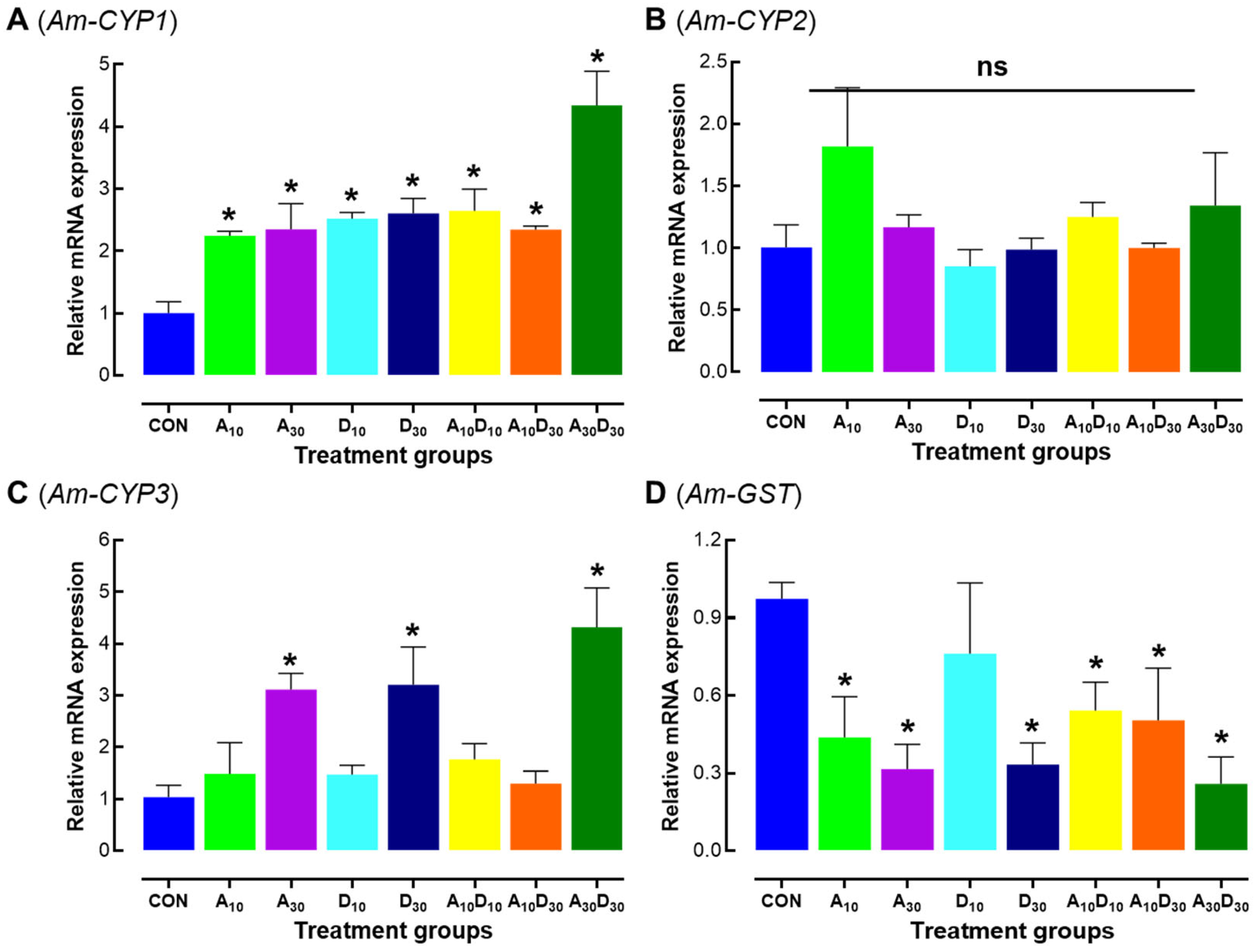
2.6. Effects of Amitraz, Diotefuran, and Their Mixture on Detoxification-Related Genes Expression
3. Discussion
4. Materials and Methods
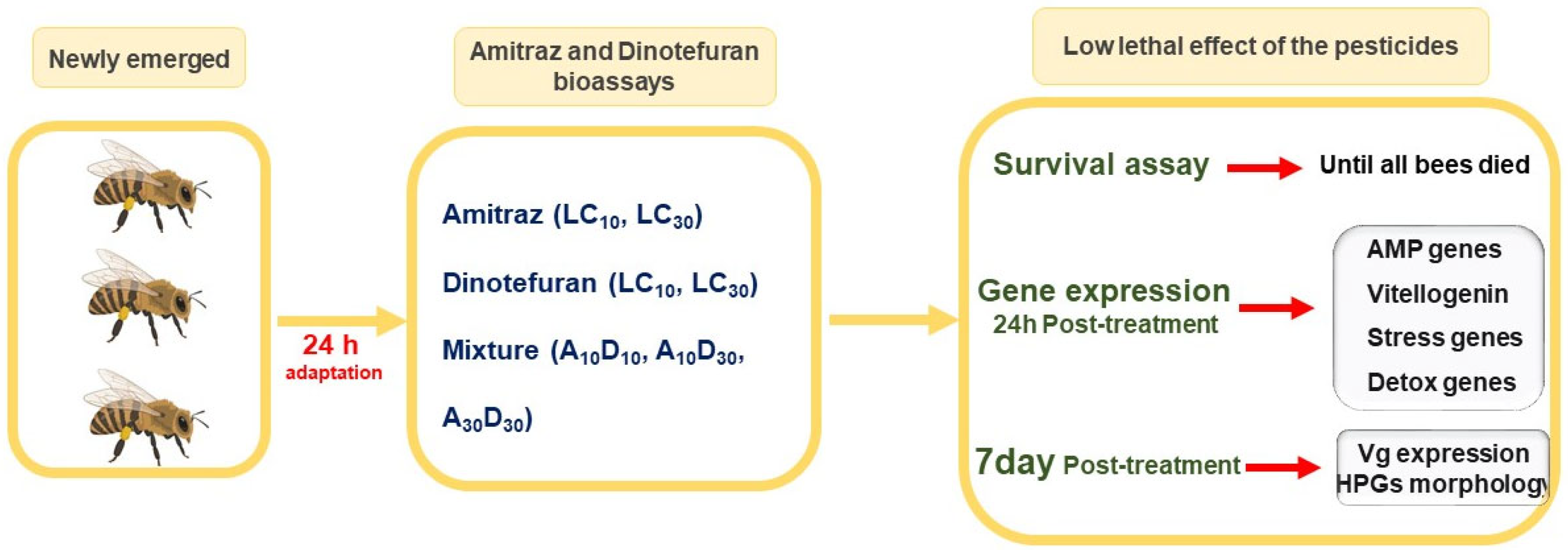
4.1. Experimental Design
4.2. Honey Bees
4.3. Chemicals
4.4. Acute Toxicity Bioassay
4.5. Survival and Longevity Experiment
4.6. Measurement of Secretion Glands
4.7. RNA Extraction and RT-qPCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.; et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Caron, D.; Pettis, J. A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. J. Apic. Res. 2011, 50, 1–10. [Google Scholar] [CrossRef]
- Steinhauer, N.A.; Rennich, K.; Wilson, M.E.; Caron, D.M.; Lengerich, E.J.; Pettis, J.S.; Rose, R.; Skinner, J.A.; Tarpy, D.R.; Wilkes, J.T.; et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2014, 53, 1–18. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Li, G.; Zhao, H.; Liu, Z.; Wang, H.; Xu, B.; Guo, X. The wisdom of honeybee defenses against environmental stresses. Front. Microbiol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Alaux, C.; Brunet, J.L.; Dussaubat, C.; Mondet, F.; Tchamitchan, S.; Cousin, M.; Brillard, J.; Baldy, A.; Belzunces, L.P.; Le Conte, Y. Interactions between Nosema microspores and a neonicotinoid weaken honey bees (Apis mellifera). Environ. microbiol. 2010, 12, 774–782. [Google Scholar] [CrossRef]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2, 326. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; VanEngelsdorp, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey Bee Toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Burley, L.M.; Fell, R.D.; Saacke, R.G. Survival of honey bee (Hymenoptera: Apidae) spermatozoa incubated at room temperature from drones exposed to miticides. J. Econ. Entomol. 2008, 101, 1081–1087. [Google Scholar] [CrossRef]
- Gregorc, A.; Evans, J.D.; Scharf, M.; Ellis, J.D. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 2012, 58, 1042–1049. [Google Scholar] [CrossRef]
- Farooqui, T. A potential link among biogenic amines-based pesticides, learning and memory, and colony collapse disorder: A unique hypothesis. Neurochem. Int. 2013, 62, 122–136. [Google Scholar] [CrossRef]
- Andrione, M.; Vallortigara, G.; Antolini, R.; Haase, A. Neonicotinoid induced impairment of odor coding in the honey bee. Sci. Rep. 2016, 6, 38110. [Google Scholar] [CrossRef]
- Forfert, N.; Troxler, A.; Retschnig, G.; Gauthier, L.; Straub, L.; Moritz, R.F.; Neumann, P.; Williams, G.R. Neonicotinoid pesticides can reduce honeybee colony genetic diversity. PLoS ONE 2017, 12, e0186109. [Google Scholar] [CrossRef]
- S’anchez-Bayo, F.; Goulson, D.; Pennacchio, F.; Nazzi, F.; Goka, K.; Desneux, N. Are bee diseases linked to pesticides? A brief review. Environ. Int. 2016, 89, 7–11. [Google Scholar] [CrossRef]
- O’Neal, S.T.; Anderson, T.D.; Wu-Smart, J.Y. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 2018, 26, 57–62. [Google Scholar] [CrossRef]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and future prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.C.; Chang, H.C.; Wu, W.Y.; Chen, Y.W. Impaired Olfactory Associative Behavior of Honeybee Workers Due to Contamination of Imidacloprid in the Larval Stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yañez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, Y.; Sun, J.; Imran, M.; Yang, H.; Wu, J.; Zou, Y.; Li-Byarlay, H.; Luo, S. Impact of acute oral exposure to thiamethoxam on the homing, flight, learning acquisition and short-term retention of Apis cerana. Pest Manag. Sci. 2019, 75, 2975–2980. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Cang, T.; Tang, T.; Guo, M.; Zhou, B.; Zhu, G.; Zhao, M. Toxicological effect and molecular mechanism of the chiral neonicotinoid dinotefuran in honeybees. Environ. Sci. Technol. 2021, 56, 1104–1112. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, Y.; Ma, W.; Liu, J.; Jiang, Y. The transcriptomic landscape of molecular effects after sublethal exposure to dinotefuran on Apis mellifera. Insects 2021, 12, 898. [Google Scholar] [CrossRef]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Ellis, J.D. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci. Rep. 2018, 8, 5635. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Zhang, X.; Wei, Q.; Kang, W.; Wang, F.; Liu, F.; Zhao, M.; Xu, S.; Han, B. Enantioselective toxicity of the neonicotinoid dinotefuran on honeybee (Apis mellifera) larvae. Sci. Total Environ. 2024, 944, 174014. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Shi, T.; Xu, S.; Lu, B.; Qin, H.; Yu, L. Synergistic toxicity and physiological impact of thiamethoxam alone or in binary mixtures with three commonly used insecticides on honeybee. Apidologie 2020, 51, 395–405. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.C.; Li, W. Interaction patterns and combined toxic effects of acetamiprid in combination with seven pesticides on honey bee (Apis mellifera L.). Ecotox. Environ. Saf. 2020, 190, 110100. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, W.; Li, X.; Wang, D.; Weng, H.; Zhu, Y.C.; Wang, Y. Mixture toxic effects of thiacloprid and cyproconazole on honey bees (Apis mellifera L.). Sci. Total Environ. 2023, 870, 161700. [Google Scholar] [CrossRef] [PubMed]
- Begna, T.; Ulziibayar, D.; Bisrat, D.; Jung, C. Acute and sublethal effects of acetamiprid alone and in mixture with emamectin benzoate on honeybee, Apis mellifera. J. Asia-Pac. Entomol. 2023, 26, 102125. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef]
- Bonzini, S.; Tremolada, P.; Bernardinelli, I.; Colombo, M.; Vighi, M. Predicting pesticide fate in the hive (part 1): Experimentally determined τ-fluvalinate residues in bees, honey and wax. Apidologie 2011, 42, 378–390. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.; Yang, H.; He, X.; Yan, W.; Zeng, Z. The adverse impact on lifespan, immunity, and forage behavior of worker bees (Apis mellifera Linnaeus 1758) after exposure to flumethrin. Sci. Total Environ. 2023, 858, 160146. [Google Scholar] [CrossRef]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef]
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J. Agrochemicals interact synergistically to increase bee mortality. Nature 2021, 596, 389–392. [Google Scholar] [CrossRef]
- Bommuraj, V.; Chen, Y.; Birenboim, M.; Barel, S.; Shimshoni, J.A. Concentration-and time-dependent toxicity of commonly encountered pesticides and pesticide mixtures to honeybees (Apis mellifera L.). Chemosphere 2021, 266, 128974. [Google Scholar] [CrossRef]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 2021, 11, 6821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Yao, J.; Adamczyk, J.; Luttrell, R. Synergistic toxicity and physiological impact of imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera). PLoS ONE 2017, 12, e0176837. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Fennern, E.; Havukainen, H. Vitellogenin in honey bee behavior and lifespan. In Honey Bee Neurobiology and Behavior: A Tribute to Randolf Menzel; Springer: Dordrecht, The Netherlands, 2012; pp. 17–29. [Google Scholar]
- Chaimanee, V.; Evans, J.D.; Chen, Y.; Jackson, C.; Pettis, J.S. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 2016, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Castelli, L.; Balbuena, S.; Branchiccela, B.; Zunino, P.; Liberti, J.; Engel, P.; Antúnez, K. Impact of chronic exposure to sublethal doses of glyphosate on honey bee immunity, gut microbiota and infection by pathogens. Microorganisms 2021, 9, 845. [Google Scholar] [CrossRef]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E., Jr.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, e62. [Google Scholar] [CrossRef]
- Shi, T.F.; Wang, Y.F.; Liu, F.; Qi, L.; Yu, L.S. Sublethal effects of the neonicotinoid insecticide thiamethoxam on the transcriptome of the honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2017, 110, 2283–2289. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Ji, W.; Wang, K.; Gull, S.; Li, J.; Chen, L.; Ji, T.; Liu, J. Physiological effects of field concentrations and sublethal concentrations of sulfoxaflor on Apis mellifera. Pest Manag. Sci. 2024, 80, 5941–5953. [Google Scholar] [CrossRef]
- Corona, M.; Velarde, R.A.; Remolina, S.; Moran-Lauter, A.; Wang, Y.; Hughes, K.A.; Robinson, G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. USA 2007, 104, 7128–7133. [Google Scholar] [CrossRef]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef]
- Tahir, F.; Goblirsch, M.; Adamczyk, J.; Karim, S.; Alburaki, M. Honey bee Apis mellifera L. responses to oxidative stress induced by pharmacological and pesticidal compounds. Front. Bee Sci. 2023, 1, 1275862. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Rana, S.; Sarkar, S.; Smith, B.; Basu, P. Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 2015, 46, 107–129. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, S.A.; Khan, K.A.; Li, J. Novel insight into the development and function of hypopharyngeal glands in honey bees. Front. Physiol. 2021, 11, 615830. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Cheng, F.; Pan, L.; Wang, Z. Expression profile of microRNAs during development of the hypopharyngeal gland in honey bee, Apis mellifera. Int. J. Mol. Sci. 2022, 23, 12970. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; Huang, Z.Y.; Lorenz, M.W.; Bienefeld, K. Regulation of hypopharyngeal gland activity and oogenesis in honey bee (Apis mellifera) workers. J. Insect Physiol. 2009, 55, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Zaluski, R.; Justulin, L.A., Jr.; Orsi, R.D.O. Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Sci. Rep. 2017, 7, 15217. [Google Scholar] [CrossRef]
- de Castro, M.B.A.; Martinez, L.C.; Cossolin, J.F.S.; Serra, R.S.; Serrão, J.E. Cytotoxic effects on the midgut, hypopharyngeal, glands and brain of Apis mellifera honey bee workers exposed to chronic concentrations of lambda-cyhalothrin. Chemosphere 2020, 248, 126075. [Google Scholar] [CrossRef]
- Hassanyar, A.K.; Huang, J.; Nie, H.; Li, Z.; Hussain, M.; Rizwan, M.; Su, S. The association between the hypopharyngeal glands and the molecular mechanism which honey bees secrete royal jelly. J. Apic. Res. 2025, 64, 281–296. [Google Scholar] [CrossRef]
- Chaves, A.; Faita, M.R.; Nodari, R.O. Effects of fungicides on the ultrastructure of the hypopharyngeal glands and the strength of the hives of Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae). Toxicol. Appl. Pharmacol. 2023, 459, 116340. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Nowak, I.; Górczyńska, A. Effects of insecticides and microbiological contaminants on Apis mellifera health. Molecules 2021, 26, 5080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, G.; Cui, X.; Wang, H.; Liu, Z.; Yang, Y.; Xu, B. Review on effects of some insecticides on honey bee health. Pestic. Biochem. Physiol. 2022, 188, 105219. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Inverteb. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Wiseman, S.; Sun, J.; Cutler, G.C.; Aboul-Soud, M.; Naiem, E.; Mona, M.; Seif, A.; Giesy, J.P. Effects of environmentally-relevant mixtures of four common organophosphorus insecticides on the honey bee (Apis mellifera L.). J. Insect Physiol. 2015, 82, 85–91. [Google Scholar] [CrossRef]
- Bartling, M.T.; Thümecke, S.; Russert, J.H.; Vilcinskas, A.; Lee, K.Z. Exposure to low doses of pesticides induces an immune response and the production of nitric oxide in honeybees. Sci. Rep. 2021, 11, 6819. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef]
- Sukkar, D.; Wagner, L.; Bonnefoy, A.; Falla-Angel, J.; Laval-Gilly, P. Imidacloprid and amitraz differentially alter antioxidant enzymes in honeybee (Apis mellifera) hemocytes when exposed to microbial pathogen-associated molecular patterns. Sci. Total Environ. 2025, 969, 178868. [Google Scholar] [CrossRef]
- Palmer, M.J.; Moffat, C.; Saranzewa, N.; Harvey, J.; Wright, G.A.; Connolly, C.N. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. commun. 2013, 4, 1634. [Google Scholar] [CrossRef]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.P.; Classen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Human, H.; Brodschneider, R.; Dietemann, V.; Dively, G.; Ellis, J.D.; Forsgren, E.; Fries, I.; Hatjina, F.; Hu, F.L.; Jaffé, R.; et al. Miscellaneous standard methods for Apis mellifera research. J. Apic. Res. 2013, 52, 1–53. [Google Scholar] [CrossRef]
- Omar, E.; Abd-Ella, A.A.; Khodairy, M.M.; Moosbeckhofer, R.; Crailsheim, K.; Brodschneider, R. Influence of different pollen diets on the development of hypopharyngeal glands and size of acid gland sacs in caged honey bees (Apis mellifera). Apidologie 2017, 48, 425–436. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data analysis using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Treatment | LC10 a (95% CL b) | LC30 (95% CL) | LC50 (95% CL) | Slope ± SE | X2 |
|---|---|---|---|---|---|
| Amitraz | 179.0 (88.6–253.1) | 301.4 (199.4–399.2) | 432.3 (320.6–596.9) | 3.3 ± 0.4 | 46.4 |
| Dinotefuran | 1.027 (0.082–2.118) | 2.088 (0.426–3.464) | 3.413 (1.274–5.093) | 2.5 ± 0.4 | 45.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeily, M.; Begna, T.; Jang, H.; Kwon, S.; Jung, C. Synergistic Effects of Amitraz and Dinotefuran on Honey Bee Health: Impacts on Survival, Gene Expression, and Hypopharyngeal Gland Morphology. Int. J. Mol. Sci. 2025, 26, 6850. https://doi.org/10.3390/ijms26146850
Esmaeily M, Begna T, Jang H, Kwon S, Jung C. Synergistic Effects of Amitraz and Dinotefuran on Honey Bee Health: Impacts on Survival, Gene Expression, and Hypopharyngeal Gland Morphology. International Journal of Molecular Sciences. 2025; 26(14):6850. https://doi.org/10.3390/ijms26146850
Chicago/Turabian StyleEsmaeily, Mojtaba, Tekalign Begna, Hyeonjeong Jang, Sunho Kwon, and Chuleui Jung. 2025. "Synergistic Effects of Amitraz and Dinotefuran on Honey Bee Health: Impacts on Survival, Gene Expression, and Hypopharyngeal Gland Morphology" International Journal of Molecular Sciences 26, no. 14: 6850. https://doi.org/10.3390/ijms26146850
APA StyleEsmaeily, M., Begna, T., Jang, H., Kwon, S., & Jung, C. (2025). Synergistic Effects of Amitraz and Dinotefuran on Honey Bee Health: Impacts on Survival, Gene Expression, and Hypopharyngeal Gland Morphology. International Journal of Molecular Sciences, 26(14), 6850. https://doi.org/10.3390/ijms26146850








