Protective Effect of a Hexapeptide Derived from Rotifer-Specific SCO-Spondin Against Beta-Amyloid Toxicity
Abstract
1. Introduction
2. Results
2.1. The Effect of Short Peptides Derived from Rotifer-Specific Proteins Against agg-Aβ-Toxicity In Vitro and In Vivo
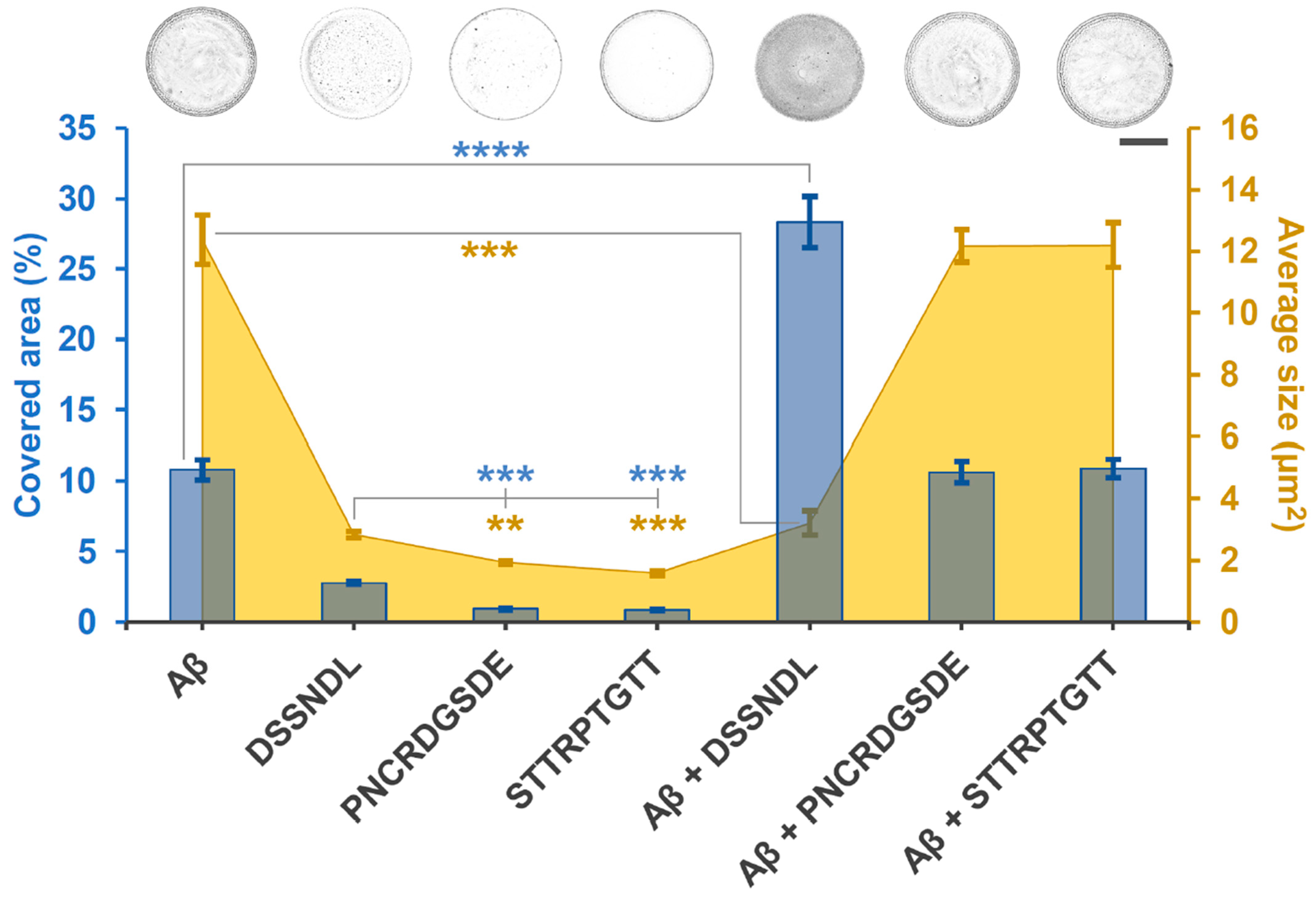
2.2. Suspected Interaction Between agg-Aβ and Short Peptides Derived from Rotifer-Specific Proteins
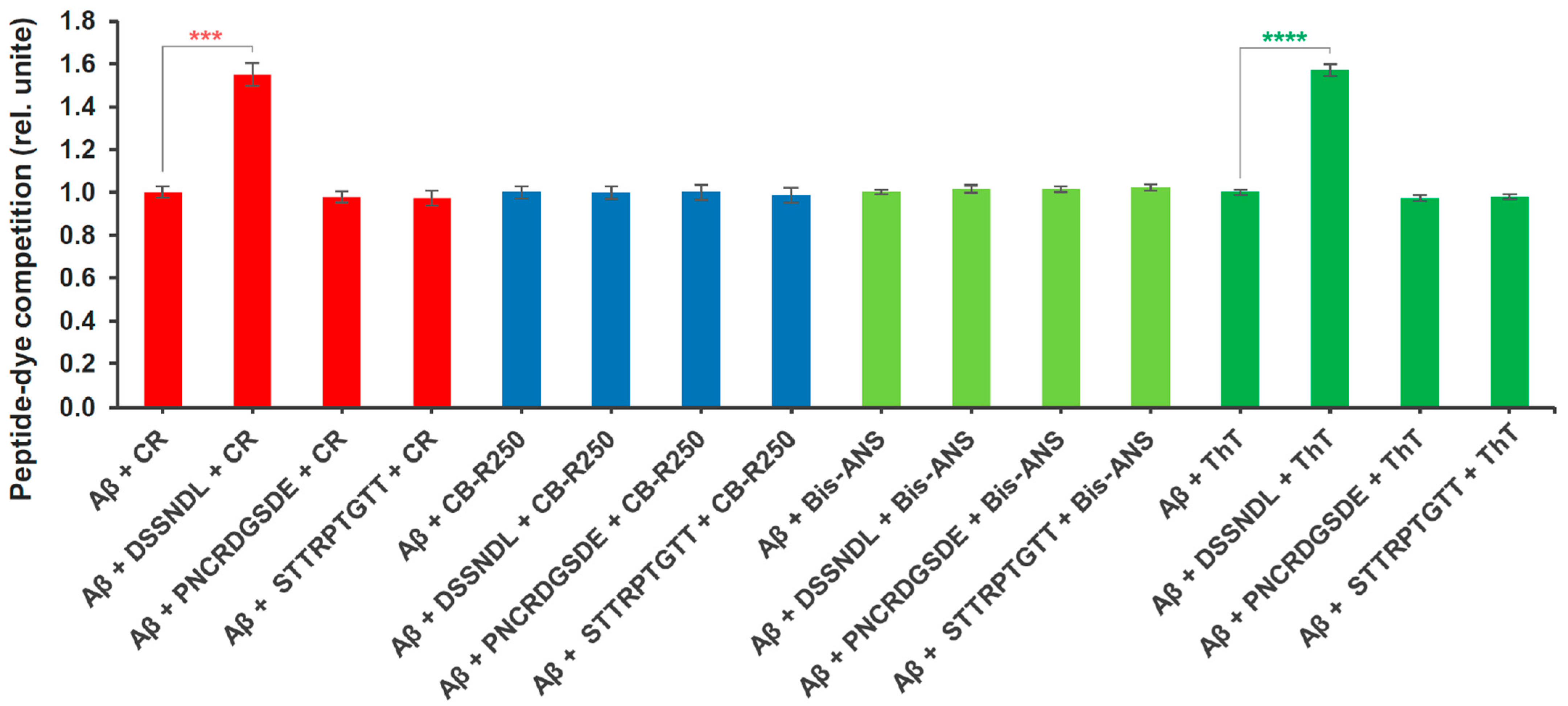
2.3. Molecular Competition Between Rotifer-Specific Peptides and Amyloid-Specific Dyes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Rotifer-Specific Peptide Synthesis
4.3. Preparation of the Amyloid Aggregates
4.4. In Vitro and In Vivo Cultures
4.5. Viability Assays
4.6. Derived Short Peptide-Amyloid Interaction Assay Based on Stagogram Optical Analysis
4.7. Binding Competition of Derived Short Peptides with Amyloid-Specific Dyes
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Saeed, M.; Alharbi, A.M.; Barreto, G.E.; Ashraf, G.M.; Choi, I. Peptide Based Therapeutics and Their Use for the Treatment of Neurodegenerative and Other Diseases. Biomed. Pharmacother. 2018, 103, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, B.H. Therapeutic Peptides for CNS Indications: Progress and Challenges. Bioorg. Med. Chem. 2018, 26, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Laxio Arenas, J.; Kaffy, J.; Ongeri, S. Peptides and Peptidomimetics as Inhibitors of Protein–Protein Interactions Involving β-Sheet Secondary Structures. Curr. Opin. Chem. Biol. 2019, 52, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, N.; Tang, H.; Wang, Y.; Liang, X.; Li, Y.; Davis, T.P.; Ke, P.C. Exploring Peptido-Nanocomposites in the Context of Amyloid Diseases. Angew. Chem. Int. Ed. 2024, 63, e202309958. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-N.; Liu, C.; Zhao, M.; Eisenberg, D.; Nowick, J.S. Amyloid β-Sheet Mimics That Antagonize Protein Aggregation and Reduce Amyloid Toxicity. Nat. Chem. 2012, 4, 927–933. [Google Scholar] [CrossRef]
- Maity, D. Inhibition of Amyloid Protein Aggregation Using Selected Peptidomimetics. ChemMedChem 2023, 18, e202200499. [Google Scholar] [CrossRef]
- Soto, C.; Sigurdsson, E.M.; Morelli, L.; Asok Kumar, R.; Castaño, E.M.; Frangione, B. β-Sheet Breaker Peptides Inhibit Fibrillogenesis in a Rat Brain Model of Amyloidosis: Implications for Alzheimer’s Therapy. Nat. Med. 1998, 4, 822–826. [Google Scholar] [CrossRef]
- Datki, Z.; Papp, R.; Zádori, D.; Soós, K.; Fülöp, L.; Juhász, A.; Laskay, G.; Hetényi, C.; Mihalik, E.; Zarándi, M.; et al. In Vitro Model of Neurotoxicity of Aβ 1-42 and Neuroprotection by a Pentapeptide: Irreversible Events during the First Hour. Neurobiol. Dis. 2004, 17, 507–515. [Google Scholar] [CrossRef]
- Tjernberg, L.O.; Näslund, J.; Lindqvist, F.; Johansson, J.; Karlström, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of -Amyloid Fibril Formation by a Pentapeptide Ligand. J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef]
- Fülöp, L.; Zarándi, M.; Datki, Z.; Soós, K.; Penke, B. β-Amyloid-Derived Pentapeptide RIIGL a Inhibits Aβ 1-42 Aggregation and Toxicity. Biochem. Biophys. Res. Commun. 2004, 324, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hetényi, C.; Szabó, Z.; Klement, É.; Datki, Z.; Körtvélyesi, T.; Zarándi, M.; Penke, B. Pentapeptide Amides Interfere with the Aggregation of β-Amyloid Peptide of Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2002, 292, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.J. Alzheimer’s Disease Plaques and Tangles: Cemeteries of a Pyrrhic Victory of the Immune Defence Network against Herpes Simplex Infection at the Expense of Complement and Inflammation-Mediated Neuronal Destruction. Neurochem. Int. 2011, 58, 301–320. [Google Scholar] [CrossRef]
- Gobron, S.; Monnerie, H.; Meiniel, R.; Creveaux, I.; Lehmann, W.; Lamalle, D.; Dastugue, B.; Meiniel, A. SCO-Spondin: A New Member of the Thrombospondin Family Secreted by the Subcommissural Organ Is a Candidate in the Modulation of Neuronal Aggregation. J. Cell Sci. 1996, 109, 1053–1061. [Google Scholar] [CrossRef]
- Monnerie, H.; Dastugue, B.; Meiniel, A. Effect of Synthetic Peptides Derived from SCO-Spondin Conserved Domains on Chick Cortical and Spinal-Cord Neurons in Cell Cultures. Cell Tissue Res. 1998, 293, 407–418. [Google Scholar] [CrossRef]
- Krishtal, J.; Metsla, K.; Bragina, O.; Tõugu, V.; Palumaa, P. Toxicity of Amyloid-β Peptides Varies Depending on Differentiation Route of SH-SY5Y Cells. J. Alzheimers Dis. 2019, 71, 879–887. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Shi, Z.; Fan, W.; Liu, H.; Deng, J.; Deng, J. Experimental Study on the Neurotoxic Effect of β-Amyloid on the Cytoskeleton of PC12 Cells. Int. J. Mol. Med. 2018, 41, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In Vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Kakinen, A.; Javed, I.; Davis, T.P.; Ke, P.C. In Vitro and in Vivo Models for Anti-Amyloidosis Nanomedicines. Nanoscale Horiz. 2021, 6, 95–119. [Google Scholar] [CrossRef]
- Guil, N. Molecular Approach to Micrometazoans. Are They Here, There and Everywhere? In Biogeography of Microscopic Organisms; Fontaneto, D., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 284–306. ISBN 978-0-521-76670-8. [Google Scholar]
- Snell, T.W. Rotifers as Models for the Biology of Aging. Int. Rev. Hydrobiol. 2014, 99, 84–95. [Google Scholar] [CrossRef]
- Rico-Martínez, R.; Arzate-Cárdenas, M.A.; Robles-Vargas, D.; Pérez-Legaspi, I.A.; Alvarado-Flores, J.; Santos-Medrano, G.E. Rotifers as Models in Toxicity Screening of Chemicals and Environmental Samples. Invertebrates—Experimental Models in Toxicity Screening, IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Pérez-Legaspi, I.A.; Rico-Martínez, R. Acute Toxicity Tests on Three Species of the Genus Lecane (Rotifera: Monogononta). In Rotifera IX; Sanoamuang, L., Segers, H., Shiel, R.J., Gulati, R.D., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 375–381. ISBN 978-94-010-3820-1. [Google Scholar]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Sinka, R.; Galik, B.; Galik-Olah, Z. Particle-Dependent Reproduction and Exogenic Biopolymer Secretion of Protozoa Co-Cultured Rotifers. Int. J. Biol. Macromol. 2022, 211, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Acs, E.; Balazs, E.; Sovany, T.; Csoka, I.; Zsuga, K.; Kalman, J.; Galik-Olah, Z. Exogenic Production of Bioactive Filamentous Biopolymer by Monogonant Rotifers. Ecotoxicol. Environ. Saf. 2021, 208, 111666. [Google Scholar] [CrossRef]
- Datki, Z.; Olah, Z.; Hortobagyi, T.; Macsai, L.; Zsuga, K.; Fulop, L.; Bozso, Z.; Galik, B.; Acs, E.; Foldi, A.; et al. Exceptional in Vivo Catabolism of Neurodegeneration-Related Aggregates. Acta Neuropathol. Commun. 2018, 6, 6. [Google Scholar] [CrossRef]
- Balazs, E.; Galik-Olah, Z.; Galik, B.; Bozso, Z.; Kalman, J.; Datki, Z. Neurodegeneration-Related Beta-Amyloid as Autocatabolism-Attenuator in a Micro-in Vivo System. IBRO Rep. 2020, 9, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Datki, Z.; Balazs, E.; Galik, B.; Sinka, R.; Zeitler, L.; Bozso, Z.; Kalman, J.; Hortobagyi, T.; Galik-Olah, Z. The Interacting Rotifer-Biopolymers Are Anti- and Disaggregating Agents for Human-Type Beta-Amyloid in Vitro. Int. J. Biol. Macromol. 2022, 201, 262–269. [Google Scholar] [CrossRef]
- Datki, Z.; Darula, Z.; Vedelek, V.; Hunyadi-Gulyas, E.; Dingmann, B.J.; Vedelek, B.; Kalman, J.; Urban, P.; Gyenesei, A.; Galik-Olah, Z.; et al. Biofilm Formation Initiating Rotifer-Specific Biopolymer and Its Predicted Components. Int. J. Biol. Macromol. 2023, 253, 127157. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, V.; Maurelia, F.; González, M.; Aguayo, J.; Caprile, T. SCO-Spondin, a Giant Matricellular Protein That Regulates Cerebrospinal Fluid Activity. Fluids Barriers CNS 2021, 18, 45. [Google Scholar] [CrossRef]
- Gobron, S.; Creveaux, I.; Meiniel, R.; Didier, R.; Dastugue, B.; Meiniel, A. SCO-Spondin Is Evolutionarily Conserved in the Central Nervous System of the Chordate Phylum. Neuroscience 1999, 88, 655–664. [Google Scholar] [CrossRef]
- Wójcik, P.; Berlicki, Ł. Peptide-Based Inhibitors of Protein–Protein Interactions. Bioorg. Med. Chem. Lett. 2016, 26, 707–713. [Google Scholar] [CrossRef]
- Oriá, A.P.; Lacerda, A.D.J.; Raposo, A.C.S.; Araújo, N.L.L.C.; Portela, R.; Mendonça, M.A.; Masmali, A.M. Comparison of Electrolyte Composition and Crystallization Patterns in Bird and Reptile Tears. Front. Vet. Sci. 2020, 7, 574. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.; Adams, D.L.; Tang, C.-M. Common Benzothiazole and Benzoxazole Fluorescent DNA Intercalators for Studying Alzheimer Aβ 1-42 and Prion Amyloid Peptides. BioTechniques 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Murube, J. Tear Crystallization Test: Two Centuries of History. Ocul. Surf. 2004, 2, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Jacob, R.F.; Mason, R.P. Quantifying Amyloid β-Peptide (Aβ) Aggregation Using the Congo Red-Aβ (CR–Aβ) Spectrophotometric Assay. Anal. Biochem. 1999, 266, 66–76. [Google Scholar] [CrossRef]
- Zayas-Santiago, A.; Díaz-García, A.; Nuñez-Rodríguez, R.; Inyushin, M. Accumulation of Amyloid Beta in Human Glioblastomas. Clin. Exp. Immunol. 2020, 202, 325–334. [Google Scholar] [CrossRef]
- Sulatsky, M.I.; Sulatskaya, A.I.; Povarova, O.I.; Antifeeva, I.A.; Kuznetsova, I.M.; Turoverov, K.K. Effect of the Fluorescent Probes ThT and ANS on the Mature Amyloid Fibrils. Prion 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an Amyloid Dye: Fibril Quantification, Optimal Concentration and Effect on Aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- Gupta, A.; Goyal, R. Amyloid Beta Plaque: A Culprit for Neurodegeneration. Acta Neurol. Belg. 2016, 116, 445–450. [Google Scholar] [CrossRef]
- Varga, E.; Juhász, G.; Bozsó, Z.; Penke, B.; Fülöp, L.; Szegedi, V. Amyloid-Β1-42 Disrupts Synaptic Plasticity by Altering Glutamate Recycling at the Synapse. J. Alzheimers Dis. 2015, 45, 449–456. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef]
- Shin, Y.J.; Evitts, K.M.; Jin, S.; Howard, C.; Sharp-Milgrom, M.; Schwarze-Taufiq, T.; Kinoshita, C.; Young, J.E.; Zheng, Y. Amyloid Beta Peptides (Aβ) from Alzheimer’s Disease Neuronal Secretome Induce Endothelial Activation in a Human Cerebral Microvessel Model. Neurobiol. Dis. 2023, 181, 106125. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of Amyloid-β Peptide across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Tachibana, M.; Kanekiyo, T.; Bu, G. Role of LRP1 in the Pathogenesis of Alzheimer’s Disease: Evidence from Clinical and Preclinical Studies. J. Lipid Res. 2017, 58, 1267–1281. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Z.; Ren, W.; Chen, L.; Xu, C.; Li, M.; Fan, S.; Xu, Y.; Chen, M.; Zheng, F.; et al. LDL Receptor-Related Protein 1 (LRP1), a Novel Target for Opening the Blood-Labyrinth Barrier (BLB). Signal Transduct. Target. Ther. 2022, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.H.; Doninck, K.V.; Mandigo, M.L.; Meselson, M. Degenerate Tetraploidy Was Established before Bdelloid Rotifer Families Diverged. Mol. Biol. Evol. 2009, 26, 375–383. [Google Scholar] [CrossRef]
- Kikuyama, S.; Toyoda, F.; Ohmiya, Y.; Matsuda, K.; Tanaka, S.; Hayashi, H. Sodefrin: A Female-Attracting Peptide Pheromone in Newt Cloacal Glands. Science 1995, 267, 1643–1645. [Google Scholar] [CrossRef]
- Van Bocxlaer, I.; Maex, M.; Treer, D.; Janssenswillen, S.; Janssens, R.; Vandebergh, W.; Proost, P.; Bossuyt, F. Beyond Sodefrin: Evidence for a Multi-Component Pheromone System in the Model Newt Cynops Pyrrhogaster (Salamandridae). Sci. Rep. 2016, 6, 21880. [Google Scholar] [CrossRef]
- Taneja, V.; Verma, M.; Vats, A. Toxic Species in Amyloid Disorders: Oligomers or Mature Fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138. [Google Scholar] [CrossRef]
- Cascella, R.; Bigi, A.; Cremades, N.; Cecchi, C. Effects of Oligomer Toxicity, Fibril Toxicity and Fibril Spreading in Synucleinopathies. Cell. Mol. Life Sci. 2022, 79, 174. [Google Scholar] [CrossRef]
- Schaeffer, V.; Meyer, L.; Patte-Mensah, C.; Eckert, A.; Mensah-Nyagan, A.G. Dose-Dependent and Sequence-Sensitive Effects of Amyloid-β Peptide on Neurosteroidogenesis in Human Neuroblastoma Cells. Neurochem. Int. 2008, 52, 948–955. [Google Scholar] [CrossRef]
- Sondag, C.M.; Dhawan, G.; Combs, C.K. Beta Amyloid Oligomers and Fibrils Stimulate Differential Activation of Primary Microglia. J. Neuroinflam. 2009, 6, 1. [Google Scholar] [CrossRef]
- Fülöp, L.; Zarándi, M.; Soós, K.; Penke, B. Self-Assembly of Alzheimer’s Disease-Related Amyloid Peptides into Highly Ordered Nanostructures. Nanopages 2006, 1, 69–83. [Google Scholar] [CrossRef]
- Pellarin, R.; Caflisch, A. Interpreting the Aggregation Kinetics of Amyloid Peptides. J. Mol. Biol. 2006, 360, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Tanaka, Y.; Kiyono, M.; Chino, M.; Chikuma, T.; Hoshi, K.; Ikeshima, H. Dependence pH and Proposed Mechanism for Aggregation of Alzheimer’s Disease-Related Amyloid-β(1–42) Protein. J. Mol. Struct. 2015, 1094, 109–117. [Google Scholar] [CrossRef]
- Balupuri, A.; Choi, K.-E.; Kang, N.S. Aggregation Mechanism of Alzheimer’s Amyloid β-Peptide Mediated by α-Strand/α-Sheet Structure. Int. J. Mol. Sci. 2020, 21, 1094. [Google Scholar] [CrossRef]
- Yu, M.; Ryan, T.M.; Ellis, S.; Bush, A.I.; Triccas, J.A.; Rutledge, P.J.; Todd, M.H. Neuroprotective Peptide-Macrocycle Conjugates Reveal Complex Structure-Activity Relationships in Their Interactions with Amyloid β. Metallomics 2014, 6, 1931–1940. [Google Scholar] [CrossRef]
- Zarándi, M.; Soós, K.; Fülöp, L.; Bozsó, Z.; Datki, Z.; Tóth, G.K.; Penke, B. Synthesis of Aβ[1-42] and Its Derivatives with Improved Efficiency. J. Pept. Sci. 2007, 13, 94–99. [Google Scholar] [CrossRef]
- Gera, J.; Szögi, T.; Bozsó, Z.; Fülöp, L.; Barrera, E.E.; Rodriguez, A.M.; Méndez, L.; Delpiccolo, C.M.L.; Mata, E.G.; Cioffi, F.; et al. Searching for Improved Mimetic Peptides Inhibitors Preventing Conformational Transition of Amyloid-Β42 Monomer. Bioorganic Chem. 2018, 81, 211–221. [Google Scholar] [CrossRef]
- Solé, V.A. Untersuchung Iiber Die Bewegung Der Teilchen Im Stagogramm Und Influenzstagogramm. Kolloid-Z. 1957, 151, 55–62. [Google Scholar] [CrossRef]
- Buell, A.K.; Dobson, C.M.; Knowles, T.P.J.; Welland, M.E. Interactions between Amyloidophilic Dyes and Their Relevance to Studies of Amyloid Inhibitors. Biophys. J. 2010, 99, 3492–3497. [Google Scholar] [CrossRef]
- Dinh, H.; Kovács, Z.Z.A.; Kis, M.; Kupecz, K.; Sejben, A.; Szűcs, G.; Márványkövi, F.; Siska, A.; Freiwan, M.; Pósa, S.P.; et al. Role of the Kisspeptin-KISS1R Axis in the Pathogenesis of Chronic Kidney Disease and Uremic Cardiomyopathy. GeroScience 2023, 46, 2463–2488. [Google Scholar] [CrossRef] [PubMed]
- Bozso, Z.; Penke, B.; Simon, D.; Laczkó, I.; Juhász, G.; Szegedi, V.; Kasza, Á.; Soós, K.; Hetényi, A.; Wéber, E.; et al. Controlled in Situ Preparation of Aβ(1–42) Oligomers from the Isopeptide “Iso-Aβ(1–42)”, Physicochemical and Biological Characterization. Peptides 2010, 31, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Borbély, E.; Horváth, J.; Furdan, S.; Bozsó, Z.; Penke, B.; Fülöp, L. Simultaneous Changes of Spatial Memory and Spine Density after Intrahippocampal Administration of Fibrillar A β1–42 to the Rat Brain. BioMed Res. Int. 2014, 2014, 345305. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Datki, Z.; Sinka, R.; Dingmann, B.J.; Galik, B.; Szabo, A.; Galik-Olah, Z.; Toth, G.K.; Bozso, Z. Protective Effect of a Hexapeptide Derived from Rotifer-Specific SCO-Spondin Against Beta-Amyloid Toxicity. Int. J. Mol. Sci. 2025, 26, 5109. https://doi.org/10.3390/ijms26115109
Datki Z, Sinka R, Dingmann BJ, Galik B, Szabo A, Galik-Olah Z, Toth GK, Bozso Z. Protective Effect of a Hexapeptide Derived from Rotifer-Specific SCO-Spondin Against Beta-Amyloid Toxicity. International Journal of Molecular Sciences. 2025; 26(11):5109. https://doi.org/10.3390/ijms26115109
Chicago/Turabian StyleDatki, Zsolt, Rita Sinka, Brian J. Dingmann, Bence Galik, Antal Szabo, Zita Galik-Olah, Gabor K. Toth, and Zsolt Bozso. 2025. "Protective Effect of a Hexapeptide Derived from Rotifer-Specific SCO-Spondin Against Beta-Amyloid Toxicity" International Journal of Molecular Sciences 26, no. 11: 5109. https://doi.org/10.3390/ijms26115109
APA StyleDatki, Z., Sinka, R., Dingmann, B. J., Galik, B., Szabo, A., Galik-Olah, Z., Toth, G. K., & Bozso, Z. (2025). Protective Effect of a Hexapeptide Derived from Rotifer-Specific SCO-Spondin Against Beta-Amyloid Toxicity. International Journal of Molecular Sciences, 26(11), 5109. https://doi.org/10.3390/ijms26115109





