Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines
Abstract
1. Introduction
2. Results
2.1. The Comet Assay
2.2. The Micronucleus Assay
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Genotoxicity Assays
4.2.1. The Comet Assay
4.2.2. The Micronucleus Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bresson, C.; Lamouroux, C.; Sandre, C.; Tabarant, M.; Gault, N.; Poncy, J.L.; Lefaix, J.C.; Den Auwer, C.; Speziae, R.; Gaigeot, M.-P.; et al. An interdisciplinary approach to investigate the impact of cobalt in a human keratinocyte cell line. Biochimie 2008, 88, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- González-Montaña, J.-R.; Escalera-Valente, F.; Alonso, A.J.; Lomillos, J.M.; Robles, R.; Alonso, M.E. Relationship between Vitamin B12 and Cobalt Metabolism in Domestic Ruminant: An Update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef]
- Ma, Y.; Lin, W.; Ruan, Y.; Lu, H.; Fan, S.; Chen, D.; Huang, Y.; Zhang, T.; Pi, J.; Xu, J.-F. Advances of Cobalt Nanomaterials as Anti-Infection Agents, Drug Carriers, and Immunomodulators for Potential Infectious Disease Treatment. Pharmaceutics 2022, 14, 2351. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A. Selected aspects of the action of cobalt ions in the human body. Centr Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 1–32. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Chromium (VI) Compounds. Monograph 100C; WHO: Geneva, Switzerland, 1990. [Google Scholar]
- Vincent, J.B. New Evidence against Chromium as an Essential Trace Element. J. Nutr. 2017, 147, 2212–2219. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef]
- Bawiec, P.; Sawicki, J.; Łasińska-Pracuta, P.; Czop, M.; Sowa, I.; Helon, P.; Pietrzak, K.; Koch, W. In Vitro Evaluation of Bioavailability of Cr from Daily Food Rations and Dietary Supplements from the Polish Market. Nutrients 2024, 16, 1022. [Google Scholar] [CrossRef] [PubMed]
- Talab, A.T.; Abdollahzad, H.; Nachvak, S.M.; Pasdar, Y.; Eghtesadi, S.; Izadi, A.; Aghdashi, M.A.; Mohammad Hossseini Azar, M.R.; Moradi, S.; Mehaki, B.; et al. Effects of Chromium Picolinate Supplementation on Cardiometabolic Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Clin. Nutr. Res. 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, E.; Jurkowska, K.; Piwowar, A. Chromium (III) and chromium (VI) as important players in the induction of genotoxicity–current view. Ann. Agric. Environ. Med. 2021, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Watts, M.J. Chromium speciation in foodstuffs: A review. Food Chem. 2018, 250, 105–112. [Google Scholar] [CrossRef]
- Obied, B.; Richard, S.; Zahavi, A.; Fixler, D.; Girshevitz, O.; Goldenberg-Cohen, N. Structure Function Correlation in Cobalt Induced Brain Toxicity. Cells 2024, 13, 1765. [Google Scholar] [CrossRef]
- Gómez-Arnaiz, S.; Tate, R.J.; Grant, M.H. Cytotoxicity of cobalt chloride in brain cell lines—A comparison between astrocytoma and neuroblastoma cells. Toxicol. Vitr. 2020, 68, 104958. [Google Scholar] [CrossRef]
- Gómez-Arnaiz, S.; Tate, R.J.; Grant, M.H. Cobalt Neurotoxicity: Transcriptional Effect of Elevated Cobalt Blood Levels in the Rodent Brain. Toxics 2022, 10, 59. [Google Scholar] [CrossRef]
- Chimeh, U.; Zimmerman, M.A.; Gilyazova, N.; Li, P.A. B355252, a novel small molecule, confers neuroprotection against cobalt chloride toxicity in mouse hippocampal cells through altering mitochondrial dynamics and limiting autophagy induction. Int. J. Med. Sci. 2018, 15, 1384–1396. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Y.; Zhang, C.; Wu, C.; Xu, X.; Xiao, K.; Zhao, Z.; Zhang, H. The Impact of Cobalt Species on the Hazardous Characteristics of Cobalt-Leaching Residue: A Case Study from Guangdong Province, China. Water 2024, 16, 2953. [Google Scholar] [CrossRef]
- Hedberg, Y.S. Chromium and leather: A review on the chemistry of relevance for allergic contact dermatitis to chromium. J. Leather Sci. Eng. 2020, 2, 20. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Chemical properties and toxicity of chromium (III) nutritional supplements. Chem. Res. Toxicol. 2008, 21, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lison, D.; van den Brule, S.; Van Maele-Fabry, G. Cobalt and its compounds: Update on genotoxic and carcinogenic activities. Crit. Rev. Toxicol. 2018, 48, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Smith, L.J.; Holmes, A.L.; Zheng, T.; Wise, J.P., Sr. The cytotoxicity and genotoxicity of soluble and particulate cobalt in human lung epithelial cells. Environ. Mol. Mutagen. 2016, 57, 282–287. [Google Scholar] [CrossRef]
- Pereira, S.C.; Oliveira, P.F.; Oliveira, S.R.; Pereira, M.d.L.; Alves, M.G. Impact of Environmental and Lifestyle Use of Chromium on Male Fertility: Focus on Antioxidant Activity and Oxidative Stress. Antioxidants 2021, 10, 1365. [Google Scholar] [CrossRef]
- Cannas, D.; Loi, E.; Serra, M.; Firinu, D.; Valera, P.; Zavattari, P. Relevance of Essential Trace Elements in Nutrition and Drinking Water for Human Health and Autoimmune Disease Risk. Nutrients 2020, 12, 2074. [Google Scholar] [CrossRef]
- Czarnek, K.; Tatarczak-Michalewska, M.; Wójcik, G.; Szopa, A.; Majerek, D.; Fila, K.; Hamitoglu, M.; Gogacz, M.; Blicharska, E. Nutritional Risks of Heavy Metals in the Human Diet—Multi-Elemental Analysis of Energy Drinks. Nutrients 2024, 16, 4306. [Google Scholar] [CrossRef]
- Wu, C.-W.; Jiang, S.-J.; Sahayam, A.C.; Huang, Y.-L. Determination of cobalt compounds in dietary supplements using liquid chromatography inductively coupled plasma mass spectrometry. Spectrochim. Acta Part. B At. Spectrosc. 2019, 154, 70–74. [Google Scholar] [CrossRef]
- Leśniewicz, A.; Grzesiak, M.; Żyrnicki, W.; Borkowska-Burnecka, J. Mineral Composition and Nutritive Value of Isotonic and Energy Drinks. Biol. Trace Elem. Res. 2016, 170, 485–495. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A.; Welna, M.; Pohl, P. Determination of Elements in Energy Drinks by ICP OES with Minimal Sample Preparation. J. Braz. Chem. Soc. 2013, 24, 1606–1612. [Google Scholar] [CrossRef]
- Kilic, S.; Cengiz, M.F.; Kilic, M. Monitoring of Metallic Contaminants in Energy Drinks Using ICP-MS. Environ. Monit. Assess. 2018, 190, 202. [Google Scholar] [CrossRef] [PubMed]
- Szczęsny, G.; Kopec, M.; Kowalewski, Z.L. Toxicity, Irritation, and Allergy of Metal Implants: Historical Perspective and Modern Solutions. Coatings 2025, 15, 361. [Google Scholar] [CrossRef]
- Tomova, Z.; Tomov, D.; Davcheva, D.; Uzunova, Y. Salivary Chromium and Cobalt Concentrations in Patients with Dental Metallic Restorations—A Pilot Study. Dent. J. 2024, 12, 362. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Sansone, V. The Effects on Bone Cells of Metal Ions Released from Orthopaedic Implants. A Review. Clin. Cases Min. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular Analysis of Chromium and Cobalt-Related Toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- Toh, W.; Tan, X.; Bhowmik, A.; Liu, E.; Tor, S. Tribochemical Characterization and Tribocorrosive Behavior of CoCrMo Alloys: A Review. Materials 2017, 11, 30. [Google Scholar] [CrossRef]
- Hannemann, F.; Hartmann, A.; Schmitt, J.; Lützner, J.; Seidler, A.; Campbell, P.; Delaunay, C.P.; Drexler, H.; Ettema, H.B.; García-Cimbrelo, E.; et al. European Multidisciplinary Consensus Statement on the Use and Monitoring of Metal-on-Metal Bearings for Total Hip Replacement and Hip Resurfacing. Orthop. Traumatol. Surg. Res. 2013, 99, 263–271. [Google Scholar] [CrossRef]
- Glaß, H.; Jonitz-Heincke, A.; Petters, J.; Lukas, J.; Bader, R.; Hermann, A. Corrosion Products from Metallic Implants Induce ROS and Cell Death in Human Motoneurons In Vitro. J. Funct. Biomater. 2023, 14, 392. [Google Scholar] [CrossRef]
- Catelas, I.; Petit, A.; Vali, H.; Fragiskatos, C.; Meilleur, R.; Zukor, D.J.; Antoniou, J.; Huk, O.L. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials 2005, 26, 2441–2453. [Google Scholar] [CrossRef]
- Filon, F.L.; D’Agostin, F.; Crosera, M.; Adami, G.; Bovenzi, M.; Maina, G. In vitro absorption of metal powders through intact and damaged human skin. Toxicol. Vitr. 2009, 23, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Eastmond, D.A.; MacGregor, J.T.; Slesinki, R.S. Trivalent Chromium: Assessing the genotoxic risk of the essential trace element and widely used human and animal nutritional supplement. Crit. Rev. Toxicol. 2008, 38, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Kierstin, V.; Grawe, P.; Karlsson, O.M.; Abramsson-Zetterberg, L.A.G.; Hellman, B.E. Evaluation of the potential genotoxicity of chromium picolinate in mammalian cells in vivo and in vitro. Food Chem. Toxicol. 2007, 45, 1097–1106. [Google Scholar] [CrossRef]
- Andrews, R.E.; Shah, K.M.; Wilkinson, J.M.; Gartland, A. Effect of cobalt and chromium ions at clinically equivalent concentration after meta-on-metal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone 2011, 49, 717–723. [Google Scholar] [CrossRef]
- Pulido, M.D.; Parrish, A.R. Metal-induced apoptosis: Mechanisms. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2003, 533, 227–241. [Google Scholar] [CrossRef]
- Ebert, B.; Jelkmann, W. Intolerability of cobalt salt as erythropoietic agent. Drug Test. Anal. 2014, 6, 185–189. [Google Scholar] [CrossRef]
- Battaglia, V.; Compagnone, A.; Bandino, A.; Bragadin, M.; Rosii, C.A.; Zanetti, F.; Colombatto, S.; Grillo, M.A.; Toninello, A. Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int. J. Biochem. Cell Biol. 2009, 41, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, W.; Zhao, X.; Liu, Y.; Cheng, Z.; Liu, Y.; Liu, J. Oxidative stress and histological alterations of chicken brain induced by oral administration of chromium(III). Biol. Trace Elem. Res. 2016, 173, 185–193. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, A.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterials 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Bucher, J.R.; Hailey, J.R.; Roycroft, J.R.; Hanseman, J.K.; Sillis, R.C.; Grumbein, S.L.; Mellick, P.W.; Chou, B.J. Inhalation toxicity and carcinogenicity studiem of cobalt sulfate. Toxicol. Sci. 1999, 49, 56–67. [Google Scholar] [CrossRef]
- Ducros, V. Chromium metabolism. Biol. Trace Elem. Res. 1992, 32, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Figgitt, M.; Newson, N.; Lesliec, I.J.; Fisher, J.; Ingham, E.; Case, C.P. The genotoxicity of physiological concentrations of chromium (Cr(III) and Cr(VI)) and cobalt (Co(II)): An in vitro study. Mutat. Res. 2010, 688, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bestic, J.M.; Berquist, T.H. Current Concepts in Hip Arthroplasty Imaging: Metal-on-Metal Prostheses, Their Complications, and Imaging Strategies. Semin. Roentgenol. 2013, 48, 178–186. [Google Scholar] [CrossRef]
- Ceriotti, L.; Pontia, F.; Broggi, F.; Kob, A.; Drechsler, S.; Thedinga, E.; Colpo, P.; Sabbioni, E.; Ehret, R.; Rossi, F. Real-time assessment of cytotoxicity by impedance measurement on a 96-well plate. Sens. Actuator B Chem. 2007, 123, 769–778. [Google Scholar] [CrossRef]
- Smith, L.J.; Holmes, A.L.; Kandpal, S.K.; Mason, M.D.; Zheng, T.; Wise, S.P. The cytotoxicity and genotoxicity of soluble and particulate cobalt in human lung fibroblast cells. Toxicol. Appl. Pharmacol. 2014, 278, 259–265. [Google Scholar] [CrossRef]
- Karovic, O.; Tonazzini, I.; Robola, N.; Edstrom, E.; Lovdahl, C.; Fredholm, B.B.; Dare’, E. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1α. Biochem. Pharmacol. 2007, 73, 694–708. [Google Scholar] [CrossRef]
- Ortega, R.; Bresson, C.; Fraysse, A.; Sandre, C.; Devec, G.; Gombert, C.; Tabarant, M.; Bleuet, P.; Seznece, H.; Simionovici, A.; et al. Cobalt distribution in keratinocyte cells indicates nuclear and perinuclear accumulation and interaction with magnesium and zinc homeostasis. Toxicol. Lett. 2009, 188, 26–32. [Google Scholar] [CrossRef]
- Ermolli, M.; Menne’, C.; Pozzi, G.; Serra, M.A.; Clerici, L.A. Nickel, cobalt and chromium-induced cytotoxicity and intracellular accumulation in human hacat keratinocytes. Toxicology 2001, 159, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schmuki, M.K.; Park, J. Engineering biocompatible implant surfaces, Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Czarnek, K.; Siwicki, A.K. Genotoxicity of chromium (III) and cobalt (II) and interactions between them. Curr. Issues Pharm. Med. Sci. 2021, 34, 142–148. [Google Scholar] [CrossRef]
- Gajski, G.; Jelčić, Z.; Oreščić, Ž.; Oreščanin, V.; Gerič, M.; Kollar, R.; Garaj-Vrhovac, V. Physico-chemical characterization and the in vitro genotoxicity of medical implants metal alloy (TiAlV and CoCrMo) and polyethylene particles in human lymphocytes. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foregin body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Asmuss, M.; Mullenders, L.H.; Eker, A.; Hartwig, A. Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis 2000, 21, 2097–2104. [Google Scholar] [CrossRef]
- Finley, B.L.; Monnot, A.D.; Paustenbach, D.J.; Gaffney, S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharmacol. 2012, 64, 491–503. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Skalny, A.V.; Suliburska, J.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 2017, 41, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Hu, F.B. Role of chromium in human health and in diabetes. Diabetes Care 2004, 27, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Burgaz, S.; Demircigil, G.Ç.; Yılmazer, M.; Erta, N.; Kemaloglu, Y.; Burgaz, Y. Assessment of cytogenetic damage in lymphocytes and in exfoliated nasal cells of dental laboratory technicians exposed to chromium, cobalt, and nikel. Mutat. Res. Fund. Mol. Mech. Mutagen. 2002, 521, 47–56. [Google Scholar] [CrossRef]
- Christova, T.Y.; Gorneva, G.A.; Taxirov, S.I.; Duridanova, B.; Setchenska, M.S. Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma–Bering mice: Protective role of heme oxygenase. Toxicol. Lett. 2003, 138, 235–242. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Huk, O.L.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Petit, A.; Tabrizian, M. The molecular structure of complexes formed by chromium or cobalt ions in simulated physiological fluids. Biomaterials 2009, 30, 460467. [Google Scholar] [CrossRef]
- Gault, N.; Sandre, C.; Poncy, J.-L.; Moulin, C.; Lefaix, J.-L.; Bresson, C. Cobalt toxicity: Chemical and radiological combined effects on HaCaT keratinocyte cell line. Toxicol. Vitr. 2010, 24, 92–98. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhu, Y.; Zhang, Y. Interaction of chromium(III) or chromium(VI) with catalase and its effect on the structure and function of catalase: An in vitro study. Food Chem. 2018, 244, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Liu, J.; Malkas, L.H.; Catalano, J.; Algaharu, S.; Hickey, R.J. Chromium reduces the in vitro activity and fidelity of DNA replication Mediatel by the human cell DNA replication mediated by the human cell DNA synhesome. Toxicol. Appl. Pharmacol. 2009, 236, 154–165. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.J.; Ceryak, S.; Patierno, S.R. Complexities of chromium carcinogenesis: Role of cellular response repair and recovery mechanisms. Mutat. Res. 2003, 533, 3–36. [Google Scholar] [CrossRef]
- Snow, E.T. A possibile role for chromium(III) in genotoxicity. Environ. Health Perspect. 1991, 92, 75–81. [Google Scholar] [CrossRef]
- Terpiłowska, A.; Siwicki, A.K. Cell cycle and transmembrane mitochondrial potential analysis after treatment with chromium(III), iron(III), molybdenum(III) or nickel(II) and their mixtures. Toxicol. Res. 2019, 8, 188–195. [Google Scholar] [CrossRef]
- Seoane, A.I.; Dulout, F.N. Genotoxic ability of cadium, chromium and nickel salts studiem by kineto chore staining in the cytokinesis–blocked micronucleus assay. Mutat. Res. 2001, 490, 99–106. [Google Scholar] [CrossRef]
- Manygoats, K.R.; Yazzie, M.; Stearns, D.M. Ultrastructural damage in chromium picolinate-treated cells: A TEM study. J. Biol. Inorg. Chem. 2002, 7, 791–798. [Google Scholar] [CrossRef]
- Shrivastava, H.Y.; Ravikumar, T.; Shanmugasundaram, N.; Babu, M.; Nair, B.U. Cytotoxicity studiem of chromiu(III) complexes on human dermal fibroblasts. Free Radic. Biol. Med. 2005, 38, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Staniek, H.; Kostrzewska-Poczekaj, M.; Arndt, M.; Szyfterb, K.; Krejpcio, Z. Genotoxicity assessment of chromium(III) propionate complex in the rat model using the comet assay. Food Chem. Toxicol. 2010, 48, 89–92. [Google Scholar] [CrossRef]
- Hepburn, D.D.D.; Vincent, J.B. Tissue and subcellular distribution of chromium picolinate with time after entering the bloodstream. J. Inorg. Biochem. 2003, 94, 86–93. [Google Scholar] [CrossRef]
- Fan, W.-T.; Zhao, X.-N.; Cheng, J.; Liu, Y.-H.; Liu, J.-Z. Oxidative Stress and Hepatocellular Injury Induced by Oral Administration of Cr3+ in Chicken. J. Biochem. Mol. Toxicol. 2015, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Terpiłowska, S.; Siwicki, A.K. Pro- and antioxidant activity of chromium(III), iron(III), molybdenum(III) or nickel(II) and their mixtures. Chem. Biol. Interact. 2019, 298, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Mwale, F.; Tkaczyk, C.; Antoniou, J.; Zukor, D.J.; Huk, O.L. Induction of protein oxidation by kobalt and chromium ions in human U937 macrophages. Biomaterials 2005, 26, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Evangelou, A. The role of oxidative stress in mechanisms of metal– induced carcinogenesis. Crit. Rev. Oncol. Hematol. 2002, 142, 93–103. [Google Scholar] [CrossRef]
- Frís, M.; Sánchez de Rojas, M.I. Total and soluble chromium, nickel and cobalt content in the main materials used in the manufacturing of Spanish commercial cements. Cement Concr. Res. 2002, 32, 435–440. [Google Scholar] [CrossRef]
- Galanis, A.; Karapetsas, A.; Sandaltzopoulos, R. Metal-induced carcinogenesis, oxidative stress and hypoxia signalling. Mutat. Res. Fund. Mol. Mech. Mutagen. 2009, 674, 31–35. [Google Scholar] [CrossRef]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
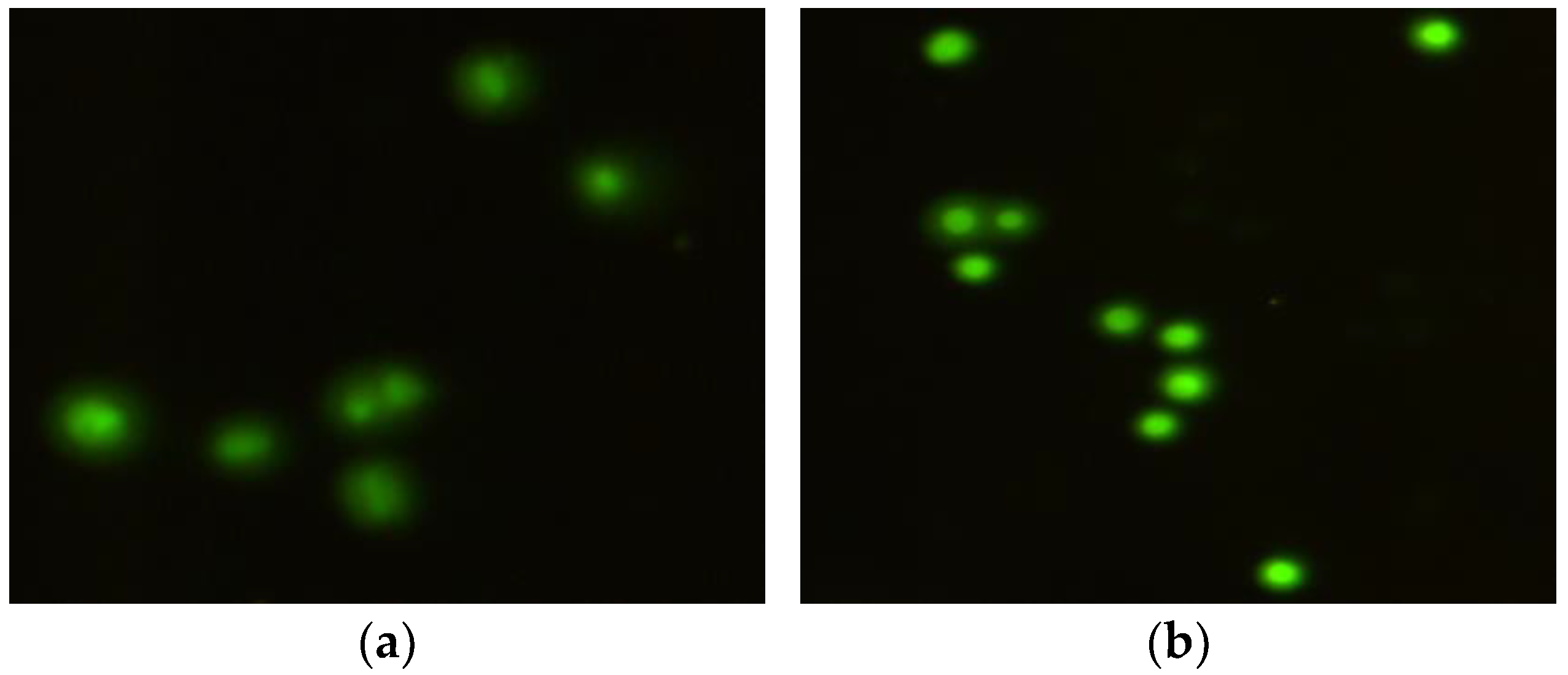


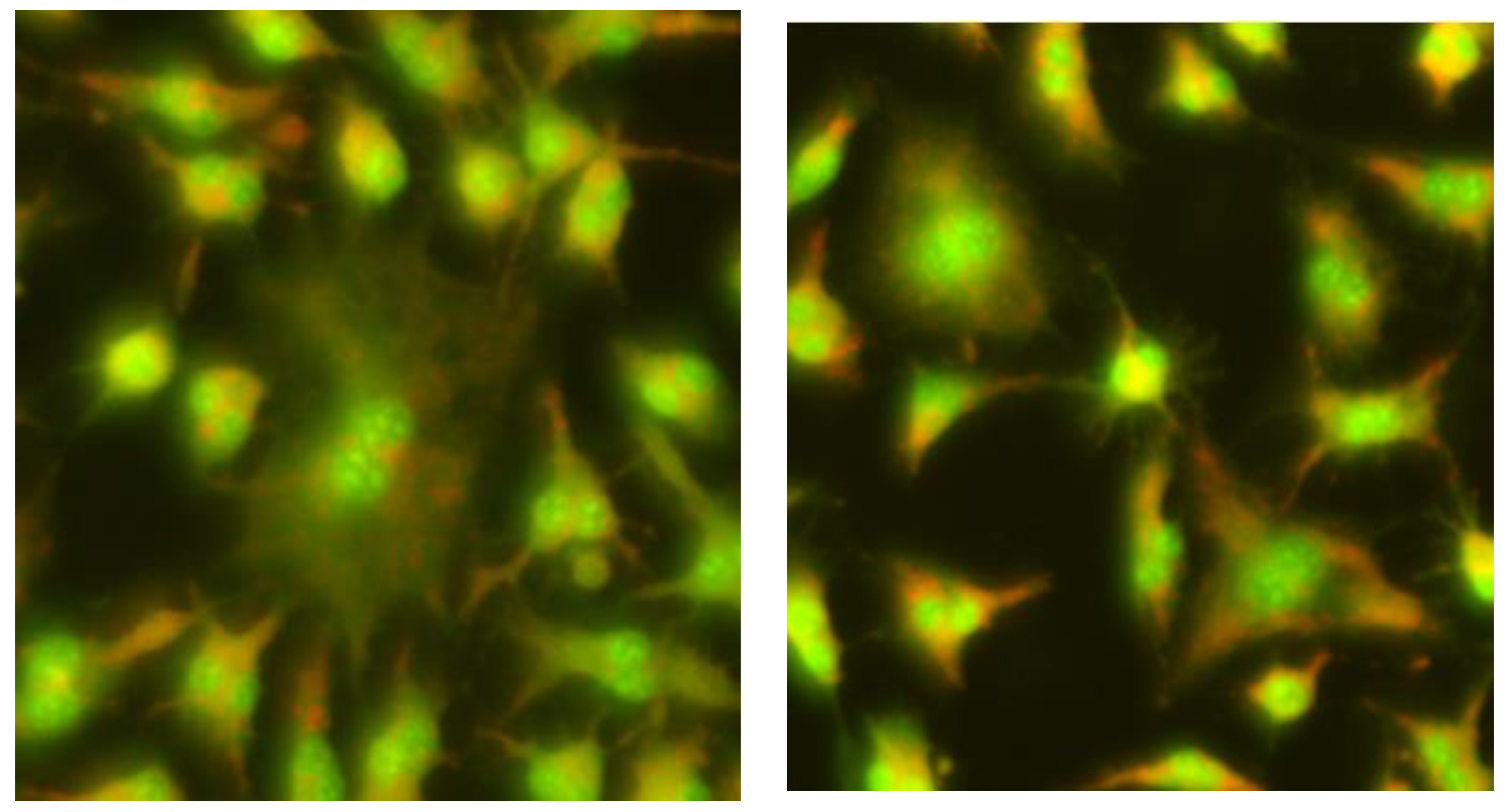
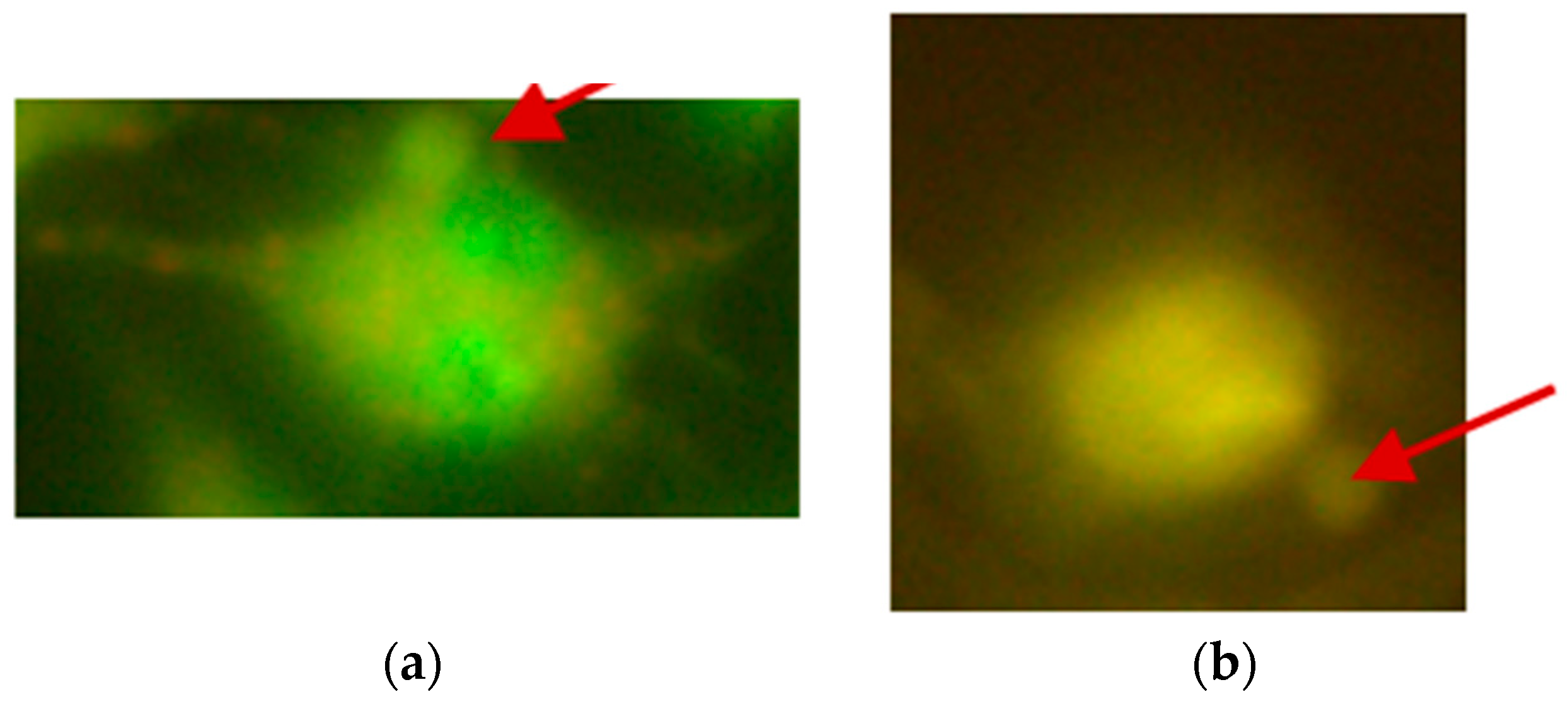
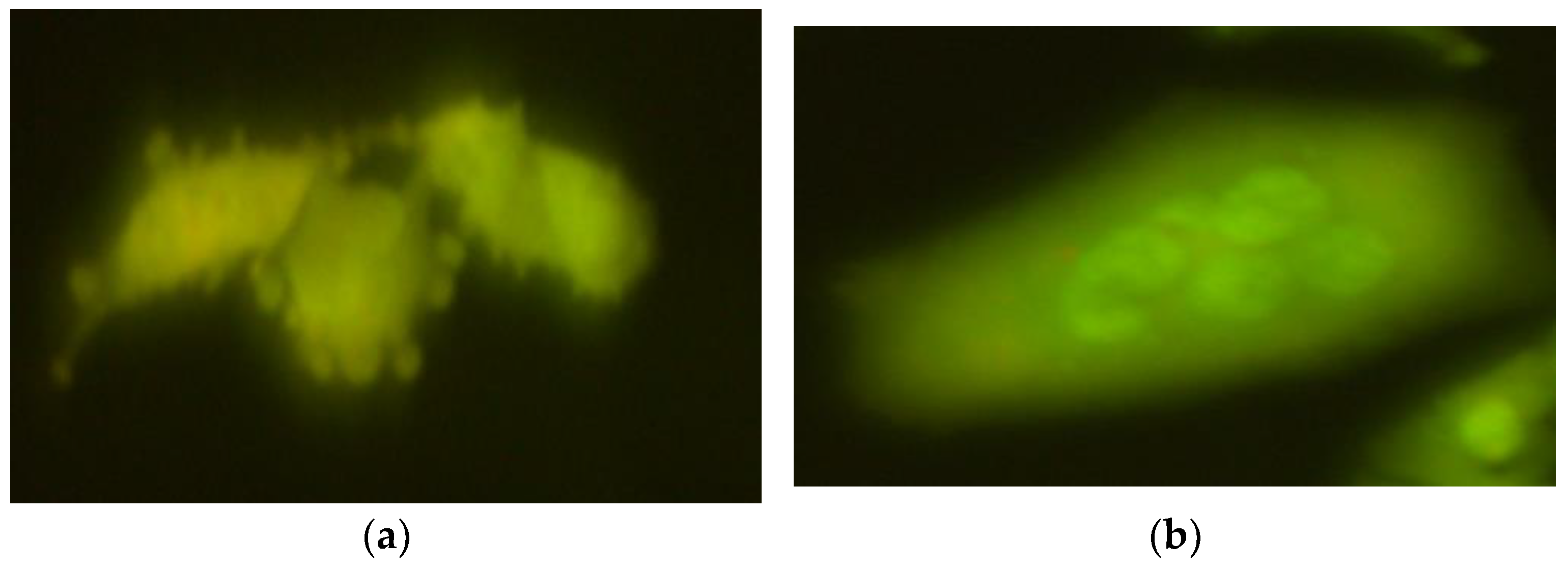


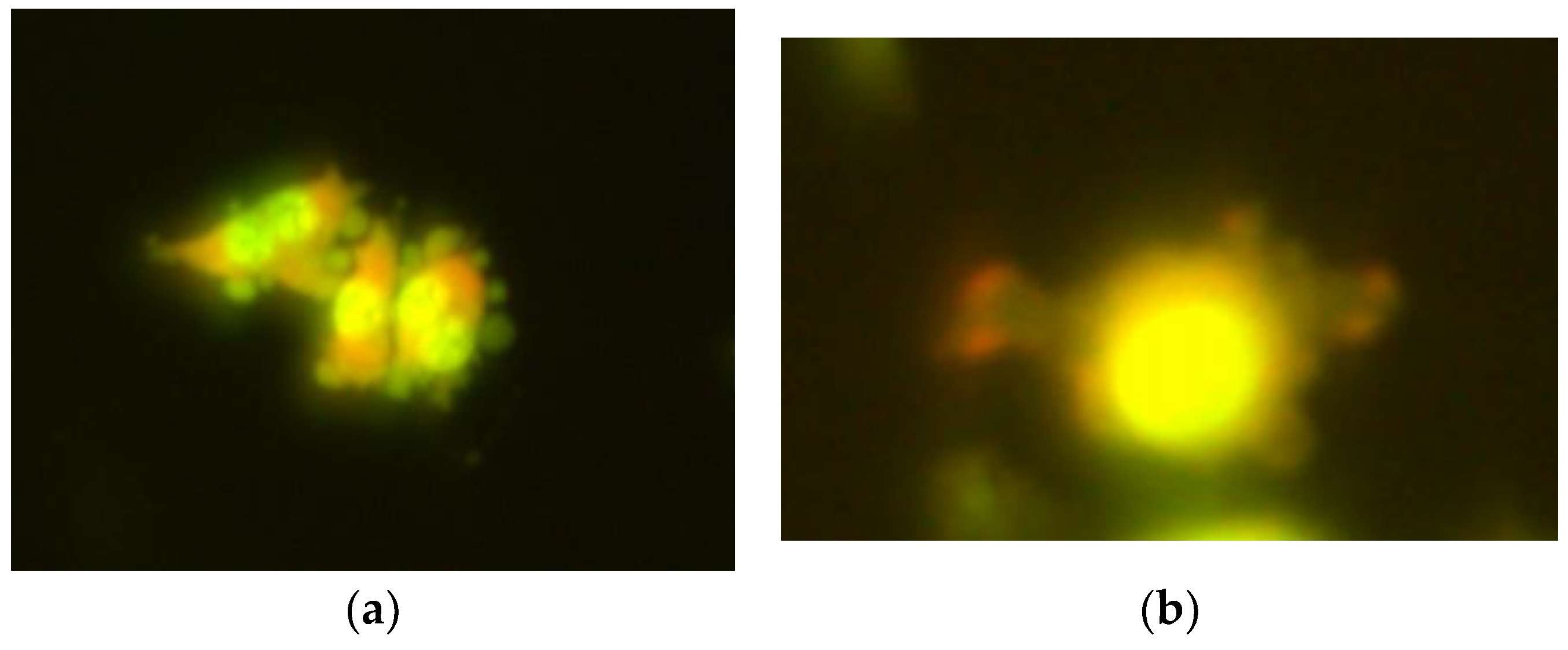
| Concentration [µM] | Mean [%] Tail DNA ± SD | |
|---|---|---|
| CrCl3 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 2 ± 0.0 | |
| 200 | 3 ± 0.2 | |
| 400 | 4 ± 0.3 * | |
| 600 | 5 ± 0.4 * | |
| 800 | 6 ± 0.6 * | |
| 1000 | 7 ± 0.6 * | |
| 1200 | 11 ± 1.0 * | |
| 1400 | 13 ± 1.0 * | |
| CoCl2 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 0 ± 0.0 | |
| 200 | 0 ± 0.0 | |
| 400 | 2 ± 0.2 * | |
| 600 | 4 ± 0.3 * | |
| 800 | 75 ± 6.5 * | |
| 1000 | 84 ± 7.4 * | |
| 1200 | 98 ± 8.5 * | |
| 1400 | 99 ± 8.1 * | |
| CrCl3 × 6H2O and CoCl2 × 6H2O mixtures | ||
| 200 µM CrCl3 × 6H2O | 1000 µM CoCl2 × 6H2O | 10 ± 1.0 *1,2 |
| 1000 µM CrCl3 × 6H2O | 200 µM CoCl2 × 6H2O | 7 ± 0.6 * |
| Concentration [µM] | Mean [%] Tail DNA ± SD | |
|---|---|---|
| CrCl3 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 2 ± 0.0 | |
| 200 | 3 ± 0.0 | |
| 400 | 3 ± 0.3 * | |
| 600 | 3 ± 0.3 * | |
| 800 | 3 ± 0.3 * | |
| 1000 | 4 ± 0.3 * | |
| 1200 | 6 ± 0.5 * | |
| 1400 | 9 ± 0.9 * | |
| CoCl2 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 0 ± 0.0 | |
| 200 | 0 ± 0.0 | |
| 400 | 0 ± 0.0 | |
| 600 | 1 ± 0.1 | |
| 800 | 10 ± 1.0 * | |
| 1000 | 97 ± 8.9 * | |
| 1200 | 98 ± 8.3 * | |
| 1400 | 100 ± 9.1 * | |
| CrCl3 × 6H2O and CoCl2 × 6H2O mixtures | ||
| 200 µM CrCl3 × 6H2O | 1000 µM CoCl2 × 6H2O | 17 ± 1.0 *1,2 |
| 1000 µM CrCl3 × 6H2O | 200 µM CoCl2 × 6H2O | 5 ± 0.4 * |
| Concentration [µM] | BNMN‰ | |
|---|---|---|
| CrCl3 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 0 ± 0.0 | |
| 200 | 2 ± 0.0 | |
| 400 | 6 ± 0.5 * | |
| 600 | 12 ± 1.0 * | |
| 800 | 13 ± 1.0 * | |
| 1000 | 22 ± 1.9 * | |
| 1200 | apoptosis | |
| 1400 | apoptosis | |
| CoCl2 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 1 ± 0.0 | |
| 200 | 3 ± 0.2 *a | |
| 400 | 4 ± 0.4 *a | |
| 600 | apoptosis | |
| 800 | apoptosis | |
| 1000 | apoptosis | |
| 1200 | apoptosis | |
| 1400 | apoptosis | |
| CrCl3 × 6H2O and CoCl2 × 6H2O mixtures | ||
| 200 µM CrCl3 × 6H2O | 1000 µM CoCl2 × 6H2O | 20 ± 1.8 *1,2 |
| 1000 µM CrCl3 × 6H2O | 200 µM CoCl2 × 6H2O | 30 ± 0.2 *3,4 |
| Concentration [µM] | BNMN‰ | |
|---|---|---|
| CrCl3 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 3 ± 0.0 | |
| 200 | 4 ± 0.3 * | |
| 400 | 5 ± 0.4 * | |
| 600 | 10 ± 1.0 * | |
| 800 | 10 ± 1.0 * | |
| 1000 | 16 ± 0.9 * | |
| 1200 | apoptosis | |
| 1400 | apoptosis | |
| CoCl2 × 6H2O | ||
| 0 | 0 ± 0.0 | |
| 100 | 1 ± 0.0 | |
| 200 | 2 ± 0.2 * | |
| 400 | apoptosis | |
| 600 | apoptosis | |
| 800 | apoptosis | |
| 1000 | apoptosis | |
| 1200 | apoptosis | |
| 1400 | apoptosis | |
| CrCl3 × 6H2O and CoCl2 × 6H2O mixtures | ||
| 200 µM CrCl3 × 6H2O | 1000 µM CoCl2 × 6H2O | 30 ± 2.8 *1,2 |
| 1000 µM CrCl3 × 6H2O | 200 µM CoCl2 × 6H2O | 20 ± 2.0 *3,4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnek, K.; Tatarczak-Michalewska, M.; Blicharska, E.; Siwicki, A.K.; Maciejewski, R. Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines. Int. J. Mol. Sci. 2025, 26, 5056. https://doi.org/10.3390/ijms26115056
Czarnek K, Tatarczak-Michalewska M, Blicharska E, Siwicki AK, Maciejewski R. Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines. International Journal of Molecular Sciences. 2025; 26(11):5056. https://doi.org/10.3390/ijms26115056
Chicago/Turabian StyleCzarnek, Katarzyna, Małgorzata Tatarczak-Michalewska, Eliza Blicharska, Andrzej K. Siwicki, and Ryszard Maciejewski. 2025. "Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines" International Journal of Molecular Sciences 26, no. 11: 5056. https://doi.org/10.3390/ijms26115056
APA StyleCzarnek, K., Tatarczak-Michalewska, M., Blicharska, E., Siwicki, A. K., & Maciejewski, R. (2025). Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines. International Journal of Molecular Sciences, 26(11), 5056. https://doi.org/10.3390/ijms26115056






