Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of mb-mRNAs
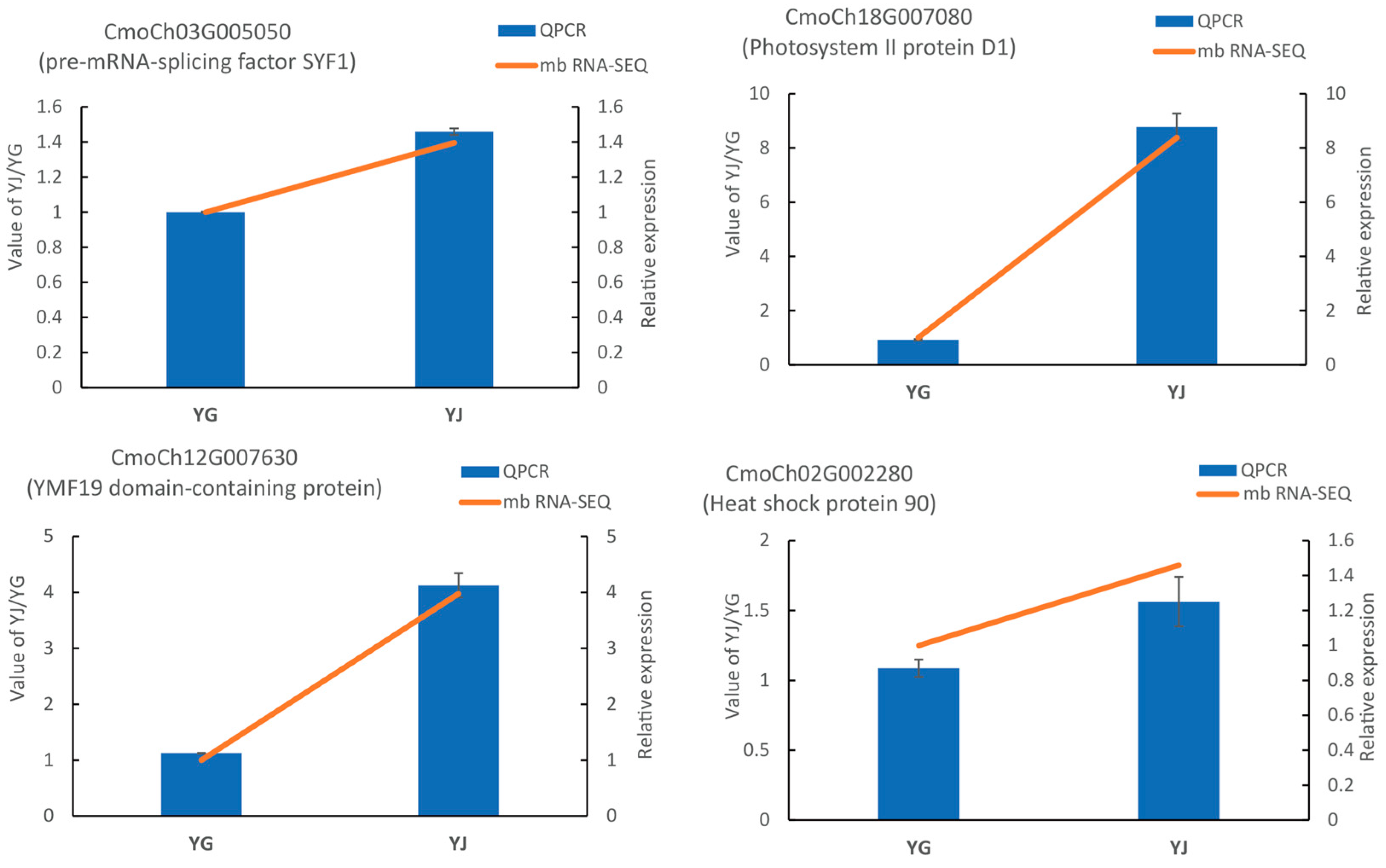
2.2. Validation of mb-mRNAs
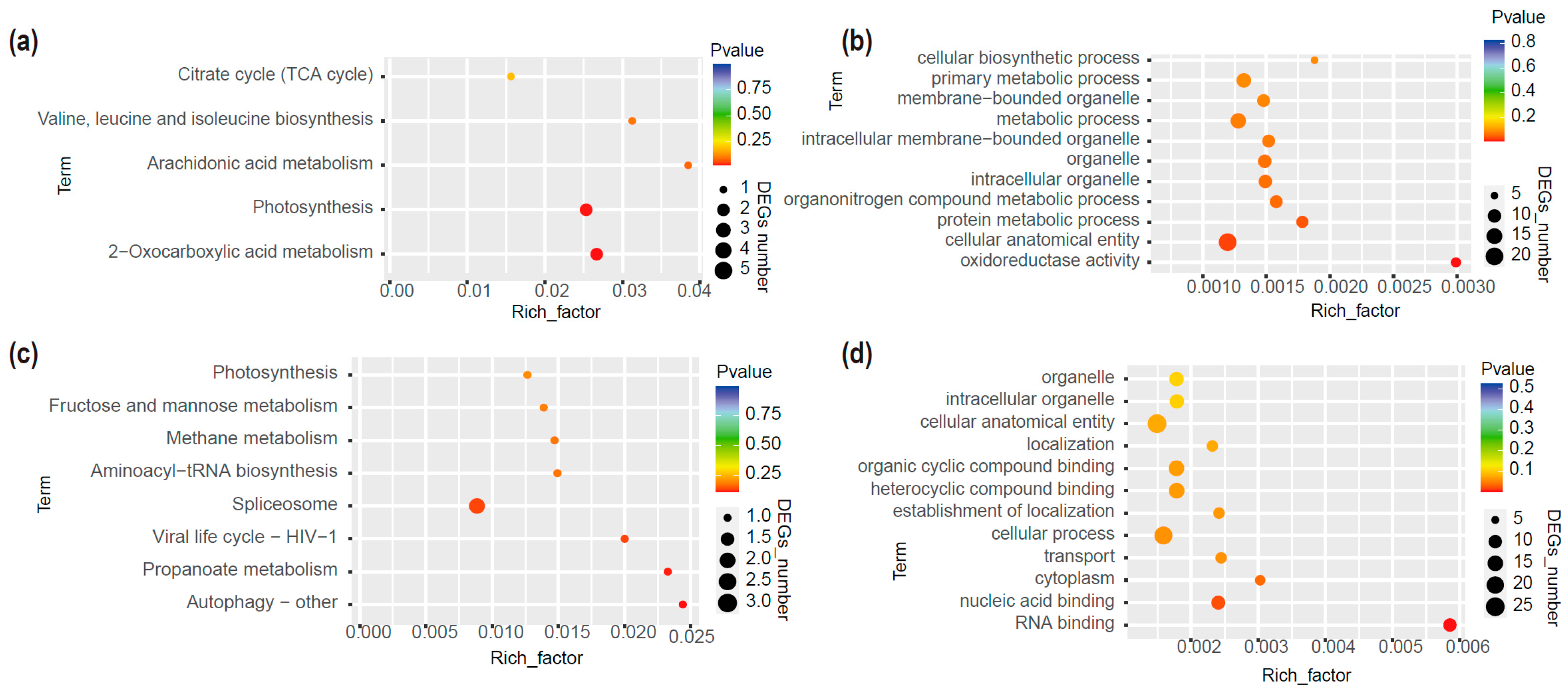
2.3. Identification of Putative mb-mRNAs
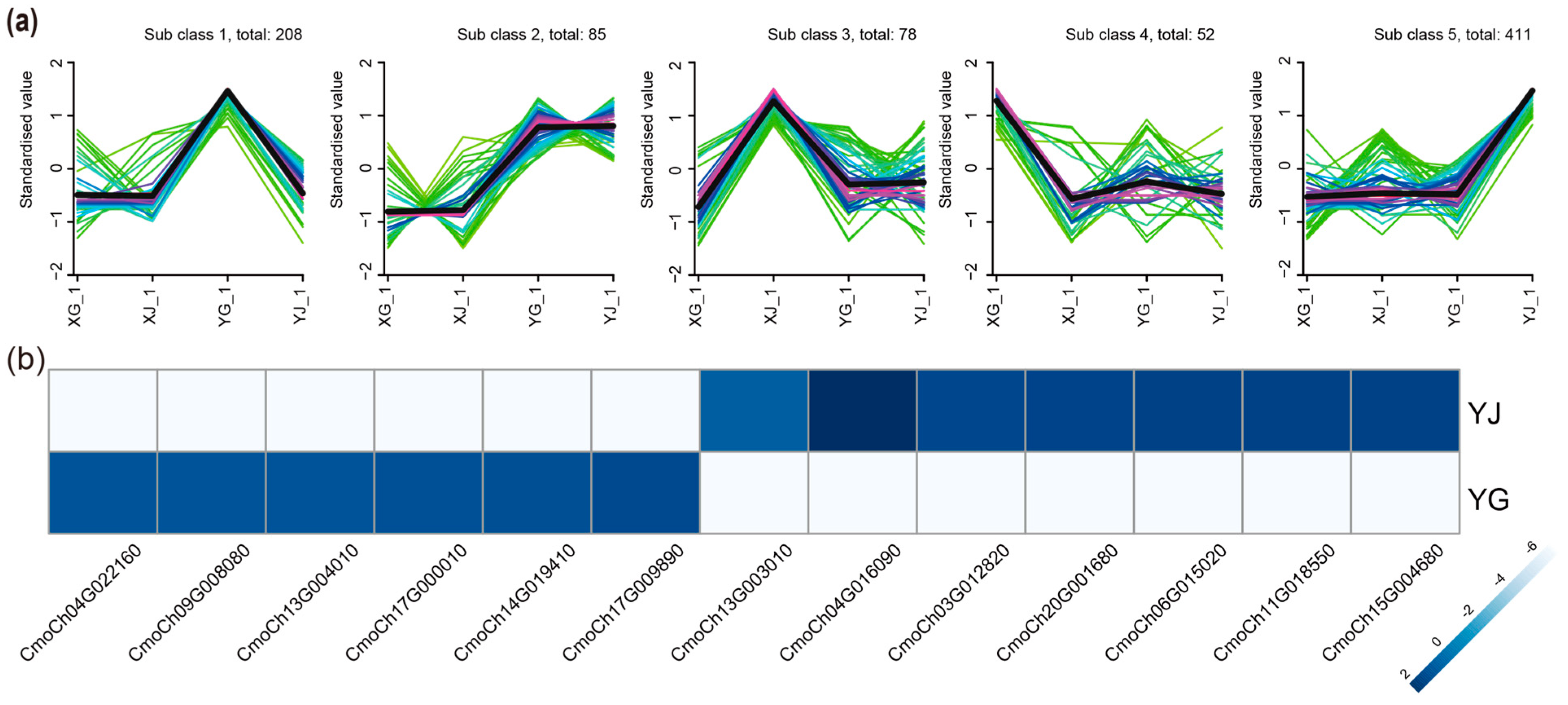
2.4. Identification of Specific mb-mRNAs by Mfuzz
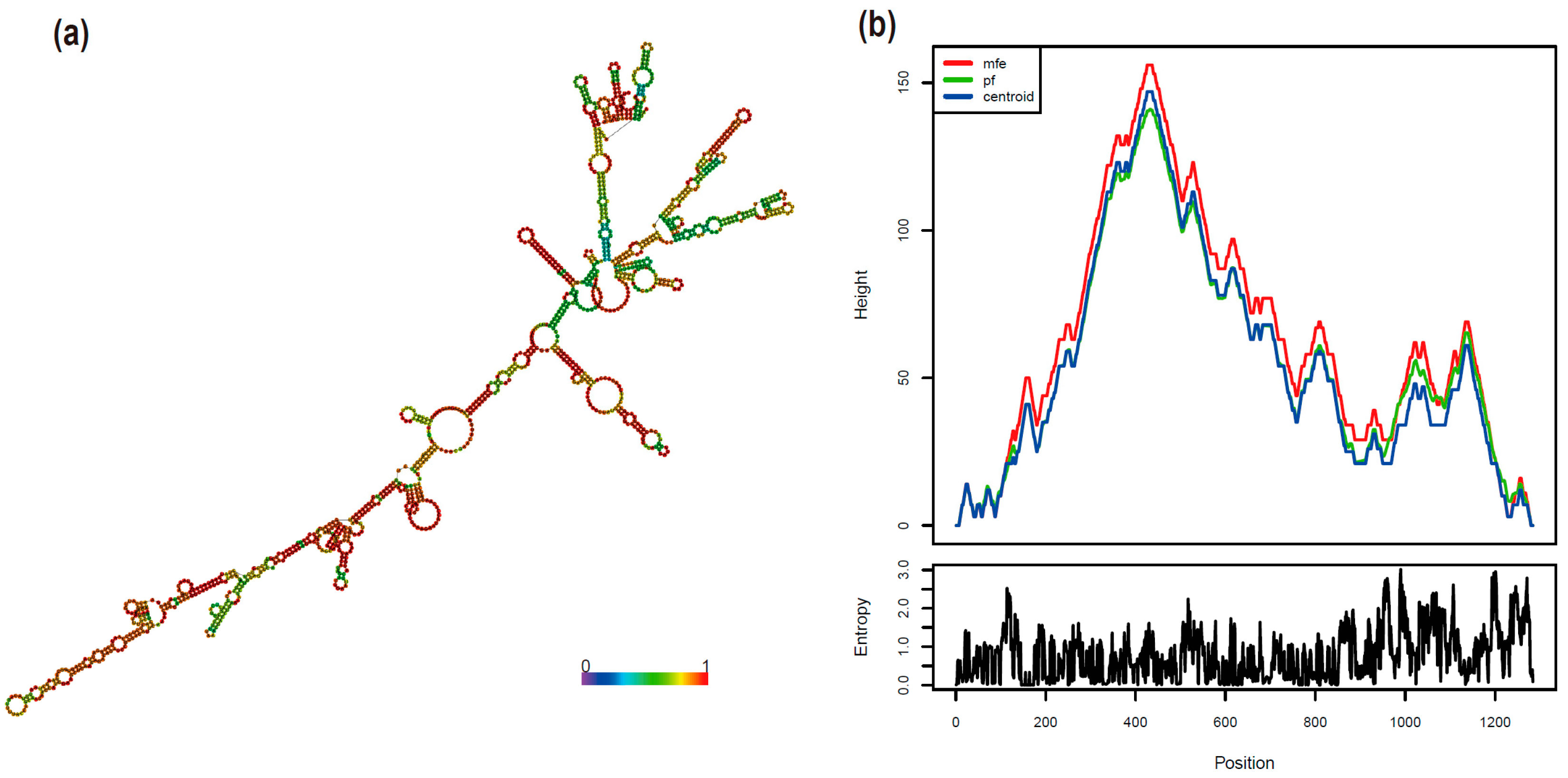
2.5. The Structure of mb-mRNA
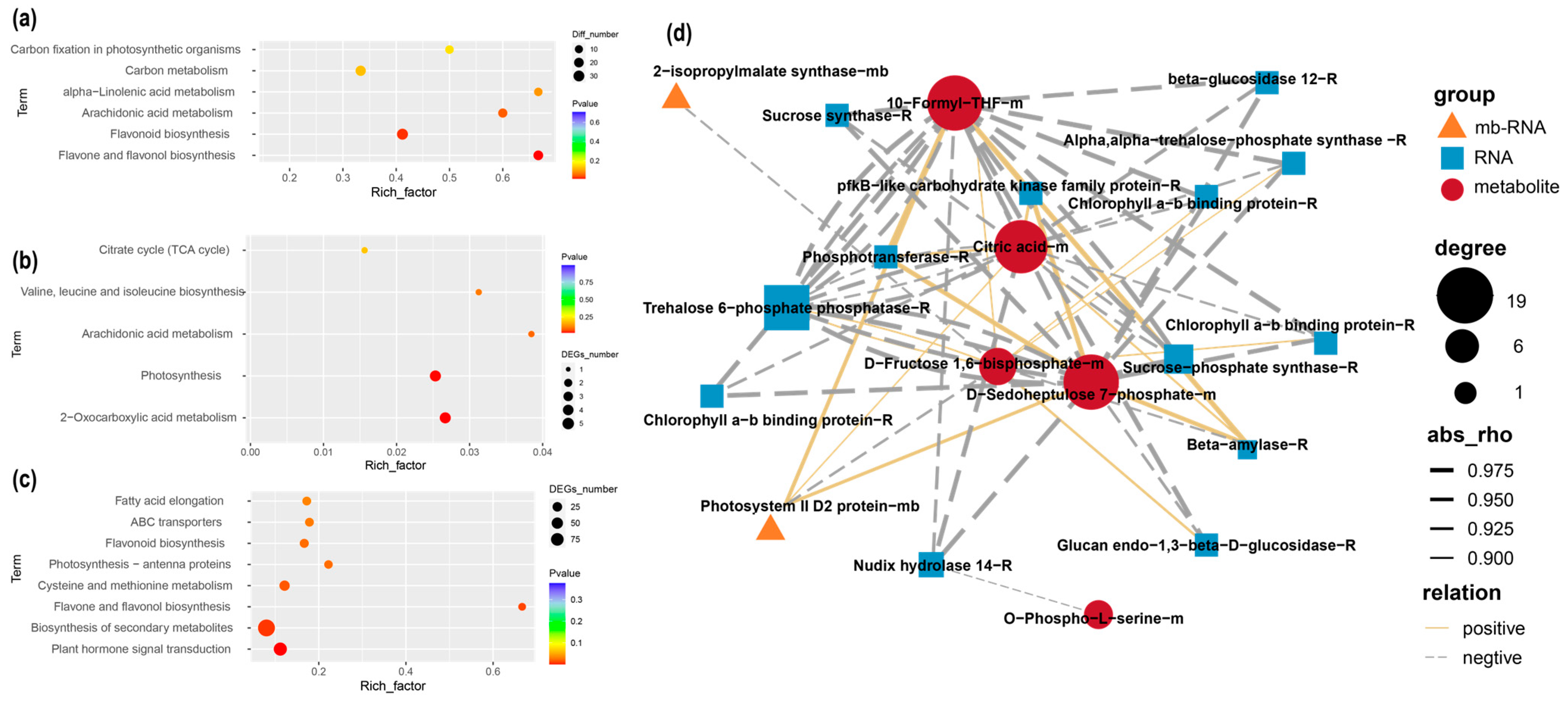
2.6. Integrated Analysis of Transcript and Metabolite Profiles
3. Materials and Methods
3.1. Identification of mb-mRNAs by RNA-Seq
3.2. RT-PCR
3.3. Bioinformatics Analysis
3.4. RNA Secondary Structure Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaion, L.A.; Braz, L.T.; Carvalho, R.F. Grafting in Vegetable Crops: A Great Technique for Agriculture. Int. J. Veg. Sci. 2017, 24, 85–102. [Google Scholar] [CrossRef]
- Grieneisen, M.L.; Aegerter, B.J.; Scott Stoddard, C.; Zhang, M. Yield and fruit quality of grafted tomatoes, and their potential for soil fumigant use reduction. A meta-analysis. Agron. Sustain. Dev. 2018, 38, 29. [Google Scholar] [CrossRef]
- Milenkovic, L.; Mastilovic, J.; Kevresan, Z.; Bajic, A.; Gledic, A.; Stanojevic, L.; Cvetkovic, D.; Sunic, L.; Ilic, Z.S. Effect of shading and grafting on yield and quality of tomato. J. Sci. Food. Agric. 2020, 100, 623–633. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, C.; Li, X.; Wang, T.; Lu, X.; Liu, Z.; Gao, L.; Zhang, W. Identification of Long-Distance Transmissible mRNA between Scion and Rootstock in Cucurbit Seedling Heterografts. Int. J. Mol. Sci. 2020, 21, 5253. [Google Scholar] [CrossRef]
- Davoudi, M.; Song, M.; Zhang, M.; Chen, J.; Lou, Q. Long-distance control of pumpkin rootstock over cucumber scion under drought stress as revealed by transcriptome sequencing and mobile mRNAs identifications. Hortic. Res. 2022, 9, uhab033. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Q.; Zhang, R.; Liu, M.; Wang, C.; Liu, Z.; Xiang, C.; Lu, X.; Zhang, X.; Li, X.; et al. Rootstock-scion exchanging mRNAs participate in the pathways of amino acids and fatty acid metabolism in cucumber under early chilling stress. Hortic. Res. 2022, 9, uhac031. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Zhou, X.; Pan, M.; Xu, J.; Liu, M.; Wang, M.; Liu, G.; Xu, T.; Wang, Y.; et al. Grafting with rootstocks promotes phenolic compound accumulation in grape berry skin during development based on integrative multi-omics analysis. Hortic. Res. 2022, 9, uhac055. [Google Scholar] [CrossRef]
- Chu, L.; Liu, D.; Li, C.; Liu, J. Dwarfing of fruit trees: From old cognitions to new insights. Hortic. Adv. 2025, 3, 7. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Liu, J.; Zhao, T.; Jia, L.; Chen, Z. Advances in understanding the graft healing mechanism: A review of factors and regulatory pathways. Hortic. Res. 2024, 11, uhae175. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2022, 34, 535–556. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, L.; Huang, Y.; He, J.; He, F.; Dong, Y. Unveiling the significance of Target of Rapamycin (TOR) signalling in grafting. Veg. Res. 2024, 4, e004. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Yan, Y.; Zhang, Y.; Shi, Y.; Chu, Y.; Li, Y.; Bao, E.; Zhou, X.; Wu, X.; et al. Additional far-red light promotes the healing and quality of double-root-cutting grafted watermelon seedlings. Sci. Hortic. 2024, 331, 113132. [Google Scholar] [CrossRef]
- Miao, L.; Li, Q.; Sun, T.S.; Chai, S.; Wang, C.; Bai, L.; Sun, M.; Li, Y.; Qin, X.; Zhang, Z.; et al. Sugars promote graft union development in the heterograft of cucumber onto pumpkin. Hortic. Res. 2021, 8, 146. [Google Scholar] [CrossRef]
- Haroldsen, V.M.; Szczerba, M.W.; Aktas, H.; Lopez-Baltazar, J.; Odias, M.J.; Chi-Ham, C.L.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L.T. Mobility of Transgenic Nucleic Acids and Proteins within Grafted Rootstocks for Agricultural Improvement. Front. Plant Sci. 2012, 3, 39. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the pieces: Uncovering the molecular basis for long-distance communication through plant grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Wang, M.; Zhou, H.; Gan, Z.; Zeng, R.; Ye, L.; Zhou, J.; Zhang, J.; Hu, C. Mobility of FLOWERING LOCUS T protein as a systemic signal in trifoliate orange and its low accumulation in grafted juvenile scions. Hortic. Res. 2022, 9, uhac056. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xing, N.; Wu, X.; Wu, X.; Wang, B.; Lu, Z.; Xu, P.; Tao, Y.; Li, G.; et al. A universal pipeline for mobile mRNA detection and insights into heterografting advantages under chilling stress. Hortic. Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, X.; Dong, H.; Wang, X.; Ye, T.; Wang, Q.; Duan, J.; Yu, M.; Zhang, T.; Du, N.; et al. gamma-aminobutyric acid contributes to a novel long-distance signaling in figleaf gourd rootstock-induced cold tolerance of grafted cucumber seedlings. Plant Physiol. Biochem. 2024, 216, 109168. [Google Scholar] [CrossRef]
- Ning, K.; Cai, X.; Yan, L.; Zhou, W.; Xie, A.; Wang, Y.; Xu, P. Transcriptomic and Metabolomic Analysis Reveals Improved Fruit Quality in Grafted Watermelon. Horticulturae 2024, 10, 1269. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xu, Z.; Ma, H.; Hao, Y.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. Characteristics of long-distance mobile mRNAs from shoot to root in grafted plant species. Hortic. Plant J. 2024, 10, 25–37. [Google Scholar] [CrossRef]
- Ahmad, D.; Ying, Y.; Bao, J. Understanding starch biosynthesis in potatoes for metabolic engineering to improve starch quality: A detailed review. Carbohydr. Polym. 2024, 346, 122592. [Google Scholar] [CrossRef]
- Rensink, W.; Hart, A.; Liu, J.; Ouyang, S.; Zismann, V.; Buell, C.R. Analyzing the potato abiotic stress transcriptome using expressed sequence tags. Genome 2005, 48, 598–605. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, Q.; Tang, M.; Gao, L.; Wang, Y.; Wang, J.; Liu, Z.; Li, X.; Mao, L.; Jia, R.Z.; et al. TBC1D23 mediates Golgi-specific LKB1 signaling. Nat. Commun. 2024, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Z.; Guo, X.; Tian, D.; Li, C.; Lin, M.; Hu, C.; Yan, J. Genome-Wide Identification and Analysis of Maize DnaJ Family Genes in Response to Salt, Heat, and Cold at the Seedling Stage. Plants 2024, 13, 2488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lei, P.; Jiang, Y.; Ji, X.; Meng, F. PmRad23d Interacts with PmSRC2 and PmCAR4 to Mediate the Abscisic Acid-Dependent Drought Response in Prunus mira Koehne. Plant Cell Environ. 2025, 48, 4178–4195. [Google Scholar] [CrossRef]
- Yu, W.; Dai, Y.; Chen, J.; Liang, A.; Wu, Y.; Suo, Q.; Chen, Z.; Yan, X.; Wang, C.; Lai, H.; et al. Upregulation of the glycine-rich protein-encoding gene GhGRPL enhances plant tolerance to abiotic and biotic stressors by promoting secondary cell wall development. J. Integr. Agric. 2024, 23, 3311–3327. [Google Scholar] [CrossRef]
- Yang, L.; Perrera, V.; Saplaoura, E.; Apelt, F.; Bahin, M.; Kramdi, A.; Olas, J.; Mueller-Roeber, B.; Sokolowska, E.; Zhang, W.; et al. m(5)C Methylation Guides Systemic Transport of Messenger RNA over Graft Junctions in Plants. Curr. Biol. 2019, 29, 2465–2476. [Google Scholar] [CrossRef]
- Shinde, H.; Dudhate, A.; Kadam, U.S.; Hong, J.C. RNA methylation in plants: An overview. Front. Plant Sci. 2023, 14, 1132959. [Google Scholar] [CrossRef]
- Huang, N.C.; Yu, T.S. The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J. 2009, 59, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Shireen, F.; Nawaz, M.A.; Xiong, M.; Ahmad, A.; Sohail, H.; Chen, Z.; Abouseif, Y.; Huang, Y.; Bie, Z. Pumpkin rootstock improves the growth and development of watermelon by enhancing uptake and transport of boron and regulating the gene expression. Plant Physiol. Biochem. 2020, 154, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Li, L.; Gao, P.; Li, H.; Shao, Q.; Shu, S.; Sun, J.; Guo, S. Effects of grafting with pumpkin rootstock on carbohydrate metabolism in cucumber seedlings under Ca (NO3)2 stress. Plant Physiol. Biochem. 2015, 87, 124–132. [Google Scholar] [CrossRef]
- Davoudi, M.; Chen, J.; Lou, Q. Genome-Wide Identification and Expression Analysis of Heat Shock Protein 70 (HSP70) Gene Family in Pumpkin (Cucurbita moschata) Rootstock under Drought Stress Suggested the Potential Role of these Chaperones in Stress Tolerance. Int. J. Mol. Sci. 2022, 23, 1918. [Google Scholar] [CrossRef]
- Sun, J.; Cao, H.; Cheng, J.; He, X.; Sohail, H.; Niu, M.; Huang, Y.; Bie, Z. Pumpkin CmHKT1;1 Controls Shoot Na+ Accumulation via Limiting Na+ Transport from Rootstock to Scion in Grafted Cucumber. Int. J. Mol. Sci. 2018, 19, 2648. [Google Scholar] [CrossRef] [PubMed]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication Between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
| SampleID | Pumpkin Alignment | Watermelon Alignment | ||||
|---|---|---|---|---|---|---|
| Clean Reads | Mapped Reads | Mapped Rate (%) | Clean Reads | Mapped Reads | Mapped Rate (%) | |
| XG_1 | 8,828,672 | 32,064 | 0.36 | 60,528,066 | 51,699,394 | 85.41 |
| XG_2 | 9,883,596 | 37,851 | 0.38 | 65,356,312 | 55,472,716 | 84.88 |
| XG_3 | 11,348,068 | 53,431 | 0.47 | 72,865,870 | 61,517,802 | 84.43 |
| XJ_1 | 5,499,504 | 47,294 | 0.86 | 91,199,184 | 85,699,680 | 93.97 |
| XJ_2 | 5,855,008 | 46,574 | 0.8 | 84,643,282 | 78,788,274 | 93.08 |
| XJ_3 | 3,536,176 | 32,162 | 0.91 | 58,043,296 | 54,507,120 | 93.91 |
| YG_1 | 67,836,182 | 244,339 | 0.36 | 450,405,130 | 382,568,948 | 84.94 |
| YG_2 | 71,149,316 | 270,573 | 0.38 | 462,696,550 | 391,547,234 | 84.62 |
| YG_3 | 69,412,674 | 276,379 | 0.4 | 453,891,800 | 384,479,126 | 84.71 |
| YJ_1 | 16,256,512 | 495,277 | 3.05 | 462,670,224 | 446,413,712 | 96.49 |
| YJ_2 | 15,682,756 | 241,877 | 1.54 | 418,630,324 | 402,947,568 | 96.25 |
| YJ_3 | 19,086,908 | 315,904 | 1.66 | 495,488,346 | 476,401,438 | 96.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, K.; Zhou, W.; Cai, X.; Yan, L.; Ma, Y.; Xie, A.; Wang, Y.; Xu, P. Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement. Int. J. Mol. Sci. 2025, 26, 5121. https://doi.org/10.3390/ijms26115121
Ning K, Zhou W, Cai X, Yan L, Ma Y, Xie A, Wang Y, Xu P. Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement. International Journal of Molecular Sciences. 2025; 26(11):5121. https://doi.org/10.3390/ijms26115121
Chicago/Turabian StyleNing, Kang, Weixin Zhou, Xiaoqi Cai, Leiyan Yan, Yuanchang Ma, An Xie, Yuhong Wang, and Pei Xu. 2025. "Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement" International Journal of Molecular Sciences 26, no. 11: 5121. https://doi.org/10.3390/ijms26115121
APA StyleNing, K., Zhou, W., Cai, X., Yan, L., Ma, Y., Xie, A., Wang, Y., & Xu, P. (2025). Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement. International Journal of Molecular Sciences, 26(11), 5121. https://doi.org/10.3390/ijms26115121





