Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women
Abstract
1. Introduction
2. Synthesis and Menopause-Related Changes of Sex Hormones
3. Menopausal Symptoms and Menopause-Related CVD
4. Sex Differences in Vascular Function
5. Estrogen Receptors (ERs) in the Vasculature
6. Cardiovascular Effects of E2
6.1. Effects of E2 on ECs
6.2. Effects of E2 on VSMCs
6.3. E2 and ECM
7. E2-Based MHT and CVD
7.1. MHT Type and CVD
7.2. MHT Dose and CVD
7.3. MHT Route of Administration and CVD
7.4. MHT and Menopausal Changes in ERs
7.5. MHT Timing and Duration
7.6. MHT and Preexisting Conditions in Post-MW
8. Influence of Hormonal Environment
8.1. MHT and Gonadotropins
8.2. Ovarian Versus Locally Produced Sex Hormones
8.3. MHT and Sex Hormone-Binding Globulin (SHBG)
9. Vascular Effects of Progesterone (P4)
10. Vascular Effects of Testosterone (T)
11. Discussion and Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar]
- Global Cardiovascular Risk Consortium; Magnussen, C.; Ojeda, F.M.; Leong, D.P.; Alegre-Diaz, J.; Amouyel, P.; Aviles-Santa, L.; De Bacquer, D.; Ballantyne, C.M.; Bernabé-Ortiz, A.; et al. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar]
- Vaura, F.; Palmu, J.; Aittokallio, J.; Kauko, A.; Niiranen, T. Genetic, Molecular, and Cellular Determinants of Sex-Specific Cardiovascular Traits. Circ. Res. 2022, 130, 611–631. [Google Scholar] [CrossRef]
- Vaidakis, N. Conceptual controversies regarding the terms Gender and Sex. Psychiatriki 2020, 31, 271–274. [Google Scholar] [CrossRef]
- Ratnu, V.S.; Emami, M.R.; Bredy, T.W. Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J. Neurosci. Res. 2017, 95, 301–310. [Google Scholar] [CrossRef]
- Liszewski, W.; Peebles, J.K.; Yeung, H.; Arron, S. Persons of Nonbinary Gender—Awareness, Visibility, and Health Disparities. N. Engl. J. Med. 2018, 379, 2391–2393. [Google Scholar] [CrossRef]
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef]
- Ober, C.; Loisel, D.A.; Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008, 9, 911–922. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Thurston, R.C.; El Khoudary, S.R. Trajectories of Blood Pressure in Midlife Women: Does Menopause Matter? Circ. Res. 2022, 130, 312–322. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am. J. Med. 2005, 118 (Suppl. 12B), 64–73. [Google Scholar] [CrossRef]
- Hyvärinen, M.; Karvanen, J.; Juppi, H.-K.; Karppinen, J.E.; Tammelin, T.H.; Kovanen, V.; Aukee, P.; Sipilä, S.; Rantalainen, T.; Laakkonen, E.K. Menopausal symptoms and cardiometabolic risk factors in middle-aged women: A cross-sectional and longitudinal study with 4-year follow-up. Maturitas 2023, 174, 39–47. [Google Scholar] [CrossRef]
- Zhu, B.T.; Han, G.Z.; Shim, J.Y.; Wen, Y.; Jiang, X.R. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 2006, 147, 4132–4150. [Google Scholar] [CrossRef]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8 (Suppl. 1), 3–63. [Google Scholar] [CrossRef]
- Smiley, D.A.; Khalil, R.A. Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Curr. Med. Chem. 2009, 16, 1863–1887. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Chaikittisilpa, S.; Sriprasert, I.; Rafatnia, A.; Nadadur, M.; Mishell, D.R., Jr. Circulating androgen levels before and after oophorectomy in premenopausal and postmenopausal women. Climacteric 2019, 22, 169–174. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Shafrir, A.L.; Rice, M.; Hankinson, S.E.; Eliassen, A.H.; Tworoger, S.S.; Narod, S.A. The relationship between bilateral oophorectomy and plasma hormone levels in postmenopausal women. Horm. Cancer 2015, 6, 54–63. [Google Scholar] [CrossRef]
- Hemsell, D.L.; Grodin, J.M.; Brenner, P.F.; Siiteri, P.K.; MacDonald, P.C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J. Clin. Endocrinol. Metab. 1974, 38, 476–479. [Google Scholar] [CrossRef]
- Labrie, F.; Martel, C.; Balser, J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: Role of the ovary? Menopause 2011, 18, 30–43. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Rothman, M.S.; Carlson, N.E.; Xu, M.; Wang, C.; Swerdloff, R.; Lee, P.; Goh, V.H.; Ridgway, E.C.; Wierman, M.E. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids 2011, 76, 177–182. [Google Scholar] [CrossRef]
- Nunes, E.; Gallardo, E.; Morgado-Nunes, S.; Fonseca-Moutinho, J. Steroid hormone levels in postmenopausal hysterectomised women with and without ovarian conservation: The continuous endocrine function of the ovaries. J. Obstet. Gynaecol. 2023, 43, 2141618. [Google Scholar] [CrossRef]
- Mai, P.L.; Miller, A.; Black, A.; Falk, R.T.; Boggess, J.F.; Tucker, K.; Stuckey, A.R.; Rodriguez, G.C.; Wong, C.; Amatruda, T.T.; et al. Effect of risk-reducing salpingo-oophorectomy on sex steroid hormone serum levels among postmenopausal women: An NRG Oncology/Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 2022, 227, 61.e1–61.e18. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; Kritz-Silverstein, D.; von Muhlen, D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2000, 85, 645–651. [Google Scholar]
- Rannevik, G.; Jeppsson, S.; Johnell, O.; Bjerre, B.; Laurell-Borulf, Y.; Svanberg, L. A longitudinal study of the perimenopausal transition: Altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 1995, 21, 103–113. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Cui, J.; Dennerstein, L.; Hopper, J.L. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J. Clin. Endocrinol. Metab. 2000, 85, 2832–2838. [Google Scholar] [CrossRef]
- Thurston, R.C.; Chang, Y.; Kline, C.E.; Swanson, L.M.; El Khoudary, S.R.; Jackson, E.A.; Derby, C.A. Trajectories of Sleep Over Midlife and Incident Cardiovascular Disease Events in the Study of Women’s Health Across the Nation. Circulation 2024, 149, 545–555. [Google Scholar] [CrossRef]
- Avis, N.E.; Crawford, S.L.; Greendale, G.; Bromberger, J.T.; Everson-Rose, S.A.; Gold, E.B.; Hess, R.; Joffe, H.; Kravitz, H.M.; Tepper, P.G.; et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 2015, 175, 531–539. [Google Scholar] [CrossRef]
- Gupta, P.; Harte, A.; Sturdee, D.W.; Sharma, A.; Barnett, A.H.; Kumar, S.; McTernan, P.G. Effects of menopausal status on circulating calcitonin gene-related peptide and adipokines: Implications for insulin resistance and cardiovascular risks. Climacteric 2008, 11, 364–372. [Google Scholar] [CrossRef]
- Herber-Gast, G.; Brown, W.J.; Mishra, G.D. Hot flushes and night sweats are associated with coronary heart disease risk in midlife: A longitudinal study. BJOG 2015, 122, 1560–1567. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Colpani, V.; Kunutsor, S.; Chowdhury, S.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0157417. [Google Scholar] [CrossRef]
- Randolph, J.F., Jr.; Sowers, M.; Bondarenko, I.; Gold, E.B.; Greendale, G.A.; Bromberger, J.T.; Brockwell, S.E.; Matthews, K.A. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J. Clin. Endocrinol. Metab. 2005, 90, 6106–6112. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, R.; Li, C.; Tao, M. Sleep disorder, an independent risk associated with arterial stiffness in menopause. Sci. Rep. 2017, 7, 1904. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Matthews, K.A.; Cursio, J.F.; Hollenberg, S.M.; Sutton-Tyrrell, K.; Bromberger, J.T.; Everson-Rose, S.A. Depressive symptoms are related to progression of coronary calcium in midlife women: The Study of Women’s Health Across the Nation (SWAN) Heart Study. Am. Heart J. 2011, 161, 1186–1191.e1. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.-F.; Dobson, A.J.; Pandeya, N.; Anderson, D.J.; Kuh, D.; Hardy, R.; Brunner, E.J.; Avis, N.E.; Gold, E.B.; et al. Vasomotor menopausal symptoms and risk of cardiovascular disease: A pooled analysis of six prospective studies. Am. J. Obstet. Gynecol. 2020, 223, 898.e1–898.e16. [Google Scholar] [CrossRef]
- Thurston, R.C.; Vlachos, H.E.A.; Derby, C.A.; Jackson, E.A.; Brooks, M.M.; Matthews, K.A.; Harlow, S.; Joffe, H.; El Khoudary, S.R. Menopausal Vasomotor Symptoms and Risk of Incident Cardiovascular Disease Events in SWAN. J. Am. Heart Assoc. 2021, 10, e017416. [Google Scholar] [CrossRef]
- Armeni, A.; Anagnostis, P.; Armeni, E.; Mili, N.; Goulis, D.; Lambrinoudaki, I. Vasomotor symptoms and risk of cardiovascular disease in peri- and postmenopausal women: A systematic review and meta-analysis. Maturitas 2023, 171, 13–20. [Google Scholar] [CrossRef]
- Hitchcock, C.L.; Elliott, T.G.; Norman, E.G.; Stajic, V.; Teede, H.; Prior, J.C. Hot flushes and night sweats differ in associations with cardiovascular markers in healthy early postmenopausal women. Menopause 2012, 19, 1208–1214. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Santoro, N.; Chen, H.-Y.; Tepper, P.G.; Brooks, M.M.; Thurston, R.C.; Janssen, I.; Harlow, S.D.; Barinas-Mitchell, E.; Selzer, F.; et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur. J. Prev. Cardiol. 2016, 23, 694–703. [Google Scholar] [CrossRef]
- Ji, H.; Kwan, A.C.; Chen, M.T.; Ouyang, D.; Ebinger, J.E.; Bell, S.P.; Niiranen, T.J.; Bello, N.A.; Cheng, S. Sex Differences in Myocardial and Vascular Aging. Circ. Res. 2022, 130, 566–577. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- George, J.; Rapsomaniki, E.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; Herrett, E.; Smeeth, L.; Timmis, A.; Hemingway, H. How Does Cardiovascular Disease First Present in Women and Men? Incidence of 12 Cardiovascular Diseases in a Contemporary Cohort of 1,937,360 People. Circulation 2015, 132, 1320–1328. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- da Silva, J.S.; Montagnoli, T.L.; de Sa, M.P.L.; Zapata-Sudo, G. Heart Failure in Menopause: Treatment and New Approaches. Int. J. Mol. Sci. 2022, 23, 15140. [Google Scholar] [CrossRef]
- Willemars, M.M.A.; Nabben, M.; Verdonschot, J.A.J.; Hoes, M.F. Evaluation of the Interaction of Sex Hormones and Cardiovascular Function and Health. Curr. Heart Fail. Rep. 2022, 19, 200–212. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.; Fauser, B.C.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol 2016, 1, 767–776. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.-F.; Dobson, A.J.; Pandeya, N.; Giles, G.G.; Bruinsma, F.; Brunner, E.J.; Kuh, D.; Hardy, R.; Avis, N.E.; et al. Age at natural menopause and risk of incident cardiovascular disease: A pooled analysis of individual patient data. Lancet Public Health 2019, 4, e553–e564. [Google Scholar] [CrossRef]
- Bots, S.H.; Peters, S.A.E.; Woodward, M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2017, 2, e000298. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Shaw, L.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Pepine, C.J.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006, 47, S21–S29. [Google Scholar]
- Epstein, A.M.; Weissman, J.S.; Schneider, E.C.; Gatsonis, C.; Leape, L.L.; Piana, R.N. Race and gender disparities in rates of cardiac revascularization: Do they reflect appropriate use of procedures or problems in quality of care? Med. Care 2003, 41, 1240–1255. [Google Scholar] [CrossRef]
- Rao, S.V.; Kaul, P.; Newby, L.K.; Lincoff, A.M.; Hochman, J.; Harrington, R.A.; Mark, D.B.; Peterson, E.D. Poverty, process of care, and outcome in acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 1948–1954. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Dubey, R.K.; Oparil, S.; Imthurn, B.; Jackson, E.K. Sex hormones and hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Nwankwo, T.; Yoon, S.S.; Burt, V.; Gu, Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief 2013, 133, 1–8. [Google Scholar]
- Ma, H.; Liu, F.; Li, J.; Chen, J.; Cao, J.; Chen, S.; Liu, X.; Yang, X.; Huang, K.; Shen, C.; et al. Sex Differences in Associations Between Socioeconomic Status and Incident Hypertension Among Chinese Adults. Hypertension 2023, 80, 783–791. [Google Scholar] [CrossRef]
- Mancusi, C.; Gerdts, E.; De Simone, G.; Abdelhai, Y.M.; Lønnebakken, M.T.; Boman, K.; Wachtell, K.; Dahlöf, B.; Devereux, R.B. Impact of isolated systolic hypertension on normalization of left ventricular structure during antihypertensive treatment (the LIFE study). Blood Press. 2014, 23, 206–212. [Google Scholar] [CrossRef][Green Version]
- Picone, D.S.; Stoneman, E.; Cremer, A.; Schultz, M.G.; Otahal, P.; Hughes, A.D.; Black, J.A.; Bos, W.J.; Chen, C.-H.; Cheng, H.-M.; et al. Sex Differences in Blood Pressure and Potential Implications for Cardiovascular Risk Management. Hypertension 2022, 80, 316–324. [Google Scholar] [CrossRef]
- Ji, H.; Niiranen, T.J.; Rader, F.; Henglin, M.; Kim, A.; Ebinger, J.E.; Claggett, B.; Merz, C.N.B.; Cheng, S. Sex Differences in Blood Pressure Associations With Cardiovascular Outcomes. Circulation 2021, 143, 761–763. [Google Scholar] [CrossRef]
- Wei, Y.C.; George, N.I.; Chang, C.W.; Hicks, K.A. Assessing Sex Differences in the Risk of Cardiovascular Disease and Mortality per Increment in Systolic Blood Pressure: A Systematic Review and Meta-Analysis of Follow-Up Studies in the United States. PLoS ONE 2017, 12, e0170218. [Google Scholar] [CrossRef]
- Roeters van Lennep, J.E.; Heida, K.Y.; Bots, M.L.; Hoek, A. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2016, 23, 178–186. [Google Scholar] [CrossRef]
- Xu, Z.; Chung, H.F.; Dobson, A.J.; Wilson, L.F.; Hickey, M.; Mishra, G.D. Menopause, hysterectomy, menopausal hormone therapy and cause-specific mortality: Cohort study of UK Biobank participants. Hum. Reprod. 2022, 37, 2175–2185. [Google Scholar] [CrossRef]
- Dam, V.; van der Schouw, Y.T.; Onland-Moret, N.C.; Groenwold, R.H.H.; Peters, S.A.E.; Burgess, S.; Wood, A.M.; Chirlaque, M.-D.; Moons, K.G.M.; Oliver-Williams, C.; et al. Association of menopausal characteristics and risk of coronary heart disease: A pan-European case-cohort analysis. Int. J. Epidemiol. 2019, 48, 1275–1285. [Google Scholar] [CrossRef]
- Zhu, D.; Chung, H.-F.; Dobson, A.J.; Pandeya, N.; Brunner, E.J.; Kuh, D.; Greenwood, D.C.; Hardy, R.; Cade, J.E.; Giles, G.G.; et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: Pooled analysis of individual data from 10 international studies. Hum. Reprod. 2020, 35, 1933–1943. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Honigberg, M.; Wang, D.; Parekh, J.K.; Bielawski, K.; Courchesne, P.; Larson, M.D.; Levy, D.; Murabito, J.M.; Ho, J.E.; et al. Protein Biomarkers of Early Menopause and Incident Cardiovascular Disease. J. Am. Heart Assoc. 2023, 12, e028849. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Finneran, P.; Klarin, D.; Bhatt, D.L.; Januzzi, J.L., Jr.; Scott, N.S.; Natarajan, P. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA 2019, 322, 2411–2421. [Google Scholar] [CrossRef]
- Matthews, C.A. Management Strategies for the Ovaries at the Time of Hysterectomy for Benign Disease. Obstet. Gynecol. Clin. North. Am. 2016, 43, 539–549. [Google Scholar] [CrossRef]
- Fattet, A.-J.; Toupance, S.; Thornton, S.N.; Monnin, N.; Guéant, J.-L.; Benetos, A.; Koscinski, I. Telomere length in granulosa cells and leukocytes: A potential marker of female fertility? A systematic review of the literature. J. Ovarian Res. 2020, 13, 96. [Google Scholar] [CrossRef]
- Ollila, M.-M.; Arffman, R.K.; Korhonen, E.; Morin-Papunen, L.; Franks, S.; Junttila, J.; Piltonen, T.T. Women with PCOS have an increased risk for cardiovascular disease regardless of diagnostic criteria-a prospective population-based cohort study. Eur. J. Endocrinol. 2023, 189, 96–105. [Google Scholar] [CrossRef]
- Eissa, M.A.; Gohar, E.Y. Aromatase enzyme: Paving the way for exploring aromatization for cardio-renal protection. Biomed. Pharmacother. 2023, 168, 115832. [Google Scholar] [CrossRef]
- Lim, J.; Hashemian, M.; Blechter, B.; Roger, V.L.; Wong, J.Y.Y. Pre-diagnostic free androgen and estradiol levels influence heart failure risk in both women and men: A prospective cohort study in the UK Biobank. Eur. J. Heart Fail. 2024, 26, 540–550. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Postigo, A.; Martinez-Selles, M. Sex Influence on Heart Failure Prognosis. Front. Cardiovasc. Med. 2020, 7, 616273. [Google Scholar] [CrossRef]
- Frost, A.E.; Badesch, D.B.; Barst, R.J.; Benza, R.L.; Elliott, C.G.; Farber, H.W.; Krichman, A.; Liou, T.G.; Raskob, G.E.; Wason, P.; et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US Contemporary Registries. Chest 2010, 139, 128–137. [Google Scholar] [CrossRef]
- Batton, K.A.; Austin, C.O.; Bruno, K.A.; Burger, C.D.; Shapiro, B.P.; Fairweather, D. Sex differences in pulmonary arterial hypertension: Role of infection and autoimmunity in the pathogenesis of disease. Biol. Sex. Differ. 2018, 9, 15. [Google Scholar] [CrossRef]
- Kumar, A.; McCullough, L. Cerebrovascular disease in women. Ther. Adv. Neurol. Disord. 2021, 14, 1756286420985237. [Google Scholar] [CrossRef]
- Leppert, M.H.; Burke, J.F.; Lisabeth, L.D.; Madsen, T.E.; Kleindorfer, D.O.; Sillau, S.; Schwamm, L.H.; Daugherty, S.L.; Bradley, C.J.; Ho, P.M.; et al. Systematic Review of Sex Differences in Ischemic Strokes Among Young Adults: Are Young Women Disproportionately at Risk? Stroke 2022, 53, 319–327. [Google Scholar] [CrossRef]
- Ekker, M.S.; Verhoeven, J.I.; Vaartjes, I.; van Nieuwenhuizen, K.M.; Klijn, C.J.M.; de Leeuw, F.E. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology 2019, 92, e2444–e2454. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef]
- de Vries, T.; Boucherie, D.M.; Chan, K.Y.; Rubio-Beltran, E.; Labastida-Ramirez, A.; Labruijere, S.; Gupta, S.; van den Bogaerdt, A.; Vincent, A.; Dammers, R.; et al. Sex differences in CGRP-induced vasodilation of human middle meningeal arteries but not human coronary arteries: Implications for migraine. Cephalalgia 2024, 44, 3331024241254088. [Google Scholar] [CrossRef]
- Cox, D.A.; Cohen, M.L. Selective enhancement of 5-hydroxytryptamine-induced contraction of porcine coronary artery by oxidized low-density lipoprotein. J. Pharmacol. Exp. Ther. 1996, 276, 1095–1103. [Google Scholar] [CrossRef]
- Stallone, J.N.; Crofton, J.T.; Share, L. Sexual dimorphism in vasopressin-induced contraction of rat aorta. Am. J. Physiol. 1991, 260, H453–H458. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef]
- Kingma, J.G., Jr.; Laher, I. Effect of endothelin on sex-dependent regulation of tone in coronary resistance vessels. Biochem. Biophys. Res. Commun. 2021, 540, 56–60. [Google Scholar] [CrossRef]
- Chan, M.V.; Bubb, K.J.; Noyce, A.; Villar, I.C.; Duchene, J.; Hobbs, A.J.; Scotland, R.S.; Ahluwalia, A. Distinct endothelial pathways underlie sexual dimorphism in vascular auto-regulation. Br. J. Pharmacol. 2012, 167, 805–817. [Google Scholar] [CrossRef]
- Moreau, K.L.; Hildreth, K.L.; Meditz, A.L.; Deane, K.D.; Kohrt, W.M. Endothelial function is impaired across the stages of the menopause transition in healthy women. J. Clin. Endocrinol. Metab. 2012, 97, 4692–4700. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pechlaner, R.; Cai, J.; Yuan, H.; Huang, Z.; Yang, G.; Wang, J.; Chen, Z.; Kiechl, S.; Xu, Q. Trajectories of Age-Related Arterial Stiffness in Chinese Men and Women. J. Am. Coll. Cardiol. 2020, 75, 870–880. [Google Scholar] [CrossRef]
- Blackwell, J.A.; Silva, J.F.; Louis, E.M.; Savu, A.; Largent-Milnes, T.M.; Brooks, H.L.; Pires, P.W. Cerebral arteriolar and neurovascular dysfunction after chemically induced menopause in mice. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H845–H860. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Denger, S.; Reid, G.; Gannon, F. Upstream open reading frames regulate the translation of the multiple mRNA variants of the estrogen receptor alpha. J. Biol. Chem. 2002, 277, 37131–37138. [Google Scholar] [CrossRef]
- Scobie, G.A.; Macpherson, S.; Millar, M.R.; Groome, N.P.; Romana, P.G.; Saunders, P.T. Human oestrogen receptors: Differential expression of ER alpha and beta and the identification of ER beta variants. Steroids 2002, 67, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.A. Estrogen receptor-beta: Recent lessons from in vivo studies. Mol. Endocrinol. 2007, 21, 1–13. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Ström, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Menazza, S.; Murphy, E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef]
- Gerhard, M.; Ganz, P. How do we explain the clinical benefits of estrogen? From bedside to bench. Circulation 1995, 92, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Dan, P.; Cheung, J.C.; Scriven, D.R.; Moore, E.D. Epitope-dependent localization of estrogen receptor-alpha, but not -beta, in en face arterial endothelium. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1295–H1306. [Google Scholar] [CrossRef] [PubMed]
- Razandi, M.; Oh, P.; Pedram, A.; Schnitzer, J.; Levin, E.R. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol. Endocrinol. 2002, 16, 100–115. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 2006, 7, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Rubanyi, G.M.; Freay, A.D.; Kauser, K.; Sukovich, D.; Burton, G.; Lubahn, D.B.; Couse, J.F.; Curtis, S.W.; Korach, K.S. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J. Clin. Investig. 1997, 99, 2429–2437. [Google Scholar] [CrossRef]
- Mendelsohn, M.E. Genomic and nongenomic effects of estrogen in the vasculature. Am. J. Cardiol. 2002, 90, 3F–6F. [Google Scholar] [CrossRef]
- Kim-Schulze, S.; McGowan, K.A.; Hubchak, S.C.; Cid, M.C.; Martin, M.B.; Kleinman, H.K.; Greene, G.L.; Schnaper, H.W. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation 1996, 94, 1402–1407. [Google Scholar] [CrossRef]
- Hodges, Y.K.; Tung, L.; Yan, X.D.; Graham, J.D.; Horwitz, K.B.; Horwitz, L.D. Estrogen receptors alpha and beta: Prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation 2000, 101, 1792–1798. [Google Scholar] [CrossRef]
- Andersson, C.; Lydrup, M.L.; Ferno, M.; Idvall, I.; Gustafsson, J.; Nilsson, B.O. Immunocytochemical demonstration of oestrogen receptor beta in blood vessels of the female rat. J. Endocrinol. 2001, 169, 241–247. [Google Scholar] [CrossRef]
- Pare, G.; Krust, A.; Karas, R.H.; Dupont, S.; Aronovitz, M.; Chambon, P.; Mendelsohn, M.E. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ. Res. 2002, 90, 1087–1092. [Google Scholar] [CrossRef]
- Jeanes, H.L.; Tabor, C.; Black, D.; Ederveen, A.; Gray, G.A. Oestrogen-mediated cardioprotection following ischaemia and reperfusion is mimicked by an oestrogen receptor (ER)alpha agonist and unaffected by an ER beta antagonist. J. Endocrinol. 2008, 197, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Ghisletti, S.; Meda, C.; Maggi, A.; Vegeto, E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell Biol. 2005, 25, 2957–2968. [Google Scholar] [CrossRef]
- Meng, Q.; Bi, Y.; Feng, H.; Ding, X.; Zhang, S.; Chen, Q.; Wang, L.; Zhang, Q.; Li, Y.; Tong, H.; et al. Activation of estrogen receptor alpha inhibits TLR4 signaling in macrophages and alleviates the instability of atherosclerotic plaques in the postmenopausal stage. Int. Immunopharmacol. 2023, 116, 109825. [Google Scholar] [CrossRef]
- Meng, Q.; Li, Y.; Ji, T.; Chao, Y.; Li, J.; Fu, Y.; Wang, S.; Chen, Q.; Chen, W.; Huang, F.; et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor alpha-mediated autophagy. J. Adv. Res. 2020, 28, 149–164. [Google Scholar] [CrossRef]
- Christian, R.C.; Liu, P.Y.; Harrington, S.; Ruan, M.; Miller, V.M.; Fitzpatrick, L.A. Intimal estrogen receptor (ER)beta, but not ERalpha expression, is correlated with coronary calcification and atherosclerosis in pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 2006, 91, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Kim, S.K.; Karas, R.H.; Kuiper, G.G.; Gustafsson, J.A.; Mendelsohn, M.E. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ. Res. 1998, 83, 224–229. [Google Scholar] [CrossRef]
- Xing, D.; Feng, W.; Miller, A.P.; Weathington, N.M.; Chen, Y.-F.; Novak, L.; Blalock, J.E.; Oparil, S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2607–H2612. [Google Scholar] [CrossRef] [PubMed]
- Hodges, Y.K.; Richer, J.K.; Horwitz, K.B.; Horwitz, L.D. Variant estrogen and progesterone receptor messages in human vascular smooth muscle. Circulation 1999, 99, 2688–2693. [Google Scholar] [CrossRef]
- Saito, K.; Cui, H. Estrogen Receptor Alpha Splice Variants, Post-Translational Modifications, and Their Physiological Functions. Cells 2023, 12, 895. [Google Scholar] [CrossRef]
- Garcia Pedrero, J.M.; Zuazua, P.; Martinez-Campa, C.; Lazo, P.S.; Ramos, S. The naturally occurring variant of estrogen receptor (ER) ERDeltaE7 suppresses estrogen-dependent transcriptional activation by both wild-type ERalpha and ERbeta. Endocrinology 2003, 144, 2967–2976. [Google Scholar] [CrossRef]
- Perkins, M.S.; Louw-du Toit, R.; Africander, D. A comparative characterization of estrogens used in hormone therapy via estrogen receptor (ER)-alpha and -beta. J. Steroid Biochem. Mol. Biol. 2017, 174, 27–39. [Google Scholar] [CrossRef]
- Rich, R.L.; Hoth, L.R.; Geoghegan, K.F.; Brown, T.A.; LeMotte, P.K.; Simons, S.P.; Hensley, P.; Myszka, D.G. Kinetic analysis of estrogen receptor/ligand interactions. Proc. Natl. Acad. Sci. USA 2002, 99, 8562–8567. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Ivory, A.; Greene, A.S. Distinct roles of estrone and estradiol in endothelial colony-forming cells. Physiol. Rep. 2023, 11, e15818. [Google Scholar] [CrossRef] [PubMed]
- Visniauskas, B.; Kilanowski-Doroh, I.; Ogola, B.O.; Mcnally, A.B.; Horton, A.C.; Sugi, A.I.; Lindsey, S.H. Estrogen-mediated mechanisms in hypertension and other cardiovascular diseases. J. Hum. Hypertens. 2023, 37, 609–618. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef]
- Haas, E.; Meyer, M.R.; Schurr, U.; Bhattacharya, I.; Minotti, R.; Nguyen, H.H.; Heigl, A.; Lachat, M.; Genoni, M.; Barton, M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension 2007, 49, 1358–1363. [Google Scholar] [CrossRef]
- Ogola, B.O.; Abshire, C.M.; Visniauskas, B.; Kiley, J.X.; Horton, A.C.; Clark-Patterson, G.L.; Kilanowski-Doroh, I.; Diaz, Z.; Bicego, A.N.; McNally, A.B.; et al. Sex differences in vascular aging and impact of GPER deletion. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H336–H349. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Wang, X.; Zhang, S.; Li, Z.; Liu, J. Improvement of Cardiovascular Function in Aging Females by the Prolonged Activation of G Protein-Coupled Estrogen Receptor. J. Cardiovasc. Transl. Res. 2023, 16, 371–381. [Google Scholar] [CrossRef]
- Gohar, E.Y. G protein-coupled estrogen receptor 1 as a novel regulator of blood pressure. Am. J. Physiol. Renal Physiol. 2020, 319, F612–F617. [Google Scholar] [CrossRef] [PubMed]
- Sbert-Roig, M.; Bauzá-Thorbrügge, M.; Galmés-Pascual, B.M.; Capllonch-Amer, G.; García-Palmer, F.J.; Lladó, I.; Proenza, A.M.; Gianotti, M. GPER mediates the effects of 17beta-estradiol in cardiac mitochondrial biogenesis and function. Mol. Cell. Endocrinol. 2016, 420, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. Molecular and cellular basis of cardiovascular gender differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.P.; Feng, W.; Xing, D.; Weathington, N.M.; Blalock, J.E.; Chen, Y.-F.; Oparil, S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 2004, 110, 1664–1669. [Google Scholar] [CrossRef]
- Cassavaugh, J.; Qureshi, N.; Csizmadia, E.; Longhi, M.S.; Matyal, R.; Robson, S.C. Regulation of Hypoxic-Adenosinergic Signaling by Estrogen: Implications for Microvascular Injury. Pharmaceuticals 2023, 16, 422. [Google Scholar] [CrossRef]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Sabbatini, A.R.; Kararigas, G. Estrogen-related mechanisms in sex differences of hypertension and target organ damage. Biol. Sex. Differ. 2020, 11, 31. [Google Scholar] [CrossRef]
- Jin, X.; Bin Kim, W.; Kim, M.-N.; Jung, W.W.; Kang, H.K.; Hong, E.-H.; Kim, Y.S.; Shim, W.J.; Han, H.C.; Colwell, C.S.; et al. Oestrogen inhibits salt-dependent hypertension by suppressing GABAergic excitation in magnocellular AVP neurons. Cardiovasc. Res. 2020, 117, 2263–2274. [Google Scholar] [CrossRef]
- Reslan, O.M.; Yin, Z.; do Nascimento, G.R.; Khalil, R.A. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J. Cardiovasc. Pharmacol. 2013, 62, 26–40. [Google Scholar] [CrossRef]
- Haynes, M.P.; Li, L.; Sinha, D.; Russell, K.S.; Hisamoto, K.; Baron, R.; Collinge, M.; Sessa, W.C.; Bender, J.R. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 2003, 278, 2118–2123. [Google Scholar] [CrossRef]
- Brosnihan, K.B.; Li, P.; Figueroa, J.P.; Ganten, D.; Ferrario, C.M. Estrogen, nitric oxide, and hypertension differentially modulate agonist-induced contractile responses in female transgenic (mRen2)27 hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1995–H2001. [Google Scholar] [CrossRef] [PubMed]
- Simoncini, T.; Mannella, P.; Fornari, L.; Caruso, A.; Willis, M.Y.; Garibaldi, S.; Baldacci, C.; Genazzani, A.R. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 2004, 145, 5745–5756. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tan, W.; Guo, Y.; Luo, M.; Shang, F.-F.; Xia, Y.; Luo, S. Progesterone promotes endothelial nitric oxide synthase expression through enhancing nuclear progesterone receptor-SP-1 formation. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H341–H348. [Google Scholar] [CrossRef]
- Yu, J.; Akishita, M.; Eto, M.; Ogawa, S.; Son, B.-K.; Kato, S.; Ouchi, Y.; Okabe, T. Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: Role of phosphatidylinositol 3-kinase/akt pathway. Endocrinology 2010, 151, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Akishita, M.; Eto, M.; Koizumi, H.; Hashimoto, R.; Ogawa, S.; Tanaka, K.; Ouchi, Y.; Okabe, T. Src kinase-mediates androgen receptor-dependent non-genomic activation of signaling cascade leading to endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 2012, 424, 538–543. [Google Scholar] [CrossRef]
- Rubio-Gayosso, I.; Garcia-Ramirez, O.; Gutierrez-Serdan, R.; Guevara-Balcazar, G.; Muñoz-García, O.; Morato-Cartajena, T.; Zamora-Garza, M.; Ceballos-Reyes, G. Testosterone inhibits bradykinin-induced intracellular calcium kinetics in rat aortic endothelial cells in culture. Steroids 2002, 67, 393–397. [Google Scholar] [CrossRef]
- Sobrino, A.; Oviedo, P.J.; Novella, S.; Laguna-Fernandez, A.; Bueno, C.; García-Pérez, M.A.; Tarín, J.J.; Cano, A.; Hermenegildo, C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-alpha. J. Mol. Endocrinol. 2010, 44, 237–246. [Google Scholar] [CrossRef]
- da Costa, D.T.; Goncalves, L.T.; Giesen, J.A.S.; Dos Santos, R.L. Progesterone modulates endothelium-dependent coronary vascular reactivity in SHR. J. Mol. Endocrinol. 2021, 66, 171–180. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Additive effects of low concentrations of estradiol-17beta and progesterone on nitric oxide production by human vascular endothelial cells through shared signaling pathways. J. Steroid Biochem. Mol. Biol. 2016, 165, 258–267. [Google Scholar] [CrossRef]
- Scotland, R.S.; Madhani, M.; Chauhan, S.; Moncada, S.; Andresen, J.; Nilsson, H.; Hobbs, A.J.; Ahluwalia, A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: Key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 2005, 111, 796–803. [Google Scholar] [CrossRef]
- Ruamyod, K.; Watanapa, W.B.; Shayakul, C. Testosterone rapidly increases Ca2+-activated K(+) currents causing hyperpolarization in human coronary artery endothelial cells. J. Steroid Biochem. Mol. Biol. 2017, 168, 118–126. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, T.R.; Giesen, J.A.S.; Rouver, W.N.; Costa, E.D.; Grando, M.D.; Lemos, V.S.; Bendhack, L.M.; dos Santos, R.L. Effects of progesterone treatment on endothelium-dependent coronary relaxation in ovariectomized rats. Life Sci. 2020, 247, 117391. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Jackson, E.K. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am. J. Physiol. Renal Physiol. 2001, 280, F365–F388. [Google Scholar] [CrossRef]
- Wassmann, K.; Wassmann, S.; Nickenig, G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ. Res. 2005, 97, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.M.; Alves-Lopes, R.; Alves, J.V.; Servian, C.P.; Mestriner, F.L.; Carneiro, F.S.; Lobato, N.d.S.; Tostes, R.C. Testosterone Contributes to Vascular Dysfunction in Young Mice Fed a High Fat Diet by Promoting Nuclear Factor E2-Related Factor 2 Downregulation and Oxidative Stress. Front. Physiol. 2022, 13, 837603. [Google Scholar] [CrossRef]
- Lu, Q.; Schnitzler, G.R.; Ueda, K.; Iyer, L.K.; Diomede, O.I.; Andrade, T.; Karas, R.H. ER Alpha Rapid Signaling Is Required for Estrogen Induced Proliferation and Migration of Vascular Endothelial Cells. PLoS ONE 2016, 11, e0152807. [Google Scholar] [CrossRef]
- Nikhil, K.; Sharan, S.; Wishard, R.; Palla, S.R.; Krishna Peddinti, R.; Roy, P. Pterostilbene carboxaldehyde thiosemicarbazone, a resveratrol derivative inhibits 17beta-Estradiol induced cell migration and proliferation in HUVECs. Steroids 2016, 108, 17–30. [Google Scholar] [CrossRef]
- Liu, H.; Tao, Y.; Chen, M.; Yu, J.; Li, W.-J.; Tao, L.; Li, Y.; Li, F. 17beta-Estradiol Promotes Angiogenesis of Rat Cardiac Microvascular Endothelial Cells In Vitro. Med. Sci. Monit. 2018, 24, 2489–2496. [Google Scholar] [CrossRef]
- Cai, J.; Hong, Y.; Weng, C.; Tan, C.; Imperato-McGinley, J.; Zhu, Y.S. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1210–H1221. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Liu, P.; Li, P.; Xu, X.; Chen, Y.; Cheng, Y.; Zhu, D.; Fu, X. 17beta-estradiol promotes angiogenesis through non-genomic activation of Smad1 signaling in endometriosis. Vascul Pharmacol. 2022, 142, 106932. [Google Scholar] [CrossRef]
- Crews, J.K.; Khalil, R.A. Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.G.; Khalil, R.A. Decreased [Ca2+](i) during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J. Pharmacol. Exp. Ther. 1999, 291, 44–52. [Google Scholar] [CrossRef]
- English, K.M.; Jones, R.D.; Jones, T.H.; Morice, A.H.; Channer, K.S. Testosterone acts as a coronary vasodilator by a calcium antagonistic action. J. Endocrinol. Investig. 2002, 25, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Jones, R.D.; Jones, T.H.; Channer, K.S.; Peers, C. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 2006, 147, 2675–2680. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone: A vascular hormone in health and disease. J. Endocrinol. 2013, 217, R47–R71. [Google Scholar] [CrossRef]
- Mazzuca, M.Q.; Mata, K.M.; Li, W.; Rangan, S.S.; Khalil, R.A. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]I in mesenteric microvessels of female rat. J. Pharmacol. Exp. Ther. 2015, 352, 291–304. [Google Scholar] [CrossRef]
- Pang, Y.; Thomas, P. Involvement of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in mPRalpha (PAQR7)-mediated progesterone induction of vascular smooth muscle relaxation. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E453–E466. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Togaibayeva, A.A.; Kannan, M.S.; Miller, V.M.; Fitzpatrick, L.A.; Sieck, G.C. Estrogen increases Ca2+ efflux from female porcine coronary arterial smooth muscle. Am. J. Physiol. 1999, 276, H926–H934. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 376, 375–383. [Google Scholar] [CrossRef]
- Deenadayalu, V.; Puttabyatappa, Y.; Liu, A.T.; Stallone, J.N.; White, R.E. Testosterone-induced relaxation of coronary arteries: Activation of BKCa channels via the cGMP-dependent protein kinase. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H115–H123. [Google Scholar] [CrossRef]
- White, R.E.; Darkow, D.J.; Lang, J.L. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ. Res. 1995, 77, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, M.; Yildiz, O.; Ulusoy, H.B.; Yildirim, V. Testosterone relaxes isolated human radial artery by potassium channel opening action. J. Pharmacol. Sci. 2007, 103, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, C.A.; Khalil, R.A. Gender-related distinctions in protein kinase C activity in rat vascular smooth muscle. Am. J. Physiol. Cell Physiol. 2001, 280, C34–C45. [Google Scholar] [CrossRef]
- Herkert, O.; Kuhl, H.; Busse, R.; Schini-Kerth, V.B. The progestin levonorgestrel induces endothelium-independent relaxation of rabbit jugular vein via inhibition of calcium entry and protein kinase C: Role of cyclic AMP. Br. J. Pharmacol. 2000, 130, 1911–1918. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Budzyn, K.; Marley, P.D.; Sobey, C.G. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke 2004, 35, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, Y.; Simoncini, T.; Xu, S. 17beta-Estradiol inhibits TNF-alpha-induced proliferation and migration of vascular smooth muscle cells via suppression of TRAIL. Gynecol. Endocrinol. 2016, 32, 581–586. [Google Scholar] [CrossRef]
- Cutini, P.H.; Massheimer, V.L. Role of progesterone on the regulation of vascular muscle cells proliferation, migration and apoptosis. Steroids 2010, 75, 355–361. [Google Scholar] [CrossRef]
- Hsu, S.-P.; Chen, T.-H.; Chou, Y.-P.; Chen, L.-C.; Kuo, C.-T.; Lee, T.-S.; Lin, J.-J.; Chang, N.-C.; Lee, W.-S. Extra-nuclear activation of progesterone receptor in regulating arterial smooth muscle cell migration. Atherosclerosis 2011, 217, 83–89. [Google Scholar] [CrossRef]
- Hu, W.-P.; Xie, L.; Hao, S.-Y.; Wu, Q.-H.; Xiang, G.-L.; Li, S.-Q.; Liu, D. Protective effects of progesterone on pulmonary artery smooth muscle cells stimulated with Interleukin 6 via blocking the shuttling and transcriptional function of STAT3. Int. Immunopharmacol. 2021, 102, 108379. [Google Scholar] [CrossRef]
- Somjen, D.; Kohen, F.; Jaffe, A.; Amir-Zaltsman, Y.; Knoll, E.; Stern, N. Effects of gonadal steroids and their antagonists on DNA synthesis in human vascular cells. Hypertension 1998, 32, 39–45. [Google Scholar] [CrossRef]
- Lopes, R.A.M.; Neves, K.B.; Pestana, C.R.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Dworatzek, E.; Fritschka, S.; Pham, T.H.; Regitz-Zagrosek, V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc. Res. 2010, 85, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, C.S.; Garr, E.; Godsland, I.F.; Stevenson, J.C. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim. Biophys. Acta 1998, 1406, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Li, W.; Tran, V.; Khalil, R.A. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem. Pharmacol. 2013, 86, 734–747. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; O’Mahony, F.; Lubahn, D.; Levin, E.R. Estrogen receptor-beta prevents cardiac fibrosis. Mol. Endocrinol. 2010, 24, 2152–2165. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Lin, M.; Groban, L. Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol. Cell Biochem. 2015, 405, 135–148. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Nishizawa, M.; Nakagawa, A.; Nakano, S.; Kigoshi, T.; Uchida, K. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002, 530, 129–132. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology 2020, 161, bqaa127. [Google Scholar] [CrossRef]
- Annibalini, G.; Agostini, D.; Calcabrini, C.; Martinelli, C.; Colombo, E.; Guescini, M.; Tibollo, P.; Stocchi, V.; Sestili, P. Effects of sex hormones on inflammatory response in male and female vascular endothelial cells. J. Endocrinol. Investig. 2014, 37, 861–869. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef]
- Bordallo, J.; Cantabrana, B.; Suarez, L.; Sanchez, M. Testosterone inhibits cAMP-phosphodiesterases in heart extracts from rats and increases cAMP levels in isolated left atria. Pharmacology 2011, 87, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Golden, K.L.; Marsh, J.D.; Jiang, Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm. Metab. Res. 2004, 36, 197–202. [Google Scholar] [PubMed]
- Furukawa, T.; Kurokawa, J. Non-genomic regulation of cardiac ion channels by sex hormones. Cardiovasc. Hematol. Disord. Drug Targets 2008, 8, 245–251. [Google Scholar] [CrossRef]
- Moshal, K.S.; Zhang, Z.; Roder, K.; Kim, T.Y.; Cooper, L.; Patedakis Litvinov, B.; Lu, Y.; Reddy, V.; Terentyev, D.; Choi, B.R.; et al. Progesterone modulates SERCA2a expression and function in rabbit cardiomyocytes. Am. J. Physiol. Cell Physiol. 2014, 307, C1050–C1057. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Pan, D.; Xu, T.; Luo, Y.; Wu, W.; Wu, P.; Zhu, H.; Li, D. Estrogen inhibits endoplasmic reticulum stress and ameliorates myocardial ischemia/reperfusion injury in rats by upregulating SERCA2a. Cell Commun. Signal 2022, 20, 38. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.-S.; Li, L.; Long, Y.; Wang, M.; Yang, H.-Z.; Li, C.-D.; Wang, Y.; Li, S.-S.; Chen, X.; et al. Progesterone Changes the Pregnancy-Induced Adaptation of Cardiomyocyte Kv2.1 Channels via MicroRNA-29b. Cardiovasc Ther 2022, 2022, 7145699. [Google Scholar]
- Feridooni, H.A.; MacDonald, J.K.; Ghimire, A.; Pyle, W.G.; Howlett, S.E. Acute exposure to progesterone attenuates cardiac contraction by modifying myofilament calcium sensitivity in the female mouse heart. Am. J. Physiol. Heart Circ. Physiol. 2016, 312, H46–H59. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Ding, L.; Ruan, Y.; Qin, W.; Lin, Y.; Xi, C.; Lu, Y.; Dou, L.; Zhu, Y.; Cao, Y.; et al. SIRT1 functions as an important regulator of estrogen-mediated cardiomyocyte protection in angiotensin II-induced heart hypertrophy. Oxid. Med. Cell Longev. 2014, 2014, 713894. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Levin, E.R. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J. Biol. Chem. 2005, 280, 26339–26348. [Google Scholar] [CrossRef]
- Altamirano, F.; Oyarce, C.; Silva, P.; Toyos, M.; Wilson, C.; Lavandero, S.; Uhlén, P.; Estrada, M. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J. Endocrinol. 2009, 202, 299–307. [Google Scholar] [CrossRef]
- Duran, J.; Oyarce, C.; Pavez, M.; Valladares, D.; Basualto-Alarcon, C.; Lagos, D.; Barrientos, G.; Troncoso, M.F.; Ibarra, C.; Estrada, M. GSK-3beta/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PLoS ONE 2016, 11, e0168255. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Contreras-Ferrat, A.; Venegas, N.; Osorio-Fuentealba, C.; Pávez, M.; Montoya, K.; Durán, J.; Maass, R.; Lavandero, S.; Estrada, M. Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J. Cell Physiol. 2013, 228, 2399–2407. [Google Scholar] [CrossRef]
- Rattanasopa, C.; Phungphong, S.; Wattanapermpool, J.; Bupha-Intr, T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 2015, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pingili, A.K.; Kara, M.; Khan, N.S.; Estes, A.M.; Lin, Z.; Li, W.; Gonzalez, F.J.; Malik, K.U. 6beta-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension 2015, 65, 1279–1287. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Dominic, P.; Stokes, K.Y. Uncovering sex-specific mechanisms of action of testosterone and redox balance. Redox Biol. 2020, 31, 101490. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Likes, C.E., 3rd; Luz, A.L.; Mao, L.; Yeh, J.S.; Wei, Z.; Kuchibhatla, M.; Ilkayeva, O.R.; Koves, T.R.; Price, T.M. A Mitochondrial Progesterone Receptor Increases Cardiac Beta-Oxidation and Remodeling. J. Endocr. Soc. 2019, 3, 446–467. [Google Scholar] [CrossRef]
- Lan, C.; Cao, N.; Chen, C.; Qu, S.; Fan, C.; Luo, H.; Zeng, A.; Yu, C.; Xue, Y.; Ren, H.; et al. Progesterone, via yes-associated protein, promotes cardiomyocyte proliferation and cardiac repair. Cell Prolif. 2020, 53, e12910. [Google Scholar] [CrossRef]
- Patten, R.D.; Pourati, I.; Aronovitz, M.J.; Baur, J.; Celestin, F.; Chen, X.; Michael, A.; Haq, S.; Nuedling, S.; Grohe, C.; et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004, 95, 692–699. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, S.; Weng, X.; Huang, J.; Zhao, H.; Dai, X.; Bai, X.; Bao, X.; Zhao, C.; Zeng, M.; et al. Estrogen deficiency accelerates postmenopausal atherosclerosis by inducing endothelial cell ferroptosis through inhibiting NRF2/GPX4 pathway. FASEB J. 2023, 37, e22992. [Google Scholar] [CrossRef]
- Geraldes, P.; Sirois, M.G.; Bernatchez, P.N.; Tanguay, J.F. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation: Role of p38 and p42/44 mitogen-activated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, D.M.; Badar, D.M.; Panza, J.A.; Quyyumi, A.A.; Cannon, R.O., 3rd. Acute vascular effects of estrogen in postmenopausal women. Circulation 1994, 90, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, N.; Nadler, E.; Barnea, O.; Shavit, G.; Ayalon, D. Acute effects of 17 beta-estradiol on the rat heart. Am. J. Obstet. Gynecol. 1994, 171, 844–848. [Google Scholar] [CrossRef]
- Kauser, K.; Rubanyi, G.M. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension 1995, 25, 517–523. [Google Scholar] [CrossRef]
- Bernstein, S.R.; Kelleher, C.; Khalil, R.A. Gender-based research underscores sex differences in biological processes, clinical disorders and pharmacological interventions. Biochem. Pharmacol. 2023, 215, 115737. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.N.; Subramanian, R.R.; Sundell, C.L.; Tracey, W.R.; Pollock, J.S.; Harrison, D.G.; Marsden, P.A. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2479–2488. [Google Scholar] [CrossRef]
- Forte, P.; Kneale, B.J.; Milne, E.; Chowienczyk, P.J.; Johnston, A.; Benjamin, N.; Ritter, J.M. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension 1998, 32, 730–734. [Google Scholar] [CrossRef]
- Kublickiene, K.; Svedas, E.; Landgren, B.M.; Crisby, M.; Nahar, N.; Nisell, H.; Poston, L. Small artery endothelial dysfunction in postmenopausal women: In vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J. Clin. Endocrinol. Metab. 2005, 90, 6113–6122. [Google Scholar] [CrossRef]
- Thompson, L.P.; Pinkas, G.; Weiner, C.P. Chronic 17beta-estradiol replacement increases nitric oxide-mediated vasodilation of guinea pig coronary microcirculation. Circulation 2000, 102, 445–451. [Google Scholar] [CrossRef]
- Smith, A.M.; Jones, R.D.; Channer, K.S. The influence of sex hormones on pulmonary vascular reactivity: Possible vasodilator therapies for the treatment of pulmonary hypertension. Curr. Vasc. Pharmacol. 2006, 4, 9–15. [Google Scholar] [CrossRef]
- Geary, G.G.; Krause, D.N.; Duckles, S.P. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H511–H519. [Google Scholar] [CrossRef] [PubMed]
- Sumi, D.; Hayashi, T.; Jayachandran, M.; Iguchi, A. Estrogen prevents destabilization of endothelial nitric oxide synthase mRNA induced by tumor necrosis factor alpha through estrogen receptor mediated system. Life Sci. 2001, 69, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, A.N.; Jun, S.S.; Chen, Z.; German, Z.; Yuhanna, I.S.; Sherman, T.S.; Shaul, P.W. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ. Res. 1997, 81, 355–362. [Google Scholar] [CrossRef]
- Knot, H.J.; Lounsbury, K.M.; Brayden, J.E.; Nelson, M.T. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. Am. J. Physiol. 1999, 276, H961–H969. [Google Scholar] [CrossRef]
- Asunción-Alvarez, D.; Palacios, J.; Ybañez-Julca, R.O.; Rodriguez-Silva, C.N.; Nwokocha, C.; Cifuentes, F.; Greensmith, D.J. Calcium signaling in endothelial and vascular smooth muscle cells: Sex differences and the influence of estrogens and androgens. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H950–H970. [Google Scholar] [CrossRef]
- Tran, Q.K.; VerMeer, M.; Burgard, M.A.; Hassan, A.B.; Giles, J. Hetero-oligomeric Complex between the G Protein-coupled Estrogen Receptor 1 and the Plasma Membrane Ca2+-ATPase 4b. J. Biol. Chem. 2015, 290, 13293–13307. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Haynes, M.P.; Sinha, D.; Clerisme, E.; Bender, J.R. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 5930–5935. [Google Scholar] [CrossRef]
- Hisamoto, K.; Ohmichi, M.; Kurachi, H.; Hayakawa, J.; Kanda, Y.; Nishio, Y.; Adachi, K.; Tasaka, K.; Miyoshi, E.; Fujiwara, N.; et al. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2001, 276, 3459–3467. [Google Scholar] [CrossRef]
- Guo, X.; Razandi, M.; Pedram, A.; Kassab, G.; Levin, E.R. Estrogen induces vascular wall dilation: Mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J. Biol. Chem. 2005, 280, 19704–19710. [Google Scholar] [CrossRef]
- Browner, N.C.; Dey, N.B.; Bloch, K.D.; Lincoln, T.M. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J. Biol. Chem. 2004, 279, 46631–46636. [Google Scholar] [CrossRef]
- Tan, E.; Gurjar, M.V.; Sharma, R.V.; Bhalla, R.C. Estrogen receptor-alpha gene transfer into bovine aortic endothelial cells induces eNOS gene expression and inhibits cell migration. Cardiovasc. Res. 1999, 43, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuhanna, I.S.; Galcheva-Gargova, Z.; Karas, R.H.; Mendelsohn, M.E.; Shaul, P.W. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Investig. 1999, 103, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Darblade, B.; Pendaries, C.; Krust, A.; Dupont, S.; Fouque, M.J.; Rami, J.; Chambon, P.; Bayard, F.; Arnal, J.F. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ. Res. 2002, 90, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Widder, J.; Pelzer, T.; von Poser-Klein, C.; Hu, K.; Jazbutyte, V.; Fritzemeier, K.-H.; Hegele-Hartung, C.; Neyses, L.; Bauersachs, J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertension 2003, 42, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moriarty, K.; Bender, J.R. Vascular cell signaling by membrane estrogen receptors. Steroids 2008, 73, 864–869. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Yuhanna, I.S.; Anderson, R.G.; Mendelsohn, M.E.; Shaul, P.W. ERbeta has nongenomic action in caveolae. Mol. Endocrinol. 2002, 16, 938–946. [Google Scholar]
- Park, J.S.; Lee, G.H.; Jin, S.W.; Pham, T.H.; Thai, T.N.; Kim, J.Y.; Kim, C.Y.; Han, E.H.; Hwang, Y.P.; Choi, C.Y.; et al. G protein-coupled estrogen receptor regulates the KLF2-dependent eNOS expression by activating of Ca2+ and EGFR signaling pathway in human endothelial cells. Biochem. Pharmacol. 2021, 192, 114721. [Google Scholar] [CrossRef]
- Brandes, R.P.; Mugge, A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997, 60, 391–396. [Google Scholar] [CrossRef]
- Yung, L.M.; Wong, W.T.; Tian, X.Y.; Leung, F.P.; Yung, L.H.; Chen, Z.Y.; Yao, X.; Lau, C.W.; Huang, Y. Inhibition of renin-angiotensin system reverses endothelial dysfunction and oxidative stress in estrogen deficient rats. PLoS ONE 2011, 6, e17437. [Google Scholar] [CrossRef]
- Hernández, I.; Delgado, J.L.; Díaz, J.; Quesada, T.; Teruel, M.J.G.; Llanos, M.C.; Carbonell, L.F. 17beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1599–R1605. [Google Scholar] [CrossRef]
- Barbacanne, M.; Rami, J.; Michel, J.; Souchard, J.; Philippe, M.; Besombes, J.; Bayard, F.; Arnal, J. Estradiol increases rat aorta endothelium-derived relaxing factor (EDRF) activity without changes in endothelial NO synthase gene expression: Possible role of decreased endothelium-derived superoxide anion production. Cardiovasc. Res. 1999, 41, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.H.; Schroeter, M.R.; Hecker, M. 17beta-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 2001, 15, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Gragasin, F.S.; Xu, Y.; Arenas, I.A.; Kainth, N.; Davidge, S.T. Estrogen reduces angiotensin II-induced nitric oxide synthase and NAD(P)H oxidase expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Huang, Y.; Fan, H.; Zhao, Y.; Ma, L.; Lan, Y.; Li, C.; Chen, P.; Lou, Z.; Zhou, J. 17beta-Estradiol inhibits hydrogen peroxide-induced senescence and apoptosis in human umbilical vein endothelial cells by regulating the THBS1/TGF-beta/Smad axis. Mol. Cell. Endocrinol. 2024, 580, 112111. [Google Scholar] [CrossRef]
- Calkin, A.C.; Sudhir, K.; Honisett, S.; Williams, M.R.; Dawood, T.; Komesaroff, P.A. Rapid potentiation of endothelium-dependent vasodilation by estradiol in postmenopausal women is mediated via cyclooxygenase 2. J. Clin. Endocrinol Metab. 2002, 87, 5072–5075. [Google Scholar] [CrossRef]
- O’Sullivan, M.G.; Goodrich, J.A.; Adams, M.R. Increased prostacyclin synthesis by atherosclerotic arteries from estrogen-treated monkeys. Life Sci. 2001, 69, 395–401. [Google Scholar] [CrossRef]
- Egan, K.M.; Lawson, J.A.; Fries, S.; Koller, B.; Rader, D.J.; Smyth, E.M.; FitzGerald, G.A. COX-2-derived prostacyclin confers atheroprotection on female mice. Science 2004, 306, 1954–1957. [Google Scholar] [CrossRef]
- Jun, S.S.; Chen, Z.; Pace, M.C.; Shaul, P.W. Estrogen upregulates cyclooxygenase-1 gene expression in ovine fetal pulmonary artery endothelium. J. Clin. Investig. 1998, 102, 176–183. [Google Scholar] [CrossRef]
- Sherman, T.S.; Chambliss, K.L.; Gibson, L.L.; Pace, M.C.; Mendelsohn, M.E.; Pfister, S.L.; Shaul, P.W. Estrogen acutely activates prostacyclin synthesis in ovine fetal pulmonary artery endothelium. Am. J. Respir. Cell Mol. Biol. 2002, 26, 610–616. [Google Scholar] [CrossRef]
- Jiang, C.W.; Sarrel, P.M.; Lindsay, D.C.; Poole-Wilson, P.A.; Collins, P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br. J. Pharmacol. 1991, 104, 1033–1037. [Google Scholar] [CrossRef]
- Case, J.; Davison, C.A. Estrogen alters relative contributions of nitric oxide and cyclooxygenase products to endothelium-dependent vasodilation. J. Pharmacol. Exp. Ther. 1999, 291, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kahonen, M.; Tolvanen, J.P.; Sallinen, K.; Wu, X.; Porsti, I. Influence of gender on control of arterial tone in experimental hypertension. Am. J. Physiol. 1998, 275, H15–H22. [Google Scholar] [PubMed]
- Liu, M.Y.; Hattori, Y.; Fukao, M.; Sato, A.; Sakuma, I.; Kanno, M. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br. J. Pharmacol. 2001, 132, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, I.; Liu, M.; Sato, A.; Hayashi, T.; Iguchi, A.; Kitabatake, A.; Hattori, Y. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: Influence of oestrogen. Br. J. Pharmacol. 2002, 135, 48–54. [Google Scholar] [CrossRef]
- SenthilKumar, G.; Katunaric, B.; Bordas-Murphy, H.; Young, M.; Doren, E.L.; Schulz, M.E.; Widlansky, M.E.; Freed, J.K. 17beta-Estradiol promotes sex-specific dysfunction in isolated human arterioles. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H330–H337. [Google Scholar] [CrossRef]
- Xu, X.; Yan, Q.; Liu, X.; Li, P.; Li, X.; Chen, Y.; Simoncini, T.; Liu, J.; Zhu, D.; Fu, X. 17beta-Estradiol nongenomically induces vascular endothelial H(2)S release by promoting phosphorylation of cystathionine gamma-lyase. J. Biol. Chem. 2019, 294, 15577–15592. [Google Scholar] [CrossRef]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Tschugguel, W.; Stonek, F.; Zhegu, Z.; Dietrich, W.; Schneeberger, C.; Stimpfl, T.; Waldhoer, T.; Vycudilik, W.; Huber, J.C. Estrogen increases endothelial carbon monoxide, heme oxygenase 2, and carbon monoxide-derived cGMP by a receptor-mediated system. J. Clin. Endocrinol. Metab. 2001, 86, 3833–3839. [Google Scholar] [CrossRef]
- Riddle, M.A.; Walker, B.R. Regulation of endothelial BK channels by heme oxygenase-derived carbon monoxide and caveolin-1. Am. J. Physiol. Cell Physiol. 2012, 303, C92–C101. [Google Scholar] [CrossRef]
- Hughes, J.M.; Riddle, M.A.; Paffett, M.L.; Gonzalez Bosc, L.V.; Walker, B.R. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1439–H1450. [Google Scholar] [CrossRef]
- Naik, J.S.; Walker, B.R. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H220–H228. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.M.; Ghatei, M.A.; McNeill, J.G.; Collins, P. 17beta-estradiol decreases endothelin-1 levels in the coronary circulation of postmenopausal women with coronary artery disease. Circulation 2000, 102, 1617–1622. [Google Scholar] [CrossRef]
- Akishita, M.; Kozaki, K.; Eto, M.; Yoshizumi, M.; Ishikawa, M.; Toba, K.; Orimo, H.; Ouchi, Y. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem. Biophys. Res. Commun. 1998, 251, 17–21. [Google Scholar] [CrossRef] [PubMed]
- David, F.L.; Carvalho, M.H.C.; Cobra, A.L.; Nigro, D.; Fortes, Z.B.; Rebouças, N.A.; Tostes, R.C. Ovarian hormones modulate endothelin-1 vascular reactivity and mRNA expression in DOCA-salt hypertensive rats. Hypertension 2001, 38, 692–696. [Google Scholar] [CrossRef]
- Ba, Z.F.; Lu, A.; Shimizu, T.; Szalay, L.; Schwacha, M.G.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. 17beta-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma-hemorrhage. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H245–H250. [Google Scholar] [CrossRef]
- Morey, A.K.; Razandi, M.; Pedram, A.; Hu, R.M.; Prins, B.A.; Levin, E.R. Oestrogen and progesterone inhibit the stimulated production of endothelin-1. Biochem. J. 1998, 330 Pt 3, 1097–1105. [Google Scholar] [CrossRef]
- Bilsel, A.S.; Moini, H.; Tetik, E.; Aksungar, F.; Kaynak, B.; Ozer, A. 17Beta-estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc. Res. 2000, 46, 579–584. [Google Scholar] [CrossRef]
- Takeda-Matsubara, Y.; Nakagami, H.; Iwai, M.; Cui, T.-X.; Shiuchi, T.; Akishita, M.; Nahmias, C.; Ito, M.; Horiuchi, M. Estrogen activates phosphatases and antagonizes growth-promoting effect of angiotensin II. Hypertension 2002, 39, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Gillespie, D.G.; Mi, Z.; Rosselli, M.; Keller, P.J.; Jackson, E.K. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension 2000, 35, 262–266. [Google Scholar] [CrossRef]
- Teoh, J.P.; Li, X.; Simoncini, T.; Zhu, D.; Fu, X. Estrogen-Mediated Gaseous Signaling Molecules in Cardiovascular Disease. Trends Endocrinol. Metab. 2020, 31, 773–784. [Google Scholar] [CrossRef]
- Sivritas, D.; Becher, M.U.; Ebrahimian, T.; Arfa, O.; Rapp, S.; Bohner, A.; Mueller, C.F.; Umemura, T.; Wassmann, S.; Nickenig, G.; et al. Antiproliferative effect of estrogen in vascular smooth muscle cells is mediated by Kruppel-like factor-4 and manganese superoxide dismutase. Basic. Res. Cardiol. 2011, 106, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Su, S.-C.; Chiang, C.-F.; Chien, C.-Y.; Hsu, C.-C.; Yu, T.-Y.; Huang, S.-M.; Shieh, Y.-S.; Kao, H.-W.; Tsai, C.-S.; et al. Estrogen modulates vascular smooth muscle cell function through downregulation of SIRT1. Oncotarget 2017, 8, 110039–110051. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yu, X.; Szynkarski, C.K.; Meng, C.; Zhou, B.; Barhoumi, R.; White, R.E.; Heaps, C.L.; Stallone, J.N.; Han, G. Activation of GPER Induces Differentiation and Inhibition of Coronary Artery Smooth Muscle Cell Proliferation. PLoS ONE 2013, 8, e64771. [Google Scholar]
- Mueck, A.O.; Seeger, H. 2-Methoxyestradiol--biology and mechanism of action. Steroids 2010, 75, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Tofovic, S.P.; Jones, T.J.; Bilan, V.P.; Jackson, E.K.; Petrusevska, G. Synergistic therapeutic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascular remodeling. J. Cardiovasc. Pharmacol. 2010, 56, 475–483. [Google Scholar] [CrossRef]
- Perez-Cremades, D.; Mompeon, A.; Vidal-Gomez, X.; Hermenegildo, C.; Novella, S. miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. Int. J. Mol. Sci. 2018, 19, 473. [Google Scholar] [CrossRef]
- Jiang, C.; Sarrel, P.M.; Poole-Wilson, P.A.; Collins, P. Acute effect of 17 beta-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am. J. Physiol. 1992, 263, H271–H275. [Google Scholar] [CrossRef]
- Kakucs, R.; Varbiro, S.; Nadasy, G.L.; Monos, E.; Szekacs, B. Acute, nongenomic vasodilatory action of estradiol is attenuated by chronic estradiol treatment. Exp. Biol. Med. 2001, 226, 538–542. [Google Scholar] [CrossRef]
- Murphy, J.G.; Khalil, R.A. Gender-specific reduction in contractility and [Ca2+](i) in vascular smooth muscle cells of female rat. Am. J. Physiol. Cell Physiol. 2000, 278, C834–C844. [Google Scholar] [CrossRef]
- Johnson, B.D.; Zheng, W.; Korach, K.S.; Scheuer, T.; Catterall, W.A.; Rubanyi, G.M. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J. Gen. Physiol. 1997, 110, 135–140. [Google Scholar] [CrossRef]
- Bowles, D.K. Gender influences coronary L-type Ca2+ current and adaptation to exercise training in miniature swine. J. Appl. Physiol. 2001, 91, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ram, J.L.; Standley, P.R.; Sowers, J.R. 17 beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am. J. Physiol. 1994, 266, C975–C980. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Zegarra, L.A.; Espejo, M.S.; Ibañez, A.M.; Rando, M.E.; Pagola, L.E.; De Giusti, V.C.; Aiello, E.A. Activation of G Protein-Coupled Estrogen Receptor (GPER) Negatively Modulates Cardiac Excitation-Contraction Coupling (ECC) through the PI3K/NOS/NO Pathway. Int. J. Mol. Sci. 2024, 25, 8993. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Yang, L.; Adzika, G.K.; Machuki, J.O.; Shi, M.; Sun, Q.; Sun, H. Estrogen Protects Vasomotor Functions in Rats During Catecholamine Stress. Front. Cardiovasc. Med. 2021, 8, 679240. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Zhao, T.; Meng, Q.Y.; Zhao, L.; Li, X.C. Estrogen and estrogen receptor affects MMP2 and MMP9 expression through classical ER pathway and promotes migration of lower venous vascular smooth muscle cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1460–1467. [Google Scholar]
- Ambhore, N.S.; Kalidhindi, R.S.R.; Pabelick, C.M.; Hawse, J.R.; Prakash, Y.S.; Sathish, V. Differential estrogen-receptor activation regulates extracellular matrix deposition in human airway smooth muscle remodeling via NF-kappaB pathway. FASEB J. 2019, 33, 13935–13950. [Google Scholar] [CrossRef]
- Medzikovic, L.; Aryan, L.; Eghbali, M. Connecting sex differences, estrogen signaling, and microRNAs in cardiac fibrosis. J. Mol. Med. 2019, 97, 1385–1398. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Li, Y.; Li, P.; Zhang, Y.; Xu, G.; Sun, K.; Huang, Y. Estrogen inhibits TGF‑beta1‑stimulated cardiac fibroblast differentiation and collagen synthesis by promoting Cdc42. Mol. Med. Rep. 2024, 30, 123. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Wang, X.; Zhao, D.; Zhang, J.; Jiang, L.; Duan, W.; Wang, X.; Hong, Z.; Li, Z.; et al. GPR30 Alleviates Pressure Overload-Induced Myocardial Hypertrophy in Ovariectomized Mice by Regulating Autophagy. Int. J. Mol. Sci. 2023, 24, 904. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Y.; Liu, X.; Chen, L.; Zhang, G.; Li, Y. G-protein coupled receptor 30 attenuates myocardial hypertrophy by reducing oxidative stress and apoptosis in Ang II-treated mice. Peptides 2022, 157, 170878. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Connor, E.; Bush, T.L. Estrogen and coronary heart disease in women. JAMA 1991, 265, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Imthurn, B.; Zacharia, L.C.; Jackson, E.K. Hormone replacement therapy and cardiovascular disease: What went wrong and where do we go from here? Hypertension 2004, 44, 789–795. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H., Jr.; Kim, S.C.; Hall, R.R.; Cochran, V.C.; Lawhorn, S.L.; McCallister, B.D. Estrogen replacement therapy after coronary angioplasty in women. J. Am. Coll. Cardiol. 1997, 29, 1–5. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S.; et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Smith, N.L.; Heckbert, S.R.; Lemaitre, R.N.; Reiner, A.P.; Lumley, T.; Weiss, N.S.; Larson, E.B.; Rosendaal, F.R.; Psaty, B.M. Esterified estrogens and conjugated equine estrogens and the risk of venous thrombosis. JAMA 2004, 292, 1581–1587. [Google Scholar] [CrossRef]
- Cushman, M.; Kuller, L.H.; Prentice, R.; Rodabough, R.J.; Psaty, B.M.; Stafford, R.S.; Sidney, S.; Rosendaal, F.R. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004, 292, 1573–1580. [Google Scholar] [CrossRef]
- Grodstein, F.; Manson, J.E.; Stampfer, M.J. Postmenopausal hormone use and secondary prevention of coronary events in the nurses’ health study. a prospective, observational study. Ann. Intern. Med. 2001, 135, 1–8. [Google Scholar] [CrossRef]
- Hendrix, S.L.; Wassertheil-Smoller, S.; Johnson, K.C.; Howard, B.V.; Kooperberg, C.; Rossouw, J.E.; Trevisan, M.; Aragaki, A.; Baird, A.E.; Bray, P.F.; et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 2006, 113, 2425–2434. [Google Scholar] [PubMed]
- Viscoli, C.M.; Brass, L.M.; Kernan, W.N.; Sarrel, P.M.; Suissa, S.; Horwitz, R.I. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001, 345, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Stefanick, M.L.; Legault, C.; Tracy, R.P.; Howard, G.; Kessler, C.M.; Lucas, D.L.; Bush, T.L. Distribution and correlates of plasma fibrinogen in middle-aged women. Initial findings of the Postmenopausal Estrogen/Progestin Interventions (PEPI) study. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Lin, F.; Vittinghoff, E.; Bittner, V. The relation of postmenopausal hormone therapy to serum uric acid and the risk of coronary heart disease events: The Heart and Estrogen-Progestin Replacement Study (HERS). Ann. Epidemiol. 2006, 16, 138–145. [Google Scholar] [CrossRef]
- Angerer, P.; Stork, S.; Kothny, W.; Schmitt, P.; von Schacky, C. Effect of oral postmenopausal hormone replacement on progression of atherosclerosis: A randomized, controlled trial. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 262–268. [Google Scholar] [CrossRef]
- Herrington, D.M.; Howard, T.D.; Hawkins, G.A.; Reboussin, D.M.; Xu, J.; Zheng, S.L.; Brosnihan, K.B.; Meyers, D.A.; Bleecker, E.R. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N. Engl. J. Med. 2002, 346, 967–974. [Google Scholar] [CrossRef]
- Vickers, M.R.; MacLennan, A.H.; Lawton, B.; Ford, D.; Martin, J.; Meredith, S.K.; DeStavola, B.L.; Rose, S.; Dowell, A.; Wilkes, H.C.; et al. Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): A randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ 2007, 335, 239. [Google Scholar] [CrossRef]
- Waters, D.D.; Alderman, E.L.; Hsia, J.; Howard, B.V.; Cobb, F.R.; Rogers, W.J.; Ouyang, P.; Thompson, P.; Tardif, J.C.; Higginson, L.; et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: A randomized controlled trial. JAMA 2002, 288, 2432–2440. [Google Scholar] [CrossRef]
- Canonico, M.; Oger, E.; Plu-Bureau, G.; Conard, J.; Meyer, G.; Lévesque, H.; Trillot, N.; Barrellier, M.T.; Wahl, D.; Emmerich, J.; et al. Hormone therapy and venous thromboembolism among postmenopausal women: Impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation 2007, 115, 840–845. [Google Scholar]
- Barrett-Connor, E.; Mosca, L.; Collins, P.; Geiger, M.J.; Grady, D.; Kornitzer, M.; McNabb, M.A.; Wenger, N.K. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N. Engl. J. Med. 2006, 355, 125–137. [Google Scholar]
- Hwang, J.; Mack, W.J.; Xiang, M.; Sevanian, A.; Lobo, R.A.; Hodis, H.N. Long-term effect of estrogen replacement on plasma nitric oxide levels: Results from the estrogen in the prevention of atherosclerosis trial (EPAT). Atherosclerosis 2005, 181, 375–380. [Google Scholar] [CrossRef]
- Miller, V.M.; Black, D.M.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hodis, H.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; Naftolin, F.; et al. Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J. Cardiovasc. Transl. Res. 2009, 2, 228–239. [Google Scholar] [CrossRef]
- Harman, S.M.; Black, D.M.; Naftolin, F.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hopkins, P.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann. Intern. Med. 2014, 161, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Tosakulwong, N.B.; Lesnick, T.G.; Kara, F.; Kendall-Thomas, J.B.; Kapoor, E.M.; Fields, J.A.; James, T.T.; Lobo, R.A.; Manson, J.E.M.; et al. Cardiometabolic outcomes in Kronos Early Estrogen Prevention Study continuation: 14-year follow-up of a hormone therapy trial. Menopause 2024, 31, 10–17. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Henderson, V.W.; Shoupe, D.; Budoff, M.J.; Hwang-Levine, J.; Li, Y.; Feng, M.; Dustin, L.; Kono, N.; et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N. Engl. J. Med. 2016, 374, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Hanes, V.; Chauhan, V.; Pitt, B. Effects of a new hormone therapy, drospirenone and 17-beta-estradiol, in postmenopausal women with hypertension. Hypertension 2006, 48, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.L.; Rubinow, D.R.; Watkins, L.; Hinderliter, A.L.; Caughey, M.C.; Girdler, S.S. The Effect of Perimenopausal Transdermal Estradiol and Micronized Progesterone on Markers of Risk for Arterial Disease. J. Clin. Endocrinol. Metab. 2020, 105, e2050–e2060. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Aye, M.; Rigby, A.; Thatcher, N.; Dargham, S.; Kilpatrick, E.; Atkin, S. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Yuk, J.; Kim, G.S.; Byun, Y.S.; Yang, S.; Kim, M.; Yoon, S.; Seo, Y.; Kim, B.G. Effect of menopausal hormonal therapy on cardiovascular risks in Korean postmenopausal women: A nationwide cohort study. BJOG 2024, 131, 1306–1317. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Grady, D.; Sashegyi, A.; Anderson, P.W.; Cox, D.A.; Hoszowski, K.; Rautaharju, P.; Harper, K.D.; MORE Investigators (Multiple Outcomes of Raloxifene Evaluation). Raloxifene and cardiovascular events in osteoporotic postmenopausal women: Four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 2002, 287, 847–857. [Google Scholar] [CrossRef]
- Douglas, G.; Cruz, M.N.; Poston, L.; Gustafsson, J.A.; Kublickiene, K. Functional characterization and sex differences in small mesenteric arteries of the estrogen receptor-beta knockout mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R112–R120. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Vinh, A.; Arumugam, T.V.; Drummond, G.R.; Sobey, C.G. G protein-coupled estrogen receptor 1: A novel target to treat cardiovascular disease in a sex-specific manner? Br. J. Pharmacol. 2021, 178, 3849–3863. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Dalais, F.S.; Kotsopoulos, D.; Liang, Y.L.; Davis, S.; McGrath, B.P. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001, 86, 3053–3060. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Woo, J. Effect of soy protein and isoflavones on blood pressure and endothelial cytokines: A 6-month randomized controlled trial among postmenopausal women. J. Hypertens. 2013, 31, 384–392. [Google Scholar] [CrossRef]
- Husain, D.; Khanna, K.; Puri, S.; Haghighizadeh, M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J. Am. Coll. Nutr. 2015, 34, 42–48. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ho, S.C.; Chen, Y.M.; Tomlinson, B.; Ho, S.; To, K.; Woo, J. Effect of whole soy and purified daidzein on ambulatory blood pressure and endothelial function--a 6-month double-blind, randomized controlled trial among Chinese postmenopausal women with prehypertension. Eur. J. Clin. Nutr. 2015, 69, 1161–1168. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Glisic, M.; Shahzad, S.; Brown, E.; Pellegrino Baena, C.; Chadni, M.; Chowdhury, R.; Franco, O.H.; Muka, T. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: A systematic review. Hum. Reprod. Update 2019, 25, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Pickar, J.H.; Yeh, I.T.; Wheeler, J.E.; Cunnane, M.F.; Speroff, L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: Two-year substudy results. Fertil. Steril. 2003, 80, 1234–1240. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Cheng, X.; Fan, K.; Gao, T.; Qi, X.; Gao, S.; Zheng, G.; Dong, H. High estrogen induces trans-differentiation of vascular smooth muscle cells to a macrophage-like phenotype resulting in aortic inflammation via inhibiting VHL/HIF1a/KLF4 axis. Aging 2024, 16, 9876–9898. [Google Scholar] [CrossRef]
- Lewandowski, K.C.; Komorowski, J.; Mikhalidis, D.P.; Bienkiewicz, M.; Tan, B.K.; O’callaghan, C.J.; Lewinski, A.; Prelevic, G.; Randeva, H.S. Effects of hormone replacement therapy type and route of administration on plasma matrix metalloproteinases and their tissue inhibitors in postmenopausal women. J. Clin. Endocrinol. Metab. 2006, 91, 3123–3130. [Google Scholar] [CrossRef]
- Kaunitz, A.M.; Manson, J.E. Management of Menopausal Symptoms. Obstet. Gynecol. 2015, 126, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.S.; Tuomikoski, P.; Lyytinen, H.; Korhonen, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Vaginal estradiol use and the risk for cardiovascular mortality. Hum. Reprod. 2016, 31, 804–809. [Google Scholar] [CrossRef]
- Goodman, M.P. Are all estrogens created equal? A review of oral vs. transdermal therapy. J. Womens Health 2012, 21, 161–169. [Google Scholar] [CrossRef]
- Turgeon, J.L.; Carr, M.C.; Maki, P.M.; Mendelsohn, M.E.; Wise, P.M. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr. Rev. 2006, 27, 575–605. [Google Scholar] [CrossRef]
- Goldstajn, M.S.; Mikus, M.; Ferrari, F.A.; Bosco, M.; Uccella, S.; Noventa, M.; Torok, P.; Terzic, S.; Lagana, A.S.; Garzon, S. Effects of transdermal versus oral hormone replacement therapy in postmenopause: A systematic review. Arch. Gynecol. Obstet. 2023, 307, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Cherbuin, N. Lipid profile differences during menopause: A review with meta-analysis. Menopause 2019, 26, 1327–1333. [Google Scholar] [CrossRef]
- Canonico, M. Hormone therapy and hemostasis among postmenopausal women: A review. Menopause 2014, 21, 753–762. [Google Scholar] [CrossRef]
- Scarabin, P.Y. Progestogens and venous thromboembolism in menopausal women: An updated oral versus transdermal estrogen meta-analysis. Climacteric 2018, 21, 341–345. [Google Scholar] [CrossRef]
- Gouva, L.; Tsatsoulis, A. The role of estrogens in cardiovascular disease in the aftermath of clinical trials. Hormones 2004, 3, 171–183. [Google Scholar] [CrossRef]
- Ho, J.Y.-P.; Chen, M.-J.; Sheu, W.H.-H.; Yi, Y.-C.; Tsai, A.C.-W.; Guu, H.-F.; Ho, E.S.-C. Differential effects of oral conjugated equine estrogen and transdermal estrogen on atherosclerotic vascular disease risk markers and endothelial function in healthy postmenopausal women. Hum. Reprod. 2006, 21, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, F.; Dea, K.; Duh, M.S.; Kahler, K.H.; Rolli, M.; Lefebvre, P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause 2018, 25, 1297–1305. [Google Scholar] [CrossRef]
- Donovitz, G.S. Low complication rates of testosterone and estradiol implants for androgen and estrogen replacement therapy in over 1 million procedures. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211015238. [Google Scholar] [CrossRef]
- Glaser, R.; York, A.E.; Dimitrakakis, C. Beneficial effects of testosterone therapy in women measured by the validated Menopause Rating Scale (MRS). Maturitas 2011, 68, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Renke, G.; Tostes, F. Cardiovascular Safety and Benefits of Testosterone Implant Therapy in Postmenopausal Women: Where Are We? Pharmaceuticals 2023, 16, 619. [Google Scholar] [CrossRef]
- Alevizaki, M.; Saltiki, K.; Cimponeriu, A.; Kanakakis, I.; Xita, N.; Alevizaki, C.C.; Georgiou, I.; Sarika, H.-L. Severity of cardiovascular disease in postmenopausal women: Associations with common estrogen receptor alpha polymorphic variants. Eur. J. Endocrinol. 2007, 156, 489–496. [Google Scholar] [CrossRef]
- Emre, A.; Sahin, S.; Erzik, C.; Nurkalem, Z.; Oz, D.; Cirakoglu, B.; Yesilcimen, K.; Ersek, B. Effect of hormone replacement therapy on plasma lipoproteins and apolipoproteins, endothelial function and myocardial perfusion in postmenopausal women with estrogen receptor-alpha IVS1-397 C/C genotype and established coronary artery disease. Cardiology 2006, 106, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ogueta, S.B.; Schwartz, S.D.; Yamashita, C.K.; Farber, D.B. Estrogen receptor in the human eye: Influence of gender and age on gene expression. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1906–1911. [Google Scholar]
- Isola, J.V.V.; Ko, S.; Ocanas, S.R.; Stout, M.B. Role of Estrogen Receptor alpha in Aging and Chronic Disease. Adv. Geriatr. Med. Res. 2023, 5, e230005. [Google Scholar]
- Gurrala, R.; Kilanowski-Doroh, I.M.; Hutson, D.D.; Ogola, B.O.; Zimmerman, M.A.; Katakam, P.V.G.; Satou, R.; Mostany, R.; Lindsey, S.H. Alterations in the estrogen receptor profile of cardiovascular tissues during aging. Geroscience 2021, 43, 433–442. [Google Scholar] [CrossRef]
- Kim, K.H.; Young, B.D.; Bender, J.R. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol. Cell Endocrinol. 2014, 389, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Haynes, M.P.; Bender, J.R. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2024, 292, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Huang, Y.; Su, H.; Ma, Y.; Tao, Y.; Liao, D.J.; Liu, Y.; Feng, Z. Identification of a novel human estrogen receptor-alpha splice variant able to enhance malignant biological behaviors of breast cancer cells. Oncol. Lett. 2018, 15, 5339–5344. [Google Scholar]
- Pagano, M.T.; Ortona, E.; Dupuis, M.L. A Role for Estrogen Receptor alpha36 in Cancer Progression. Front Endocrinol 2020, 11, 506. [Google Scholar] [CrossRef]
- Costa, T.J.; Jiménez-Altayó, F.; Echem, C.; Akamine, E.H.; Tostes, R.; Vila, E.; Dantas, A.P.; de Carvalho, M.H.C. Late Onset of Estrogen Therapy Impairs Carotid Function of Senescent Females in Association with Altered Prostanoid Balance and Upregulation of the Variant ERalpha36. Cells 2019, 8, 1217. [Google Scholar] [CrossRef]
- Post, W.S.; Goldschmidt-Clermont, P.J.; Wilhide, C.C.; Heldman, A.W.; Sussman, M.S.; Ouyang, P.; Milliken, E.E.; Issa, J.P. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc. Res. 1999, 43, 985–991. [Google Scholar] [CrossRef]
- Somani, Y.B.; Pawelczyk, J.A.; De Souza, M.J.; Kris-Etherton, P.M.; Proctor, D.N. Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H395–H404. [Google Scholar] [CrossRef]
- Liu, P.Y.; Christian, R.C.; Ruan, M.; Miller, V.M.; Fitzpatrick, L.A. Correlating androgen and estrogen steroid receptor expression with coronary calcification and atherosclerosis in men without known coronary artery disease. J. Clin. Endocrinol. Metab. 2005, 90, 1041–1046. [Google Scholar] [CrossRef]
- Novensà, L.; Novella, S.; Medina, P.; Segarra, G.; Castillo, N.; Heras, M.; Hermenegildo, C.; Dantas, A.P. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PLoS ONE 2011, 6, e25335. [Google Scholar] [CrossRef]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Pinna, C.; Cignarella, A.; Sanvito, P.; Pelosi, V.; Bolego, C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension 2008, 51, 1210–1217. [Google Scholar] [CrossRef]
- Kalesnykas, G.; Roschier, U.; Puolivali, J.; Wang, J.; Miettinen, R. The effect of aging on the subcellular distribution of estrogen receptor-alpha in the cholinergic neurons of transgenic and wild-type mice. Eur. J. Neurosci. 2005, 21, 1437–1442. [Google Scholar] [CrossRef]
- Wynne, F.L.; Payne, J.A.; Cain, A.E.; Reckelhoff, J.F.; Khalil, R.A. Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats. Hypertension 2004, 43, 405–412. [Google Scholar] [CrossRef]
- Forabosco, A.; Criscuolo, M.; Coukos, G.; Uccelli, E.; Weinstein, R.; Spinato, S.; Botticelli, A.; Volpe, A. Efficacy of hormone replacement therapy in postmenopausal women with oral discomfort. Oral. Surg. Oral. Med. Oral. Pathol. 1992, 73, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjanen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Parés, D.; Iglesias, M.; Pera, M.; Pascual, M.; Torner, A.; Baró, T.; Alonso, S.; Grande, L. Expression of estrogen and progesterone receptors in the anal canal of women according to age and menopause. Dis. Colon. Rectum 2010, 53, 1687–1691. [Google Scholar] [CrossRef]
- Dieudonne, M.N.; Leneveu, M.C.; Giudicelli, Y.; Pecquery, R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: Regional specificities and regulation by estrogens. Am. J. Physiol. Cell Physiol. 2004, 286, C655–C661. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.J.; Andersson, T.C.; Šimonytė, K.; Söderström, I.; Mattsson, C.; Seckl, J.R.; Olsson, T. Association of 11beta-hydroxysteroid dehydrogenase type I expression and activity with estrogen receptor beta in adipose tissue from postmenopausal women. Menopause 2012, 19, 1347–1352. [Google Scholar] [CrossRef]
- Ahmed, F.; Kamble, P.G.; Hetty, S.; Fanni, G.; Vranic, M.; Sarsenbayeva, A.; Kristófi, R.; Almby, K.; Svensson, M.K.; Pereira, M.J.; et al. Role of Estrogen and Its Receptors in Adipose Tissue Glucose Metabolism in Pre- and Postmenopausal Women. J. Clin. Endocrinol. Metab. 2022, 107, e1879–e1889. [Google Scholar] [CrossRef]
- Park, Y.M.; Erickson, C.; Bessesen, D.; Van Pelt, R.E.; Cox-York, K. Age- and menopause-related differences in subcutaneous adipose tissue estrogen receptor mRNA expression. Steroids 2017, 121, 17–21. [Google Scholar] [CrossRef]
- Ishunina, T.A.; Kruijver, F.P.; Balesar, R.; Swaab, D.F. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J. Clin. Endocrinol. Metab. 2000, 85, 3283–3291. [Google Scholar]
- Chen, G.D.; Oliver, R.H.; Leung, B.S.; Lin, L.Y.; Yeh, J. Estrogen receptor alpha and beta expression in the vaginal walls and uterosacral ligaments of premenopausal and postmenopausal women. Fertil. Steril. 1999, 71, 1099–1102. [Google Scholar] [CrossRef]
- Gebhart, J.B.; Rickard, D.J.; Barrett, T.J.; Lesnick, T.G.; Webb, M.J.; Podratz, K.C.; Spelsberg, T.C. Expression of estrogen receptor isoforms alpha and beta messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am. J. Obstet. Gynecol. 2001, 185, 1325–1330; discussion 1330–1331. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, H.; Fujimoto, J.; Aoki, I.; Tamaya, T. Expression of estrogen receptor alpha and beta in myometrium of premenopausal and postmenopausal women. Steroids 2003, 68, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; El-Alfy, M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J. Clin. Endocrinol. Metab. 2000, 85, 4835–4840. [Google Scholar] [PubMed]
- Hodis, H.N.; Mack, W.J.; Azen, S.P.; Lobo, R.A.; Shoupe, D.; Mahrer, P.R.; Faxon, D.P.; Cashin-Hemphill, L.; Sanmarco, M.E.; French, W.J.; et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N. Engl. J. Med. 2003, 349, 535–545. [Google Scholar] [CrossRef]
- Carmel, S. Health and Well-Being in Late Life: Gender Differences Worldwide. Front. Med. 2019, 6, 218. [Google Scholar] [CrossRef]
- Chen, K.; Xu, M.; Lu, F.; He, Y. Development of Matrix Metalloproteinases-Mediated Extracellular Matrix Remodeling in Regenerative Medicine: A Mini Review. Tissue Eng. Regen. Med. 2023, 20, 661–670. [Google Scholar] [CrossRef]
- Wang, D.; Brady, T.; Santhanam, L.; Gerecht, S. The extracellular matrix mechanics in the vasculature. Nat. Cardiovasc. Res. 2023, 2, 718–732. [Google Scholar] [CrossRef]
- Cho, L.; Kaunitz, A.M.; Faubion, S.S.; Hayes, S.N.; Lau, E.S.; Pristera, N.; Scott, N.; Shifren, J.L.; Shufelt, C.L.; Stuenkel, C.A.; et al. Rethinking Menopausal Hormone Therapy: For Whom, What, When, and How Long? Circulation 2023, 147, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Skouby, S.O.; Pan, K.; Thompson, J.R.; Komm, B.S.; Mirkin, S. Effects of conjugated estrogens/bazedoxifene on lipid and coagulation variables: A randomized placebo- and active-controlled trial. Menopause 2015, 22, 640–649. [Google Scholar] [CrossRef]
- Marko, K.I.; Simon, J.A. Clinical trials in menopause. Menopause 2018, 25, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Nita, A.R.; Knock, G.A.; Heads, R.J. Signalling mechanisms in the cardiovascular protective effects of estrogen: With a focus on rapid/membrane signalling. Curr. Res. Physiol. 2021, 4, 103–118. [Google Scholar] [CrossRef] [PubMed]
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef]
- Mehta, J.; Manson, J.E. Menopausal hormone therapy and hypertension: Minimizing risk. Menopause 2021, 28, 1201–1202. [Google Scholar] [CrossRef]
- Shimbo, D.; Wang, L.; Lamonte, M.J.; Allison, M.; Wellenius, G.A.; Bavry, A.A.; Martin, L.W.; Aragaki, A.; Newman, J.D.; Swica, Y.; et al. The effect of hormone therapy on mean blood pressure and visit-to-visit blood pressure variability in postmenopausal women: Results from the Women’s Health Initiative randomized controlled trials. J. Hypertens. 2014, 32, 2071–2081; discussion 2081. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Herrington, D.M.; Espeland, M.A.; Crouse, J.R., 3rd; Robertson, J.; Riley, W.A.; McBurnie, M.A.; Burke, G.L. Estrogen replacement and brachial artery flow-mediated vasodilation in older women. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1955–1961. [Google Scholar] [CrossRef]
- Mikkola, T.S.; Clarkson, T.B. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc. Res. 2002, 53, 605–619. [Google Scholar] [CrossRef]
- Mori, T.; Durand, J.; Chen, Y.; Thompson, J.A.; Bakir, S.; Oparil, S. Effects of short-term estrogen treatment on the neointimal response to balloon injury of rat carotid artery. Am. J. Cardiol. 2000, 85, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, Z.; Bischoff, J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.; Davis, M.; Elgendy, I.; Epps, K.; Lindley, K.J.; Mehta, P.K.; Michos, E.D.; Minissian, M.; Pepine, C.; Vaccarino, V.; et al. Summary of Updated Recommendations for Primary Prevention of Cardiovascular Disease in Women: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2602–2618. [Google Scholar] [CrossRef]
- Abramenko, N.; Vellieux, F.; Tesařová, P.; Kejík, Z.; Kaplánek, R.; Lacina, L.; Dvořánková, B.; Rösel, D.; Brábek, J.; Tesař, A.; et al. Estrogen Receptor Modulators in Viral Infections Such as SARS-CoV-2: Therapeutic Consequences. Int. J. Mol. Sci. 2021, 22, 6551. [Google Scholar] [CrossRef]
- Randolph, J.F., Jr.; Zheng, H.; Sowers, M.R.; Crandall, C.; Crawford, S.; Gold, E.B.; Vuga, M. Change in follicle-stimulating hormone and estradiol across the menopausal transition: Effect of age at the final menstrual period. J. Clin. Endocrinol. Metab. 2011, 96, 746–754. [Google Scholar] [CrossRef]
- Ausmanas, M.K.; Tan, D.A.; Jaisamrarn, U.; Tian, X.W.; Holinka, C.F. Estradiol, FSH and LH profiles in nine ethnic groups of postmenopausal Asian women: The Pan-Asia Menopause (PAM) study. Climacteric 2007, 10, 427–437. [Google Scholar] [CrossRef]
- Kling, J.M.; Dowling, N.M.; Bimonte-Nelson, H.A.; Gleason, C.E.; Kantarci, K.; Manson, J.E.; Taylor, H.S.; Brinton, E.A.; Lobo, R.A.; Cedars, M.I.; et al. Impact of menopausal hormone formulations on pituitary-ovarian regulatory feedback. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R912–R920. [Google Scholar] [CrossRef]
- Costa, R.; Tuomainen, T.P.; Virtanen, J.; Niskanen, L.; Bertone-Johnson, E. Associations of reproductive factors with postmenopausal follicle stimulating hormone. Womens Midlife Health 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Freeman, K.; Ayala, A.; Mullen, M.; Sun, Z.; Rhee, J.W. Cardiovascular Impact of Androgen Deprivation Therapy: From Basic Biology to Clinical Practice. Curr. Oncol. Rep. 2023, 25, 965–977. [Google Scholar] [CrossRef]
- Labrie, F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015, 145, 133–138. [Google Scholar] [CrossRef]
- Purohit, A.; Reed, M.J. Regulation of estrogen synthesis in postmenopausal women. Steroids 2002, 67, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Harada, N.; Sasano, H. Aromatase in atherosclerotic lesions of human aorta. J. Steroid Biochem. Mol. Biol. 2001, 79, 67–74. [Google Scholar] [CrossRef]
- Ishunina, T.A.; Fischer, D.F.; Swaab, D.F. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Vicennati, V.; Bertazzo, D.; Casimirri, F.; Pascal, G.; Tortelli, O.; Labate, A.M.M. Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-Menopause-Health Group. Metabolism 1997, 46, 5–9. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Sriprasert, I.; Karim, R.; Hwang-Levine, J.; Mack, W.J.; Hodis, H.N. Concentrations of endogenous sex steroid hormones and SHBG in healthy postmenopausal women. J. Steroid Biochem. Mol. Biol. 2022, 223, 106080. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Hodis, H.N.; Stanczyk, F.Z.; Lobo, R.A.; Mack, W.J. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J. Clin. Endocrinol. Metab. 2008, 93, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pieber, D.; Allport, V.C.; Hills, F.; Johnson, M.; Bennett, P.R. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol. Hum. Reprod. 2001, 7, 875–879. [Google Scholar] [CrossRef]
- Leslie, K.K.; Stein, M.P.; Kumar, N.S.; Dai, D.; Stephens, J.; Wandinger-Ness, A.; Glueck, D.H. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol. Oncol. 2005, 96, 32–41. [Google Scholar] [CrossRef][Green Version]
- Vazquez, F.; Rodriguez-Manzaneque, J.C.; Lydon, J.P.; Edwards, D.P.; O’Malley, B.W.; Iruela-Arispe, M.L. Progesterone regulates proliferation of endothelial cells. J. Biol. Chem. 1999, 274, 2185–2192. [Google Scholar] [CrossRef]
- Nisolle, M.; Gillerot, S.; Casanas-Roux, F.; Squifflet, J.; Berliere, M.; Donnez, J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum. Reprod. 1999, 14, 2844–2850. [Google Scholar] [CrossRef]
- Pedroza, D.A.; Subramani, R.; Lakshmanaswamy, R. Classical and Non-Classical Progesterone Signaling in Breast Cancers. Cancers 2020, 12, 2440. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Battaglia, A.; Grossini, E.; Mary, D.A.S.G.; Stoker, J.B.; Surico, N.; Vacca, G. The effect of progesterone on coronary blood flow in anaesthesized pigs. Exp. Physiol. 2001, 86, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Rupnow, H.L.; Phernetton, T.M.; Shaw, C.E.; Modrick, M.L.; Bird, I.M.; Magness, R.R. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1699–H1705. [Google Scholar] [CrossRef]
- Selles, J.; Polini, N.; Alvarez, C.; Massheimer, V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life Sci. 2001, 69, 815–827. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y. Protective actions of progesterone in the cardiovascular system: Potential role of membrane progesterone receptors (mPRs) in mediating rapid effects. Steroids 2013, 78, 583–588. [Google Scholar] [CrossRef]
- Giesen, J.A.S.; Rouver, W.D.N.; Costa, E.D.; Lemos, V.S.; Dos Santos, R.L. Sex differences in progesterone-induced relaxation in the coronary bed from normotensive rats. J. Mol. Endocrinol. 2019, 64, 91–102. [Google Scholar] [CrossRef]
- Cox, M.W.; Fu, W.; Chai, H.; Paladugu, R.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Effects of progesterone and estrogen on endothelial dysfunction in porcine coronary arteries. J. Surg. Res. 2005, 124, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-H.; Fan, Y.-Y.; Yang, C.-R.; Gao, X.-R.; Zhang, L.-L.; Hu, Y.; Wang, Y.-Q.; Jun, H. Progesterone amplifies oxidative stress signal and promotes NO production via H2O2 in mouse kidney arterial endothelial cells. J. Steroid Biochem. Mol. Biol. 2015, 155, 104–111. [Google Scholar] [CrossRef]
- Faulkner, J.L.; Kennard, S.; Huby, A.C.; Antonova, G.; Lu, Q.; Jaffe, I.Z.; Patel, V.S.; Fulton, D.J.R.; Belin de Chantemèle, E.J. Progesterone Predisposes Females to Obesity-Associated Leptin-Mediated Endothelial Dysfunction via Upregulating Endothelial MR (Mineralocorticoid Receptor) Expression. Hypertension 2019, 74, 678–686. [Google Scholar] [CrossRef]
- Nickenig, G.; Strehlow, K.; Wassmann, S.; Bäumer, A.T.; Albory, K.; Sauer, H.; Böhm, M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation 2000, 102, 1828–1833. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H. Progestogens and target tissues: Vascular systems. Maturitas 2009, 62, 356–361. [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone for treatment of symptomatic menopausal women. Climacteric 2018, 21, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, C.L.; Prior, J.C. Oral micronized progesterone for vasomotor symptoms--a placebo-controlled randomized trial in healthy postmenopausal women. Menopause 2012, 19, 886–893. [Google Scholar] [CrossRef]
- Casanova, G.; Spritzer, P.M. Effects of micronized progesterone added to non-oral estradiol on lipids and cardiovascular risk factors in early postmenopause: A clinical trial. Lipids Health Dis. 2012, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Kaemmle, L.M.; Stadler, A.; Janka, H.; von Wolff, M.; Stute, P. The impact of micronized progesterone on cardiovascular events—A systematic review. Climacteric 2022, 25, 327–336. [Google Scholar] [CrossRef]
- Lobo, R.A.; Kaunitz, A.M.; Santoro, N.; Bernick, B.; Graham, S.; Mirkin, S. Metabolic and cardiovascular effects of TX-001HR in menopausal women with vasomotor symptoms. Climacteric 2019, 22, 610–616. [Google Scholar] [CrossRef]
- Koh, K.K.; Jin, D.K.; Yang, S.H.; Lee, S.-K.; Hwang, H.Y.; Kang, M.H.; Kim, W.; Kim, D.S.; Choi, I.S.; Shin, E.K. Vascular effects of synthetic or natural progestagen combined with conjugated equine estrogen in healthy postmenopausal women. Circulation 2001, 103, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Coksuer, H.; Koplay, M.; Oghan, F.; Coksuer, C.; Keskin, N.; Ozveren, O. Effects of estradiol-drospirenone hormone treatment on carotid artery intima-media thickness and vertigo/dizziness in postmenopausal women. Arch. Gynecol. Obstet. 2011, 283, 1045–1051. [Google Scholar] [CrossRef]
- Vitale, C.; Mammi, C.; Gambacciani, M.; Russo, N.; Spoletini, I.; Fini, M.; Volterrani, M.; Rosano, G.M. Effect of hormone replacement therapy with the anti-mineralocorticoid progestin Drospirenone compared to tibolone on endothelial function and central haemodynamics in post-menopausal women. Int. J. Cardiol. 2017, 227, 217–221. [Google Scholar] [CrossRef]
- Preston, R.A.; White, W.B.; Pitt, B.; Bakris, G.; Norris, P.M.; Hanes, V. Effects of drospirenone/17-beta estradiol on blood pressure and potassium balance in hypertensive postmenopausal women. Am. J. Hypertens. 2005, 18, 797–804. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Leo, S.; De Franciscis, P.; Colacurci, N.; Paolisso, G. Short-term effects of low-dose estrogen/drospirenone vs low-dose estrogen/dydrogesterone on glycemic fluctuations in postmenopausal women with metabolic syndrome. Age 2014, 36, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Shala, B.A.; Miller, L.A.; Lerner, J.L.; McBrayer, J.D. Progestin enhances vasoconstrictor responses in postmenopausal women receiving estrogen replacement therapy. Menopause 2018, 25, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.; Mueck, A.O.; Teichmann, A.T.; Lippert, T.H. Effect of sequential estrogen/progestin treatment on biochemical vasoactive markers in postmenopausal women comparing oral and transdermal application. Clin. Exp. Obstet. Gynecol. 2000, 27, 17–20. [Google Scholar]
- Viinikka, L.; Orpana, A.; Puolakka, J.; Pyorala, T.; Ylikorkala, O. Different effects of oral and transdermal hormonal replacement on prostacyclin and thromboxane A2. Obstet Gynecol 1997, 89, 104–107. [Google Scholar] [CrossRef]
- Clarkson, T.B.; Anthony, M.S.; Wagner, J.D. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J. Clin. Endocrinol. Metab. 2001, 86, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Anthony, M.S.; Honoré, E.K.; Herrington, D.M.; Morgan, T.M.; Register, T.C.; Clarkson, T.B. Regression of atherosclerosis in female monkeys. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 827–836. [Google Scholar] [CrossRef]
- Azizian, H.; Khaksari, M.; Asadikaram, G.; Esmailidehaj, M.; Shahrokhi, N. Progesterone eliminates 17beta-estradiol-Mediated cardioprotection against diabetic cardiovascular dysfunction in ovariectomized rats. Biomed. J. 2021, 44, 461–470. [Google Scholar] [CrossRef]
- Levine, R.L.; Chen, S.J.; Durand, J.; Chen, Y.F.; Oparil, S. Medroxyprogesterone attenuates estrogen-mediated inhibition of neointima formation after balloon injury of the rat carotid artery. Circulation 1996, 94, 2221–2227. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Yanes, L.L.; Iliescu, R.; Fortepiani, L.A.; Granger, J.P. Testosterone supplementation in aging men and women: Possible impact on cardiovascular-renal disease. Am. J. Physiol. Renal Physiol. 2005, 289, F941–F948. [Google Scholar] [CrossRef]
- Jiroutek, M.R.; Chen, M.H.; Johnston, C.C.; Longcope, C. Changes in reproductive hormones and sex hormone-binding globulin in a group of postmenopausal women measured over 10 years. Menopause 1998, 5, 90–94. [Google Scholar] [CrossRef]
- Gonzales, R.J.; Ansar, S.; Duckles, S.P.; Krause, D.N. Androgenic/estrogenic balance in the male rat cerebral circulation: Metabolic enzymes and sex steroid receptors. J. Cereb. Blood Flow. Metab. 2007, 27, 1841–1852. [Google Scholar] [CrossRef]
- Oh, H.; Wild, R.A.; Manson, J.E.; Bea, J.W.; Shadyab, A.H.; Pfeiffer, R.M.; Saquib, N.; Underland, L.; Anderson, G.L.; Xu, X.; et al. Obesity, Height, and Serum Androgen Metabolism among Postmenopausal Women in the Women’s Health Initiative Observational Study. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.K. New perspectives on Mars and Venus: Unravelling the role of androgens in gender differences in cardiovascular biology and disease. Heart Lung Circ. 2007, 16, 185–192. [Google Scholar] [CrossRef]
- Wynne, F.L.; Khalil, R.A. Testosterone and coronary vascular tone: Implications in coronary artery disease. J. Endocrinol. Investig. 2003, 26, 181–186. [Google Scholar] [CrossRef]
- Higashiura, K.; Mathur, R.S.; Halushka, P.V. Gender-related differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth-muscle cells. J. Cardiovasc. Pharmacol. 1997, 29, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, X.; Yang, M.; Song, M.; Wang, K.; Giovannucci, E.; Ma, H.; Jin, G.; Hu, Z.; Shen, H.; et al. Sex-specific associations of circulating testosterone levels with all-cause and cause-specific mortality. Eur. J. Endocrinol. 2021, 184, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Ba, Z.F.; Wang, P.; Kuebler, J.F.; Rue, L.W.; 3rd Bland, K.I.; Chaudry, I.H. Flutamide induces relaxation in large and small blood vessels. Arch. Surg. 2002, 137, 1180–1186. [Google Scholar] [CrossRef]
- Horton, A.C.; Wilkinson, M.M.; Kilanowski-Doroh, I.; Dong, Z.; Liu, J.; Ogola, B.O.; Visniauskas, B.; Lindsey, S.H. Dihydrotestosterone induces arterial stiffening in female mice. Biol. Sex. Differ. 2024, 15, 9. [Google Scholar] [CrossRef]
- Sorva, R.; Kuusi, T.; Dunkel, L.; Taskinen, M.R. Effects of endogenous sex steroids on serum lipoproteins and postheparin plasma lipolytic enzymes. J. Clin. Endocrinol. Metab. 1988, 66, 408–413. [Google Scholar] [CrossRef]
- Mäkinen, J.I.; Perheentupa, A.; Irjala, K.; Pöllänen, P.; Mäkinen, J.; Huhtaniemi, I.; Raitakari, O.T. Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis 2008, 197, 688–693. [Google Scholar] [CrossRef]
- English, K.M.; Steeds, R.P.; Jones, T.H.; Diver, M.J.; Channer, K.S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation 2000, 102, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Leonardo, F.; Pagnotta, P.; Pelliccia, F.; Panina, G.; Cerquetani, E.; della Monica, P.L.; Bonfigli, B.; Volpe, M.; Chierchia, S.L. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation 1999, 99, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Sievers, C.; Klotsche, J.; Pieper, L.; Schneider, H.J.; März, W.; Wittchen, H.U.; Stalla, G.K.; Mantzoros, C. Low testosterone levels predict all-cause mortality and cardiovascular events in women: A prospective cohort study in German primary care patients. Eur. J. Endocrinol. 2010, 163, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, A.; Agardh, C.-D.; Shakir, Y.A.; Nerbrand, C.; Nyberg, P.; Lidfeldt, J.; Samsioe, G. Could androgens protect middle-aged women from cardiovascular events? A population-based study of Swedish women: The Women’s Health in the Lund Area (WHILA) Study. Climacteric 2007, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Montalcini, T.; Gorgone, G.; Gazzaruso, C.; Sesti, G.; Perticone, F.; Pujia, A. Endogenous testosterone and endothelial function in postmenopausal women. Coron. Artery Dis. 2007, 18, 9–13. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Zhou, H.-M.; Chen, F.-F.; Liu, Y.-P.; Han, L.; Song, M.; Wang, Z.-H.; Zhang, W.; Shang, Y.-Y.; Zhong, M. Testosterone ameliorates vascular aging via the Gas6/Axl signaling pathway. Aging 2020, 12, 16111–16125. [Google Scholar] [CrossRef] [PubMed]
- Son, B.K.; Kojima, T.; Ogawa, S.; Akishita, M. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J. Endocrinol. 2019, 241, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Chatterjee, K.; Beale, C.; Poole-Wilson, P.A.; Collins, P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 1995, 91, 1154–1160. [Google Scholar] [CrossRef]
- Costarella, C.E.; Stallone, J.N.; Rutecki, G.W.; Whittier, F.C. Testosterone causes direct relaxation of rat thoracic aorta. J. Pharmacol. Exp. Ther. 1996, 277, 34–39. [Google Scholar] [CrossRef]
- Chou, T.M.; Sudhir, K.; Hutchison, S.J.; Ko, E.; Amidon, T.M.; Collins, P.; Chatterjee, K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 1996, 94, 2614–2619. [Google Scholar] [CrossRef]
- Honda, H.; Unemoto, T.; Kogo, H. Different mechanisms for testosterone-induced relaxation of aorta between normotensive and spontaneously hypertensive rats. Hypertension 1999, 34, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zhao, J.; Jin, C.-W.; Li, Y.-H.; Tang, M.-X.; Wang, Z.-H.; Zhang, W.; Zhang, Y.; Li, L.; Zhong, M. Testosterone delays vascular smooth muscle cell senescence and inhibits collagen synthesis via the Gas6/Axl signaling pathway. Age 2016, 38, 60. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.; Cardozo, L.L.Y.; Oluwatade, T.N.; Leone, C.A.; Burgos, M.; Okifo, F.; Pal, L.; Reckelhoff, J.F.; Stachenfeld, N.S. Testosterone-associated blood pressure dysregulation in women with androgen excess polycystic ovary syndrome. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H232–H243. [Google Scholar] [CrossRef]
- Wild, S.; Pierpoint, T.; McKeigue, P.; Jacobs, H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: A retrospective cohort study. Clin. Endocrinol. 2000, 52, 595–600. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Bresciani, E.; Meanti, R.; Rizzi, L.; Omeljaniuk, R.J.; Torsello, A. The role of androgens in women’s health and wellbeing. Pharmacol. Res. 2021, 171, 105758. [Google Scholar] [CrossRef]
- Kloner, R.A.; Carson, C., 3rd; Dobs, A.; Kopecky, S.; Mohler, E.R., 3rd. Testosterone and Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 67, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Lessons From the Testosterone Trials. Endocr. Rev. 2018, 39, 369–386. [Google Scholar] [CrossRef]
- Hackett, G.I. Long Term Cardiovascular Safety of Testosterone Therapy: A Review of the TRAVERSE Study. World J. Mens Health 2025, 43, 282–290. [Google Scholar] [CrossRef]
- Ghowsi, M.; Sisakhtnezhad, S.; Wang, Y. Effect of testosterone on the mRNA expression of Wnt-2 and dickkopf1 (DKK1), collagen deposition and oxidative stress in the cardiac tissue in male rats. Cell. Mol. Biol. 2023, 69, 75–81. [Google Scholar] [CrossRef]
- Nácul, A.P.; Rezende, G.P.; Gomes, D.A.Y.; Maranhão, T.; Costa, L.O.B.F.; dos Reis, F.M.; Maciel, G.A.R.; Damásio, L.C.V.d.C.; Silva, A.C.J.d.S.R.e.; Lopes, V.M.; et al. Use of androgens at different stages of life: Climacterium. Rev. Bras. Ginecol. Obstet. 2022, 44, 83–88. [Google Scholar] [CrossRef]
- Vegunta, S.; Kling, J.M.; Kapoor, E. Androgen Therapy in Women. J. Womens Health 2020, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Eckenrode, H.E.; Carwie, J.C.; Curtis, L.M. Does Gender Affirming Hormone Therapy Increase the Risk of Kidney Disease? Semin. Nephrol. 2022, 42, 151284. [Google Scholar] [CrossRef] [PubMed]
- Nota, N.M.; Wiepjes, C.M.; de Blok, C.J.M.; Gooren, L.J.G.; Kreukels, B.P.C.; den Heijer, M. Occurrence of Acute Cardiovascular Events in Transgender Individuals Receiving Hormone Therapy. Circulation 2019, 139, 1461–1462. [Google Scholar] [CrossRef]
- Caceres, B.A.; Jackman, K.B.; Edmondson, D.; Bockting, W.O. Assessing gender identity differences in cardiovascular disease in US adults: An analysis of data from the 2014-2017 BRFSS. J. Behav. Med. 2020, 43, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Frisell, T.; Cnattingius, S.; Holmberg, D.; Holmberg, M.; Kollia, N.; Skalkidou, A.; Papadopoulos, F.C. Cardiovascular outcomes in transgender individuals in Sweden after initiation of gender-affirming hormone therapy. Eur. J. Prev. Cardiol. 2022, 29, 2017–2026. [Google Scholar] [CrossRef]
- Quinn, V.P.; Nash, R.; Hunkeler, E.; Contreras, R.; Cromwell, L.; Becerra-Culqui, T.A.; Getahun, D.; Giammattei, S.; Lash, T.L.; Millman, A.; et al. Cohort profile: Study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open 2017, 7, e018121. [Google Scholar] [CrossRef]
- Panel, A. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.L.; Howard, B.V.; Thomson, C.A.; Lacroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
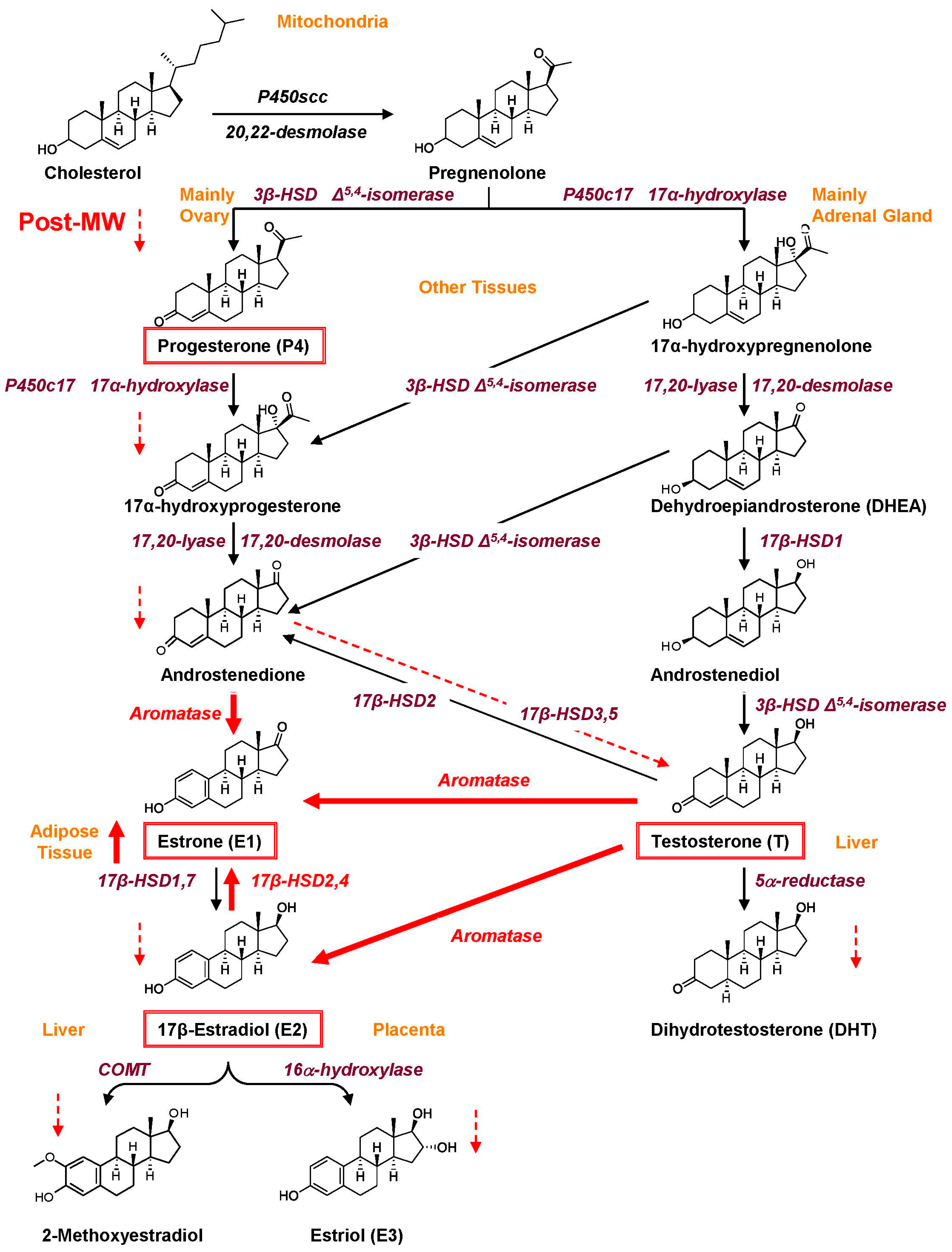

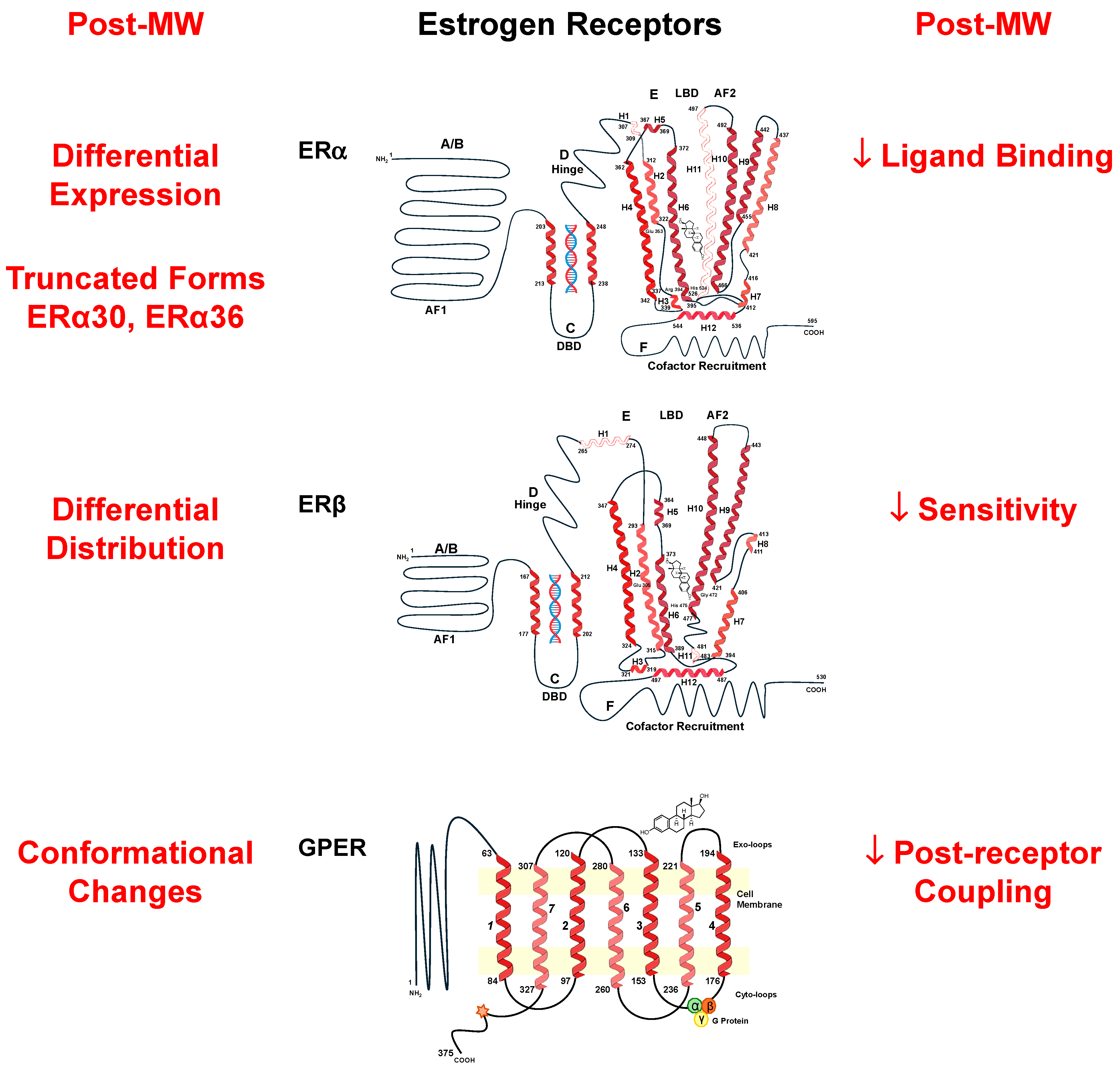
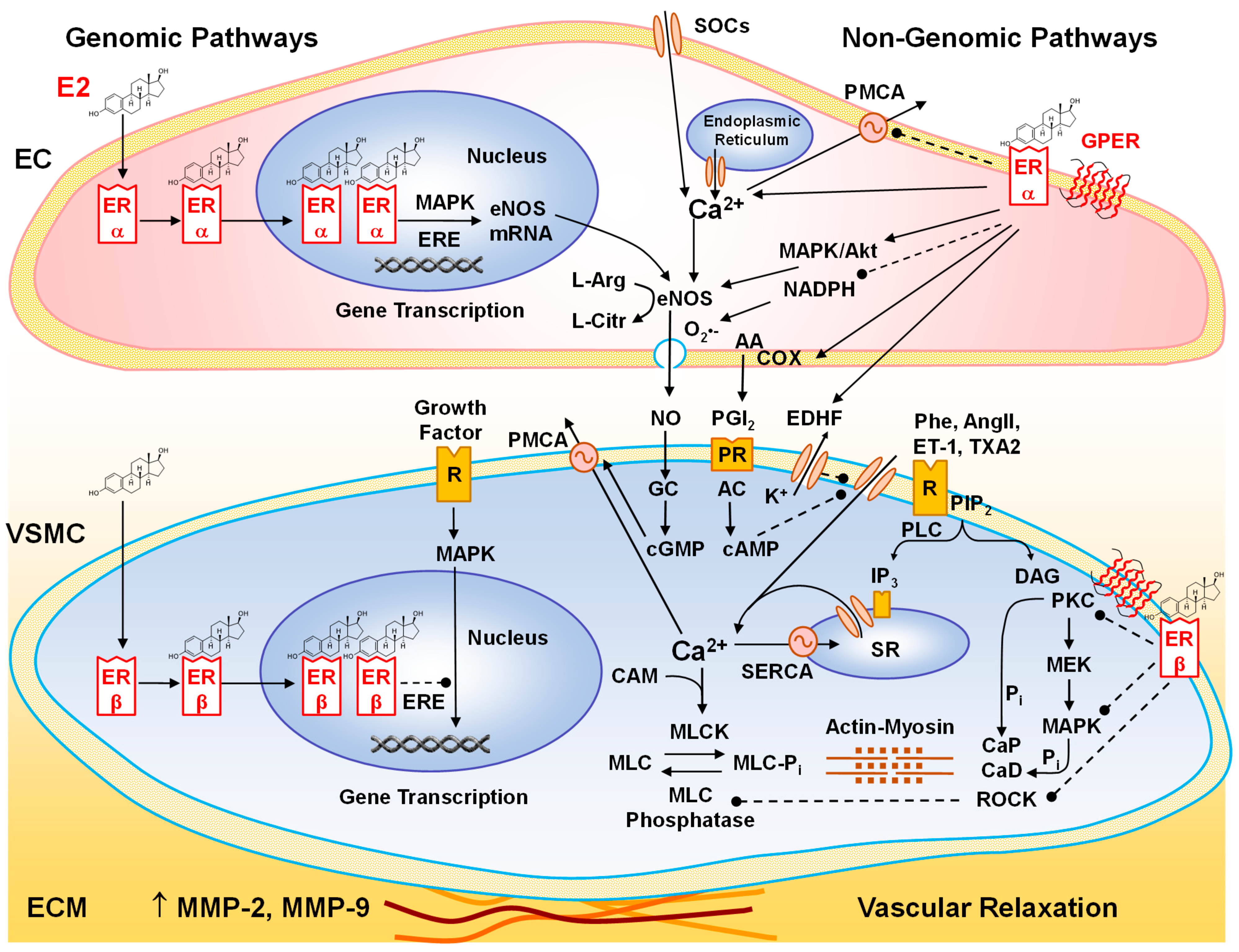

| Cell/Process | E2 | P4 | T | References |
|---|---|---|---|---|
| ECs | ||||
| Endothelium-dependent vascular relaxation | + | + | +/- | [140,141,142,143,144,145,146] |
| eNOS, NO | + | + | +/- | [140,141,142,143,144,145,146] |
| PGI2 | + | + | [147,148,149] | |
| EDHF | + | + | + | [150,151,152] |
| ROS production | - | + | + | [153,154,155] |
| Proliferation | + | + | [156,157,158,159] | |
| Migration | + | [156,157,158] | ||
| Angiogenesis | + | [158,160] | ||
| VSMCs | ||||
| Endothelium-independent vascular relaxation | + | + | + | [87,161,162] |
| [Ca2+]c, L-type Ca2+ channel | - | - | - | [161,162,163,164,165,166] |
| PMCA, SERCA | + | + | [167,168] | |
| BKCa | + | + | [169,170,171] | |
| Kv, KATP | + | [169,172] | ||
| PKC | - | - | [173,174] | |
| ROCK | - | [175] | ||
| Proliferation | - | +/- | +/- | [100,176,177,178,179,180] |
| Migration | - | +/- | [100,176,177,178] | |
| Apoptosis | + | + | [177,181] | |
| Fibroblasts | ||||
| MMP-2, MMP-9 | +/- | [182,183,184] | ||
| Fibrosis | - | [182,185,186] | ||
| Inflammation | - | - | +/- | [112,187,188,189,190] |
| Atherogenesis | - | - | [15,165] | |
| Cardiomyocytes | ||||
| β-adrenergic receptor | + | [191,192] | ||
| L-type Ca2+ channel | - | + | [191,192,193] | |
| Delayed-rectifier K+ channel | + | [193] | ||
| SERCA2a | + | + | [194,195] | |
| Kv2.1 channel | + | [196] | ||
| Contractility | - | - | + | [191,192,193,197] |
| Hypertrophy | - | + | [100,198,199,200,201] | |
| Glucose uptake | + | [202] | ||
| ATP synthesis | + | [203] | ||
| ROS Production | - | + | [203,204,205] | |
| β-oxidation, cell respiration | + | [206] | ||
| Proliferation | + | [47,207] | ||
| Apoptosis | - | [47,208] |
| Clinical Trial | Cohort Size, Age, Condition | Follow-Up Period | MHT | Outcome | Ref. |
|---|---|---|---|---|---|
| PEPI RDBPC | 875, 45–64 yr healthy | 3 yr (1991–1994) | CEE 0.625 mg CEE+MPA 2.5 mg Oral | CEE or CEE+progestin improves lipoproteins and lowers fibrinogen levels. | [303] |
| HERS RDBPC | 2763, 44–79 yr with CAD | 4.1 yr (1993–1997) | CEE 0.625 mg +MPA 2.5 mg Oral | No overall reduction in cardiovascular events. High risk of CVD in first year. | [296] |
| HERS II RDBPC Follow-up | 2321, 44–79 yr with CAD | 6.8 (2.7 yr added to HERS) (1993–1999) | CEE+MPA Oral | MHT did not reduce risk of cardiovascular events in women with CAD. | [297] |
| HERS-UA (Uric Acid) RDBPC | 2763, 44–79 yr | 4.1 yr (1993–1997) | CEE+MPA Oral | CEE+MPA lowered serum UA levels slightly, but neither baseline UA nor change in UA affected CHD risk. | [304] |
| WHI RDBPC | 16,608, 50–79 yr healthy 10,739 healthy with prior hysterectomy | 5.2 yr (1993–1998) | CEE+MPA Oral CEE Oral | Overall health risks exceeded benefits. Overall doubling of VTE events in CEE+MPA arm; 30% to 40% increased risk of stroke. | [295] |
| WEST RDBPC | 664, ~71 yr (46–91 yr) with prior ischemic stroke | 2.8 yr (1993- 1998) | E2 Oral | No reduction in mortality or recurrence of stroke. MHT should not be used for secondary prevention of cerebrovascular disease. | [302] |
| PHOREA RDBPC | 321, >55 yr (40–70 yr) | 48 weeks (1995–1996) | E2 1 mg + gestodene 0.025 mg Oral | Did not slow progression of subclinical atherosclerosis in Post-MW at increased risk. | [305] |
| ERA RDBPC | 309, ~65.8 yr with CAD | 3.2 yr (1996–1999) | CEE CEE+MPA, Oral | Increased HDL and decreased LDL but no change in coronary atherosclerosis progression. | [306] |
| WISDOM RDBPC | 5692, 50–69 yr | 1 yr (1999- 2002) | CEE CEE+MPA, Oral | MHT increased CVD and VTE risk when started many years postmenopause. | [307] |
| WAVE RDBPC | 423, >55 yr with coronary stenosis | 2.8 yr (1999–2002) | CEE+MPA Vitamin E, C Oral | No cardiovascular benefit of MHT or vitamin C and E. | [308] |
| ESTHER case-control | 271—case 610—control 45–70 yr | 6 yr (1999–2006) | Users of E2+ Progestogen Oral versus Trans-dermal | Oral but not transdermal E2 increases VTE risk. Norpregnanes may be thrombogenic. Micronized P4 and pregnanes are safe with respect to thrombotic risk. | [309] |
| RUTH RDBPC | 10,101, ~67.5 yr | 5.6 yr (2000–2005) | Raloxifene daily Oral | Did not affect risk of CVD. Benefits in reducing invasive breast cancer and vertebral fracture should be weighed against risk of VTE and stroke. | [310] |
| EPAT RDBPC | 222, ~57 yr healthy | 2 yr (2001–2003) | E2 Oral | Slower progression of subclinical atherosclerosis. | [311] |
| KEEPS DBRCT | 720, 42–58 yr, 6–36 months postmenopause | 5 yr (2005–2010) | Oral CEE 0.45 mg/d or Transdermal E2 50 μg weekly + Oral P4 200 mg/d 12 d/month | No effect on progression of carotid intima-thickness, atherosclerosis, or accrual of coronary calcium. No CAD benefits or adverse effects. | [312,313,314] |
| ELITE RDBPC | 643, <6 versus ≥10 yr postmenopause | 2005–2012 | Oral E2 1 mg/d ± Vaginal P4 gel 4% | Less progression of carotid-intima thickness and subclinical atherosclerosis when MHT was initiated within 6 yr but not ≥ 10 yr postmenopause. | [315] |
| DRSP+E2 RDBPC | 750, 45–75 yr, with HTN | 8 weeks (~2006) | DRSP 1–3 mg +E2 1 mg Oral | DRSP+E2 reduced BP in Post-MW with HTN. Decreased serum LDL-C. No increase in serum potassium. | [316] |
| E2+Micro P4 RDBPC | 172, 45–60 yr healthy | 1 yr (2010–2016) | Transdermal E2 0.1 mg patch +Micronized P4 200 mg/d oral 12 d/month | Lower diastolic BP and LDL-C. Improved cardiac autonomic baroreflex sensitivity. Prevented age-related decrease in brachial artery FMD (endothelium-dependent) and increase in stress reactivity score. | [317] |
| Soy ± isoflavone DBRCT | 200, ~55 yr, Healthy | 6 months (2012) | Oral 15 g/d soy ± 66 mg/d isoflavone for 6 months | Beneficial effect on systolic BP with soy protein plus isoflavones versus soy protein without isoflavones. | [318] |
| System | Tissue | Pre-MW | Post-MW | Function/Significance | Ref. |
|---|---|---|---|---|---|
| Eye/Vision | Retina, retinal pigment epithelium, ciliary body, iris, and lens epithelium | ERα | ↓ ERα | Alterations in ER may be involved age-related macular degeneration, idiopathic full-thickness macular hole, glaucoma, cataract, and dry eye. | [348] |
| Cardiovascular System | Coronary artery all layers, mainly VSMCs | ERβ > ERα | No change among MHT nonusers. ↓ ERα in MHT users. | Dominant role of ERβ in coronary arteries and in association with atherosclerosis and calcification. The association of ERβ and atherosclerotic severity is not linked to age. MHT may decrease ERα expression. | [108,115] |
| Aorta VSMCs | ERα ~30% ERβ ~70% | ERα ~23% ERβ 77% (MHT user) | Relative abundance of ERβ mRNA supports E2/ERβ-mediated inhibition of VSMC proliferation/migration. ERβ levels may not correlate with age. | [108] | |
| Peripheral arterioles from discarded adipose tissue | ERβ and GPER in ECs and ERα in adventitia | ↓↓ ERβ ↓↓ GPER | Continuous E2 exposure in cis- and transgender females or Post-MW may suppress EC ERβ and GPER, causing oxidative stress, decreased FMD, and microvascular damage. | [255] | |
| Peripheral veins | ERα in ECs ↓ in early versus late follicular phase of menstrual cycle | ↓↓ ERα | ERα levels positively related to eNOS expression, ser1177 phosphorylation, and endothelium-dependent brachial artery FMD and are modulated by fluctuations in E2 status during menopausal cycle and age-related E2 deficiency in Post-MW. | [361] | |
| Digestive System | Buccal mucosa, minor salivary, submandibular, and parotid glands | ERs | ↓ ERs | E2/ER-responsive tissues. MHT may be effective for menopausal mucosal diseases and oral discomfort. | [365,366] |
| Anal canal hemorrhoid tissue | ERs | No change | No differences in anal canal ERs in relation to menopausal status or age. | [367] | |
| Adipose Tissue | Visceral and subcutaneous | ERα>ERβ | ↓ ERα ↑ ERβ | ↓ ERα/ERβ ratio may affect adipose tissue metabolism in Post-MW. | [368,369,370,371] |
| Nervous System | Dorsolateral supraoptic nucleus | ERβ>ERα | ↑ ERα ↓↓ ERβ | ↓ ERβ and ↑ ERα in Post-MW may activate arginine vasopressin neurons and increase incidence of HTN and CVD. | [372] |
| Reproductive System | Vaginal wall and uterosacral ligaments | ERα and ERβ | ↓ ERα ↓↓ ERβ | Decreased ER expression, particularly ERβ, may play a role in common symptoms of vaginal atrophy and dryness in Post-MW. | [373,374] |
| Myometrium | ERβ/ERα ratio 0.6–1.5 | ↓ ERα ↑ ERβ ERβ:ERα ratio 2.5–7.6 | Altered ERα and ERβ expression may be associated with pathological myometrium growth. | [375] | |
| Ovaries | ERα in thecal cells, ERβ in granulosa cells, and ERα and ERβ in interstitial gland cells | ↓↓ ERβ | In Pre-MW, high ERβ expression in granulosa cells may be associated with growth and development of follicles, which are reduced in Post-MW. | [376] |
| Menopausal Stage | Early | Perimenopause | Late |
|---|---|---|---|
| Age (years) | 40–50 | 50–60 | >60 |
| Years since menopause | Within 10 | 10–15 | >15 |
| Smoking | - | + | + |
| Cardiovascular health | |||
| BMI | 18.5–25 (Normal) | 25–30 (Overweight) | ≥30 (Obese) |
| Physical activity | Active | Limited | Sedentary |
| BP (systolic/diastolic) mmHg | <120/<80 Normal | 120–129/<80 Elevated | ≥130/≥80 HTN |
| 10-year ASCVD risk | <5% | 5–10% | >10% |
| Pre-existing CVD | - | - | Congenital heart disease ASCVD/CAD/PAD Clotting disorders, VTE MI, stroke/TIA |
| Other pre-existing conditions | |||
| Diabetes/metabolic syndrome | - | + | ++ |
| Lipid profile Hyperlipidemia | ↑ HDL, ↓ LDL - | ↓ HDL, ↑ LDL + | ↓↓ HDL, ↑↑ LDL ++ |
| Autoimmune disease | - | + | ++ |
| Breast/uterine cancer | Low risk | High risk | Detected |
| MHT risk | Low | Intermediate | High |
| MHT guide | |||
| Route | Oral/transdermal | Transdermal preferred | Avoid oral/transdermal Topical preferred |
| Dose To control menopausal and genitourinary symptoms | E2 1 mg/d oral or 0.025–0.05 mg/d transdermal + Micronized P4 100–200 mg/d oral | E2 0.5 mg/d oral or 0.025 mg/d transdermal (preferred) + Micronized P4 100 mg/d oral | Vaginal E2 at minimal effective dose |
| Other | Transdermal E2 reduces risk of VTE/stroke | Lifestyle adjustments. Diet, exercise, lipid-lowering drugs (statins) | Consider non-hormonal treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Khalil, R.A. Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women. Int. J. Mol. Sci. 2025, 26, 5078. https://doi.org/10.3390/ijms26115078
Xia W, Khalil RA. Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women. International Journal of Molecular Sciences. 2025; 26(11):5078. https://doi.org/10.3390/ijms26115078
Chicago/Turabian StyleXia, Wenhan, and Raouf A. Khalil. 2025. "Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women" International Journal of Molecular Sciences 26, no. 11: 5078. https://doi.org/10.3390/ijms26115078
APA StyleXia, W., & Khalil, R. A. (2025). Hormone Replacement Therapy and Cardiovascular Health in Postmenopausal Women. International Journal of Molecular Sciences, 26(11), 5078. https://doi.org/10.3390/ijms26115078







