Phenethyl Acetate as an Agonist of Insect Odorant Receptor Co-Receptor (Orco): Molecular Mechanisms and Functional Insights
Abstract
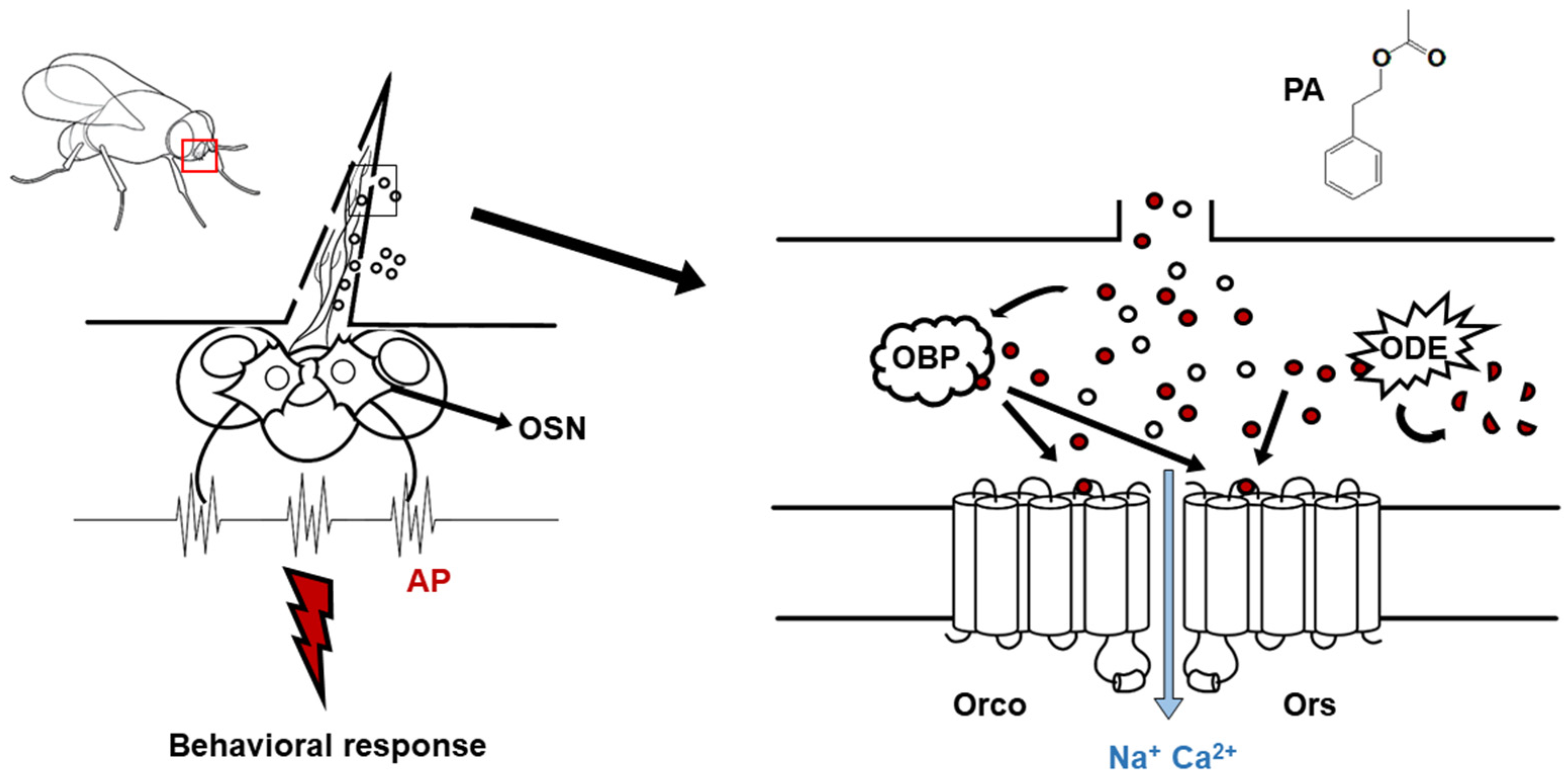
1. Introduction
2. Results
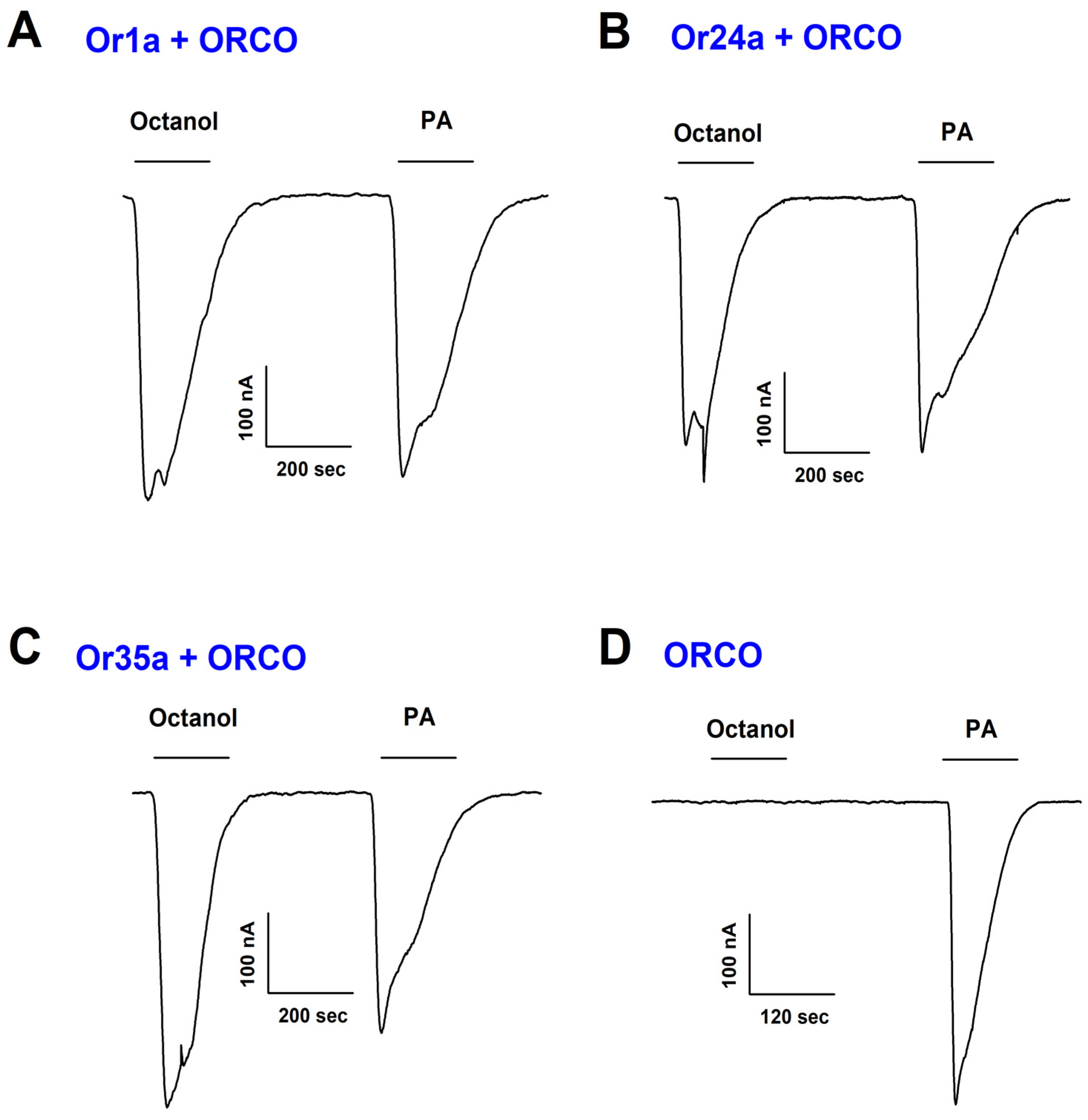
2.1. PA Activates Or1a, Or24a, and Or35a Receptors in Combination with Orco
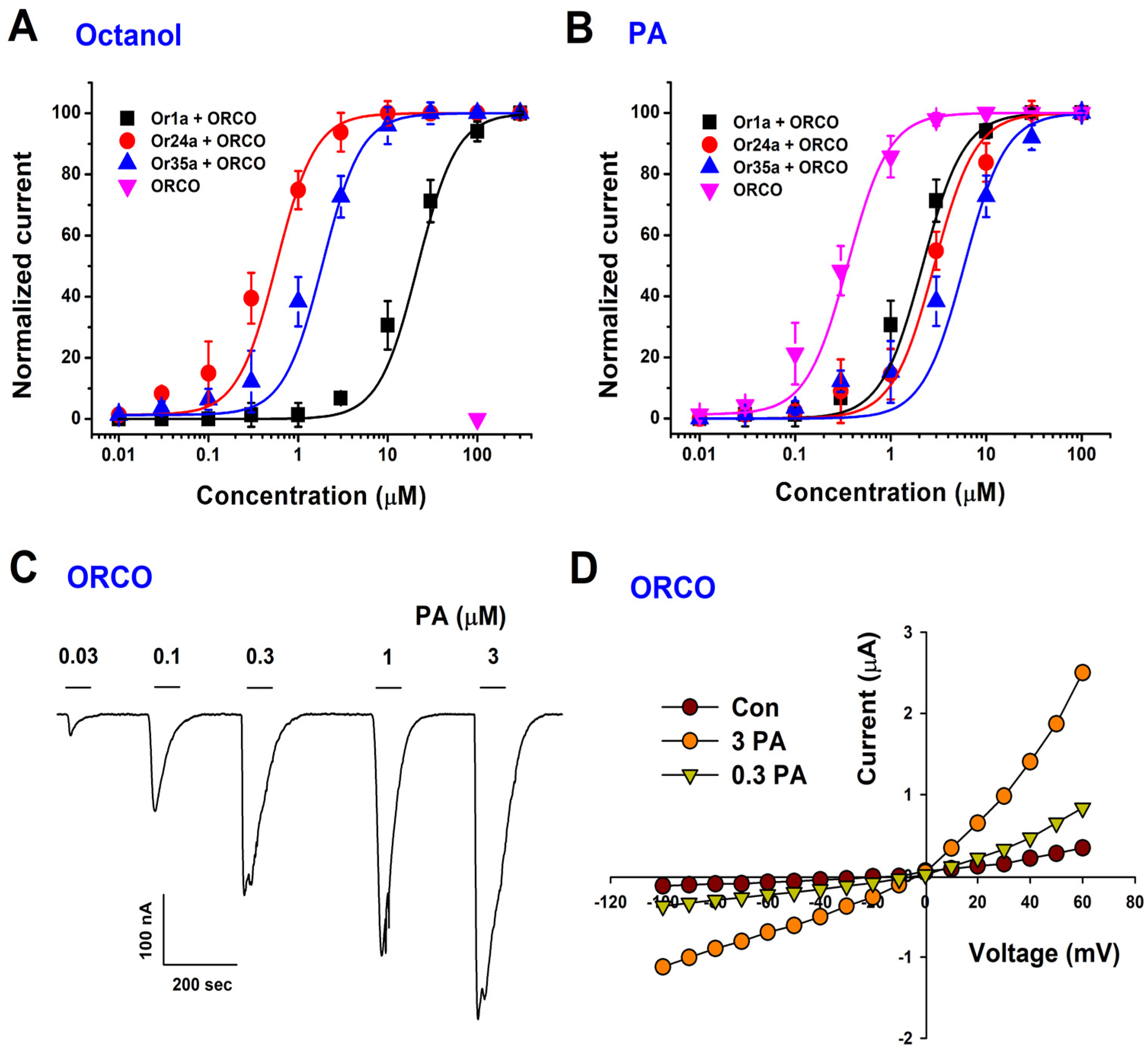
2.2. PA Activation Is Concentration-Dependent and Voltage-Independent
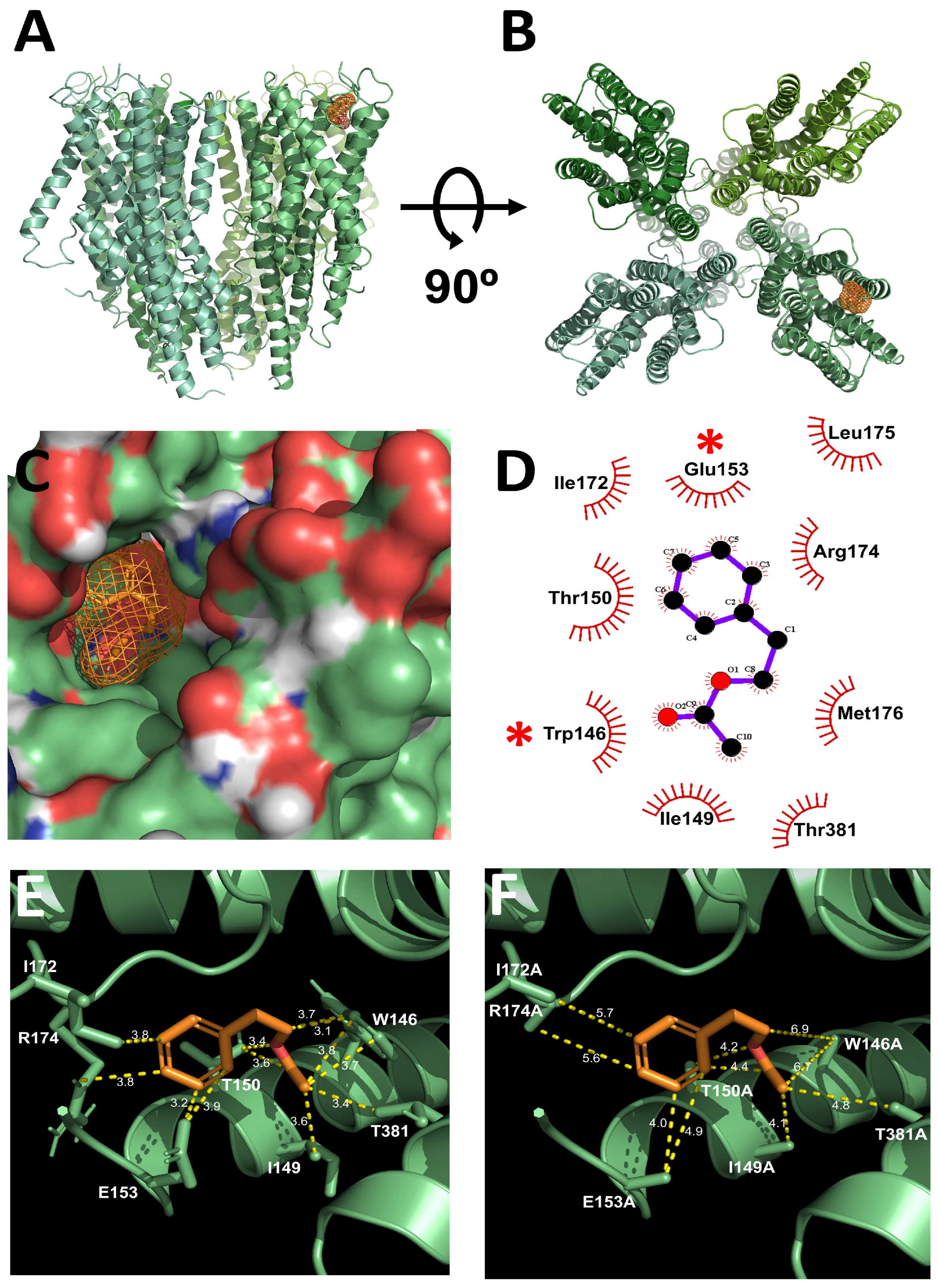
2.3. Molecular Docking Identifies PA-Binding Site in Orco
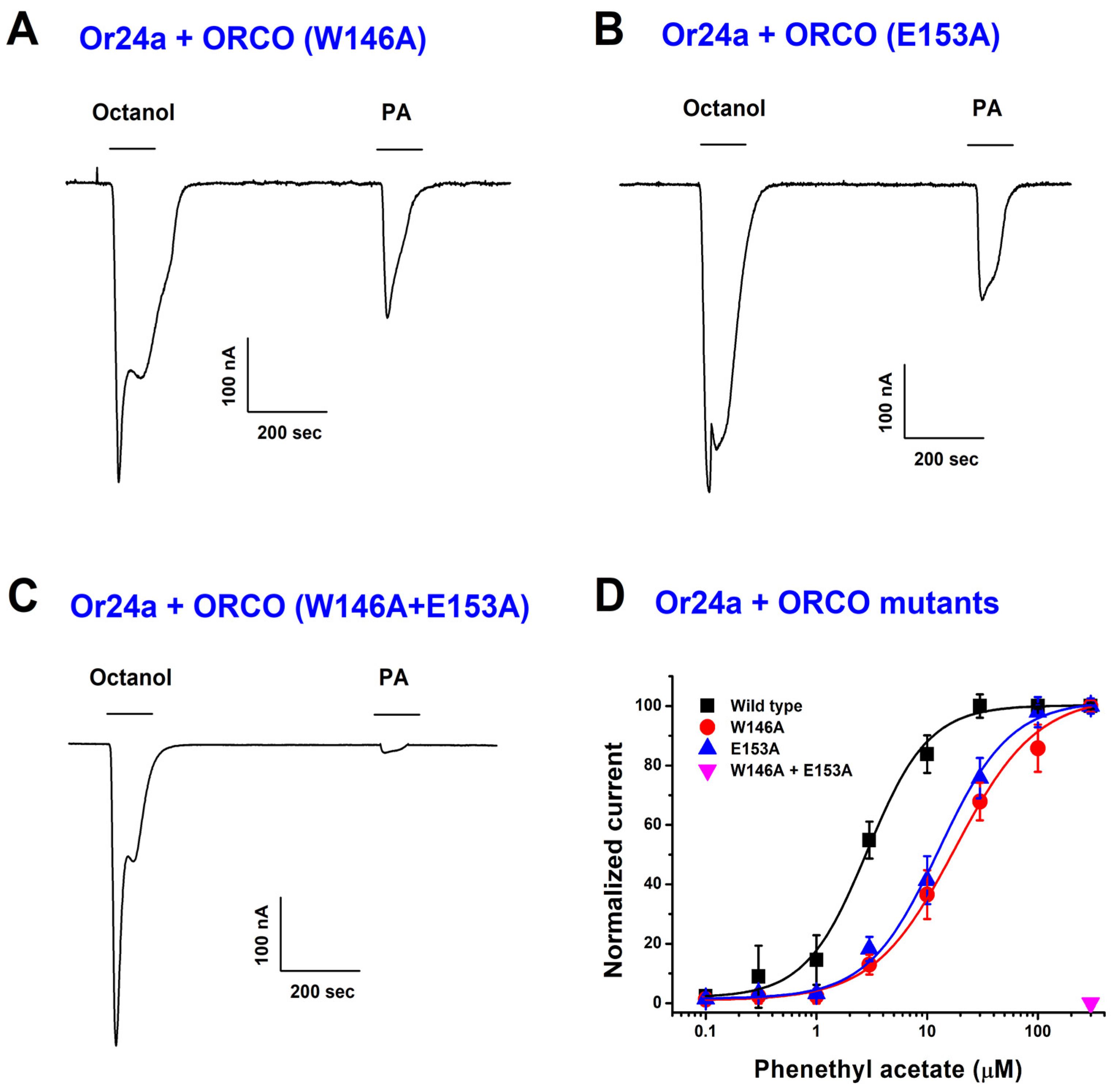
2.4. PA-Induced Activation Is Reduced in Orco Mutants
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Transcription mRNA and Injection into Xenopus Oocyte
4.3. Xenopus Oocyte Preparation
4.4. Three-Dimensional Structure Modeling and Molecular Docking Studies
4.5. Data Recording with a Two-Electrode Voltage Clamp
4.6. Site-Directed Mutagenesis for Interaction Site Validation
4.7. Data Statistics and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.B.; Liu, Y.; Yan, S.C.; Wang, G.R. Identification and functional characterization of an odorant receptor in pea aphid, Acyrthosiphon pisum. Insect Sci. 2019, 26, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Galizia, C.G.; Rössler, W. Parallel olfactory systems in insects: Anatomy and function. Annu. Rev. Entomol. 2010, 55, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.N.; Löfstedt, C.; Newcomb, R.D. Insect olfaction and the evolution of receptor tuning. Front. Ecol. Evol. 2015, 3, 53. [Google Scholar] [CrossRef]
- Kepchia, D.; Moliver, S.; Chohan, K.; Phillips, C.; Luetje, C.W. Inhibition of insect olfactory behavior by an airborne antagonist of the insect odorant receptor co-receptor subunit. PLoS ONE 2017, 12, e0177454. [Google Scholar]
- Martin, J.P.; Beyerlein, A.; Dacks, A.M.; Reisenman, C.E.; Riffell, J.A.; Lei, H.; Hildebrand, J.G. The neurobiology of insect olfaction: Sensory processing in a comparative context. Prog. Neurobiol. 2011, 95, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef]
- Keil, T.A. Morphology and development of the peripheral olfactory organs. In Insect Olfaction; Springer: Berlin/Heidelberg, Germany, 1999; pp. 5–47. [Google Scholar]
- Nichols, A.S.; Chen, S.; Luetje, C.W. Subunit contributions to insect olfactory receptor function: Channel block and odorant recognition. Chem. Senses 2011, 36, 781–790. [Google Scholar] [CrossRef]
- Grabe, V.; Sachse, S. Fundamental principles of the olfactory code. Biosystems 2018, 164, 94–101. [Google Scholar] [CrossRef]
- Richgels, P.; Rollmann, S.M. Genetic variation in odorant receptors contributes to variation in olfactory behavior in a natural population of Drosophila melanogaster. Chem. Senses 2012, 37, 229–240. [Google Scholar] [CrossRef]
- Sato, K.; Touhara, K. Insect olfaction: Receptors, signal transduction, and behavior. Chemosens. Syst. Mamm. Fishes Insects 2009, 47, 203–220. [Google Scholar]
- Butterwick, J.A.; Del Mármol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zarzo, M. The sense of smell: Molecular basis of odorant recognition. Biol. Rev. 2007, 82, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, I.; Rouquier, S.; Giorgi, D. Olfactory receptors. Cell. Mol. Life Sci. CMLS 2004, 61, 456–469. [Google Scholar] [CrossRef]
- McAfee, A.; Iovinella, I.; Gallagher-Kurtzke, Y.; Collins, T.; Higo, H.; Madilao, L.; Pelosi, P.; Foster, L.; Chapman, A. Death pheromones triggering hygienic behavior in honey bees (Apis mellifera L.). Apidologie 2018, 8, 5719. [Google Scholar]
- Swanson, J.A.; Torto, B.; Kells, S.A.; Mesce, K.A.; Tumlinson, J.H.; Spivak, M. Odorants that induce hygienic behavior in honeybees: Identification of volatile compounds in chalkbrood-infected honeybee larvae. J. Chem. Ecol. 2009, 35, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Younus, F.; Fraser, N.J.; Coppin, C.W.; Liu, J.-W.; Correy, G.J.; Chertemps, T.; Pandey, G.; Maïbèche, M.; Jackson, C.J.; Oakeshott, J.G. Molecular basis for the behavioral effects of the odorant degrading enzyme Esterase 6 in Drosophila. Sci. Rep. 2017, 7, 46188. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, A.; Chen, J. Olfactory and behavioral responses to acetate esters in red imported fire ant, Solenopsis invicta. Pest Manag. Sci. 2021, 77, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Zufall, F.; Domingos, A.I. The structure of Orco and its impact on our understanding of olfaction. J. Gen. Physiol. 2018, 150, 1602–1605. [Google Scholar] [CrossRef]
- Brand, P.; Robertson, H.M.; Lin, W.; Pothula, R.; Klingeman, W.E.; Jurat-Fuentes, J.L.; Johnson, B.R. The origin of the odorant receptor gene family in insects. elife 2018, 7, e38340. [Google Scholar] [CrossRef]
- Lee, S.; Eom, S.; Pyeon, M.; Moon, M.; Yun, J.; Lee, J.; Choi, Y.-S.; Lee, J.H. Identification of 2, 4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors. Int. J. Mol. Sci. 2024, 25, 220. [Google Scholar] [CrossRef]
- Soffan, A.; Subandiyah, S.; Makino, H.; Watanabe, T.; Horiike, T. Evolutionary analysis of the highly conserved insect odorant coreceptor (Orco) revealed a positive selection mode, implying functional flexibility. J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Drakou, C.E.; Tsitsanou, K.E.; Potamitis, C.; Fessas, D.; Zervou, M.; Zographos, S.E. The crystal structure of the AgamOBP1•Icaridin complex reveals alternative binding modes and stereo-selective repellent recognition. Cell. Mol. Life Sci. 2017, 74, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.P.; Thomas, G.H.; Parkhill, J.; Thomson, N.R. Evolutionary diversification of an ancient gene family (rhs) through C-terminal displacement. BMC Genom. 2009, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Khuri, S.; Bakker, F.T.; Dunwell, J.M. Phylogeny, function, and evolution of the cupins, a structurally conserved, functionally diverse superfamily of proteins. Mol. Biol. Evol. 2001, 18, 593–605. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Eisenhaber, B.; Eisenhaber, F. N-terminal N-myristoylation of proteins: Refinement of the sequence motif and its taxon-specific differences. J. Mol. Biol. 2002, 317, 523–540. [Google Scholar] [CrossRef]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef]
- Celniker, G.; Nimrod, G.; Ashkenazy, H.; Glaser, F.; Martz, E.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf: Using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 2013, 53, 199–206. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Erez, E.; Martz, E.; Pupko, T.; Ben-Tal, N. ConSurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010, 38, W529–W533. [Google Scholar] [CrossRef]
- Li, F.; Yan, X.; Dong, W. Purification and Properties of Alcohol Acetyltransferase from Hanseniaspora valbyensis. Food Sci. 2006, 27, 49–53. [Google Scholar]
- Tsoumani, K.T.; Belavilas-Trovas, A.; Gregoriou, M.-E.; Mathiopoulos, K.D. Anosmic flies: What Orco silencing does to olive fruit flies. BMC Genet. 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Pyeon, M.; Moon, M.; Yun, J.; Yang, J.; Yeom, H.D.; Lee, G.; Lee, J.H. Molecular Mechanisms of Nicergoline from Ergot Fungus in Blocking Human 5-HT3A Receptor. J. Microbiol. Biotechnol. 2024, 35, e2411020. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, M.; Yun, J.; Pyeon, M.; Yun, J.; Yang, J.; Yeom, H.D.; Lee, G.; Choi, Y.-S.; Lee, J.; Lee, J.H. Phenethyl Acetate as an Agonist of Insect Odorant Receptor Co-Receptor (Orco): Molecular Mechanisms and Functional Insights. Int. J. Mol. Sci. 2025, 26, 4970. https://doi.org/10.3390/ijms26114970
Moon M, Yun J, Pyeon M, Yun J, Yang J, Yeom HD, Lee G, Choi Y-S, Lee J, Lee JH. Phenethyl Acetate as an Agonist of Insect Odorant Receptor Co-Receptor (Orco): Molecular Mechanisms and Functional Insights. International Journal of Molecular Sciences. 2025; 26(11):4970. https://doi.org/10.3390/ijms26114970
Chicago/Turabian StyleMoon, Myungmi, Jihwon Yun, Minsu Pyeon, Jeongyeon Yun, Jaehui Yang, Hye Duck Yeom, Geonu Lee, Yong-Seok Choi, Jaehyeong Lee, and Junho H. Lee. 2025. "Phenethyl Acetate as an Agonist of Insect Odorant Receptor Co-Receptor (Orco): Molecular Mechanisms and Functional Insights" International Journal of Molecular Sciences 26, no. 11: 4970. https://doi.org/10.3390/ijms26114970
APA StyleMoon, M., Yun, J., Pyeon, M., Yun, J., Yang, J., Yeom, H. D., Lee, G., Choi, Y.-S., Lee, J., & Lee, J. H. (2025). Phenethyl Acetate as an Agonist of Insect Odorant Receptor Co-Receptor (Orco): Molecular Mechanisms and Functional Insights. International Journal of Molecular Sciences, 26(11), 4970. https://doi.org/10.3390/ijms26114970





