Roseburia intestinalis Modulates Immune Responses by Inducing M1 Macrophage Polarization
Abstract
1. Introduction
2. Results
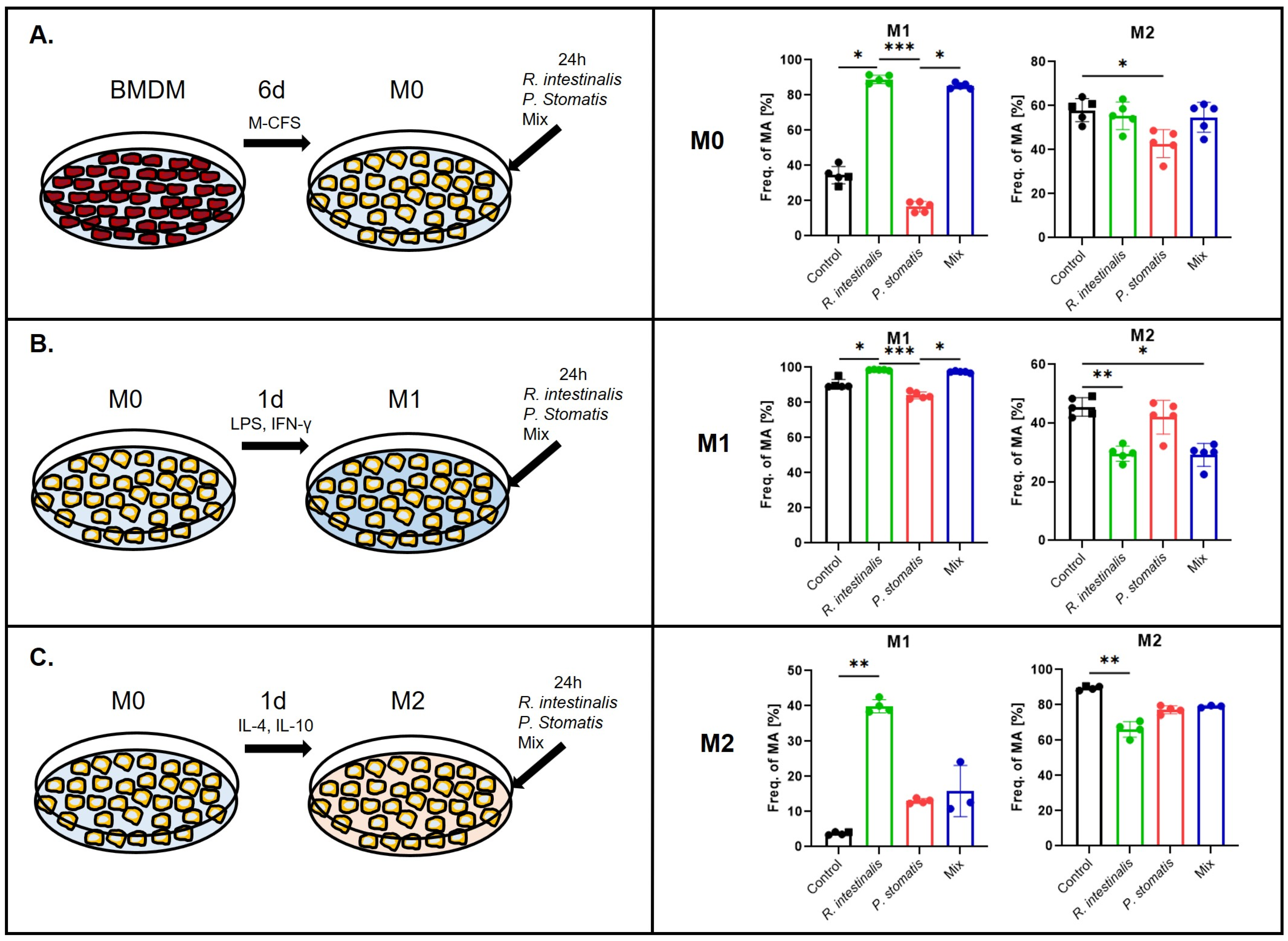
2.1. R. intestinalis Treatments Induce M1 Polarization of Macrophages
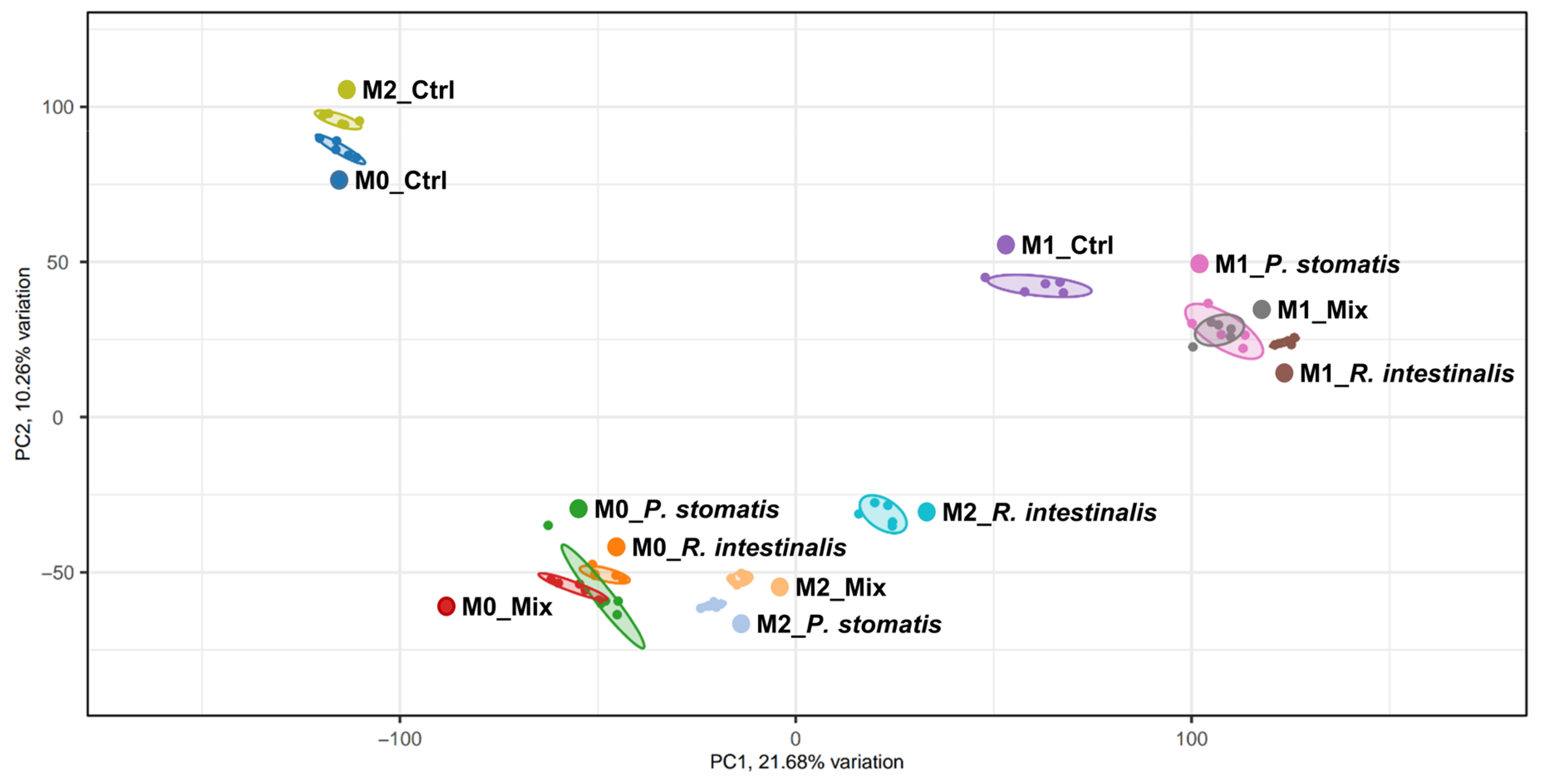
2.2. R. intestinalis Induces a Characteristic Gene Expression Pattern in Macrophages
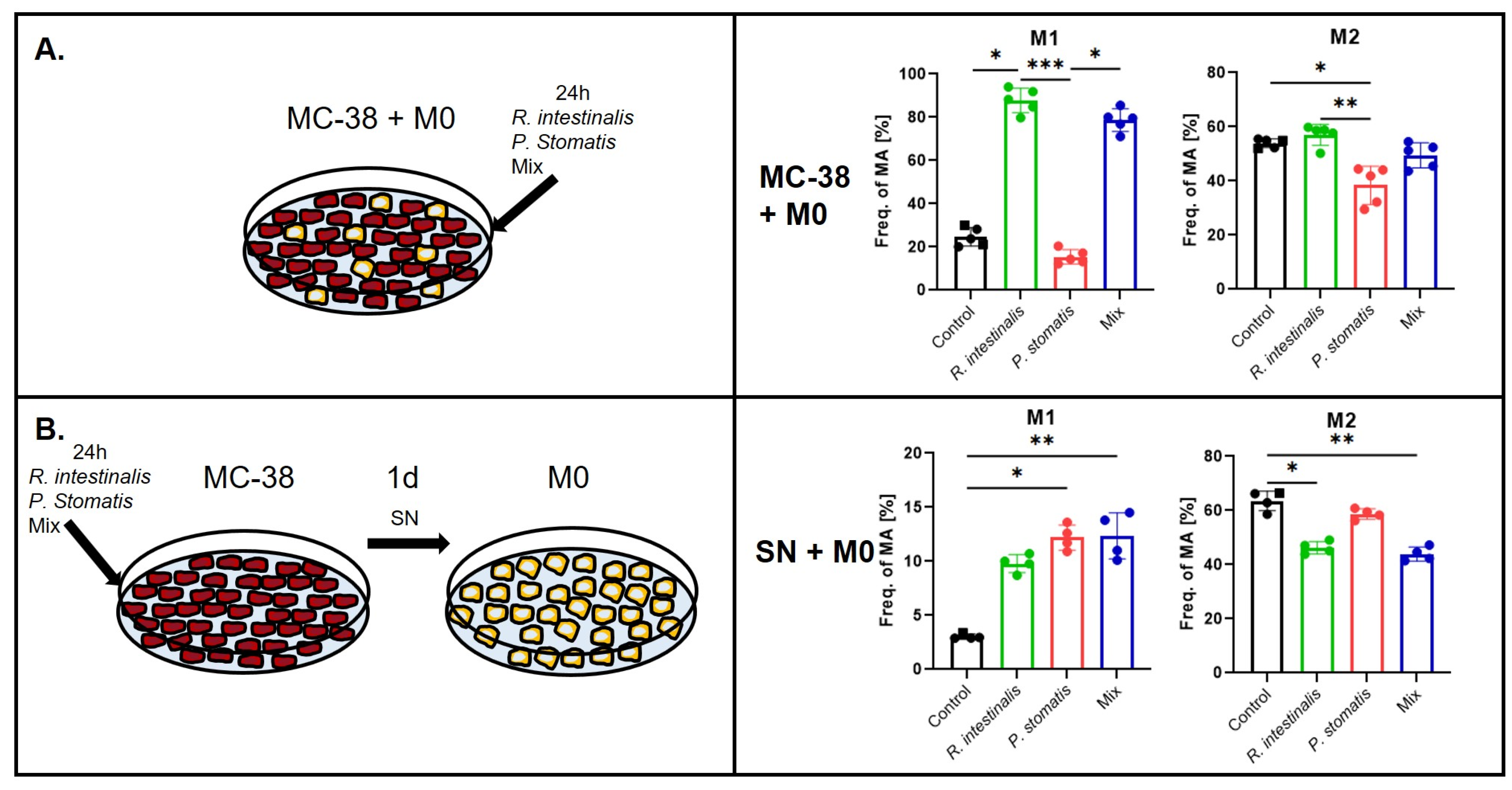
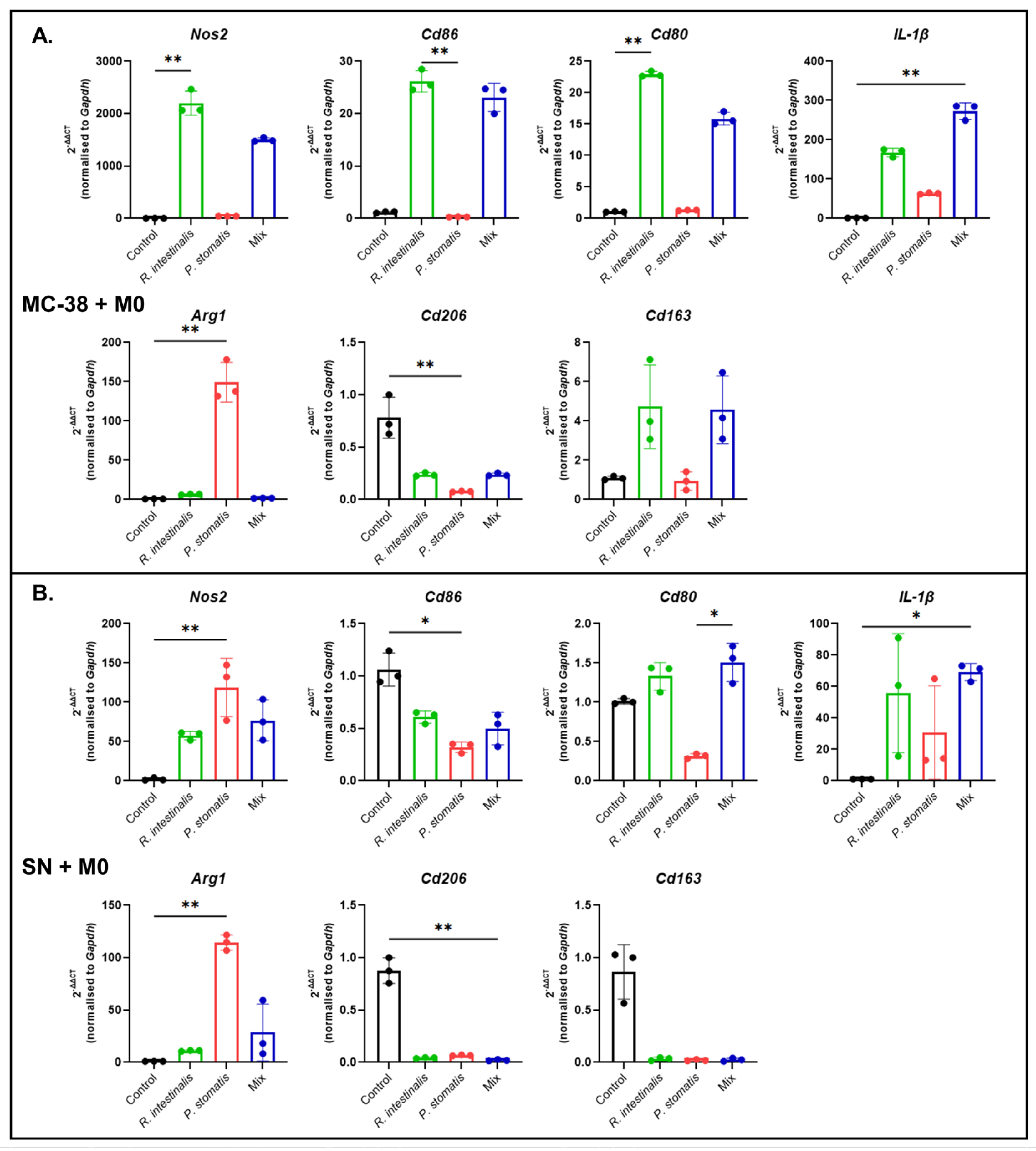
2.3. R. intestinalis Enhances M1 Macrophage Polarization After Co-Culture with MC-38 CRC Cells
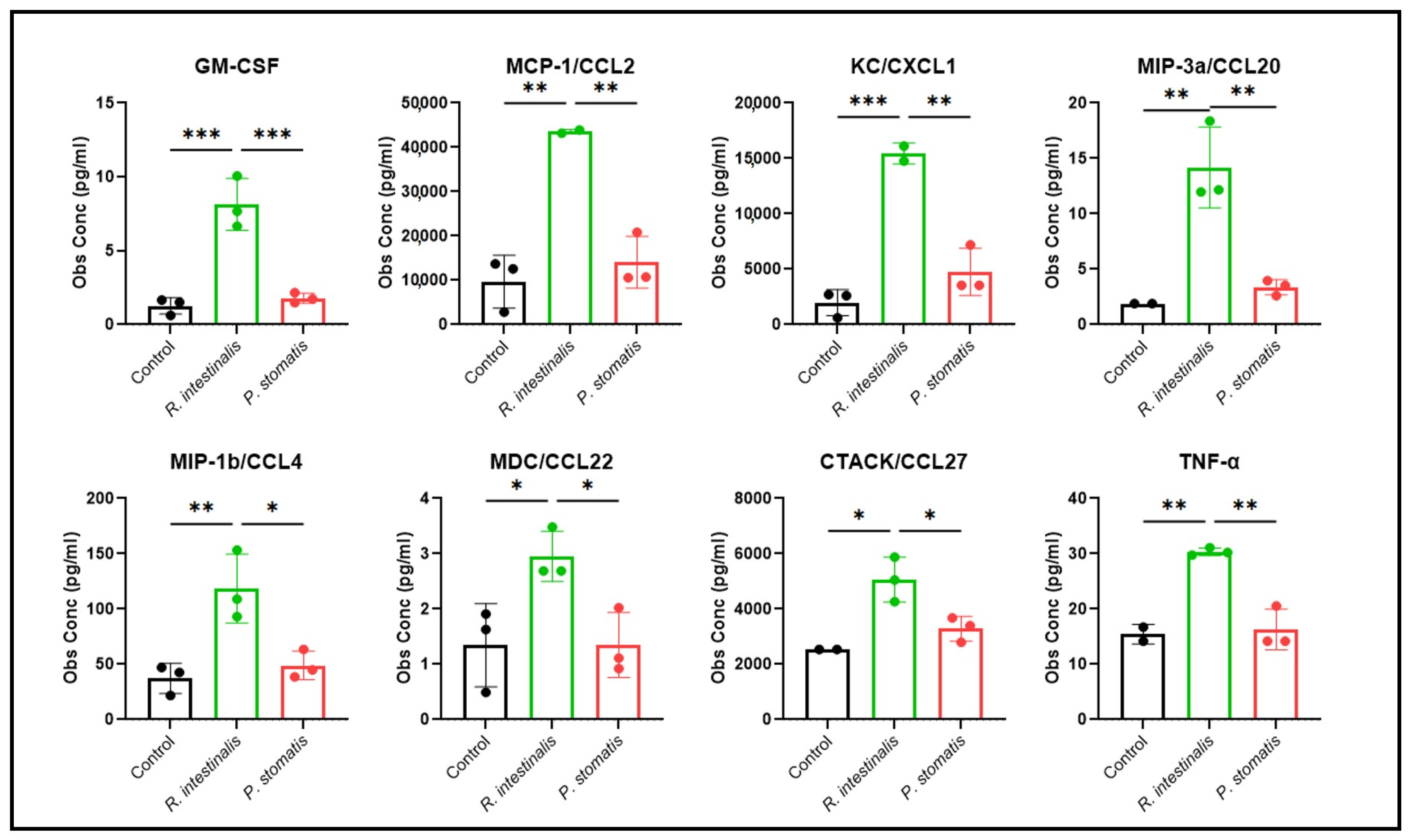
2.4. R. intestinalis Impact on MC-38 Cell Chemokine and Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Bacterial Culture and Treatment
4.2. Generation and Stimulation of Bone Marrow-Derived Macrophages
4.3. Co-Culture of BMDMs and/or MC-38 Cells with Bacteria
4.4. Flow Cytometry
4.5. RNA Extraction and RT-PCR
4.6. Bio-Plex Multiplex Immunoassay
4.7. RNA Sequencing and Data Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACK | Ammonium–chloride–potassium |
| ARG1 | Arginase 1 |
| BMDMs | Bone marrow-derived recruited macrophages |
| CRC | Colorectal cancer |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DEG | Differentially expressed genes |
| FBS | Fetal bovine serum |
| FGCZ | Functional Genomics Center Zurich |
| GI | Gastrointestinal |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GO | Gene Ontology |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MAMPs | Microbial-associated molecular patterns |
| MCP-1 | Monocyte chemotactic protein-1 |
| M-CSF | Macrophage colony-stimulating factor |
| MDSC | Myeloid-derived suppressor cells |
| MOI | Multiplicity of infection |
| NEAA | Non-essential amino acid |
| NOS2 | Nitric oxide synthase 2 |
| OD | Optical density |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death protein ligand 1 |
| PRRs | Pattern recognition receptors |
| PYG | Peptone, yeast extract and glucose |
| RNI | Reactive nitrogen intermediates |
| ROS | Reactive oxygen species |
| TAMs | Tumor-associated macrophages |
| TLR | Toll-like receptor |
| TME | Tumor microenvironment |
| TNFα | Tumor necrosis factor alpha |
| YCFA | Yeast extract, casitone and fatty acid |
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Li, J.H.; Jia, H.J.; Cai, X.H.; Zhong, H.Z.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Satokari, R. Contentious host-microbiota relationship in inflammatory bowel disease—Can foes become friends again? Scand. J. Gastroenterol. 2015, 50, 34–42. [Google Scholar] [CrossRef]
- Montalban-Arques, A.; Scharl, M. Intestinal microbiota and colorectal carcinoma: Implications for pathogenesis, diagnosis, and therapy. eBioMedicine 2019, 48, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678. [Google Scholar] [CrossRef]
- Uchino, Y.; Goto, Y.; Konishi, Y.; Tanabe, K.; Toda, H.; Wada, M.; Kita, Y.; Beppu, M.; Mori, S.; Hijioka, H.; et al. Colorectal Cancer Patients Have Four Specific Bacterial Species in Oral and Gut Microbiota in Common-A Metagenomic Comparison with Healthy Subjects. Cancers 2021, 13, 3332. [Google Scholar] [CrossRef]
- Montalban-Arques, A.; Katkeviciute, E.; Busenhart, P.; Bircher, A.; Wirbel, J.; Zeller, G.; Morsy, Y.; Borsig, L.; Glaus Garzon, J.F.; Muller, A.; et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021, 29, 1573–1588 e7. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Schwiertz, A.; Aminov, R.I.; Blaut, M.; Flint, H.J. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 2003, 69, 4320–4324. [Google Scholar] [CrossRef]
- Zhu, C.; Song, K.; Shen, Z.; Quan, Y.; Tan, B.; Luo, W.; Wu, S.; Tang, K.; Yang, Z.; Wang, X. Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T cells differentiation in colitis. Mol. Med. Rep. 2018, 17, 7567–7574. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, S.; Luo, W.; Shen, Z.; Meng, X.; Xiao, M.; Tan, B.; Nie, K.; Tong, T.; Wang, X. Roseburia intestinalis-derived flagellin ameliorates colitis by targeting miR-223-3p-mediated activation of NLRP3 inflammasome and pyroptosis. Mol. Med. Rep. 2020, 22, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Choi, Y.; Jun, J.E.; Lee, Y.B.; Jin, S.M.; Hur, K.Y.; Ko, G.P.; Lee, M.K. Additional Effect of Dietary Fiber in Patients with Type 2 Diabetes Mellitus Using Metformin and Sulfonylurea: An Open-Label, Pilot Trial. Diabetes Metab. J. 2019, 43, 422–431. [Google Scholar] [CrossRef]
- Ruff, W.E.; Dehner, C.; Kim, W.J.; Pagovich, O.; Aguiar, C.L.; Yu, A.T.; Roth, A.S.; Vieira, S.M.; Kriegel, C.; Adeniyi, O.; et al. Pathogenic Autoreactive T and B Cells Cross-React with Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe 2019, 26, 100–113 e8. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Backheds, F.; Lusis, A.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef]
- Quan, Y.S.; Song, K.R.; Zhang, Y.; Zhu, C.X.; Shen, Z.H.; Wu, S.; Luo, W.W.; Tan, B.; Yang, Z.Y.; Wang, X.Y. Roseburia intestinalis-derived flagellin is a negative regulator of intestinal inflammation. Biochem. Biophys. Res. Commun. 2018, 501, 791–799. [Google Scholar] [CrossRef]
- Ruan, G.C.; Chen, M.J.; Chen, L.; Xu, F.H.; Xiao, Z.F.; Yi, A.L.; Tian, Y.T.; Ping, Y.; Lv, L.L.; Cheng, Y.; et al. Roseburia intestinalis and Its Metabolite Butyrate Inhibit Colitis and Upregulate TLR5 through the SP3 Signaling Pathway. Nutrients 2022, 14, 3041. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, J.M.; Yuan, W.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J. Gastroenterol. Hepatol. 2018, 33, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Kibbie, J.; Lee, E.J.; Guo, K.J.; Santiago, M.L.; Austin, G.L.; Gianella, S.; Landay, A.L.; Donovan, A.M.; Frank, D.N.; et al. Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. Aids 2017, 31, 510–521. [Google Scholar] [CrossRef]
- Nicholson, K.; Bjornevik, K.; Abu-Ali, G.; Chan, J.; Cortese, M.; Dedi, B.; Jeon, M.; Xavier, R.; Huttenhower, C.; Ascherio, A.; et al. The human gut microbiota in people with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The fecal microbiota of patients with pancreatic ductal adenocarcinoma and autoimmune pancreatitis characterized by metagenomic sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Mohandas, S.; Soma, V.L.; Tran, T.D.B.; Sodergren, E.; Ambooken, T.; Goldman, D.L.; Weinstock, G.; Herold, B.C. Differences in Gut Microbiome in Hospitalized Immunocompetent vs. Immunocompromised Children, Including Those With Sickle Cell Disease. Front. Pediatr. 2020, 8, 583446. [Google Scholar] [CrossRef]
- Xu, F.; Cheng, Y.; Ruan, G.; Fan, L.; Tian, Y.; Xiao, Z.; Chen, D.; Wei, Y. New pathway ameliorating ulcerative colitis: Focus on Roseburia intestinalis and the gut-brain axis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211004469. [Google Scholar] [CrossRef]
- Bircher, A. UZH, Zurich, Switzerland. Anaerobic and aerobic cell co-culture. 2023, unpublished work.
- Lamond, A.I. Molecular biology of the cell, 4th edition. Nature 2002, 417, 383. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef]
- Nagashima, R.; Maeda, K.; Imai, Y.; Takahashi, T. Lamina propria macrophages in the human gastrointestinal mucosa: Their distribution, immunohistological phenotype, and function. J. Histochem. Cytochem. 1996, 44, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, K.; Nie, K.; Deng, M.; Luo, W.; Wu, X.; Huang, Y.; Wang, X. Assessment of the safety and probiotic properties of Roseburia intestinalis: A potential “Next Generation Probiotic”. Front. Microbiol. 2022, 13, 973046. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Orchestration of metabolism by macrophages. Cell Metab. 2012, 15, 432–437. [Google Scholar] [CrossRef]
- Benoit, M.; Desnues, B.; Mege, J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef]
- Kather, J.N.; Halama, N. Harnessing the innate immune system and local immunological microenvironment to treat colorectal cancer. Br. J. Cancer 2019, 120, 871–882. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Haruki, K.; Lau, M.C.; Vayrynen, S.A.; Zhong, R.; Dias Costa, A.; Borowsky, J.; Zhao, M.; Fujiyoshi, K.; Arima, K.; et al. The Prognostic Role of Macrophage Polarization in the Colorectal Cancer Microenvironment. Cancer Immunol. Res. 2021, 9, 8–19. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Gao, X.Y.; Zhou, S.Q.; Qin, Z.F.; Li, D.C.; Zhu, Y.P.; Ma, D.N. Upregulation of HMGB1 in tumor-associated macrophages induced by tumor cell-derived lactate further promotes colorectal cancer progression. J. Transl. Med. 2023, 21, 53. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Xiong, L.; Jiang, P.; Wang, J.; Li, C. Metabolism, metabolites, and macrophages in cancer. J. Hematol. Oncol. 2023, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Klimp, A.H.; de Vries, E.G.; Scherphof, G.L.; Daemen, T. A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002, 44, 143–161. [Google Scholar] [CrossRef]
- Erbs, G.; Newman, M.A. The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe-associated molecular patterns (MAMPs), in plant innate immunity. Mol. Plant Pathol. 2012, 13, 95–104. [Google Scholar] [CrossRef]
- Bao, W.; Wang, Y.; Fu, Y.; Jia, X.; Li, J.; Vangan, N.; Bao, L.; Hao, H.; Wang, Z. mTORC1 Regulates Flagellin-Induced Inflammatory Response in Macrophages. PLoS ONE 2015, 10, e0125910. [Google Scholar] [CrossRef]
- Kortmann, J.; Brubaker, S.W.; Monack, D.M. Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J. Immunol. 2015, 195, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, D.; Ren, C.; Su, X.; Wong, C.K.; Yang, R. A Special Network Comprised of Macrophages, Epithelial Cells, and Gut Microbiota for Gut Homeostasis. Cells 2022, 11, 307. [Google Scholar] [CrossRef]
- Shen, Z.H.; Luo, W.W.; Tan, B.; Nie, K.; Deng, M.Z.; Wu, S.; Xiao, M.W.; Wu, X.; Meng, X.R.; Tong, T.; et al. Roseburia intestinalis stimulates TLR5-dependent intestinal immunity against Crohn’s disease. eBioMedicine 2022, 85, 104285. [Google Scholar] [CrossRef]
- Toujas, L.; Delcros, J.G.; Diez, E.; Gervois, N.; Semana, G.; Corradin, G.; Jotereau, F. Human monocyte-derived macrophages and dendritic cells are comparably effective in vitro in presenting HLA class I restricted exogenous peptides. Immunology 1997, 91, 635–642. [Google Scholar] [CrossRef]
- Hennemann, B.; Rehm, A.; Kottke, A.; Meidenbauer, N.; Andreesen, R. Adoptive immunotherapy with tumor-cytotoxic macrophages derived from recombinant human granulocyte-macrophage colony-stimulating factor (rhuGM-CSF) mobilized peripheral blood monocytes. J. Immunother. 1997, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lesimple, T.; Moisan, A.; Guille, F.; Leberre, C.; Audran, R.; Drenou, B.; Toujas, L. Treatment of metastatic renal cell carcinoma with activated autologous macrophages and granulocyte-macrophage colony-stimulating factor. J. Immunother. 2000, 23, 675–679. [Google Scholar] [CrossRef]
- Yan, W.L.; Shen, K.Y.; Tien, C.Y.; Chen, Y.A.; Liu, S.J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef]
- Hanisakova, N.; Vitezova, M.; Rittmann, S.K.R. The Historical Development of Cultivation Techniques for Methanogens and Other Strict Anaerobes and Their Application in Modern Microbiology. Microorganisms 2022, 10, 412. [Google Scholar] [CrossRef]
- Toda, G.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2021, 2, 100246. [Google Scholar] [CrossRef]




| MC-38 | M0 | MC-38 + M0 | ||||
|---|---|---|---|---|---|---|
| R. intestinalis | P. stomatis | R. intestinalis | P. stomatis | R. intestinalis | P. stomatis | |
| BCA-1/CXCL13 | > | > | > | > | > | |
| CTACK/CCL27 | > * | > * | > * | > | > | |
| ENA-78/CXCL5 | > | > ** | > ** | > *** | > *** | |
| Eotaxin2/CCL24 | > | > | ||||
| Fractalkine/CX3CL | > | > ** | > ** | > ** | > ** | |
| GM-CSF | > ** | |||||
| I-309/CCL1 | > | > | > | > | > | |
| I-TAC/CXCL11 | > | > * | > ** | > | > * | |
| IFN-γ | ||||||
| IL-1β | > | > * | > | > * | > ** | |
| IL-2 | ||||||
| IL-4 | > | > ** | > ** | > | > | |
| IL-6 | > | > | ||||
| IL-10 | > ** | > ** | > ** | > | ||
| IL-16 | > | > * | ||||
| IP-10/CXCL10 | > | > | > | > * | > | |
| KC/CXCL1 | > ** | > | > * | > | > | |
| MCP-1/CCL2 | > ** | > ** | > * | > ** | > * | |
| MCP-3/CCL7 | > | > ** | > *** | > * | > * | |
| MCP-5/CCL12 | > | > ** | > ** | > **** | ||
| MDC/CCL22 | > | > ** | > * | > | > *** | |
| MIP-1a/CCL3 | > | > | > | > | > | |
| MIP-1b/CCL4 | > ** | > | > | > | ||
| MIP-3a/CCL20 | > ** | > ** | > ** | > | > * | |
| MIP-3b/CCL19 | > * | > ** | > * | > *** | ||
| RANTES/CCL5 | > | > | > | > | ||
| SCYB16/CXCL16 | > | > | ||||
| SDF-1a/CXCL12 | > *** | > *** | > ** | > **** | ||
| TARC/CCL17 | > *** | > *** | > ** | > *** | ||
| TNF-α | > ** | > * | > **** | > ** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bircher, A.; Katkeviciute, E.; Morsy, Y.; Lang, S.; Montalban-Arques, A.; Scharl, M. Roseburia intestinalis Modulates Immune Responses by Inducing M1 Macrophage Polarization. Int. J. Mol. Sci. 2025, 26, 5049. https://doi.org/10.3390/ijms26115049
Bircher A, Katkeviciute E, Morsy Y, Lang S, Montalban-Arques A, Scharl M. Roseburia intestinalis Modulates Immune Responses by Inducing M1 Macrophage Polarization. International Journal of Molecular Sciences. 2025; 26(11):5049. https://doi.org/10.3390/ijms26115049
Chicago/Turabian StyleBircher, Anna, Egle Katkeviciute, Yasser Morsy, Silvia Lang, Ana Montalban-Arques, and Michael Scharl. 2025. "Roseburia intestinalis Modulates Immune Responses by Inducing M1 Macrophage Polarization" International Journal of Molecular Sciences 26, no. 11: 5049. https://doi.org/10.3390/ijms26115049
APA StyleBircher, A., Katkeviciute, E., Morsy, Y., Lang, S., Montalban-Arques, A., & Scharl, M. (2025). Roseburia intestinalis Modulates Immune Responses by Inducing M1 Macrophage Polarization. International Journal of Molecular Sciences, 26(11), 5049. https://doi.org/10.3390/ijms26115049





