Abstract
Photodynamic therapy (PDT) is a light-activated treatment that generates reactive oxygen species (ROS) to induce microbial cell death. As resistance to traditional antibiotics intensifies globally, PDT has emerged as a promising alternative or adjunctive antimicrobial strategy. Among various photosensitizers, Hypocrellin, a perylenequinone compound, has shown high ROS yield and broad-spectrum activity against bacteria and fungi. This systematic review evaluated the efficacy, safety, and therapeutic potential of Hypocrellin-mediated antimicrobial photodynamic therapy. Following PRISMA 2020 guidelines, a comprehensive literature search was conducted in PubMed, Embase, Scopus, and the Cochrane Library for studies published between 2015 and 2025. Eligible studies included in vitro and preclinical in vivo research using Hypocrellin as a photosensitizer. Quality and risk of bias were assessed using a structured nine-item checklist. Ten eligible studies, all conducted in China, were included. Hypocrellin-mediated aPDT significantly reduced microbial loads in both planktonic and biofilm states of resistant pathogens such as Candida albicans, Candida auris, Cutibacterium acnes, and Staphylococcus aureus. The treatment acted via ROS-mediated apoptosis, membrane disruption, and mitochondrial dysfunction, with minimal cytotoxicity to mammalian cells. Studies also reported enhanced efficacy when Hypocrellin was incorporated into nanocarriers, polymeric scaffolds, or combined with chemodynamic or photothermal therapies. However, substantial heterogeneity was observed in Hypocrellin concentrations, irradiation parameters, and outcome measures. Hypocrellin-based PDT exhibits potent antimicrobial activity and favorable safety in preclinical settings, supporting its potential as an alternative to conventional antibiotics. However, standardized treatment protocols and robust clinical trials are urgently needed to validate long-term safety and translational feasibility. These findings underscore the broader promise of PDT in addressing drug-resistant infections through a mechanism unlikely to induce resistance.
1. Introduction
Photodynamic therapy (PDT) is an increasingly recognized, minimally invasive treatment modality that harnesses a photosensitizer (PS), a specific wavelength of light, and molecular oxygen to produce cytotoxic reactive oxygen species (ROS) in target cells [1,2,3,4,5]. Originally developed and extensively studied in oncology and dermatology, PDT has demonstrated efficacy in treating various malignancies, as well as in managing conditions such as psoriasis and macular degeneration [6,7,8]. More recently, its potential has broadened to include a range of clinical applications, including antimicrobial photodynamic therapy (aPDT), where the goal is to eradicate or reduce pathogenic microorganisms [9,10,11,12,13,14,15]. The surge of interest in aPDT has been driven by the relentless rise in antimicrobial resistance, which has emerged as a significant global health concern [9,10,11]. As conventional antibiotics become less effective against drug-resistant pathogens, the ROS-mediated mechanism of aPDT offers a promising alternative for infection control. Unlike systemic antibiotics, the multitargeted oxidative damage inflicted by ROS on microbial cells can limit the development of resistance [9,10].
In photodynamic therapy, two primary mechanisms, Type I and Type II reactions, govern the cytotoxic effects induced by activated photosensitizers. In Type I reactions, the excited triplet state of the photosensitizer interacts directly with surrounding biomolecules or molecular oxygen through electron or hydrogen transfer, leading to the formation of highly reactive oxygen species (ROS) such as superoxide anion (O2−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2) [9,10,11,12,13]. These ROS cause oxidative damage to cellular components like membranes, proteins, and DNA. In Type II reactions, the excited photosensitizer transfers energy directly to molecular oxygen (O2), converting it into singlet oxygen (1O2), a highly reactive and short-lived form of oxygen that rapidly oxidizes cellular targets. Both pathways result in cell death, but Type II is generally considered the dominant mechanism in oxygen-rich environments, while Type I may prevail in hypoxic or oxygen-poor conditions [9,10,11,12,13]. These are shown in Figure 1. Figure 2 shows the experimental setup flowchart.
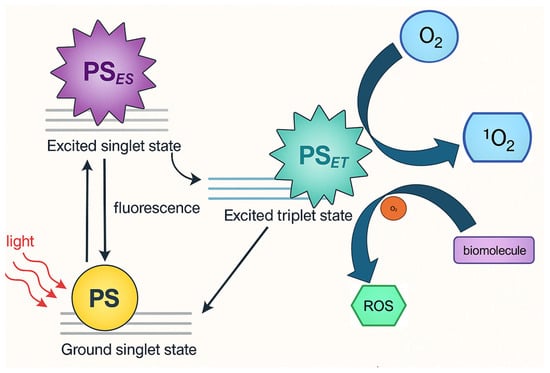
Figure 1.
Photophysical Pathways of Hypocrellin as photosensitizer in Photodynamic Therapy: Generation of Reactive Oxygen Species via Type I and Type II Mechanisms.
Figure 2.
PDT Experimental Setup.
A variety of photosensitizers, such as porphyrins, phthalocyanines, phenothiazines, and perylenequinones, has been investigated for both anticancer and antimicrobial applications [16,17]. Among these, Hypocrellin, a naturally occurring perylenequinone, has gained prominence due to its relatively high singlet oxygen yield, broad-spectrum antimicrobial effects, and comparatively low toxicity toward mammalian cells [12,18,19,20,21,22]. Despite the encouraging results, the broader PDT research community continues to explore pivotal questions surrounding the optimal design and use of Hypocrellin-based PDT. Ongoing debates focus on the most effective drug concentrations, illumination parameters (e.g., wavelength, fluence, and power density), and the merits of combining Hypocrellin with existing antimicrobials or emerging nanotechnology-based delivery systems [23,24,25,26]. Furthermore, there remains a need to clarify long-term safety, any potential off-target effects, and the stability of Hypocrellin-based formulations under diverse clinical conditions. Addressing these knowledge gaps is critical for advancing Hypocrellin-mediated PDT from an experimental approach—whether aimed at tumors or pathogens—to an established, evidence-driven therapeutic option.
Objectives
The primary objective of this systematic review is to critically evaluate the efficacy, applications, and therapeutic outcomes of Hypocrellin-mediated photodynamic therapy in antimicrobial treatment. By systematically analyzing preclinical and in vitro studies, this review aims to determine the antimicrobial effectiveness of Hypocrellin-based PDT in eradicating bacterial and fungal pathogens, particularly multidrug-resistant strains. This review will also assess variations in treatment parameters, including Hypocrellin concentration, light source wavelength, energy fluence, and irradiation duration, to identify optimal PDT protocols. Additionally, the potential synergistic effects of Hypocrellin-mediated PDT when combined with other antimicrobial agents or nanotechnology-based delivery systems will be examined. A secondary objective is to explore the safety profile of Hypocrellin-mediated PDT by analyzing cytotoxicity data on host tissues, assessing potential off-target effects, and evaluating its applicability for clinical translation. By consolidating current evidence, this systematic review seeks to establish the feasibility of Hypocrellin-mediated PDT as a viable alternative or adjunct to conventional antimicrobial therapies, guiding future research and informing clinical applications in infection management.
2. Methods
2.1. Focused Question
A systematic review was conducted using the PICO framework [27], defined as follows: In patients with microbial infections (Population), does treatment with Hypocrellin-mediated antimicrobial photodynamic therapy (Intervention), compared to light-based therapy without Hypocrellin, the use of Hypocrellin alone, or conventional antimicrobial treatments (Comparison), result in more effective eradication or reduction in microbial pathogens (Outcome)?
2.2. Search Strategy
This systematic review has been registered with PROSPERO under the ID CRD420251027986. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guidelines [28]. A comprehensive literature search was performed across major electronic databases, including PubMed/Medline, Embase, Scopus, and the Cochrane Library. The detailed search strategy is presented in Figure 1. Three independent reviewers conducted database searches using standardized search terms. To refine the selection process, electronic filters were applied to limit results to English-language studies published between 1 January 2014 and 3 December 2024. The initial screening involved reviewing titles and abstracts for alignment with predefined inclusion criteria (Table 1). This was followed by a full-text review conducted by two independent authors to extract relevant data. To broaden the scope of the review, a snowballing technique was used, examining the reference lists of selected studies to identify additional eligible literature. This review aimed to assess the potential of Hypocrellin-mediated antimicrobial photodynamic therapy as an effective strategy for microbial eradication, evaluating its role as a potential alternative or adjunct to standard antimicrobial treatments. The final study selection was guided by clearly established inclusion and exclusion criteria.

Table 1.
Search syntax used in the study.
2.3. Study Selection Process
To uphold methodological rigor and reduce the risk of bias, all retrieved records underwent an independent screening process by multiple reviewers. Titles and abstracts were carefully evaluated against the inclusion criteria, and any differences in judgment were addressed through collaborative discussion until a unanimous decision was reached (Table 2). This structured and transparent approach, consistent with PRISMA 2020 recommendations [28], ensured that only studies of high relevance and methodological integrity were included. By applying this stringent vetting process, we aimed to generate a reliable synthesis of the available evidence regarding the efficacy and clinical utility of Hypocrellin-mediated antimicrobial photodynamic therapy in managing infectious diseases.

Table 2.
Selection criteria for papers included in the systematic review.
2.4. Risk of Bias in Individual Studies
To minimize selection bias during the study screening process, titles and abstracts of all retrieved articles were reviewed independently by multiple authors. Inter-reviewer reliability was quantified using Cohen’s kappa statistic to assess the degree of agreement [29]. Any inconsistencies in the inclusion or exclusion of studies were addressed through collaborative discussion, ensuring that final decisions reflected a shared consensus. This structured approach helped safeguard the objectivity and methodological rigor of the review process.
2.5. Quality Assessment
The methodological quality of all included studies was independently evaluated by three reviewers, with attention to key aspects of aPDT design, execution, and data integrity relevant to Hypocrellin-mediated interventions. To assess risk of bias objectively, a structured scoring system was applied in which a value of 1 was assigned to each item meeting the criterion (“yes”), and 0 to items not meeting it (“no”). The following nine criteria were used for evaluation:
- Was the concentration of Hypocrellin used in the aPDT protocol explicitly stated?
- Was the source or manufacturer of Hypocrellin clearly identified?
- Was the incubation or pre-irradiation time prior to light activation described in detail?
- Were full technical specifications of the light source provided (e.g., type, wavelength, power output, fluence, power density)?
- Was a power meter used to validate the delivered light dose?
- Did the study include an appropriate negative or untreated control group?
- Were quantitative outcomes presented using valid statistical analysis?
- Was outcome reporting complete, with no missing or selectively reported data?
- Was the study free from apparent conflicts of interest or influence from funding sources?
Each study received a cumulative score out of nine based on the number of “yes” responses. Risk of bias was classified as follows: high (0–3 points), moderate (4–6 points), and low (7–9 points). Final judgments on study quality were made in line with the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions [30]. Table 3 shows the results of this quality assessment.

Table 3.
The results of the quality assessment and risk of bias across the studies.
2.6. Data Extraction
Following consensus on the final list of studies eligible for inclusion, two reviewers independently extracted key data using a standardized approach. Extracted information included bibliographic details (first author, year of publication), study design, type of microbial strains investigated, experimental and control group composition, duration of follow-up (where applicable), primary and secondary outcomes, technical specifications of the light source, concentration and formulation of Hypocrellin used, presence of any adjunctive agents, and the durations of both incubation and light exposure phases.
3. Results
3.1. Study Selection
Following the PRISMA 2020 guidelines [28], the study selection procedure is illustrated in Figure 3. An initial database search retrieved 166 records, which were reduced to 10 distinct articles after removing duplicates. Screening titles and abstracts affirmed the relevance of these 10 studies, all of which advanced to full-text review. None were excluded at that stage, leaving a final pool of 10 publications—covering the past decade—to be included in the synthesis. A comprehensive summary of each study’s characteristics and outcomes can be found in Table 4.
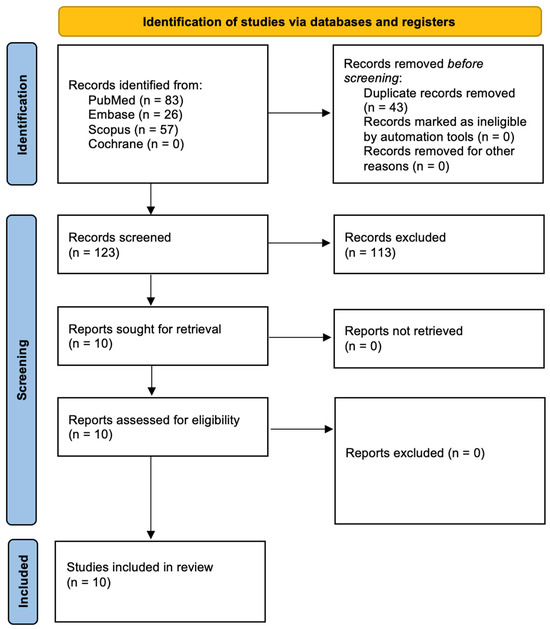
Figure 3.
Prisma 2020 flow diagram.

Table 4.
A general overview of the studies.
3.2. Data Presentation
A comprehensive overview of findings drawn from the 10 included studies is presented in Table 4, Table 5, Table 6 and Table 7. These tables supply an organized and reader-friendly synopsis of major results, research procedures, and measured outcomes pertinent to Hypocrellin-mediated aPDT.

Table 5.
Main outcomes and details from each study.
3.3. Overview of Study Characteristics
Table 4 outlines the core attributes of the studies included in this review, highlighting variations in study design, microbial targets, treatment protocols, and assessment parameters.
3.4. Characteristics of Light Sources Used in PDT
Table 6 presents the key physical properties of the light sources utilized in the studies meeting the inclusion criteria. Meanwhile, Table 7 provides a detailed overview of the characteristics of RB across the included studies.

Table 6.
Characteristics of light sources used.
Table 6.
Characteristics of light sources used.
| Author and Year | Light Source | Wavelength (nm) | Energy Density (Fluence) (J/cm2) | Power Output (mW/cm2) | Irradiation Time (s) |
|---|---|---|---|---|---|
| Niu et al., 2020 [31] | LED red light | 630 | Not stated | 5.68 | 600 |
| Niu et al., 2021 [32] | LED red light | 630 | 3 | 5.68 | Not stated |
| Yang et al., 2019 [33] | 8 W incandescent lamp | 400–780 | Not stated | Not stated | Not stated |
| Zhang et al., 2025 [34] | LED light | 470 | 30 | Not stated | Not explicitly stated |
| Jan et al., 2019 [35] | Xenon lamp with optical filter | 400–780 | 72 | 80 | 900 |
| Liu et al., 2022a [36] | 470 nm laser | 470 | Not stated | Not stated | 1800 |
| Liu et al., 2022b [37] | Laser | 470 | 180 | 100 | 1800 |
| Lan et al., 2024 [38] | 980 nm NIR laser (via UCNPs upconversion) | Not stated | Not explicitly stated | 2000 | Variable (up to 1200 s) |
| Ding et al., 2020 [39] | 671 nm laser | 671 | Not explicitly stated | 100 | Not stated |
| Guo et al., 2020 [40] | Laser (assumed, red light) | Not stated | Not explicitly stated | Not stated | Not stated |

Table 7.
Characteristics of Hypocrellin used in studies meeting eligibility criteria.
Table 7.
Characteristics of Hypocrellin used in studies meeting eligibility criteria.
| Author and Year | Hypocrellin Concentration |
|---|---|
| Niu et al., 2020 [31] | 0–1 μM |
| Niu et al., 2021 [32] | 0.25 μM |
| Yang et al., 2019 [33] | 0.5–1.0 μg/mL |
| Zhang et al., 2025 [34] | 1 μg/mL, 0.125 μg/mL, 0.03125 μg/mL |
| Jan et al., 2019 [35] | 0.1, 1, 10, and 100 μM |
| Liu et al., 2022a [36] | Not stated |
| Liu et al., 2022b [37] | 0.078–2.5 μg/mL |
| Lan et al., 2024 [38] | 0, 5, 10, 50, 100, 200, 300, 400 μM |
| Ding et al., 2020 [39] | 7.7 μM |
| Guo et al., 2020 [40] | 0.69 and 1.38 mg/L, 250 and 500 mg/L |
4. Discussion
4.1. Results in the Context of Other Evidence
Hypocrellin-mediated aPDT demonstrates broad-spectrum efficacy against both bacterial and fungal pathogens, including multidrug-resistant strains such as Candida auris and MRSA, suggesting its potential as a powerful alternative to conventional antimicrobials [31,36,40]. Upon activation with specific light wavelengths, Hypocrellin generates ROS that induce oxidative damage, apoptosis, and membrane disruption in microbial cells, with minimal toxicity to mammalian cells [33,34]. This mechanism bypasses classical resistance pathways, making it effective against both azole-sensitive and azole-resistant Candida albicans [35]. Several studies demonstrated synergistic effects when Hypocrellin is delivered via nanoparticles or polymeric scaffolds, enhancing solubility, cellular uptake, and photodynamic efficiency [38,39]. The addition of chemodynamic therapy (CDT) or photothermal therapy (PTT) further boosts therapeutic outcomes, particularly in hypoxic environments such as tumors or deep infections [38,39]. In vivo models showed reduced microbial loads, accelerated wound healing, and excellent biocompatibility, supporting clinical translational potential [37,40]. Recyclable and light-triggered nanofiber platforms allow repeated applications with sustained efficacy and safety [36]. Despite promising outcomes, optimal light parameters and Hypocrellin formulations vary across studies, highlighting the need for protocol standardization [32,34]. Importantly, Hypocrellin-based aPDT exhibited negligible dark toxicity and strong selectivity for infected tissues, further validating its safety profile [33,35]. Collectively, these findings support Hypocrellin-mediated aPDT as a versatile and effective adjunct or alternative to traditional antimicrobial therapies, especially in the context of rising drug resistance [38,40].
Hypocrellin A has demonstrated significant photodynamic antifungal activity against Candida albicans. In vitro studies revealed that HA, when activated by light, induces apoptosis in C. albicans cells through ROS generation, leading to mitochondrial dysfunction and DNA fragmentation. Furthermore, in a murine model of cutaneous C. albicans infection, HA-mediated aPDT effectively reduced fungal burden and improved skin lesions without notable toxicity [33]. Similarly, hypocrellin B (HB) has exhibited potent antibacterial effects against methicillin-resistant Staphylococcus aureus through sonodynamic action. The mechanism involves ROS production, resulting in significant bacterial inactivation. Notably, HB-mediated sonodynamic therapy did not cause substantial damage to mammalian cells, indicating its potential as a selective antibacterial agent [41]. Advancements in nano-formulations have further enhanced the therapeutic efficacy of hypocrellins. For instance, cage-modified hypocrellin has shown unprecedented activity against multidrug-resistant Candida species. This modification improves water solubility and photostability, leading to more effective light-triggered combinational photodynamic therapy [37]. Integrating natural photosensitizers like hypocrellin with nanotechnology has opened new avenues in photodynamic therapy. Nanoconjugated hypocrellin enhances therapeutic precision and efficacy by facilitating targeted delivery and con-trolled activation, thereby minimizing damage to surrounding healthy tissues [42]. The combination of HB and curcumin has demonstrated a synergistic effect in photodynamic inactivation of Staphylococcus aureus. This joint action results in a more substantial reduction in bacterial survival rates compared to individual treatments, suggesting a promising approach for controlling foodborne pathogens [43]. In vitro studies have shown that HB effectively inactivates both azole-sensitive and azole-resistant Candida albicans strains under light irradiation. This indicates the potential of HB-mediated aPDT as an alternative treatment for resistant fungal infections [35]. Photodynamic therapy has also been explored for treating Staphylococcus aureus infections, with studies indicating its efficacy as an adjunct to traditional antibiotic therapy. This approach could be particularly beneficial in addressing antibiotic-resistant strains [44]. Water-soluble HA nanoparticles have been developed to enhance the photosensitivity and therapeutic potential of HA. These nanoparticles exhibit superior water solubility and photodynamic activity, making them promising candidates for cancer therapy [45]. The production and application of hypocrellins have seen significant advancements, with studies highlighting their excellent light-induced antimicrobial activity against various pathogens, including drug-resistant strains. This positions hypocrellins as valuable agents in antimicrobial PDT [46]. Inactivation of Staphylococcus aureus by HB-mediated photodynamic action has been observed, with notable damage to bacterial cells and increased intracellular ROS levels, leading to cell death. This underscores the potential of HB as an effective photosensitizer in antimicrobial applications [47]. Hypocrellin-based multifunctional phototheranostic agents have been designed for near-infrared (NIR) imaging and therapy of glioblastoma. These agents combine chemotherapy and photodynamic therapy, offering a comprehensive approach to cancer treatment [48]. The development of recyclable, biodegradable, and light-driven antifungal nano-fibrous membranes incorporating HA has shown promise in treating Candida auris infections. These membranes provide effective antifungal activity and excellent biocompatibility [36]. Antimicrobial photodynamic therapy (aPDT) has been effective against drug-resistant pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, and Candida auris. The multi-target mechanisms of aPDT reduce the likelihood of developing resistance, making it a promising alternative to traditional antibiotics [49,50,51,52]. Enhancing the photosensitivity of HA through host–guest complexation with perylene diimide has provided a straightforward approach to improving its photodynamic efficacy. This method offers new avenues for supramolecular theranostics in cancer treatment [53]. The use of polyphenolic natural products as photosensitizers in antimicrobial photodynamic therapy has gained attention due to their safety and compatibility with the human body. These natural compounds offer a promising direction for developing effective antimicrobial treatments [54]. Transferrin-modified cancer cell membrane-coated HB nanocrystals have been developed for targeted cancer therapy. This innovative approach enhances the delivery and efficacy of HB in photodynamic applications [55]. Advancements in the production and application of hypocrellins have highlighted their potential in photodynamic therapy. These natural photosensitizers offer effective antimicrobial and anticancer properties, making them valuable in medical research [46]. The combination of chemotherapy and photodynamic therapy using HA-cisplatin-intercalated hectorite nano-formulations has shown enhanced anticancer efficacy. This synergistic approach offers a promising strategy for cancer treatment [56,57,58]. In vitro studies have demonstrated the effectiveness of HB-mediated photodynamic in-activation against Gram-positive antibiotic-resistant bacteria.
Although Hypocrellin-mediated aPDT has generally demonstrated a favorable safety profile in preclinical models, detailed reporting on specific adverse effects remains limited. Potential systemic responses, such as local inflammation, immune activation, or off-target tissue damage, are rarely quantified or discussed [33,35,36,37,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. In particular, the generation of reactive oxygen species (ROS), while central to antimicrobial efficacy, may also elicit oxidative stress in adjacent healthy tissues under suboptimal conditions. Furthermore, long-term immunological consequences, including potential immune modulation or sensitisation, have not been systematically evaluated. Future studies should incorporate comprehensive safety assessments, including histopathological analyses, systemic toxicity markers, and immunological profiling, to fully characterize the risk–benefit ratio of Hypocrellin-based therapies.
4.2. Limitations of the Evidence
Despite promising findings, the overall evidence base for Hypocrellin-mediated aPDT remains constrained by several notable limitations. A notable limitation of this review is that all included studies were conducted in China, which may limit the generalizability of the findings to other geographic regions with different microbial profiles, clinical practices, and healthcare infrastructures. Future research should prioritize international collaborations and multicenter studies to validate the efficacy and safety of Hypocrellin-mediated aPDT across diverse populations and clinical settings. Broadening the geographical scope in future reviews would also provide a more comprehensive understanding of regional variations in antimicrobial resistance and treatment response.
First, the included studies exhibit considerable heterogeneity in photosensitizer concentrations, light parameters (e.g., wavelength, fluence, irradiation time), and outcome measures, complicating direct comparisons and consensus on optimal treatment protocols. Second, much of the data derive from in vitro experiments or preclinical animal models, leaving a gap in high-level clinical evidence that would confirm real-world efficacy and safety. Many studies also utilize small sample sizes and short follow-up periods, restricting both the statistical power and the ability to assess long-term outcomes. Additionally, while most reports address basic cytotoxic effects and ROS mechanisms, few systematically investigate potential adverse events or off-target effects, especially in complex physiological environments. Lastly, although several studies highlight the benefits of integrating Hypocrellin with nanocarriers or combining photodynamic therapy with adjunctive modalities, the lack of standardized methods for evaluating synergy and clinical feasibility limits definitive conclusions about their translational potential. Consequently, these shortcomings underscore the need for more rigorous, well-designed clinical trials that incorporate standardized intervention parameters and robust reporting of safety data to fully validate Hypocrellin-mediated aPDT as a reliable antimicrobial strategy.
While the preclinical data on Hypocrellin-mediated aPDT are promising, several key challenges must be addressed to facilitate clinical translation. First, regulatory pathways for light-activated therapies remain complex, requiring rigorous demonstration of safety, efficacy, and device–drug compatibility. Second, formulation standardization is critical; variations in photosensitiser preparation, light dosimetry, and delivery systems across studies hinder reproducibility and clinical scalability. Third, patient acceptability—particularly with regard to photosensitivity, treatment duration, and light delivery methods—requires thorough evaluation in early-phase trials. Addressing these barriers through well-designed translational studies and interdisciplinary collaboration will be essential for advancing Hypocrellin-based aPDT into routine clinical practice.
A further limitation of the included studies is the inconsistent reporting of critical photophysical parameters such as light fluence, power density, and irradiation duration. These variables are essential for reproducibility and comparison across studies. While this reflects a shortcoming in the original publications, it underscores the urgent need for standardized and comprehensive reporting guidelines in photodynamic therapy research. Establishing such standards would enhance transparency, facilitate meta-analyses, and accelerate clinical translation.
4.3. Limitations of the Review Process
The primary limitation of our review process arises from the variability among the included studies, which differed considerably in methodologies, intervention protocols, and outcome measures. This heterogeneity made it challenging to conduct a quantitative synthesis; consequently, our findings are based primarily on a narrative summary. We also excluded non-English language publications, introducing a potential language bias that could omit relevant data. Additionally, gray literature and unpublished studies were not thoroughly investigated, further raising the possibility of publication bias. Although we took steps to minimize bias, such as using predefined inclusion and exclusion criteria and conducting independent screenings by multiple reviewers, variations in reporting quality, sample sizes, and study designs may still influence the reliability and generalizability of our conclusions. Finally, short follow-up durations in some studies prevent robust assessment of long-term outcomes, underscoring the need for more standardized protocols and well-designed, large-scale clinical trials to strengthen and refine the evidence base.
4.4. Implications for Practice, Policy, and Future Research
The review’s findings suggest that Hypocrellin-mediated aPDT holds considerable promise as an adjunct or alternative to conventional antimicrobial therapies, particularly for drug-resistant infections. For clinical practice, the incorporation of this modality may offer targeted, minimally invasive treatment options; however, standardized protocols regarding light parameters, photosensitizer dosing, and treatment durations are essential before widespread adoption. In terms of policy, healthcare regulators and funding bodies should prioritize and support larger-scale, multicenter clinical trials that rigorously evaluate both efficacy and safety, ensuring that emerging photodynamic therapies are integrated into treatment guidelines based on robust evidence. Future research should also focus on elucidating long-term outcomes, potential synergistic effects with other therapies, and strategies for enhancing delivery systems, all of which could help optimize patient outcomes and contribute to combating the global challenge of antimicrobial resistance.
5. Conclusions
The findings of this systematic review indicate that Hypocrellin-mediated antimicrobial photodynamic therapy holds significant promise as both an alternative and an adjunct to conventional antimicrobial treatments. By generating reactive oxygen species upon light activation, hypocrellin demonstrates potent, broad-spectrum antimicrobial activity against various drug-resistant pathogens, while exhibiting minimal toxicity toward host tissues. However, the variability in study designs, photosensitizer dosages, and light parameters underscores the need for standardized protocols and further clinical validation. Future research should focus on optimizing treatment regimens, exploring synergistic combinations with other therapeutic modalities, and confirming long-term safety and efficacy in well-designed, large-scale clinical trials.
Author Contributions
Conceptualization, J.F.-R. and R.W.; Methodology, J.F.-R., D.S. and R.W.; Software, J.F.-R.; Formal analysis, J.F.-R., K.K. (Katarzyna Kapłon), K.K. (Kornela Kotucha), M.M., A.K.-K., D.S. and R.W.; Investigation, J.F.-R., D.S. and R.W.; Writing—original draft preparation, J.F.-R., D.S. and R.W.; Writing—review and editing, J.F.-R., K.K. (Katarzyna Kapłon), K.K. (Kornela Kotucha), M.M., A.K.-K., D.S. and R.W.; Supervision, D.S. and R.W.; Funding acquisition, D.S. and R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| aPDT | Antimicrobial Photodynamic Therapy |
| ATP | Adenosine Triphosphate |
| Ca2+ | Calcium Ion |
| CDT | Chemodynamic Therapy |
| COP1T | Cage-Organic Polymer 1T |
| DNA | Deoxyribonucleic Acid |
| ECM | Extracellular Matrix |
| ERK | Extracellular signal-Regulated Kinase |
| HA | Hypocrellin A |
| HA-aPDT | Hypocrellin A-mediated Antimicrobial Photodynamic Therapy |
| HA-PDT | Hypocrellin A-mediated Photodynamic Therapy |
| HA-R-PDT | Hypocrellin A with Red Light-mediated Photodynamic Therapy |
| HB | Hypocrellin B |
| HB-aPDT | Hypocrellin B-mediated Antimicrobial Photodynamic Therapy |
| HE | Hypocrellin B derivative E |
| HE-PEG-NPs | HE-loaded PEGylated Nanoparticles |
| HF | Hypocrellin B derivative F |
| H2O2 | Hydrogen Peroxide |
| JNK | c-Jun N-terminal Kinase |
| LED | Light-Emitting Diode |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MMP | Matrix Metalloproteinase |
| MnO2 | Manganese Dioxide |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NIR | Near-Infrared |
| ORCID | Open Researcher and Contributor ID |
| PBS | Phosphate-Buffered Saline |
| PDT | Photodynamic Therapy |
| PEG | Polyethylene Glycol |
| PICO | Population, Intervention, Comparison, Outcome |
| PLA | Polylactic Acid |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PS | Photosensitizer |
| PTT | Photothermal Therapy |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIMP | Tissue Inhibitor of Metalloproteinases |
| UCNPs | Upconversion Nanoparticles |
| α-SMA | Alpha-Smooth Muscle Actin |
| μg/mL | Micrograms per Milliliter |
| μM | Micromolar |
| mPEG-PCL | methoxy Poly(ethylene glycol)-b-Poly(ε-caprolactone) |
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Antimicrobial Resistance [Internet]. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 23 March 2025).
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliveira, M.; Antunes, W.; Mota, S.; Madureira-Carvalho, Á.; Dinis-Oliveira, R.J.; Dias da Silva, D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms 2024, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations [Internet]. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 23 March 2025).
- Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eze, E.C.; Chenia, H.Y.; El Zowalaty, M.E. Acinetobacter Baumannii Biofilms: Effects of Physicochemical Factors, Virulence, Antibiotic Resistance Determinants, Gene Regulation, and Future Antimicrobial Treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter Baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef]
- Łopaciński, M.; Fiegler-Rudol, J.; Niemczyk, W.; Skaba, D.; Wiench, R. Riboflavin- and Hypericin-Mediated Antimicrobial Photodynamic Therapy as Alternative Treatments for Oral Candidiasis: A Systematic Review. Pharmaceutics 2025, 17, 33. [Google Scholar] [CrossRef]
- Dembicka-Mączka, D.; Kępa, M.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Matys, J.; Grzech-Leśniak, K.; Wiench, R. Evaluation of the Disinfection Efficacy of Er: YAG Laser Light on Single-Species Candida Biofilms—An In Vitro Study. Dent. J. 2025, 13, 88. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, M.; Liu, Z.; Wu, C.; Pan, X.; Huang, Z.; Lu, C.; Quan, G. Photodynamic therapy for cancer: Mechanisms, photosensitizers, nanocarriers, and clinical studies. MedComm 2024, 5, e603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Łopaciński, M.; Los, A.; Skaba, D.; Wiench, R. Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes. Pharmaceutics 2025, 17, 217. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martins Antunes de Melo, W.C.; Celiešiūtė-Germanienė, R.; Šimonis, P.; Stirkė, A. Antimicrobial photodynamic therapy (aPDT) for biofilm treatments. Possible synergy between aPDT and pulsed electric fields. Virulence 2021, 12, 2247–2272. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization. 2014. Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 3 February 2025).
- Ziental, D.; Mlynarczyk, D.T.; Czarczynska-Goslinska, B.; Lewandowski, K.; Sobotta, L. Photosensitizers Mediated Photodynamic Inactivation against Fungi. Nanomaterials 2021, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Baron, E.D.; Scull, H.; Hsia, A.; Berlin, J.C.; McCormick, T.; Colussi, V.; Kenney, M.E.; Cooper, K.D.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: The case experience with preclinical mechanistic and early clinical-translational studies. Toxicol. Appl. Pharmacol. 2007, 224, 290–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ScienceDirect. Hypocrellin A [Internet]. Available online: https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/hypocrellin-a (accessed on 23 March 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 12004204, Hypocrellin D. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hypocrellin-D (accessed on 1 March 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 119305, Hypocrellin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hypocrellin (accessed on 1 March 2025).
- Gao, R.; Xu, Z.; Deng, H.; Guan, Z.; Liao, X.; Zhao, Y.; Zheng, X.; Cai, Y. Enhanced hypocrellin production of Shiraia sp. SUPER-H168 by overexpression of alpha-amylase gene. PLoS ONE 2018, 13, e0196519. [Google Scholar] [CrossRef]
- Pachydaki, S.; Sobrin, L.; Miller, J.W. Photodynamic therapy and combination treatments. Int. Ophthalmol. Clin. 2007, 47, 95–115. [Google Scholar] [CrossRef]
- Merlin, J.P.J.; Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of dietary antioxidants in p53-mediated cancer chemoprevention and tumor suppression. Oxid. Med. Cell Longev. 2021, 2021, 9924328. [Google Scholar] [CrossRef]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Overcoming challenges in cancer treatment: Nano-enabled photodynamic therapy as a viable solution. WIREs Nanomed. Nanobiotechnol. 2024, 16, e1942. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Feng, Y.; Wei, S.; Zhou, J.; Yu, B.; Shen, J. Delivering a hydrophobic anticancer drug for photodynamic therapy by amorphous formulation. Bioorg. Med. Chem. Lett. 2010, 20, 6172–6174. [Google Scholar] [CrossRef] [PubMed]
- Olivo, M.; Chin, W. Perylenequinones in photodynamic therapy: Cellular versus vascular response. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO Framework to Improve Searching PubMed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Wang, G.; Guo, G.; Tong, Y.; Shi, Y. Inhibition of ROS–NF-κB-dependent autophagy enhances Hypocrellin A united LED red light-induced apoptosis in squamous carcinoma A431 cells. Cell Signal. 2020, 69, 109550. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Shi, Y.; Guo, G.; Tong, Y.; Wang, G. Antifibrotic effects of Hypocrellin A combined with LED red light irradiation on keloid fibroblasts by counteracting the TGF-β/Smad/autophagy/apoptosis signalling pathway. Photodiagn. Photodyn. Ther. 2021, 34, 102202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic antifungal activity of hypocrellin A against Candida albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Ran, X.; Gao, R.; Sun, J.; Zhuang, K.; You, Z.; Zhang, Z.; Ran, Y. Hypocrellin A-mediated photodynamic antibacterial activity against Cutibacterium acnes: An in vitro study. Photodiagn. Photodyn. Ther. 2025, 51, 104467. [Google Scholar] [CrossRef]
- Jan, A.; Liu, C.; Deng, H.; Li, J.; Ma, W.; Zeng, X.; Ji, Y. In vitro photodynamic inactivation effects of hypocrellin B on azole-sensitive and resistant Candida albicans. Photodiagn. Photodyn. Ther. 2019, 27, 419–427. [Google Scholar] [CrossRef]
- Liu, X.; Guo, C.; Zhuang, K.; Chen, W.; Zhang, M.; Dai, Y.; Tan, L. A recyclable and light-triggered nanofibrous membrane against the emerging fungal pathogen Candida auris. PLoS Pathog. 2022, 18, e1010534. [Google Scholar] [CrossRef]
- Liu, X.; Fang, R.; Feng, R.; Li, Q.; Su, M.; Hou, C.; Zhuang, K.; Dai, Y.; Lei, N.; Jiang, Y.; et al. Cage-modified hypocrellin against multidrug-resistant Candida spp. with unprecedented activity in light-triggered combinational photodynamic therapy. Drug Resist. Updates 2022, 65, 100887. [Google Scholar] [CrossRef]
- Lan, J.; Chen, S.; Chen, Z.; Luo, D.; Yu, C.; Zeng, L.; Sun, W.; Zhang, X.; Yao, X.; Wu, F.; et al. Chemo-photodynamic antitumour therapy based on Er-doped upconversion nanoparticles coated with hypocrellin B and MnO2. Biomater. Adv. 2024, 161, 213891. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, W.; Wu, J.; Zheng, X.; Ge, J.; Ren, H.; Zhang, W.; Lee, C.; Wang, P. Novel near-infrared hypocrellin derivatives for synergistic photodynamic and photothermal therapy. Chem. Asian J. 2020, 15, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-Y.; Yan, S.-Z.; Tao, X.; Yang, Q.; Li, Q.; Wang, T.-S.; Yu, S.-Q.; Chen, S.-L. Evaluation of hypocrellin A-loaded lipase sensitive polymer micelles for intervening methicillin-resistant Staphylococcus aureus antibiotic-resistant bacterial infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110230. [Google Scholar] [CrossRef]
- Wang, X.; Ip, M.; Leung, A.W.; Wang, P.; Zhang, H.; Hua, H.; Xu, C. Sonodynamic action of hypocrellin B on methicillin-resistant Staphylococcus aureus. Ultrasonics 2016, 65, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Advancing Photodynamic Therapy with Nano-Conjugated Hypocrellin: Mechanisms and Clinical Applications. Int. J. Nanomed. 2024, 19, 11023–11038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Xu, Y.; Liao, Q.; Xie, M.; Tao, H.; Wang, H.L. Synergistic effect of hypocrellin B and curcumin on photodynamic inactivation of Staphylococcus aureus. Microb. Biotechnol. 2021, 14, 692–707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez, C.; Zúñiga, T.; Palavecino, C.E. Photodynamic therapy for treatment of Staphylococcus aureus infections. Photodiagn. Photodyn. Ther. 2021, 34, 102285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, J.H.; Dong, C.; Ma, F.; Wei, S.H.; Shen, J. Water-soluble hypocrellin A nanoparticles as a photodynamic therapy delivery system. Dye. Pigment. 2009, 82, 90–94. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Q.; Tian, L.; Huang, Z.; Tang, Y.; Wen, Y.; Yu, F.; Yan, X.; Zhao, Y.; Wu, Z.; et al. Advancements and Future Prospects in Hypocrellins Production and Modification for Photodynamic Therapy. Fermentation 2024, 10, 559. [Google Scholar] [CrossRef]
- Moan, J.; Pettersen, E.O.; Christensen, T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br. J. Cancer 1979, 39, 398–407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, M.A.; Jalouli, M.; Yadab, M.K.; Al-Zharani, M. Progress in Drug Delivery Systems Based on Nanoparticles for Improved Glioblastoma Therapy: Addressing Challenges and Investigating Opportunities. Cancers 2025, 17, 701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmoudi, H.; Bahador, A.; Pourhajibagher, M.; Alikhani, M.Y. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Bacterial Infections. J. Lasers Med. Sci. 2018, 9, 154–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiegler-Rudol, J.; Zięba, N.; Turski, R.; Misiołek, M.; Wiench, R. Hypericin-Mediated Photodynamic Therapy for Head and Neck Cancers: A Systematic Review. Biomedicines 2025, 13, 181. [Google Scholar] [CrossRef]
- Kruczek-Kazibudzka, A.; Lipka, B.; Fiegler-Rudol, J.; Tkaczyk, M.; Skaba, D.; Wiench, R. Toluidine Blue and Chlorin-e6 Mediated Photodynamic Therapy in the Treatment of Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2528. [Google Scholar] [CrossRef]
- Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. [Google Scholar] [CrossRef]
- Li, R.; Yang, T.; Peng, X.; Feng, Q.; Hou, Y.; Zhu, J.; Chu, D.; Duan, X.; Zhang, Y.; Zhang, M. Enhancing the Photosensitivity of Hypocrellin A by Perylene Diimide Metallacage-Based Host–Guest Complexation for Photodynamic Therapy. Nano-Micro Lett. 2024, 16, 226. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajkhowa, S.; Hussain, S.Z.; Agarwal, M.; Zaheen, A.; Al-Hussain, S.A.; Zaki, M.E.A. Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing. Pharmaceutics 2024, 16, 1160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willis, J.A.; Cheburkanov, V.; Chen, S.; Soares, J.M.; Kassab, G.; Blanco, K.C.; Bagnato, V.S.; de Figueiredo, P.; Yakovlev, V.V. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: Combining antimicrobial photodynamic and antibiotic treatments. Proc. Natl. Acad. Sci. USA 2022, 119, e2208378119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).