Adoptive Transfer of Lepr+ Bone Marrow Cells Attenuates the Osteopetrotic Phenotype of db/db Mice
Abstract
1. Introduction
2. Results
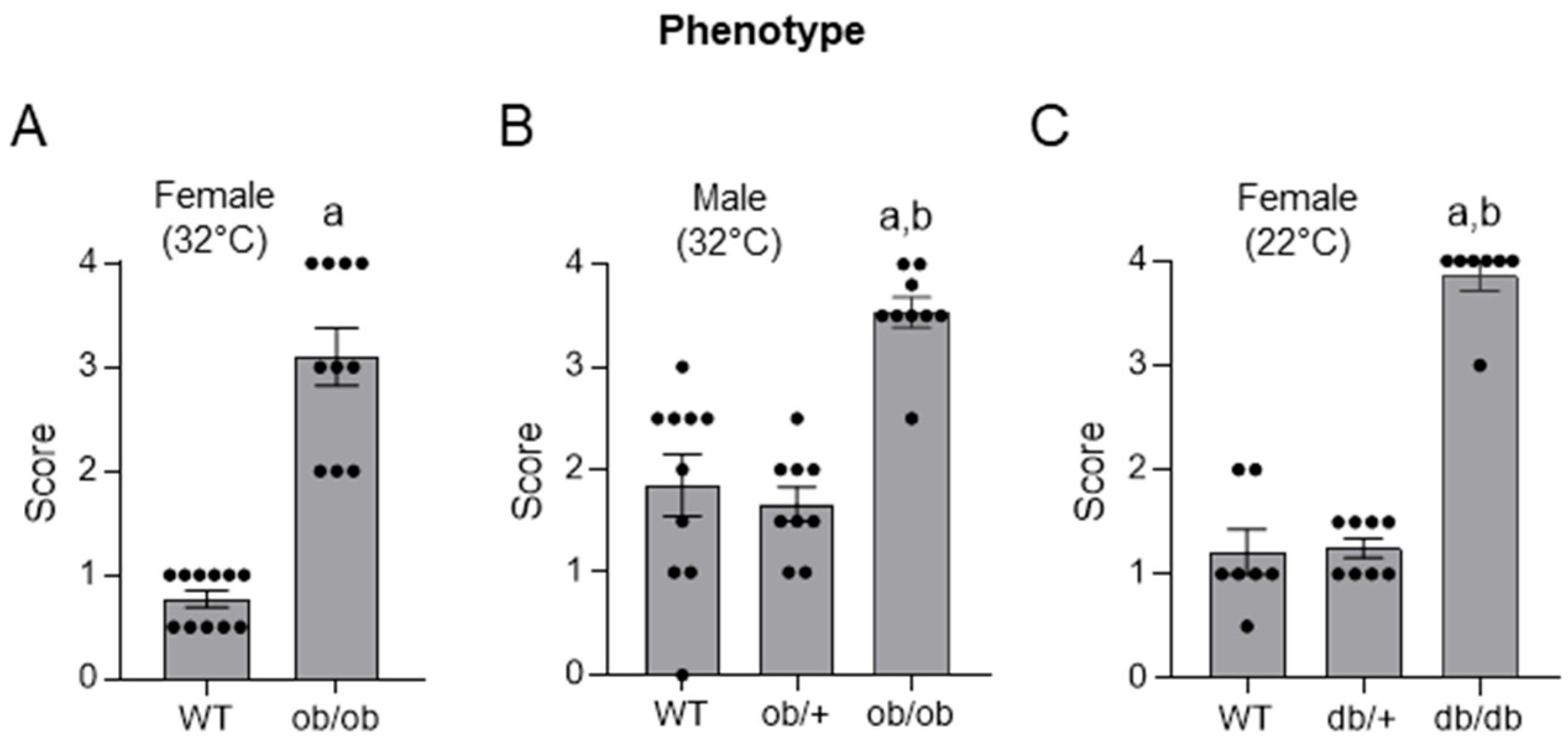
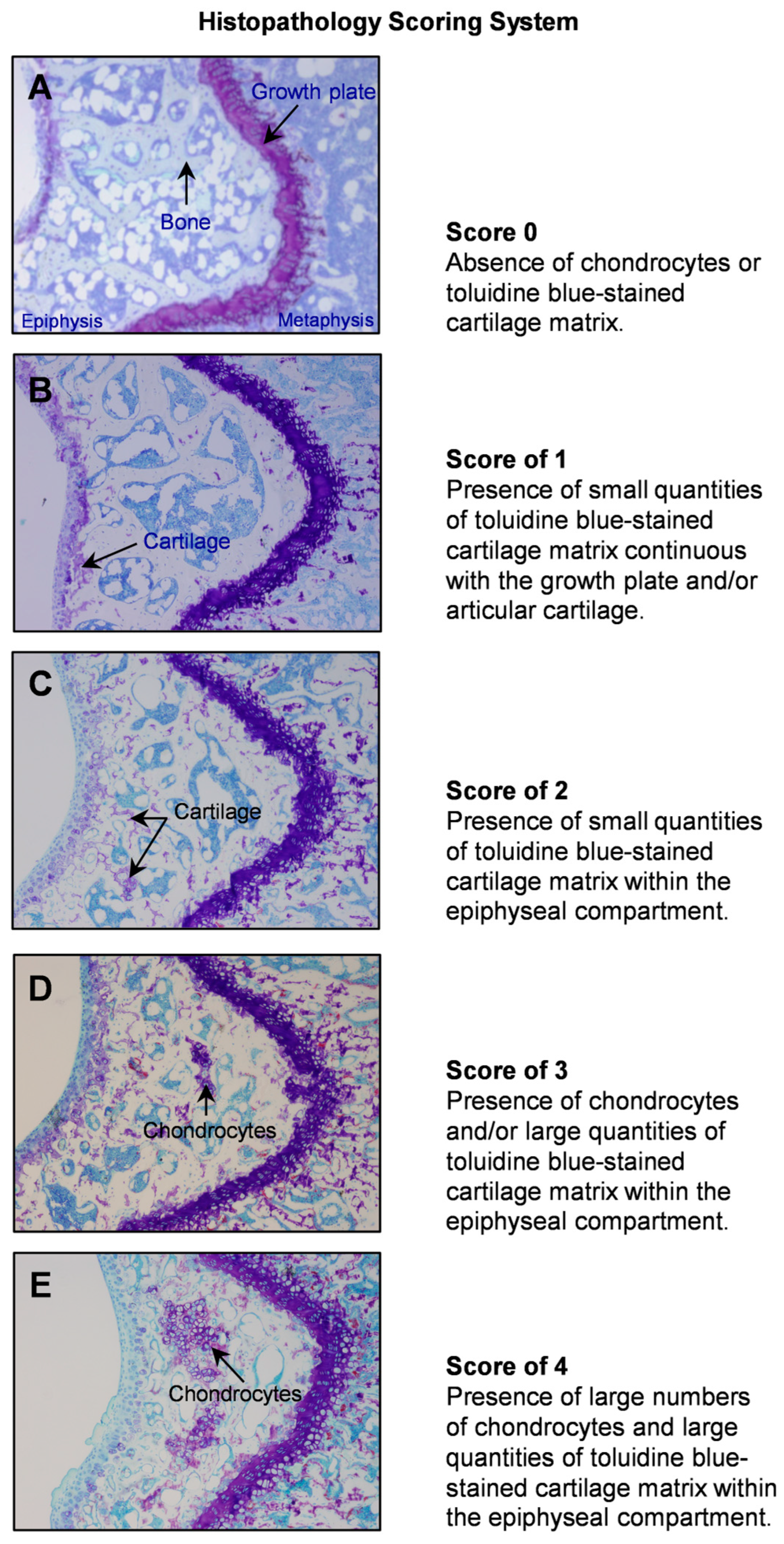
2.1. Histopathology Evaluation of Distal Femur Epiphysis in 4-Month-Old WT, ob/ob, ob/+, db/db, and db/+ Mice
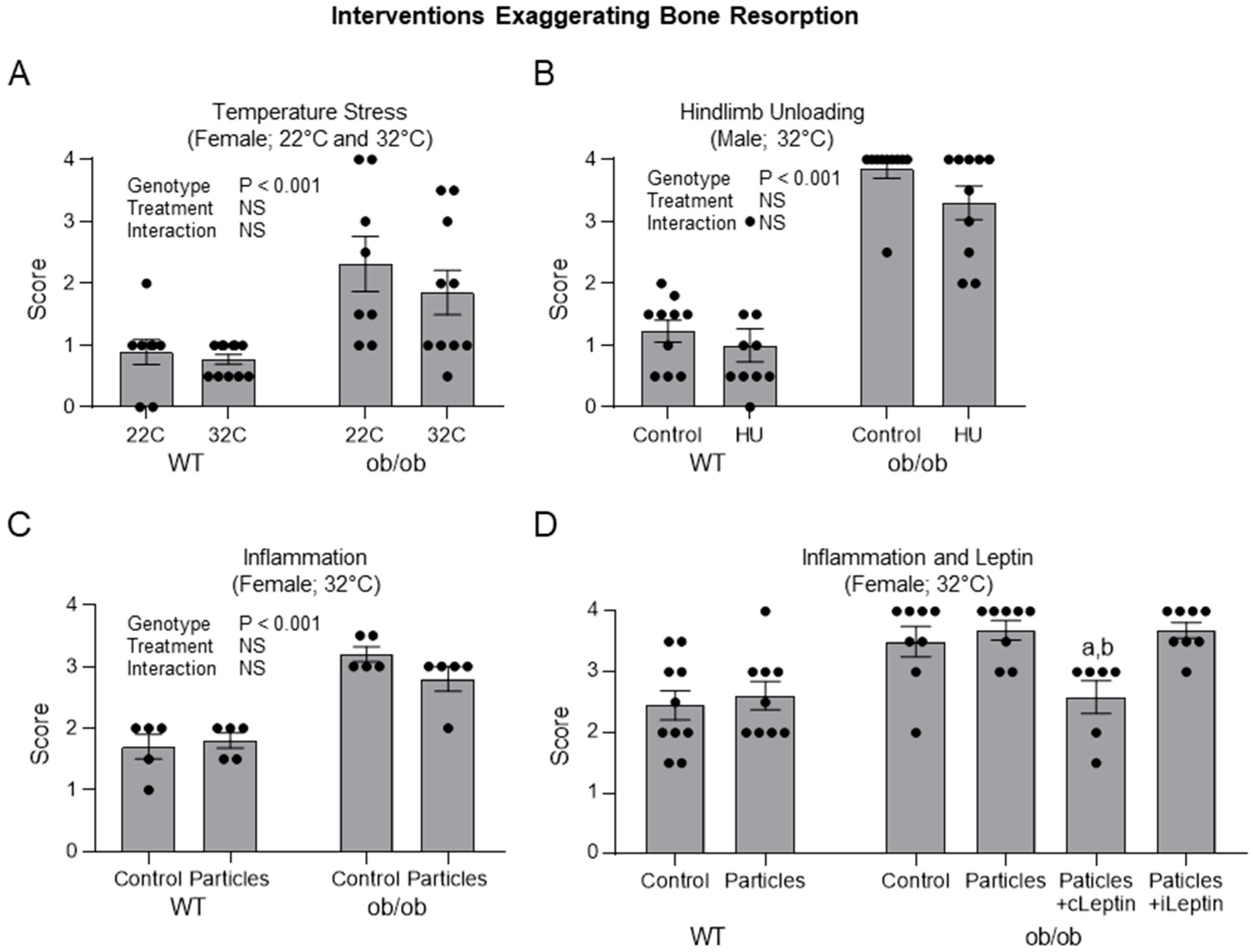
2.2. Impact of Interventions Known to Increase Bone Resorption on Histopathology Score in ob/ob Mice
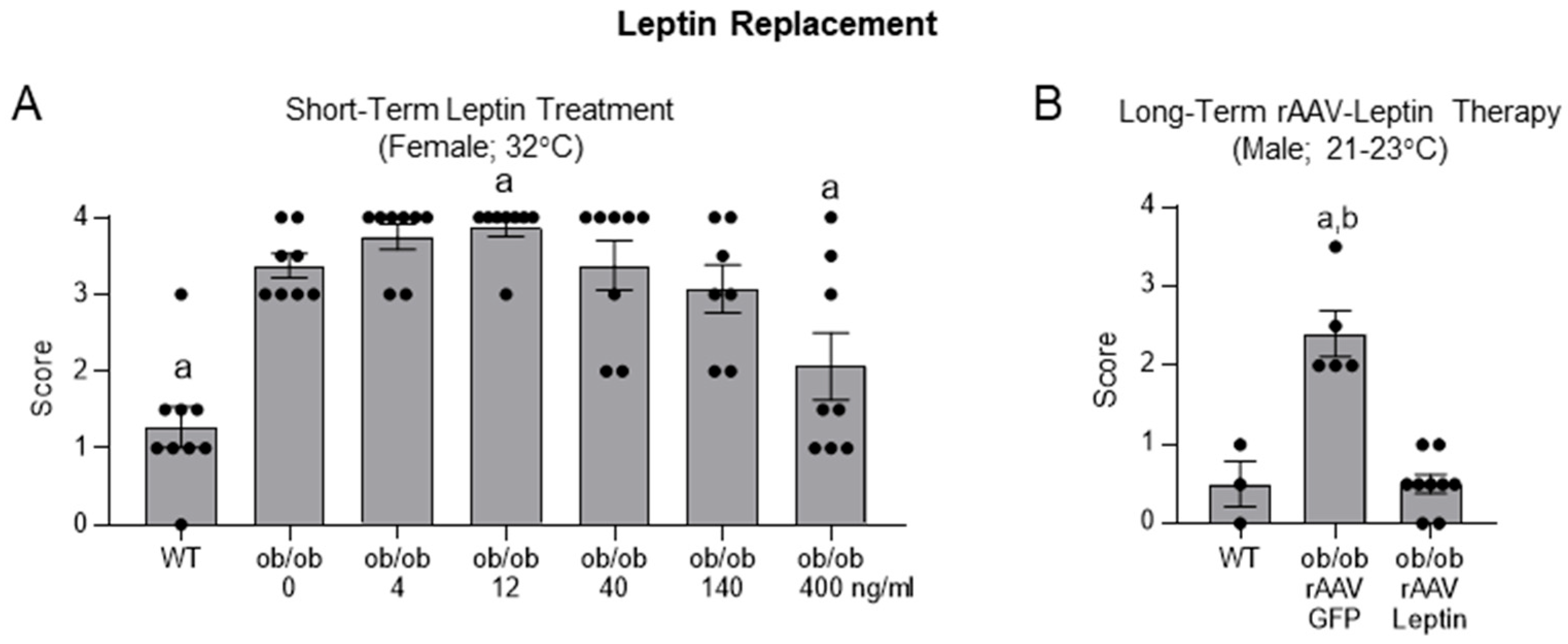
2.3. Effects of Leptin Replacement on Histopathology Score in ob/ob Mice
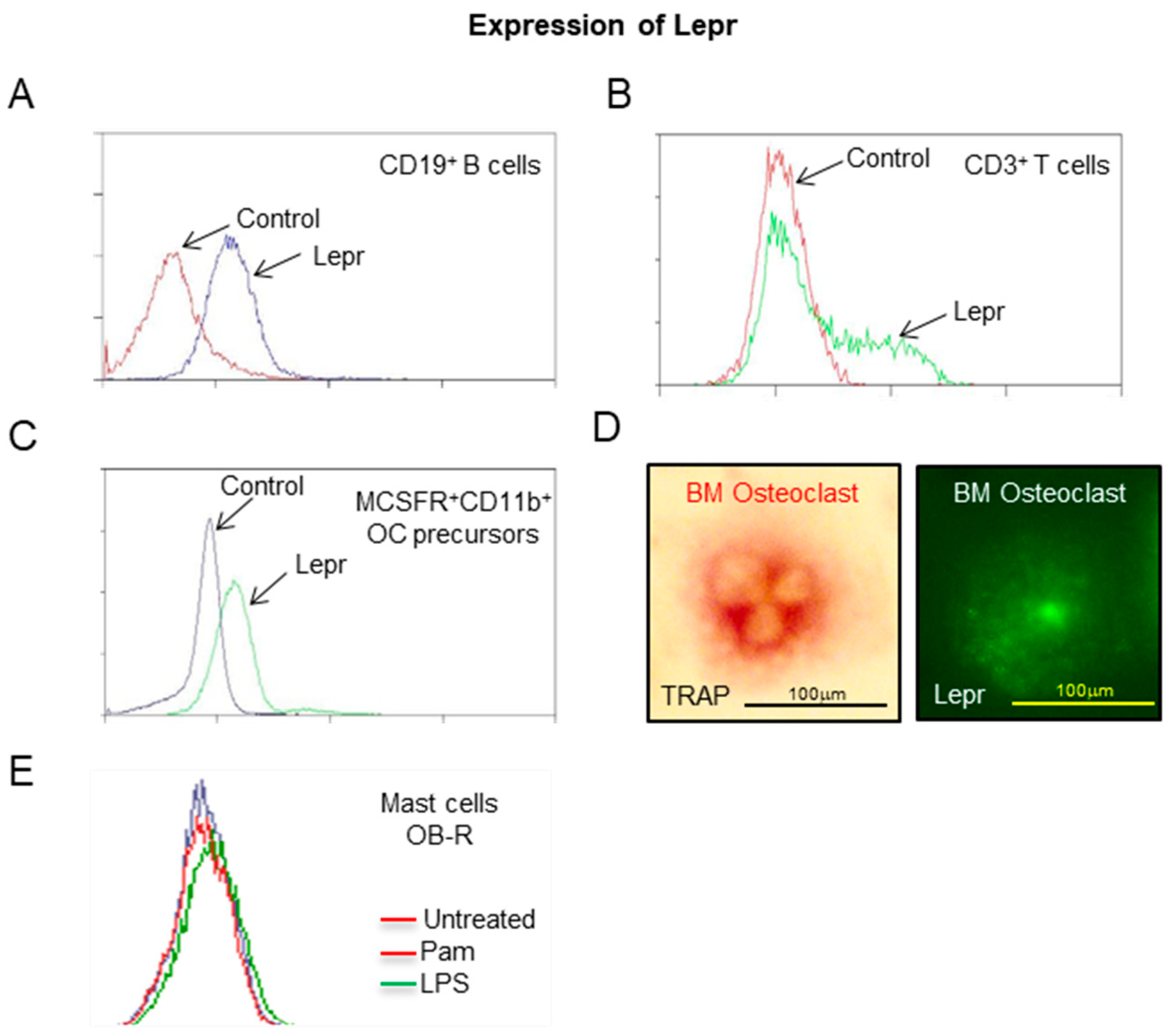
2.4. Expression of Lepr by Hematopoietic Lineage Cells
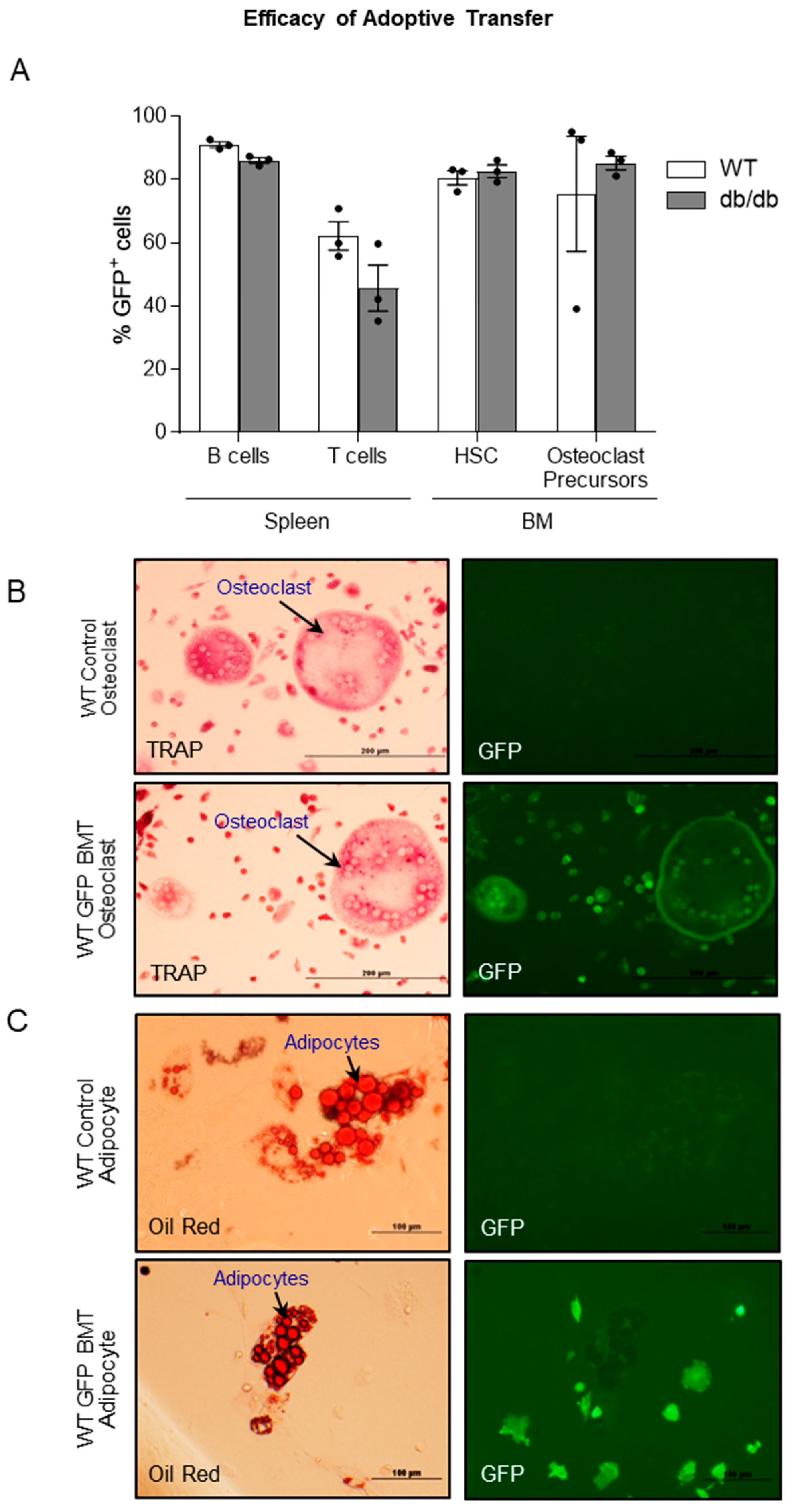
2.5. Efficacy of Adoptive Transfer of GFP-Expressing Bone Marrow Cells into WT and db/db Mice
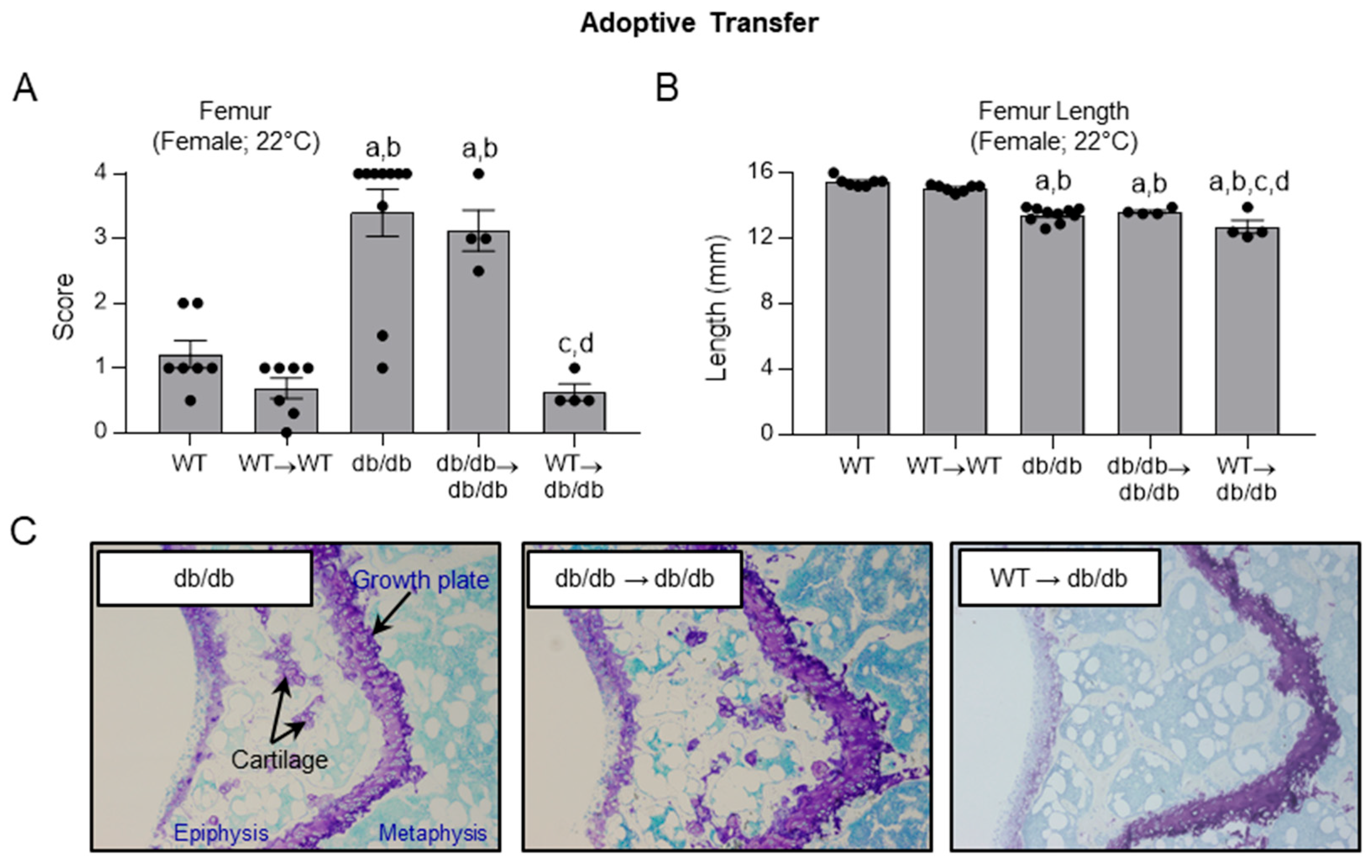
2.6. Effects of Adoptive Transfer of WT and db/db Bone Marrow Cells on Histopathology Score in WT and db/db Mice
3. Discussion
4. Methods
4.1. Mice
4.2. Evaluation of Skeletal Maturation of Mouse Long Bones
4.3. Studies
- Skeletal maturation in the distal femur epiphysis in 16-week-old WT, ob/ob, ob/+, db/db, and db/+ mice.
- B.
- Skeletal maturation in the distal femur epiphysis in ob/ob mice subjected to interventions that exaggerate bone resorption.
- C.
- Skeletal maturation in the distal femur epiphysis in ob/ob mice following leptin replacement.
- D.
- Expression of Lepr in hematopoietic lineage cells in WT mice.
- E.
- Efficacy of adoptive transfer of bone marrow cells from GFP mice into WT and db/db mice
- F.
- Efficacy of adoptive transfer of bone marrow cells from WT and db/db mice into db/db mice in advancing skeletal maturation
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwaniec, U.T.; Turner, R.T. Influence of body weight on bone mass, architecture and turnover. J. Endocrinol. 2016, 230, R115–R130. [Google Scholar] [CrossRef] [PubMed]
- Ealey, K.N.; Fonseca, D.; Archer, M.C.; Ward, W.E. Bone abnormalities in adolescent leptin-deficient mice. Regul. Pept. 2006, 136, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, Z.M.; Leinung, M.C.; Lee, D.W.; Grasso, P. Oral delivery of mouse [d-Leu-4]-OB3, a synthetic peptide amide with leptin-like activity, in male C57BL/6J wild-type and ob/ob mice: Effects on energy balance, glycaemic control and serum osteocalcin levels. Diabetes Obes. Metab. 2010, 12, 532–539. [Google Scholar] [CrossRef]
- Smith, C.K.; Romsos, D.R. Cold acclimation of obese (ob/ob) mice: Effects on skeletal muscle and bone. Metabolism 1984, 33, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.A.; Callon, K.E.; Watson, M.; Costa, J.L.; Ding, Y.; Dickinson, M.; Wang, Y.; Naot, D.; Reid, I.R.; Cornish, J. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J. Bone Miner. Res. 2011, 26, 1698–1709. [Google Scholar] [CrossRef]
- Yagasaki, Y.; Yamaguchi, T.; Watahiki, J.; Konishi, M.; Katoh, H.; Maki, K. The role of craniofacial growth in leptin deficient (ob/ob) mice. Orthod. Craniofac Res. 2003, 6, 233–241. [Google Scholar] [CrossRef]
- Gat-Yablonski, G.; Phillip, M. Leptin and regulation of linear growth. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 303–308. [Google Scholar] [CrossRef]
- Jing, D.; Luo, E.; Cai, J.; Tong, S.; Zhai, M.; Shen, G.; Wang, X.; Luo, Z. Mechanical Vibration Mitigates the Decrease of Bone Quantity and Bone Quality of Leptin Receptor-Deficient Db/Db Mice by Promoting Bone Formation and Inhibiting Bone Resorption. J. Bone Miner. Res. 2016, 31, 1713–1724. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Z.; Zhao, Y.; Jing, D.; Tang, C.; Ding, Y.; Feng, X. Effects of low-intensity pulsed electromagnetic fields on bone microarchitecture, mechanical strength and bone turnover in type 2 diabetic db/db mice. Sci. Rep. 2017, 7, 10834. [Google Scholar] [CrossRef]
- Turner, R.T.; Kalra, S.P.; Wong, C.P.; Philbrick, K.A.; Lindenmaier, L.B.; Boghossian, S.; Iwaniec, U.T. Peripheral leptin regulates bone formation. J. Bone Miner. Res. 2013, 28, 22–34. [Google Scholar] [CrossRef]
- Philbrick, K.A.; Wong, C.P.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J. Endocrinol. 2017, 232, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, K.A.; Martin, S.A.; Colagiovanni, A.R.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T. Effects of hypothalamic leptin gene therapy on osteopetrosis in leptin-deficient mice. J. Endocrinol. 2018, 236, 57–68. [Google Scholar] [CrossRef]
- Hung, J.; Al-Nakkash, L.; Broderick, T.L.; Castro, M.; Plochocki, J.H. Leptin-deficient mice have altered three-dimensional growth plate histomorphometry. Diabetol. Metab. Syndr. 2019, 11, 8. [Google Scholar] [CrossRef]
- Kishida, Y.; Hirao, M.; Tamai, N.; Nampei, A.; Fujimoto, T.; Nakase, T.; Shimizu, N.; Yoshikawa, H.; Myoui, A. Leptin regulates chondrocyte differentiation and matrix maturation during endochondral ossification. Bone 2005, 37, 607–621. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Huang, W.; Jaspan, J.B.; Maness, L.M. Leptin enters the brain by a saturable system independent of insulin. Peptides 1996, 17, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Maness, L.M.; Kastin, A.J.; Farrell, C.L.; Banks, W.A. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology 1998, 139, 4556–4562. [Google Scholar] [CrossRef]
- Trotter-Mayo, R.N.; Roberts, M.R. Leptin acts in the periphery to protect thymocytes from glucocorticoid-mediated apoptosis in the absence of weight loss. Endocrinology 2008, 149, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of Leptin on the Skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Turner, R.T.; Philbrick, K.A.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Iwaniec, U.T. Morbid obesity attenuates the skeletal abnormalities associated with leptin deficiency in mice. J. Endocrinol. 2014, 223, M1–M15. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Dube, M.G.; Boghossian, S.; Song, H.; Helferich, W.G.; Turner, R.T.; Kalra, S.P. Body mass influences cortical bone mass independent of leptin signaling. Bone 2009, 44, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J.; Moskovsky, L.; Thompson, M.S.; Grunhen, J.; Cheng, X.; Kashima, T.G.; Athanasou, N.A. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 2012, 461, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Philbrick, K.A.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Jump, D.B.; Marik, C.K.; DenHerder, J.M.; Sargent, J.L.; Turner, R.T.; et al. Thermoneutral housing attenuates premature cancellous bone loss in male C57BL/6J mice. Endocr. Connect. 2019, 8, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Keune, J.A.; Branscum, A.J.; Wong, C.P.; Iwaniec, U.T.; Turner, R.T. Effect of Leptin Deficiency on the Skeletal Response to Hindlimb Unloading in Adult Male Mice. Sci. Rep. 2019, 9, 9336. [Google Scholar] [CrossRef]
- Philbrick, K.A.; Branscum, A.J.; Wong, C.P.; Turner, R.T.; Iwaniec, U.T. Leptin Increases Particle-Induced Osteolysis in Female ob/ob Mice. Sci. Rep. 2018, 8, 14790. [Google Scholar] [CrossRef]
- Leger, J.; Fjellestad-Paulsen, A.; Bargiacchi, A.; Doyen, C.; Ecosse, E.; Carel, J.C.; Le Heuzey, M.F. Can growth hormone treatment improve growth in children with severe growth failure due to anorexia nervosa? A preliminary pilot study. Endocr. Connect. 2017, 6, 839–846. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Yaroslavsky, A.; Kochavi, B.; Toledano, A.; Segev, S.; Balawi, F.; Mitrany, E.; Stein, D. Linear growth and final height characteristics in adolescent females with anorexia nervosa. PLoS ONE 2012, 7, e45504. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Misra, M.; Miller, K.K.; Kruczek, K.; Sundaralingam, S.; Herzog, D.B.; Katzman, D.K.; Klibanski, A. Determinants of height in adolescent girls with anorexia nervosa. Pediatrics 2008, 121, e1517–e1523. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Newton, D.; Xie, D.; Isales, C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004, 34, 376–383. [Google Scholar] [CrossRef]
- Steppan, C.M.; Crawford, D.T.; Chidsey-Frink, K.L.; Ke, H.; Swick, A.G. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul. Pept. 2000, 92, 73–78. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Della-Fera, M.A.; Choi, Y.H.; Pennington, C.; Hartzell, D.; Baile, C.A. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J. Bone Miner. Res. 2005, 20, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, K.A.; Turner, R.T.; Branscum, A.J.; Wong, C.P.; Iwaniec, U.T. Paradoxical effects of partial leptin deficiency on bone in growing female mice. Anat. Rec. 2015, 298, 2018–2029. [Google Scholar] [CrossRef]
- Donaubauer, A.J.; Deloch, L.; Becker, I.; Fietkau, R.; Frey, B.; Gaipl, U.S. The Influence of Radiation on Bone and Bone Cells-Differential Effects on Osteoclasts and Osteoblasts. Int. J. Mol. Sci. 2020, 21, 6377. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Clifton, K.B.; Lorenzo, J.; Hansen, M.F.; Drissi, H. Comparative transcriptomic analysis identifies distinct molecular signatures and regulatory networks of chondroclasts and osteoclasts. Arthritis Res. Ther. 2020, 22, 168. [Google Scholar] [CrossRef]
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J.; Miller, W.P.; Petryk, A.; Rush, E.T.; Shoback, D.M.; et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123. [Google Scholar] [CrossRef]
- Su, P.H.; Yang, S.F.; Yu, J.S.; Chen, S.J.; Chen, J.Y. A polymorphism in the leptin receptor gene at position 223 is associated with growth hormone replacement therapy responsiveness in idiopathic short stature and growth hormone deficiency patients. Eur. J. Med. Genet. 2012, 55, 682–687. [Google Scholar] [CrossRef]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P.; Wenkert, D.; Clements, K.L.; McAlister, W.H.; Mumm, S. Bisphosphonate-induced osteopetrosis. N. Engl. J. Med. 2003, 349, 457–463. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. The hypertrophic chondrocyte: To be or not to be. Histol. Histopathol. 2021, 36, 1021–1036. [Google Scholar]
- Iwaniec, U.T.; Wronski, T.J.; Turner, R.T. Histological analysis of bone. Methods Mol. Biol. 2008, 447, 325–341. [Google Scholar]
- Ferguson, V.L.; Ayers, R.A.; Bateman, T.A.; Simske, S.J. Bone development and age-related bone loss in male C57BL/6J mice. Bone 2003, 33, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Philbrick, K.A.; Wong, C.P.; Branscum, A.J.; Iwaniec, U.T. Higher weight in partially leptin-resistant db/+ mice is associated with positive effects on bone. J. Endocrinol. 2023, 259, e230182. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Thermoregulation in the diabetic-obese (db/db) mouse. The role of non-shivering thermogenesis in energy balance. Pflugers Arch. 1979, 380, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Swoap, S.J.; Gutilla, M.J. Cardiovascular changes during daily torpor in the laboratory mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R769–R774. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Philbrick, K.A.; Wong, C.P.; Gordon, J.L.; Kahler-Quesada, A.M.; Olson, D.A.; Branscum, A.J.; Sargent, J.L.; DeMambro, V.E.; Rosen, C.J.; et al. Room temperature housing results in premature cancellous bone loss in growing female mice: Implications for the mouse as a preclinical model for age-related bone loss. Osteoporos. Int. 2016, 27, 3091–3101. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef]
- Wong, C.P.; Branscum, A.J.; Fichter, A.R.; Sargent, J.; Iwaniec, U.T.; Turner, R.T. Cold stress during room temperature housing alters skeletal response to simulated microgravity (hindlimb unloading) in growing female C57BL6 mice. Biochimie 2023, 210, 61–70. [Google Scholar] [CrossRef]
- Philbrick, K.A.; Wong, C.P.; Kahler-Quesada, A.M.; Olson, D.A.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T. Polyethylene particles inserted over calvarium induce cancellous bone loss in femur in female mice. Bone Rep. 2018, 9, 84–92. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Boghossian, S.; Lapke, P.D.; Turner, R.T.; Kalra, S.P. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides 2007, 28, 1012–1019. [Google Scholar] [CrossRef]
- Boghossian, S.; Dube, M.G.; Torto, R.; Kalra, P.S.; Kalra, S.P. Hypothalamic clamp on insulin release by leptin-transgene expression. Peptides 2006, 27, 3245–3254. [Google Scholar] [CrossRef]
- Bennett, B.D.; Solar, G.P.; Yuan, J.Q.; Mathias, J.; Thomas, G.R.; Matthews, W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996, 6, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martin-Romero, C.; Perez-Perez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010, 2010, 568343. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.; Ropa, J.; Aljoufi, A.; Cooper, S.; Sinn, A.; Srour, E.F.; Broxmeyer, H.E. Leptin receptor, a surface marker for a subset of highly engrafting long-term functional hematopoietic stem cells. Leukemia 2021, 35, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Papathanassoglou, E.; El-Haschimi, K.; Li, X.C.; Matarese, G.; Strom, T.; Mantzoros, C. Leptin receptor expression and signaling in lymphocytes: Kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J. Immunol. 2006, 176, 7745–7752. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, R.T.; Wong, C.P.; Philbrick, K.A.; Keune, J.A.; Labut, E.M.; Menn, S.A.; Branscum, A.J.; Iwaniec, U.T. Adoptive Transfer of Lepr+ Bone Marrow Cells Attenuates the Osteopetrotic Phenotype of db/db Mice. Int. J. Mol. Sci. 2025, 26, 5120. https://doi.org/10.3390/ijms26115120
Turner RT, Wong CP, Philbrick KA, Keune JA, Labut EM, Menn SA, Branscum AJ, Iwaniec UT. Adoptive Transfer of Lepr+ Bone Marrow Cells Attenuates the Osteopetrotic Phenotype of db/db Mice. International Journal of Molecular Sciences. 2025; 26(11):5120. https://doi.org/10.3390/ijms26115120
Chicago/Turabian StyleTurner, Russell T., Carmen P. Wong, Kenneth A. Philbrick, Jessica A. Keune, Edwin M. Labut, Scott A. Menn, Adam J. Branscum, and Urszula T. Iwaniec. 2025. "Adoptive Transfer of Lepr+ Bone Marrow Cells Attenuates the Osteopetrotic Phenotype of db/db Mice" International Journal of Molecular Sciences 26, no. 11: 5120. https://doi.org/10.3390/ijms26115120
APA StyleTurner, R. T., Wong, C. P., Philbrick, K. A., Keune, J. A., Labut, E. M., Menn, S. A., Branscum, A. J., & Iwaniec, U. T. (2025). Adoptive Transfer of Lepr+ Bone Marrow Cells Attenuates the Osteopetrotic Phenotype of db/db Mice. International Journal of Molecular Sciences, 26(11), 5120. https://doi.org/10.3390/ijms26115120




