Evaluating the Efficacy of Rose Bengal as a Photosensitizer in Antimicrobial Photodynamic Therapy Against Candida albicans: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Focused Question
2.2. Search Strategy
2.3. Selection of Studies
2.4. Risk of Bias in Individual Studies
2.5. Quality Assessment and Risk of Bias Across Studies
2.6. Data Extraction
3. Results
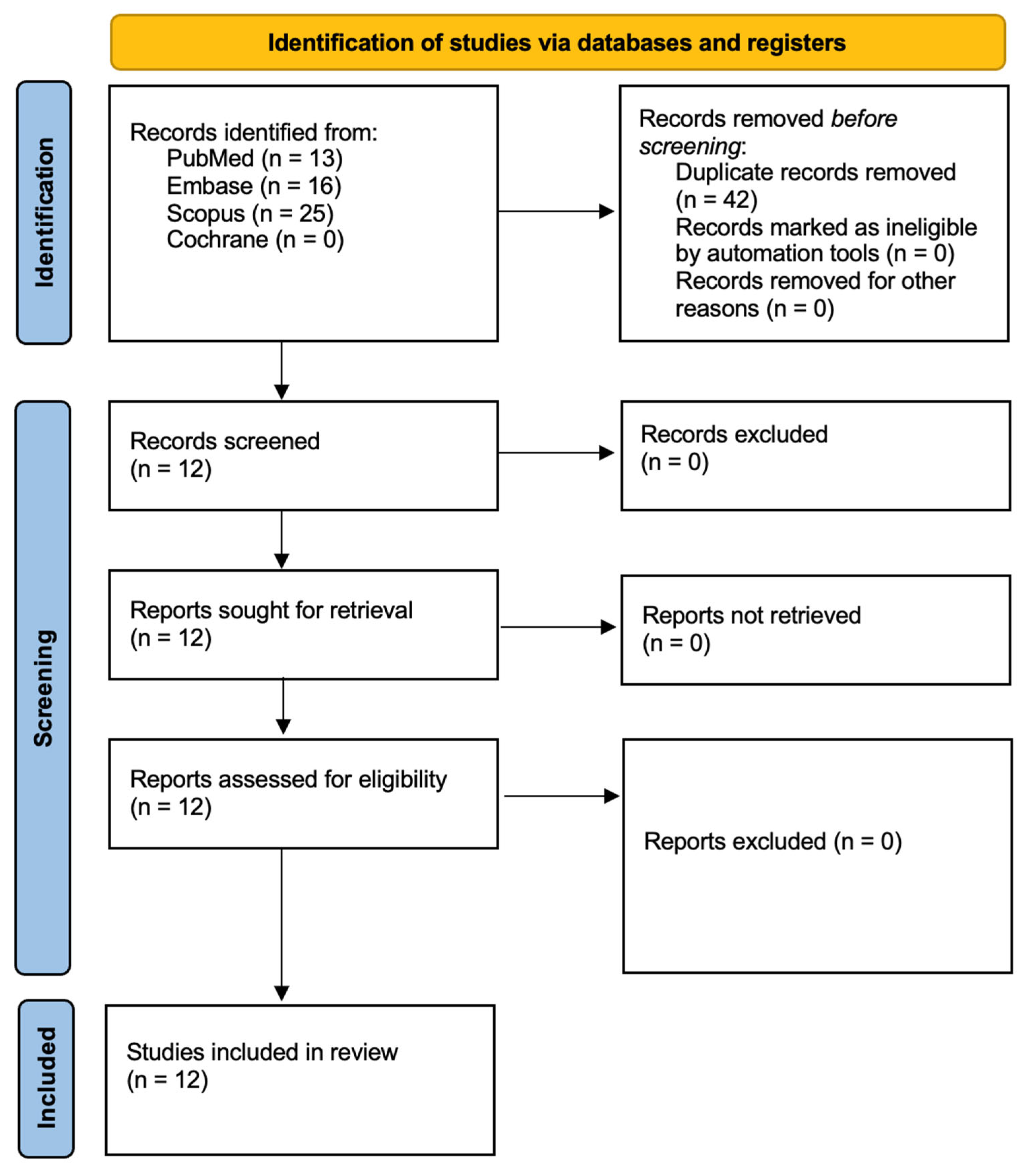
3.1. Study Selection
3.2. Data Presentation
3.3. Characteristics of Light Sources Used in aPDT
4. Discussion
4.1. Results in the Context of Other Evidence
4.2. Limitations of the Evidence
4.3. Limitations of the Review Process
4.4. Implications for Practice, Policy, and Future Research
4.5. Research Gaps and Future Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macias-Paz, I.U.; Pérez-Hernández, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cárdenas, J.E.; Reyna-Beltrán, E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol. 2023, 55, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Brizuela, M.; Raja, A. Oral Candidiasis. [Updated 4 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545282/ (accessed on 1 March 2025).
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Infections caused by dimorphic fungi and dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014; Available online: https://iris.who.int/bitstream/handle/10665/112642/9789241564748_eng.pdf (accessed on 25 February 2025).
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2025, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Martins Antunes de Melo, W.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [PubMed] [PubMed Central]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.J.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Feenstra, R.P.; Tseng, S.C. What is actually stained by rose bengal? Arch Ophthalmol. 1992, 110, 984–993. [Google Scholar] [CrossRef] [PubMed]
- de Melo, W.C.; Avci, P.; de Oliveira, M.N.; Gupta, A.; Vecchio, D.; Sadasivam, M.; Chandran, R.; Huang, Y.Y.; Yin, R.; Perussi, L.R.; et al. Photodynamic inactivation of biofilm: Taking a lightly colored approach to stubborn infection. Expert. Rev. Anti Infect. Ther. 2013, 11, 669–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Zięba, N.; Turski, R.; Misiołek, M.; Wiench, R. Hypericin-Mediated Photodynamic Therapy for Head and Neck Cancers: A Systematic Review. Biomedicines 2025, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Marchillo, K.; Spiegel, C.A.; Andes, D.R. Development and validation of an in vivo Candida albicans biofilm denture model. Infect. Immun. 2010, 78, 3650–3659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rao, H.; Choo, S.; Rajeswari Mahalingam, S.R.; Adisuri, D.S.; Madhavan, P.; Md Akim, A.; Chong, P.P. Approaches for Mitigating Microbial Biofilm-Related Drug Resistance: A Focus on Micro- and Nanotechnologies. Molecules 2021, 26, 1870. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szliszka, E.; Czuba, Z.P.; Kawczyk-Krupka, A.; Sieron-Stoltny, K.; Sieron, A.; Krol, W. Chlorin-based photodynamic therapy enhances the effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in bladder cancer cells. Med. Sci. Monit. 2012, 18, BR47–BR53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef] [PubMed]
- Kawczyk-Krupka, A.; Waśkowska, J.; Raczkowska-Siostrzonek, A.; Kościarz-Grzesiok, A.; Kwiatek, S.; Straszak, D.; Latos, W.; Koszowski, R.; Sieroń, A. Comparison of cryotherapy and photodynamic therapy in treatment of oral leukoplakia. Photodiagnosis Photodyn. Ther. 2012, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Updated August 2024; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 4 March 2025).
- Soria-Lozano, P.; Gilaberte, Y.; Paz-Cristobal, M.P.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Aporta, J.; Pérez-Laguna, V.; García-Luque, I.; Revillo, M.; Rezusta, A. In vitro effect of photodynamic therapy with different photosensitizers on cariogenic microorganisms. BMC Microbiol. 2015, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Torres-Hurtado, S.A.; Ramírez-Ramírez, J.; Larios-Morales, A.C.; Ramírez-San-Juan, J.C.; Ramos-García, R.; Espinosa-Texis, A.P.; Spezzia-Mazzocco, T. Efficient in vitro photodynamic inactivation using repetitive light energy density on Candida albicans and Trichophyton mentagrophytes. Photodiagnosis Photodyn. Ther. 2019, 26, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Alqarawi, F.K.; Tanveer, S.A.; Alshahrani, F.A.; Alshahrani, A.; AlHamdan, E.M.; Alzahrani, K.M.; Aldahiyan, N.; Naseem, M.; Vohra, F.; et al. Disinfection of acrylic denture resin polymer with Rose Bengal, Methylene blue, and Porphyrin derivative in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 35, 102362. [Google Scholar] [CrossRef]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic Enterococcus faecalis and Candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef]
- Freire, F.; da Costa, A.C.B.P.; Beltrame, M.; Junqueira, J.C. Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers Med. Sci. 2013, 28, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Gavara, R.; de Llanos, R.; Pérez-Laguna, V.; Arnau del Valle, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. Broad-spectrum photo-antimicrobial polymers based on cationic polystyrene and Rose Bengal. Front. Med. 2021, 8, 641–646. [Google Scholar] [CrossRef]
- Silva, M.P.; dos Santos, T.A.; de Barros, P.P.; de Camargo Ribeiro, F.; Junqueira, J.C.; Jorge, A.O.C. Action of antimicrobial photodynamic therapy on heterotypic biofilm: Candida albicans and Bacillus atrophaeus. Lasers Med. Sci. 2016, 31, 605–610. [Google Scholar] [CrossRef]
- Tunçcan, Ö.G.; Kalkanci, A.; Ünal, E.A.; Abdulmajed, O.; Erdoğan, M.; Dizbay, M.; Çaglar, K. The in vitro effect of antimicrobial photodynamic therapy on Candida and Staphylococcus biofilms. Turk. J. Med. Sci. 2018, 48, 873–879. [Google Scholar] [CrossRef]
- Hung, J.H.; Wang, Z.X.; Lo, Y.H.; Lee, C.N.; Chang, Y.; Chang, R.Y.; Huang, C.C.; Wong, T.W. Rose Bengal-mediated photodynamic therapy to inhibit Candida albicans. J. Vis. Exp. 2022, 181, e63558. [Google Scholar] [CrossRef]
- Arboleda, A.; Durkee, H.; Miller, D.; Aguilar, M.C.; Alawa, K.; Relhan, N.; Amescua, G.; Parel, J.-M. Variations in irradiation energy and rose bengal concentration for photodynamic antimicrobial therapy of fungal keratitis isolates. Lasers Med. Sci. 2024, 39, 72. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Hamblin, M.R.; Wang, H.; Fekrazad, R.; Wang, C.; Wen, X. Rose Bengal diacetate-mediated antimicrobial photodynamic inactivation: Potentiation by potassium iodide and acceleration of wound healing in MRSA-infected diabetic mice. BMC Microbiol. 2024, 24, 246. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.-Y.; Sarna, T.; Hamblin, M.R. Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by rose bengal in in vitro and in vivo studies. Antimicrob. Agents Chemother. 2017, 61, e00467-17. [Google Scholar] [CrossRef]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical applications of antimicrobial photodynamic therapy in dentistry. Front. Microbiol. 2023, 13, 1020995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual wavelength irradiation antimicrobial photodynamic therapy using indocyanine green and metformin doped with nano-curcumin as an efficient adjunctive endodontic treatment modality. Photodiagnosis Photodyn. Ther. 2020, 29, 101628. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; França, G.G.; de Annunzio, S.R.; Kopp, W.; Fontana, C.R.; Brighenti, F.L. Potential Use of Brazilian Green Propolis Extracts as New Photosensitizers for Antimicrobial Photodynamic Therapy against Cariogenic Microorganisms. Pathogens 2023, 12, 155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balhaddad, A.A.; Garcia, I.M.; Ibrahim, M.S.; Rolim, J.P.M.L.; Gomes, E.A.B.; Martinho, F.C.; Collares, F.M.; Xu, H.; Melo, M.A.S. Prospects on Nano-Based Platforms for Antimicrobial Photodynamic Therapy Against Oral Biofilms. Photobiomodul Photomed. Laser Surg. 2020, 38, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Chiniforush, N.; Pourhajibagher, M.; Shahabi, S.; Kosarieh, E.; Bahador, A. Can Antimicrobial Photodynamic Therapy (aPDT) Enhance the Endodontic Treatment? J. Lasers Med. Sci. 2016, 7, 76–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, M.; Xuan, W.; Hamblin, M.R.; Huang, L. Clinical aPDT’s effect on Candida albicans: Antifungal susceptibility, virulence gene expression, and correlation with leukocyte and neutrophil counts. Photodiagnosis Photodyn. Ther. 2024, 49, 104327. [Google Scholar] [CrossRef] [PubMed]
- Pereira Gonzales, F.; Maisch, T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.M.; Klein, M.I.; Jordão, C.C.; Carmello, J.C.; Bellini, A.; Pavarina, A.C. Successive applications of Antimicrobial Photodynamic Therapy effects the susceptibility of Candida albicans grown in medium with or without fluconazole. Photodiagnosis Photodyn. Ther. 2020, 32, 102018. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.M.N.; Bekmukhametova, A.; Antony, A.; Barman, S.K.; Houang, J.; Wu, M.J.; Hook, J.M.; George, L.; Wuhrer, R.; Mawad, D.; et al. Encapsulated Rose Bengal Enhances the Photodynamic Treatment of Triple-Negative Breast Cancer Cells. Molecules 2024, 29, 546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buchner, M.; García Calavia, P.; Muhr, V.; Kröninger, A.; Baeumner, A.J.; Hirsch, T.; Russell, D.A.; Marín, M.J. Photosensitiser functionalised luminescent upconverting nanoparticles for efficient photodynamic therapy of breast cancer cells. Photochem. Photobiol. Sci. 2019, 18, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xu, X.; Jin, F.; Xu, X.; Fang, T.; Du, Y. Tumor oxygenation nanoliposomes promote deep photodynamic therapy for triple-negative breast cancer. Biomater. Sci. 2024, 12, 4967–4979. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Delaney, C.; Rajendran, R.; Sherry, L.; Metcalfe, R.; Thomas, R.; McLean, W.; Williams, C.; Ramage, G. Gaining Insights from Candida Biofilm Heterogeneity: One Size Does Not Fit All. J. Fungi 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral. Biol. 2006, 51, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, H.F.; Lala, H.C.; Shepherd, M.G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 1990, 58, 1429–1436. [Google Scholar] [CrossRef]
- Reynaud, A.H.; Nygaard-Ostby, B.; Boygard, G.K.; Eribe, E.R.; Olsen, I.; Gjermo, P. Yeasts in periodontal pockets. J. Clin. Periodontol. 2001, 28, 860–864. [Google Scholar] [CrossRef]
- Li, L.; Redding, S.; Dongari-Bagtzoglou, A. Candida glabrata: An emerging oral opportunistic pathogen. J. Dent. Res. 2007, 86, 204–215. [Google Scholar] [CrossRef]
- McCormack, M.G.; Smith, A.J.; Akram, A.N.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am. J. Infect. Control. 2015, 43, 35–37. [Google Scholar] [CrossRef]
- Smith, A.J.; Robertson, D.; Tang, M.K.; Jackson, M.S.; MacKenzie, D.; Bagg, J. Staphylococcus aureus in the oral cavity: A three-year retrospective analysis of clinical laboratory data. Br. Dent. J. 2003, 195, 701–703; discussion 694. [Google Scholar] [CrossRef] [PubMed]
- Lederer, S.R.; Riedelsdorf, G.; Schiffl, H. Nasal carriage of meticillin resistant Staphylococcus aureus: The prevalence, patients at risk and the effect of elimination on outcomes among outclinic haemodialysis patients. Eur. J. Med. Res. 2007, 12, 284–288. [Google Scholar] [PubMed]
- Kenner, J.; O’Connor, T.; Piantanida, N.; Fishbain, J.; Eberly, B.; Viscount, H.; Uyehara, C.; Hospenthal, D. Rates of carriage of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in an outpatient population. Infect. Control Hosp. Epidemiol. 2003, 24, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lucet, J.C.; Regnier, B. Screening and decolonization: Does methicillin-susceptible Staphylococcus aureus hold lessons for methicillin-resistant S. aureus? Clin. Infect. Dis. 2010, 51, 585–590. [Google Scholar] [CrossRef] [PubMed]
| Source | Search Term | Filters | Number of Results |
|---|---|---|---|
| PubMed | (“Rose Bengal” [Title/Abstract] OR “Rose Bengal-mediated photodynamic therapy” [Title/Abstract] OR “Rose Bengal aPDT” [Title/Abstract]) AND (“Candida albicans” [Title/Abstract] OR “C. albicans” [Title/Abstract]) AND (“antimicrobial photodynamic therapy” [Title/Abstract] OR “aPDT” [Title/Abstract] OR “photodynamic inactivation” [Title/Abstract] OR “PACT” [Title/Abstract] OR “Photodynamic Antimicrobial Chemotherapy” [Title/Abstract]) | English language Publication years: 2014–2024 Full text | 13 |
| Embase | (‘rose bengal’:ti,ab,kw OR ‘rose bengal-mediated photodynamic therapy’:ti,ab,kw OR ‘rose bengal apdt’:ti,ab,kw) AND (‘candida albicans’:ti,ab,kw OR ‘C. albicans’:ti,ab,kw) AND (‘antimicrobial photodynamic therapy’:ti,ab,kw OR ‘apdt’:ti,ab,kw OR ‘photodynamic inactivation’:ti,ab,kw OR ‘PACT’:ti,ab,kw OR ‘photodynamic antimicrobial chemotherapy’:ti,ab,kw) | Publication years: 2014–2024 Controlled Clinical Trial Randomized Controlled Trial | 16 |
| Scopus | TITLE-ABS-KEY (“Rose Bengal” OR “Rose Bengal-mediated photodynamic therapy” OR “Rose Bengal aPDT”) AND TITLE-ABS-KEY (“Candida albicans” OR “C. albicans”) AND TITLE-ABS-KEY (“antimicrobial photodynamic therapy” OR “aPDT” OR “photodynamic inactivation” OR “PACT” OR “Photodynamic Antimicrobial Chemotherapy”) | Article Publication years: 2014–2024 | 25 |
| Cochrane | (“Rose Bengal” OR “Rose Bengal-mediated photodynamic therapy” OR “Rose Bengal aPDT”) AND (“Candida albicans” OR “C. albicans”) AND (“antimicrobial photodynamic therapy” OR “aPDT” OR “photodynamic inactivation” OR “PACT” OR “Photodynamic Antimicrobial Chemotherapy”) | Publication years: 2014–2024 | 0 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies investigating Candida elimination using Rose Bengal-mediated aPDT in both in vitro and animal models. In vitro and animal studies that examine Candida species and their susceptibility to Rose Bengal-based aPDT. Studies where Rose Bengal is used as the primary photosensitizer in aPDT for Candida treatment. Studies assessing synergistic effects of Rose Bengal-mediated aPDT in combination with other antifungal agents. Controlled studies evaluating the effects of Rose Bengal-aPDT compared to untreated controls or alternative therapeutic approaches. Comparative analyses examining the efficacy of Rose Bengal-mediated aPDT versus conventional antifungal treatments. Longitudinal studies or those with follow-up periods to assess the sustained antifungal effects of Rose Bengal-aPDT. | Grey literature sources, case reports, letters to editors, narrative or systematic reviews, books, documents, and other non-journal materials. Non-peer-reviewed sources. Studies published in languages other than English. Duplicate studies or research sharing the same ethical approval number. Studies without a control or comparison group. Research on aPDT where it is not applied as a therapeutic intervention for Candida. Studies using photosensitizers other than Rose Bengal. Studies focusing on infections other than Candida or those that do not specifically assess Candida strains. In vitro studies that do not replicate oral conditions relevant to Candida infections. |
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total Score | Bias Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soria-Lozano et al., 2015 [35] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Torres-Hurtado et al., 2019 [36] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Alhenaki et al., 2021 [37] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 8 | Low |
| Diogo et al., 2017 [38] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Freire et al., 2013 [39] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Gavara et al., 2021 [40] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Silva et al., 2016 [41] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 | Low |
| Tunçcan et al., 2018 [42] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | Moderate |
| Hung et al., 2022 [43] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | Low |
| Arboleda et al., 2024 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | Low |
| Wei et al., 2024 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | Low |
| Wen et al., 2017 [46] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 | Low |
| Author and Year | C. albicans Strain Evaluated |
|---|---|
| Soria-Lozano et al., 2015 [35] | ATCC 10231 |
| Alhenaki et al., 2021 [37] | ATCC Strain, number not specified |
| Freire et al., 2013 [39] | ATCC 18804 |
| Gavara et al., 2021 [40] | ATCC 10231 |
| Silva et al., 2016 [41] | ATCC 18804 |
| Tunçcan et al., 2018 [42] | ATCC 90028 |
| Hung et al., 2022 [43] | ATCC 10231 |
| Wei et al., 2024 [45] | ATCC MYA-2876 |
| Wen et al., 2017 [46] | CEC 749, Luciferase-expressing strain |
| Diogo et al., 2017 [38] | Pathogenic Yeast Collection of the Faculty of Medicine, University of Coimbra (FMUC), Portugal. No. YP0037 |
| Arboleda et al., 2024 [44] | Clinical isolate recovered from the cornea of a patient with culture-positive fungal keratitis. Ocular Microbiology Laboratory, Bascom Palmer Eye Institute, University of Miami. |
| Torres-Hurtado et al., 2019 [36] | Clinical isolate donated by the Department of Mycology from Benemérita Universidad Autónoma de Puebla, Mexico |
| Author and Year | Study Groups | Outcomes |
|---|---|---|
| Soria-Lozano et al., 2015 [35] | Three experimental groups based on different microorganisms: Streptococcus mutans (ATCC 35668), Streptococcus sanguis (ATCC 10556), and C. albicans (ATCC 10231). Each of these groups was subjected to aPDT using one of three photosensitizers: methylene blue, RB, or CUR in combination with white light. The study evaluated the photodynamic efficacy of each photosensitizer across these three microbial strains at varying concentrations and incubation times. | RB reduced C. albicans viability by 5 log10, but only at relatively high concentrations (≥320–640 μg/mL), and even higher concentrations were needed when longer pre-irradiation incubation times were applied. Compared to methylene blue, which was more effective at lower concentrations, RB demonstrated limited antifungal efficacy. The study concluded that MB was the most effective photosensitizer for C. albicans, while RB showed greater efficacy against Streptococcus spp., indicating that the antimicrobial efficacy of each photosensitizer is organism dependent. |
| Torres-Hurtado et al., 2019 [36] | Four experimental groups based on combinations of microorganism species and photosensitizers. C. albicans was treated with MB and RB, while Trichophyton mentagrophytes was treated only with MB. For each organism–photosensitizer pairing, the study compared single-dose versus repetitive-dose photodynamic inactivation protocols using varying concentrations of photosensitizers and light energy densities. Control groups included untreated cells, light-only exposure, and dark-incubated cells with photosensitizer but no light. | RB is highly effective as a photosensitizer in PDI of C. albicans, outperforming MB under comparable conditions. Specifically, complete inhibition of yeast growth (>99%) was achieved using RB at concentrations as low as 1 μM with 30 J/cm2 and 5 μM with 10 J/cm2 under single-dose light exposure. Moreover, by applying repetitive low-dose light exposures (e.g., 2–3 irradiations with 30-min dark incubation times), the authors were able to significantly reduce both RB concentration and light energy density while maintaining strong antifungal effects. For example, >90% inhibition was achieved using 1 μM RB and 10 J/cm2 with two exposures, which used fewer photons and one-fifth of the RB concentration compared to single-dose setups. The repetitive irradiation strategy appeared to enhance efficacy by allowing oxygen replenishment and cumulative photodynamic damage, without increasing toxicity, and could be advantageous for minimizing side effects in sensitive patients. |
| Alhenaki et al., 2021 [37] | Four groups of acrylic denture resin specimens contaminated with Streptococcus mutans, Staphylococcus aureus, Escherichia coli, and C. albicans. Group 1 was treated with Rose Bengal, Group 2 with Methylene Blue, Group 3 with a Porphyrin Derivative, and Group 4 served as the control group treated with 0.12% CHX. Each group underwent aPDT or chemical disinfection, and the effectiveness of microbial reduction was assessed by counting colony-forming units (CFU/mL). | RB was tested at a concentration of 5 µM activated by 480 nm LED light at 200 mW and 526 mW/cm2 for 180 s against Candida albicans ATCC strain on acrylic denture resin. The results showed that RB was the least effective among the photosensitizers tested, producing a mean CFU/mL (log10) of 6.15 ± 0.31, indicating limited antifungal activity. In comparison, the porphyrin derivative (PD) achieved 3.67 ± 0.18 log10 CFU/mL, and 0.12% chlorhexidine (CHX), used as a positive control, yielded 2.09 ± 0.85 log10 CFU/mL. The authors concluded that RB exhibited selective antimicrobial efficacy, being more effective against gram-positive bacteria like S. aureus and S. mutans, but not effective for inactivating C. albicans under the tested conditions |
| Diogo et al., 2017 [38] | The study groups included (1) monospecies biofilms of E. faecalis, (2) monospecies biofilms of C. albicans, and (3) mixed biofilms of both microorganisms. Each biofilm type was treated using different photosensitizers—toluidine blue O(TBO), rose bengal (RB), TMPyP, and Zn(II)chlorin e6 methyl ester (Zn(II)e6Me)—activated by appropriate LED light sources. These groups were compared to controls and to classical endodontic irrigants: 3% sodium hypochlorite (NaOCl), 2% chlorhexidine (CHX), and 17% EDTA. The treatments were assessed at two irradiation durations (60 and 90 s), with pre-incubation in darkness to ensure cellular uptake of the photosensitizers. | RB was tested at 0.1 mg/mL for aPDT against C. albicans monospecies and mixed biofilms, using green LED light at 557 nm for 60 or 90 s. While RB exhibited some capacity to reduce C. albicans biofilm biomass, its efficacy was significantly lower than Zn(II)chlorin e6 methyl ester (Zn(II)e6Me), especially after 90 s of irradiation, where Zn(II)e6Me showed superior biofilm removal (p = 0.0079). RB performed similarly to toluidine blue O (TBO) and was less effective than TMPyP and conventional irrigants like CHX and EDTA. Additionally, RB alone (without light) caused minimal disturbance to C. albicans biofilms (10% biomass reduction), confirming the need for light activation. The authors concluded that RB showed limited efficacy in aPDT against C. albicans biofilms under the tested conditions, underscoring the importance of optimizing photosensitizer selection for effective endodontic disinfection. |
| Freire et al., 2013 [39] | For planktonic cultures, six experimental groups were formed: control (PBS, P−L−), light-only (PBS+LED, P−L+), RB without light (P+L−), RB with light (P+L+), EY without light (P+L−), and EY with light (P+L+), using photosensitizer concentrations from 0.78 to 400 μM. For biofilms, six corresponding groups were tested using 200 μM of each photosensitizer. These groups allowed for comparison of the individual and combined effects of photosensitizer and light exposure on fungal viability. | RB was evaluated as a photosensitizer for photodynamic inactivation of C. albicans ATCC 18804 using a green LED (532 ± 10 nm, 16.2 J, 237 mW/cm2). RB showed significant antifungal activity against planktonic cells at concentrations of 6.25 μM or higher, achieving complete microbial reduction (100%) at 12.5, 25, and 50 μM. However, in biofilms, RB at 200 μM combined with LED produced only a 0.22 log10 CFU/mL reduction, significantly less than observed for planktonic forms. Statistical analysis confirmed a significant difference between RB + LED and control (p = 0.0057), though the effect was modest. Scanning electron microscopy also showed a visible reduction in fungal structures after RB-mediated PDI. The authors concluded that RB-PDI is effective against C. albicans planktonic cells, but less so against biofilms, likely due to structural and physiological resistance factors inherent to biofilm growth. |
| Gavara et al., 2021 [40] | The study evaluated the aPDI potential of two rose bengal-loaded cationic polystyrene resins—RB@Pmp (macroporous Amberlite IRA-900) and RB@Pgel (gel-type Amberlite IRA-400)—against five microorganisms: Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Enterococcus faecalis, and C. albicans. For each microorganism, ten experimental groups were formed: five under light exposure (RB@Pmp, RB@Pgel, corresponding unloaded resins Pmp and Pgel, and no resin) and five corresponding dark controls. These groups allowed evaluation of both photoactivated and dark antimicrobial effects of the polymers. | RB was immobilized on two types of commercial cationic polystyrene resins—Amberlite® IRA-900 (RB@Pmp) and Amberlite® IRA-400 (RB@Pgel)—to create photoactive polymeric materials for antimicrobial photodynamic inactivation (aPDI). Against C. albicans ATCC 10231, the materials exhibited modest antifungal activity, achieving reductions of 1.5 to 3.0 log10 CFU/mL after irradiation with green light (515 nm, up to 200 J/cm2). However, most of the observed effect (~2.5 log10 CFU/mL) was attributed to dark toxicity of the polymeric matrices, likely due to the presence of quaternary ammonium groups, with RB’s direct photodynamic action being minimal. This aligns with previous findings reporting scarce photoactivity of RB against C. albicans, possibly due to the yeast’s thick cell wall. The authors concluded that while the system is effective against bacteria, its antifungal efficacy is limited under the tested conditions, but could potentially be improved by increasing RB concentration or modifying the polymer matrix. |
| Silva et al., 2016 [41] | The P+L+ group was treated with RB at a concentration of 12.5 μM and exposed to LED light at 532 nm with an energy density of 16.2 J; the P+L− group received the same concentration of RB but was not exposed to LED light; the P−L+ group was treated with a 0.9% NaCl solution and exposed to LED light; and the P−L− group, serving as the control, was treated with 0.9% NaCl solution without LED exposure. | RB at 12.5 μM combined with green LED light at 532 nm and 16.2 J was tested for its effect on heterotypic biofilms of C. albicans (ATCC 18804) and Bacillus atrophaeus. The photodynamic therapy (PDT) group (P+L+) showed a 33.92% reduction in CFU/mL for C. albicans compared to the control group (P−L−). However, statistical analysis revealed no significant differences between the PDT group and the control or individual light or photosensitizer-only groups, indicating that the tested parameters were insufficient to achieve a meaningful antifungal effect. The authors concluded that this limited outcome may result from factors such as the thickness of the Candida biofilm, photosensitizer concentration, limited light penetration, or inadequate pre-irradiation time, and emphasized the need for further optimization of aPDT protocols targeting C. albicans biofilms. |
| Tunçcan et al., 2018 [42] | The study investigated the in vitro effectiveness of aPDT on biofilms formed by Staphylococcus aureus, Staphylococcus epidermidis, C. albicans, and Candida parapsilosis. Study groups were formed by combining three photosensitizers—methylene blue (MB), rose bengal (RB), and riboflavin (RBF)—with corresponding light sources: red LED (660 nm) for MB, green LED (518 nm) for RB, and UVA (370 nm) for RBF. Each microorganism was treated with one of these dye-light combinations, and outcomes were compared to untreated biofilm controls and drug-treated negative controls (amphotericin B or teicoplanin). The biofilm inhibition effects of each combination were evaluated using crystal violet staining, CFU counts, and scanning electron microscopy. | RB was evaluated as a photosensitizer aPDT against C. albicans (ATCC 90028) biofilms using green LED light at 518 nm and 0.1% RB concentration. The combination of green LED and RB produced a 22.7% biofilm inhibition index in C. albicans, indicating moderate antifungal activity compared to the most effective combination—red LED + methylene blue, which achieved 45.4% inhibition. The efficacy of green LED + RB was strain-dependent, showing no effect against C. parapsilosis and variable results against bacterial biofilms. Thus, while RB exhibited some inhibitory effect on C. albicans biofilms under specific conditions, it was less potent than methylene blue in this APDT setting, suggesting limited standalone utility and the need for optimization in future applications. |
| Hung et al., 2022 [43] | The study investigated the effectiveness of rose bengal-mediated antimicrobial photodynamic therapy (RB-aPDT) against multidrug-resistant C. albicans. The study included four experimental groups: (1) absolute control (no rose bengal, no light), (2) dark control (rose bengal without light exposure), (3) light control (light exposure without rose bengal), and (4) RB-aPDT (rose bengal with light exposure). These groups were subjected to varying incubation times and light fluences (e.g., 10, 20, and 30 J/cm2), and the fungal viability was assessed through colony-forming unit (CFU) counts after treatment. | RB-aPDT effectively inhibited multidrug-resistant C. albicans (BCRC 21538/ATCC 10231) in a dose-dependent manner using a 0.2% RB solution combined with green LED light at 540 nm. The optimal condition—30 J/cm2 light fluence—resulted in a 4-log10 (99.99%) reduction in fungal colony-forming units (CFU). Control groups (RB alone, light alone, or no treatment) showed negligible effects, confirming that fungal inhibition required both RB and light. Additionally, RB uptake by C. albicans was time-dependent, with strong red fluorescence observed inside the cells after 15–30 min of incubation. These findings indicate that RB-aPDT is a promising alternative treatment for drug-resistant C. albicans, especially due to its efficacy and the simplicity and affordability of the LED-based system used. |
| Arboleda et al., 2024 [44] | RB-aPDT on seven fungal keratitis isolates—Aspergillus fumigatus, C. albicans, Curvularia lunata, Fusarium keratoplasticum, Fusarium solani, Paecilomyces variotii, and Pseudallescheria boydii. The study groups were defined by combinations of rose bengal concentrations (0.1%, 0.05%, and 0.01%) and irradiation energy levels (5.4 J/cm2, 2.7 J/cm2 continuous, 2.7 J/cm2 pulsed, and 1.8 J/cm2), along with a non-irradiated control group for each organism. Each fungal species was tested in triplicate under these varying conditions to assess antifungal efficacy. | The researchers tested three concentrations of RB (0.1%, 0.05%, 0.01%) combined with varying green light irradiation energies (5.4, 2.7, and 1.8 J/cm2), using both continuous and pulsed light modes. C. albicans was identified as the most susceptible species, exhibiting complete growth inhibition across all ten treatment combinations, including the lowest tested RB concentration (0.01%) and minimal energy dose (1.8 J/cm2). This confirmed that C. albicans could be effectively inactivated with reduced PDAT parameters, suggesting a high sensitivity to RB-mediated photoinactivation. Notably, RB alone or light alone did not produce antifungal effects, reinforcing the requirement of both components for efficacy. These findings highlight the potential of RB-PDAT as a tailored, low-toxicity approach for treating C. albicans keratitis. |
| Wei et al., 2024 [45] | The study involved both in vitro and in vivo experimental groups to evaluate the antimicrobial and wound-healing effects of rose bengal diacetate (RBDA)-mediated photodynamic inactivation (aPDI), with and without potassium iodide (KI). In vitro, the study used microbial suspensions of MRSA, Escherichia coli, and C. albicans, divided into six groups: (1) blank control (no RBDA, no light), (2) RBDA only (no light), (3) KI only (no light), (4) RBDA + KI (no light), (5) RBDA + light, and (6) RBDA + KI + light. In vivo, diabetic mice with MRSA-infected wounds were assigned to four groups: (1) PBS control, (2) RBDA only, (3) RBDA + light, and (4) RBDA + KI + light. These groups allowed the authors to assess the individual and combined effects of RBDA, KI, and light exposure on microbial killing and wound healing. | C. albicans SC5314 (ATCC MYA-2876) was treated with Rose Bengal diacetate for aPDI using green light at 540 nm (10 J/cm2). The results showed that RBDA alone, even at 15 µM, exhibited no significant antifungal activity. However, when 100 mM potassium iodide was added, 10 µM RBDA killed 3 log10 units and 15 µM RBDA killed over 4 log10 units of C. albicans, demonstrating that KI dramatically potentiated the photodynamic killing effect. This marks the first report of RBDA mediating effective aPDI against fungal yeast, and suggests that the combination of RBDA and KI can overcome the otherwise limited efficacy of RB-based photosensitization against C. albicans. |
| Wen et al., 2017 [46] | The study investigated the potentiation of RB-aPDI by potassium iodide KI through both in vitro and in vivo experiments. In vitro, microbial suspensions of Escherichia coli, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), and C. albicans were divided into several study groups based on treatment conditions: (1) cells treated with RB and KI followed by green light (540 nm), (2) cells treated with RB only plus light, (3) cells treated with KI only plus light, (4) cells treated with RB and KI in the dark, (5) cells added after light exposure of RB and KI, and (6) cells centrifuged after RB incubation before light exposure with KI. In vivo, mice with P. aeruginosa skin infections were assigned to four groups: (1) untreated control, (2) RB + KI without light (dark control), (3) RB + light, and (4) RB + KI + light. These groups allowed the researchers to assess the individual and combined antimicrobial effects of RB, KI, and light under varying conditions. | RB demonstrated limited antifungal activity alone against C. albicans (only ~1.5 log10 reduction at 10 μM RB with 10 J/cm2 of 540 nm light), but when combined with 100 mM potassium iodide (KI), it achieved complete eradication (≥6 log10 kill) of C. albicans. The potentiation effect was only observed when the yeast cells were present during light exposure, indicating the generation of short-lived reactive iodine species such as peroxyiodide, I2•−, and HOO•. Notably, C. albicans was the only tested organism that showed visible RB fluorescence binding to the cell surface, suggesting partial photosensitizer-cell interaction is crucial for the enhanced killing. The mechanism primarily involved singlet oxygen (1O2) reacting with iodide to produce iodine species that contribute to microbial inactivation. These findings support the use of RB-KI-mediated aPDI as a highly effective strategy against C. albicans, particularly when conventional RB-PDI is insufficient. |
| Author and Year | Light Source | Wavelength (nm) | Energy Density (Fluence) (J/cm2) | Power Output (mW/cm2) | Irradiation Time (s) |
|---|---|---|---|---|---|
| Soria-Lozano et al., 2015 [35] | Metal Halide Lamp | 420–700 | 37 | 90 | Not stated |
| Torres-Hurtado et al., 2019 [36] | LED Array | 600–650, 490–540 | 10–60 (single) 3–20 (repetitive) | Not stated | Not stated |
| Alhenaki et al., 2021 [37] | LED Array | 480 | 37.5 | 526 | 180 |
| Diogo et al., 2017 [38] | LED (Red & Green) | 627, 557 | Not stated | 42 | 90 |
| Freire et al., 2013 [39] | Green LED | 532 ± 10 | Not stated | 237 | 180 |
| Gavara et al., 2021 [40] | LED (Showtec LED Par 64) | 515 ± 10 | Up to 200 | 5.8 | Not stated |
| Silva et al., 2016 [41] | Green LED Array | 510–560 | 10, 20, 30 | 10 | Not stated |
| Tunçcan et al., 2018 [42] | Green LED Array | 518 | Not stated | Not stated | Not stated |
| Hung et al., 2022 [43] | Green LED | 532 ± 10 | 16.2 | 90 | 180 |
| Arboleda et al., 2024 [44] | Red LED, Green LED, UVA Lamp | 660, 518, 370 | 5.4, 2.7, 1.8 | 3 | 300 |
| Wei et al., 2024 [45] | Green LED | 540 ± 15 | 10–20 | 20.83 | 480 |
| Wen et al., 2017 [46] | Green Light | 540 ± 15 | 10–20 | 100 | 100 |
| Author and Year | Incubation Time (Minutes) | Concentration/s of RB Used (µg/mL) |
|---|---|---|
| Soria-Lozano et al., 2015 [35] | <1, 60, 180 | 0.31–0.62, 0.62–1.25 |
| Torres-Hurtado et al., 2019 [36] | 30 | 0.51–10.18 |
| Alhenaki et al., 2021 [37] | Not stated | 5.09 |
| Diogo et al., 2017 [38] | 15 | 100 |
| Freire et al., 2013 [39] | 5 | 6.36–407.06 |
| Gavara et al., 2021 [40] | Not stated | 60 |
| Silva et al., 2016 [41] | 15 | 2000 |
| Tunçcan et al., 2018 [42] | Not stated | 1000, 500, 100 |
| Hung et al., 2022 [43] | 5 | 12.72 |
| Arboleda et al., 2024 [44] | 15 | 1000 |
| Wei et al., 2024 [45] | 120 | 407.06 |
| Wen et al., 2017 [46] | Not mentioned | Not mentioned |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiegler-Rudol, J.; Lipka, B.; Kapłon, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Evaluating the Efficacy of Rose Bengal as a Photosensitizer in Antimicrobial Photodynamic Therapy Against Candida albicans: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5034. https://doi.org/10.3390/ijms26115034
Fiegler-Rudol J, Lipka B, Kapłon K, Moś M, Skaba D, Kawczyk-Krupka A, Wiench R. Evaluating the Efficacy of Rose Bengal as a Photosensitizer in Antimicrobial Photodynamic Therapy Against Candida albicans: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(11):5034. https://doi.org/10.3390/ijms26115034
Chicago/Turabian StyleFiegler-Rudol, Jakub, Barbara Lipka, Katarzyna Kapłon, Magdalena Moś, Dariusz Skaba, Aleksandra Kawczyk-Krupka, and Rafał Wiench. 2025. "Evaluating the Efficacy of Rose Bengal as a Photosensitizer in Antimicrobial Photodynamic Therapy Against Candida albicans: A Systematic Review" International Journal of Molecular Sciences 26, no. 11: 5034. https://doi.org/10.3390/ijms26115034
APA StyleFiegler-Rudol, J., Lipka, B., Kapłon, K., Moś, M., Skaba, D., Kawczyk-Krupka, A., & Wiench, R. (2025). Evaluating the Efficacy of Rose Bengal as a Photosensitizer in Antimicrobial Photodynamic Therapy Against Candida albicans: A Systematic Review. International Journal of Molecular Sciences, 26(11), 5034. https://doi.org/10.3390/ijms26115034






