Modulation of the Antitumor Response to Metformin, Caffeine, and Sodium Dichloroacetate by the Hypoxic Microenvironment in Lung Cancer Cells
Abstract
1. Introduction
| Drug | Condition | Antitumor Effect | Mechanism | References |
|---|---|---|---|---|
| Metformin | Normoxia | Induces apoptosis Arrests the cell cycle Inhibits DNA repair Blocks mTOR/Akt | AMPK activation mTOR/Akt inhibition c-MYC reduction | [6,7,8,9,10,22,23,24,25] |
| Metformin | Hypoxia | Inhibits HIF-1α activation | Suppression of HIF-1α VEGF and MMP-2 | [11,26] |
| Caffeine | Normoxia/ Hypoxia | Induces apoptosis Inhibits metastasis Reduces VEGF Increases cisplatin Inhibits integrin | Induction of p53 Inhibition of PI3K Adenosine receptor blockade | [12,13,14,15,16,27,28,29,30] |
| DCA | Normoxia/ Hypoxia | Modifies the Warburg effect Reduces lactate/glucose consumption Slows cell growth Synergizes with chemotherapy | Modification of tumor metabolism | [17,31,32,33,34,35] |
| Combinations | ||||
| Metformin + Caffeine | In vivo | Inhibit fibrosarcoma | Decreased Ki-67 | [36] |
| Metformin + DCA | Normoxia | Additive effect Suppresses expansion Increases apoptosis Inhibits mTOR1 and MCL-1 | Inhibición de mTOR1 y MCL-1 | [33,37] |
| Metformin + DCA | Normoxia | Increases cell death | In the absence of HIF-1α | [38] |
2. Results
2.1. Oxygen Consumption
2.2. Pharmacological Effect of Metformin
2.3. Pharmacological Effect of Caffeine
2.4. Pharmacological Effect of DCA
2.5. Drug Combinations
2.6. Pharmacological Interaction of Metformin with Caffeine in Normoxia and Hypoxia Conditions
2.7. Drug Interaction of Metformin with DCA Under Normoxia and Hypoxia Conditions
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Line Selection
4.3. Drugs
4.4. Hypoxia
4.5. Cell Viability Assay
4.6. Quantification of the Pharmacological Effect and Interaction Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCA | Dichloroacetate |
| mM | Millimolar |
| gr | Gram |
| U/mL | Units per milliliter |
| μg/mL | Micrograms per milliliter |
| mg/mL | Milligrams per milliliter |
| PBS | Phosphate-Buffered Saline |
| KCl | Potassium chloride |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EDTA | Ethylenediaminetetraacetic acid |
| μL | Microliter |
| mL | Milliliter |
| G | Grams |
| nM | Nanomolar |
| nm | Nanometer |
| IQR | Interquartile range |
| p-value | Probability value |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| mTOR | Mammalian target of rapamycin |
| Akt | Also known as protein kinase B (PKB) |
| c-MYC | Proto-oncogene c-Myc |
| VEGF | Vascular endothelial growth factor |
| MMP-2 | Matrix metalloproteinase 2 |
| AMPK | AMP-activated protein kinase |
| PI3K | Phosphatidylinositol-3 kinase |
| NSCLC | Non-small cell lung cancer |
| TKIs | Tyrosine Kinase Inhibitors |
| EGFR | Epidermal growth factor receptor |
| MDR-1 | Multidrug resistance protein 1 |
| NF-κB | Nuclear factor kappa B |
References
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. Global Variations in Lung Cancer Incidence by Histological Subtype in 2020: A Population-Based Study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The Global Burden of Lung Cancer: Current Status and Future Trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Rodríguez-Lara, V.; Ramírez-Tirado, L.A.; Barrón, F.; Zatarain-Barrón, Z.L.; Flores-Estrada, D.; Arrieta, O. Characteristics of Non-Small Cell Lung Cancer: Differences by Sex and Hormonal Status in a Mexican Population. Salud Publica Mex. 2019, 61, 265–275. [Google Scholar] [CrossRef]
- Alatorre, J.A.; Campos-Gómez, S.; De la Mora, E.; Novick, D.; Cruz, A.; Iglesias-Chiesa, J.M. Treatment Patterns and Costs Associated with Stage IV Non-Small Cell Lung Cancer in a Mexican Population: A Chart Review. Pharmacoecon. Open 2020, 4, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Chiang, J.-H.; Tsai, F.-J.; Hsu, Y.-M.; Juan, Y.-N.; Yang, J.-S.; Chiu, H.-Y. Metformin Triggers the Intrinsic Apoptotic Response in Human AGS Gastric Adenocarcinoma Cells by Activating AMPK and Suppressing mTOR/AKT Signaling. Int. J. Oncol. 2019, 54, 1271–1281. [Google Scholar] [CrossRef]
- Lee, J.; Hong, E.M.; Kim, J.H.; Jung, J.H.; Park, S.W.; Koh, D.H.; Choi, M.H.; Jang, H.J.; Kae, S.H. Metformin Induces Apoptosis and Inhibits Proliferation through the AMP-Activated Protein Kinase and Insulin-like Growth Factor 1 Receptor Pathways in the Bile Duct Cancer Cells. J. Cancer 2019, 10, 1734–1744. [Google Scholar] [CrossRef]
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S.; et al. Metformin Elicits Anticancer Effects through the Sequential Modulation of DICER and C-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef]
- Goetzman, E.S.; Prochownik, E.V. The Role for Myc in Coordinating Glycolysis, Oxidative Phosphorylation, Glutaminolysis, and Fatty Acid Metabolism in Normal and Neoplastic Tissues. Front. Endocrinol. 2018, 9, 129. [Google Scholar] [CrossRef]
- Mogavero, A.; Maiorana, M.V.; Zanutto, S.; Varinelli, L.; Bozzi, F.; Belfiore, A.; Volpi, C.C.; Gloghini, A.; Pierotti, M.A.; Gariboldi, M. Metformin Transiently Inhibits Colorectal Cancer Cell Proliferation as a Result of Either AMPK Activation or Increased ROS Production. Sci. Rep. 2017, 7, 15992. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Yi, G.; Deng, M.; Liu, H.; Liang, M.; Shi, B.; Fu, X.; Chen, Y.; Chen, L.; et al. Metformin Suppresses Hypoxia-Induced Stabilization of HIF-1α through Reprogramming of Oxygen Metabolism in Hepatocellular Carcinoma. Oncotarget 2016, 7, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Daniele, N.; Ktori, C.; Anderson, K.E.; Jensen, J.; Shepherd, P.R. Direct Effects of Caffeine and Theophylline on P110 Delta and Other Phosphoinositide 3-Kinases. Differential Effects on Lipid Kinase and Protein Kinase Activities. J. Biol. Chem. 2002, 277, 37124–37130. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Imamichi, Y.; Kawajiri, S.; Fujimaki, T.; Tanida, I.; Kobayashi, H.; Sato, F.; Sato, S.; Ishikawa, K.-I.; et al. Caffeine Induces Apoptosis by Enhancement of Autophagy via PI3K/Akt/mTOR/p70S6K Inhibition. Autophagy 2011, 7, 176–187. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ma, W.-Y.; Hashimoto, T.; Bode, A.M.; Yang, C.S.; Dong, Z. Induction of Apoptosis by Caffeine Is Mediated by the P53, Bax, and Caspase 3 Pathways. Cancer Res. 2003, 63, 4396–4401. [Google Scholar]
- Dong, S.; Kong, J.; Kong, J.; Shen, Q.; Kong, F.; Sun, W.; Zheng, L. Low Concentration of Caffeine Inhibits the Progression of the Hepatocellular Carcinoma via Akt Signaling Pathway. Anticancer. Agents Med. Chem. 2015, 15, 484–492. [Google Scholar] [CrossRef]
- Meisaprow, P.; Aksorn, N.; Vinayanuwattikun, C.; Chanvorachote, P.; Sukprasansap, M. Caffeine Induces G0/G1 Cell Cycle Arrest and Inhibits Migration through Integrin Av, Β3, and FAK/Akt/c-Myc Signaling Pathway. Molecules 2021, 26, 7659. [Google Scholar] [CrossRef]
- Allen, K.T.; Chin-Sinex, H.; DeLuca, T.; Pomerening, J.R.; Sherer, J.; Watkins, J.B.; Foley, J.; Jesseph, J.M.; Mendonca, M.S. Dichloroacetate Alters Warburg Metabolism, Inhibits Cell Growth, and Increases the X-Ray Sensitivity of Human A549 and H1299 NSC Lung Cancer Cells. Free Radic. Biol. Med. 2015, 89, 263–273. [Google Scholar] [CrossRef]
- Ancel, J.; Perotin, J.-M.; Dewolf, M.; Launois, C.; Mulette, P.; Nawrocki-Raby, B.; Dalstein, V.; Gilles, C.; Deslée, G.; Polette, M.; et al. Hypoxia in Lung Cancer Management: A Translational Approach. Cancers 2021, 13, 3421. [Google Scholar] [CrossRef]
- Kopecka, J.; Salaroglio, I.C.; Perez-Ruiz, E.; Sarmento-Ribeiro, A.B.; Saponara, S.; De Las Rivas, J.; Riganti, C. Hypoxia as a Driver of Resistance to Immunotherapy. Drug Resist. Updat. 2021, 59, 100787. [Google Scholar] [CrossRef]
- Grabovsky, Y.; Tallarida, R.J. Isobolographic Analysis for Combinations of a Full and Partial Agonist: Curved Isoboles. J. Pharmacol. Exp. Ther. 2004, 310, 981–986. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.-X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram Analysis: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ashinuma, H.; Takiguchi, Y.; Kitazono, S.; Kitazono-Saitoh, M.; Kitamura, A.; Chiba, T.; Tada, Y.; Kurosu, K.; Sakaida, E.; Sekine, I.; et al. Antiproliferative Action of Metformin in Human Lung Cancer Cell Lines. Oncol. Rep. 2012, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-C.; Huang, Y.-C.; Chen, H.-J.; Tseng, S.-C.; Chiu, H.-C.; Wo, T.-Y.; Huang, Y.-J.; Weng, S.-H.; Chiou, R.Y.Y.; Lin, Y.-W. Metformin Induces Cytotoxicity by Down-Regulating Thymidine Phosphorylase and Excision Repair Cross-Complementation 1 Expression in Non-Small Cell Lung Cancer Cells. Basic. Clin. Pharmacol. Toxicol. 2013, 113, 56–65. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, F.-C.; Wang, W.; Shi, H.-S.; Li, D.; Wang, Y.-S. K-ras Gene Mutation as a Predictor of Cancer Cell Responsiveness to Metformin. Mol. Med. Rep. 2013, 8, 763–768. [Google Scholar] [CrossRef]
- Xie, W.; Wang, L.; Sheng, H.; Qiu, J.; Zhang, D.; Zhang, L.; Yang, F.; Tang, D.; Zhang, K. Metformin Induces Growth Inhibition and Cell Cycle Arrest by Upregulating MicroRNA34a in Renal Cancer Cells. Med. Sci. Monit. 2017, 23, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhou, W.; Huang, Y.; Ren, M.; Xu, F.; Wang, H. Systemic Hypoxia Potentiates Anti-Tumor Effects of Metformin in Hepatocellular Carcinoma in Mice. Acta Biochim. Biophys. Sin. 2020, 52, 421–429. [Google Scholar] [CrossRef]
- Gude, R.P.; Menon, L.G.; Rao, S.G. Effect of Caffeine, a Xanthine Derivative, in the Inhibition of Experimental Lung Metastasis Induced by B16F10 Melanoma Cells. J. Exp. Clin. Cancer Res. 2001, 20, 287–292. [Google Scholar]
- Merighi, S.; Benini, A.; Mirandola, P.; Gessi, S.; Varani, K.; Simioni, C.; Leung, E.; Maclennan, S.; Baraldi, P.G.; Borea, P.A. Caffeine Inhibits Adenosine-Induced Accumulation of Hypoxia-Inducible Factor-1alpha, Vascular Endothelial Growth Factor, and Interleukin-8 Expression in Hypoxic Human Colon Cancer Cells. Mol. Pharmacol. 2007, 72, 395–406. [Google Scholar] [CrossRef]
- Eini, H.; Frishman, V.; Yulzari, R.; Kachko, L.; Lewis, E.C.; Chaimovitz, C.; Douvdevani, A. Caffeine Promotes Anti-Tumor Immune Response during Tumor Initiation: Involvement of the Adenosine A2A Receptor. Biochem. Pharmacol. 2015, 98, 110–118. [Google Scholar] [CrossRef]
- Wang, G.; Bhoopalan, V.; Wang, D.; Wang, L.; Xu, X. The Effect of Caffeine on Cisplatin-Induced Apoptosis of Lung Cancer Cells. Exp. Hematol. Oncol. 2015, 4, 5. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, D.; Hou, B.; Liu, Q.-X.; Chen, Q.; Deng, X.-F.; Yu, Z.-B.; Dai, J.-G.; Zheng, H. Dichloroacetate Enhances the Antitumor Efficacy of Chemotherapeutic Agents via Inhibiting Autophagy in Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2018, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Al-Azawi, A.; Sulaiman, S.; Arafat, K.; Yasin, J.; Nemmar, A.; Attoub, S. Impact of Sodium Dichloroacetate Alone and in Combination Therapies on Lung Tumor Growth and Metastasis. Int. J. Mol. Sci. 2021, 22, 12553. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, X.; Ni, Z.; Zhang, Y.; Zeng, Y.; Yan, X.; Huang, Y.; He, J.; Lyu, X.; Wu, Y.; et al. Dichloroacetate and Metformin Synergistically Suppress the Growth of Ovarian Cancer Cells. Oncotarget 2016, 7, 59458–59470. [Google Scholar] [CrossRef]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Boichuk, I.V.; Solyanik, G.I. Hypoxia Enhances Antitumor Activity of Dichloroacetate. Exp. Oncol. 2014, 36, 231–235. [Google Scholar] [PubMed]
- Sanchez, W.Y.; McGee, S.L.; Connor, T.; Mottram, B.; Wilkinson, A.; Whitehead, J.P.; Vuckovic, S.; Catley, L. Dichloroacetate Inhibits Aerobic Glycolysis in Multiple Myeloma Cells and Increases Sensitivity to Bortezomib. Br. J. Cancer 2013, 108, 1624–1633. [Google Scholar] [CrossRef]
- Popović, D.J.; Lalošević, D.; Miljković, D.; Popović, K.J.; Čapo, I.; Popović, J.K. Caffeine Induces Metformin Anticancer Effect on Fibrosarcoma in Hamsters. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2461–2467. [Google Scholar] [CrossRef]
- Kim, T.S.; Lee, M.; Park, M.; Kim, S.Y.; Shim, M.S.; Lee, C.Y.; Choi, D.H.; Cho, Y. Metformin and Dichloroacetate Suppress Proliferation of Liver Cancer Cells by Inhibiting mTOR Complex 1. Int. J. Mol. Sci. 2021, 22, 10027. [Google Scholar] [CrossRef]
- Hong, S.-E.; Jin, H.-O.; Kim, H.-A.; Seong, M.-K.; Kim, E.-K.; Ye, S.-K.; Choe, T.-B.; Lee, J.K.; Kim, J.-I.; Park, I.-C.; et al. Targeting HIF-1α Is a Prerequisite for Cell Sensitivity to Dichloroacetate (DCA) and Metformin. Biochem. Biophys. Res. Commun. 2016, 469, 164–170. [Google Scholar] [CrossRef]
- Yin, X.; Wei, Z.; Song, C.; Tang, C.; Xu, W.; Wang, Y.; Xie, J.; Lin, Z.; Han, W. Metformin Sensitizes Hypoxia-Induced Gefitinib Treatment Resistance of HNSCC via Cell Cycle Regulation and EMT Reversal. Cancer Manag. Res. 2018, 10, 5785–5798. [Google Scholar] [CrossRef]
- Wang, J.-C.; Li, G.-Y.; Li, P.-P.; Sun, X.; Li, W.-M.; Li, Y.; Lu, S.-Y.; Liu, P.-J. Suppression of Hypoxia-Induced Excessive Angiogenesis by Metformin via Elevating Tumor Blood Perfusion. Oncotarget 2017, 8, 73892–73904. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.J.; Sonneveld, P.; Schoester, M.; Raaijmakers, H.G.; Nieuwenhuis, H.K.; Dekker, A.W.; Lokhorst, H.M. MDR-1 Expression and Response to Vincristine, Doxorubicin, and Dexamethasone Chemotherapy in Multiple Myeloma Refractory to Alkylating Agents. J. Clin. Oncol. 1994, 12, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.K.; Durán, G.E.; Mangili, A.; Beketic-Oreskovic, L.; Sikic, B.I. MDR 1 Activation Is the Predominant Resistance Mechanism Selected by Vinblastine in MES-SA Cells. Br. J. Cancer 2000, 83, 892–898. [Google Scholar] [CrossRef]
- Friche, E.; Skovsgaard, T.; Nissen, N.I. Anthracycline Resistance. Acta Oncol. 1989, 28, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Asakuno, K.; Kohno, K.; Uchiumi, T.; Kubo, T.; Sato, S.; Isono, M.; Kuwano, M. Involvement of a DNA Binding Protein, MDR-NF1/YB-1, in Human MDR1 Gene Expression by Actinomycin D. Biochem. Biophys. Res. Commun. 1994, 199, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, R.Z.; Duan, Z.; Lamendola, D.E.; Penson, R.T.; Seiden, M.V. Paclitaxel Resistance: Molecular Mechanisms and Pharmacologic Manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The Chemistry, Physiology and Pathology of pH in Cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Thews, O.; Riemann, A.; Nowak, M.; Gekle, M. Impact of Hypoxia-Related Tumor Acidosis on Cytotoxicity of Different Chemotherapeutic Drugs in Vitro and in Vivo. Adv. Exp. Med. Biol. 2014, 812, 51–58. [Google Scholar] [CrossRef]
- Cheng, Z.-X.; Wang, D.-W.; Liu, T.; Liu, W.-X.; Xia, W.-B.; Xu, J.; Zhang, Y.-H.; Qu, Y.-K.; Guo, L.-Q.; Ding, L.; et al. Effects of the HIF-1α and NF-κB Loop on Epithelial-Mesenchymal Transition and Chemoresistance Induced by Hypoxia in Pancreatic Cancer Cells. Oncol. Rep. 2014, 31, 1891–1898. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Toth, R.K.; Jensen, C.C.; Casillas, A.L.; Kashatus, D.F.; Warfel, N.A. PIM Kinases Alter Mitochondrial Dynamics and Chemosensitivity in Lung Cancer. Oncogene 2020, 39, 2597–2611. [Google Scholar] [CrossRef]
- Marayati, R.; Stafman, L.L.; Williams, A.P.; Bownes, L.V.; Quinn, C.H.; Aye, J.M.; Stewart, J.E.; Yoon, K.J.; Anderson, J.C.; Willey, C.D.; et al. PIM Kinases Mediate Resistance to Cisplatin Chemotherapy in Hepatoblastoma. Sci. Rep. 2021, 11, 5984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, G.; Gao, Y.; Liang, H. HOTAIR/miR-125 Axis-Mediated Hexokinase 2 Expression Promotes Chemoresistance in Human Glioblastoma. J. Cell Mol. Med. 2020, 24, 5707–5717. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of Mitochondrial Function in Situ in Permeabilized Muscle Fibers, Tissues and Cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]

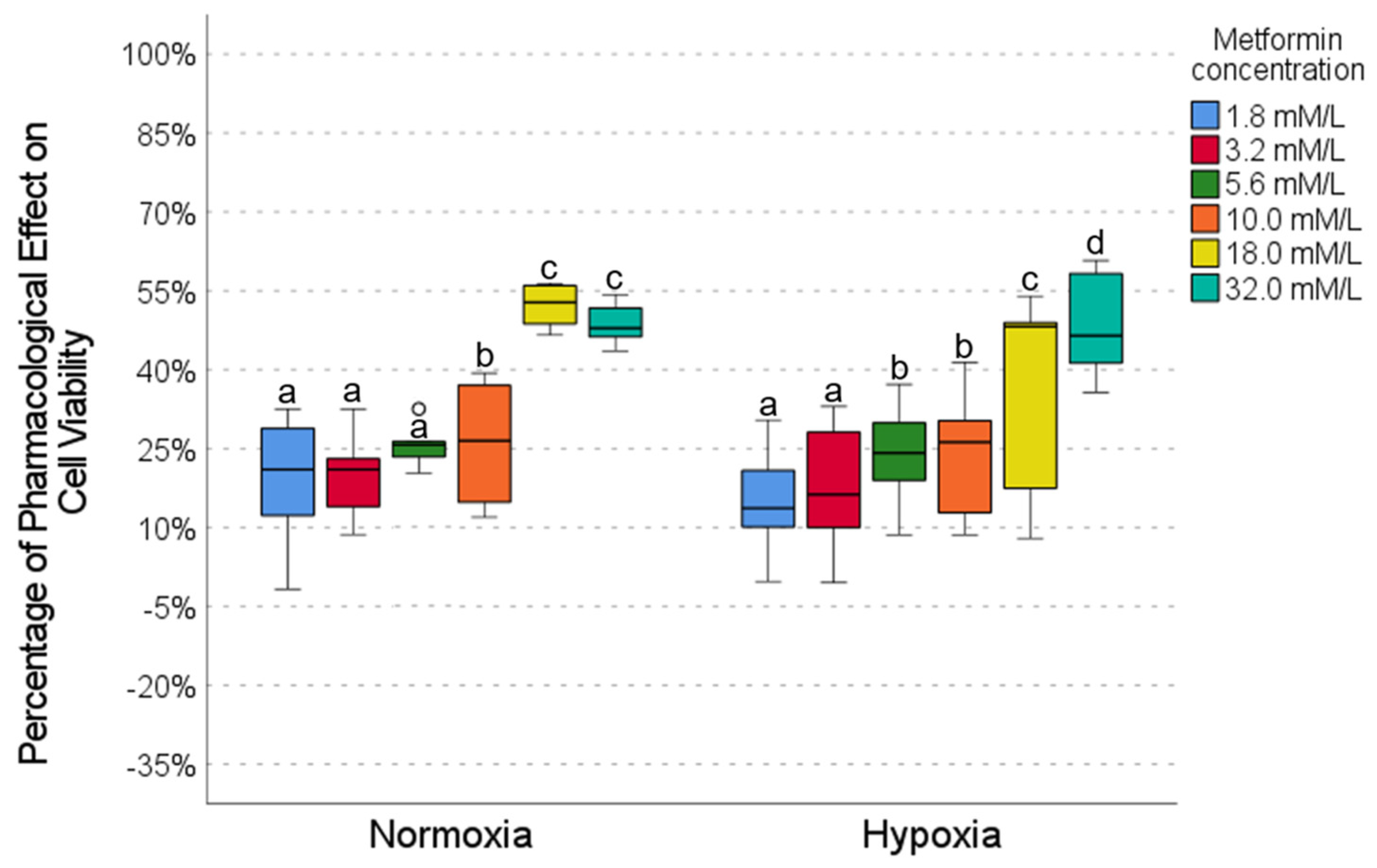
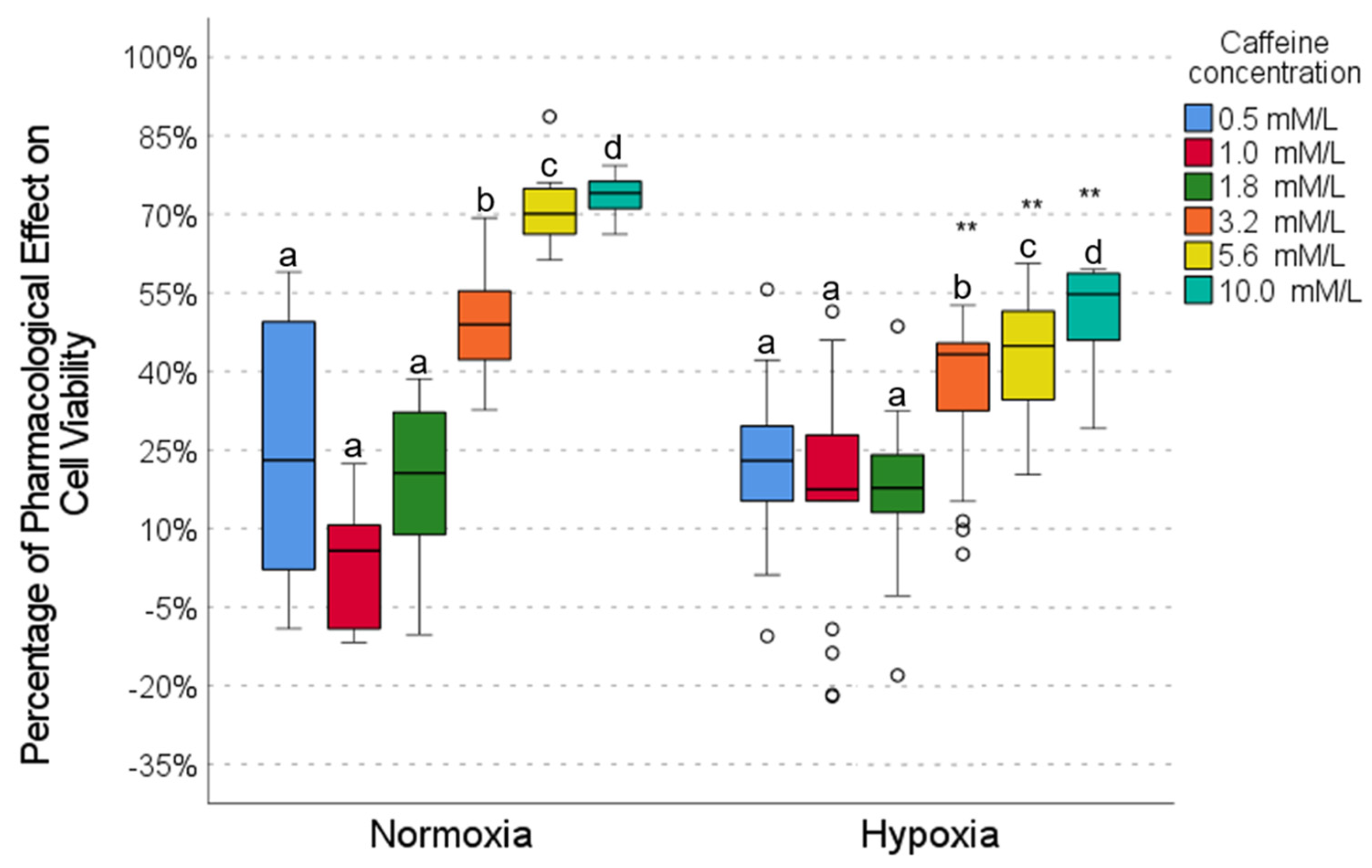
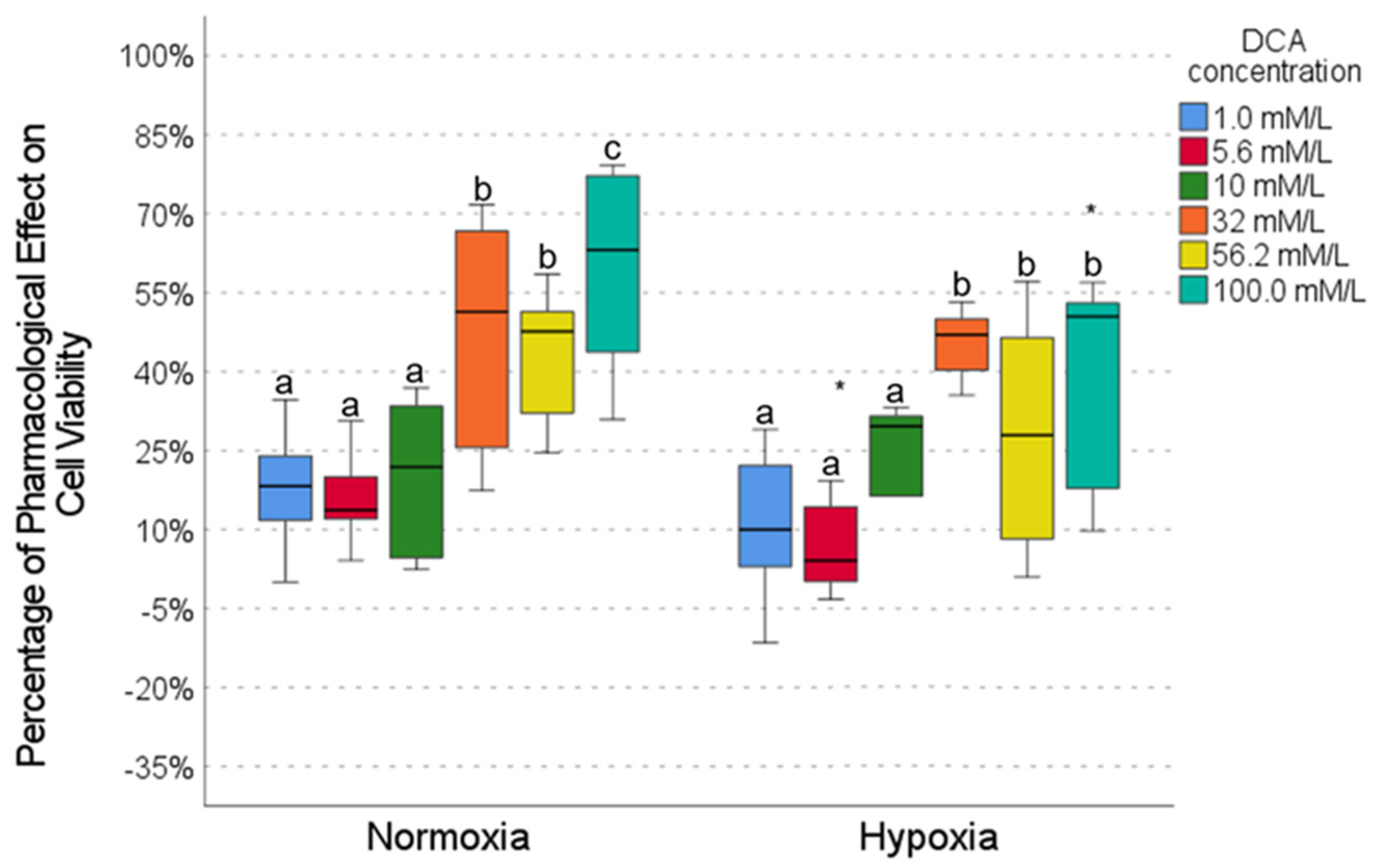
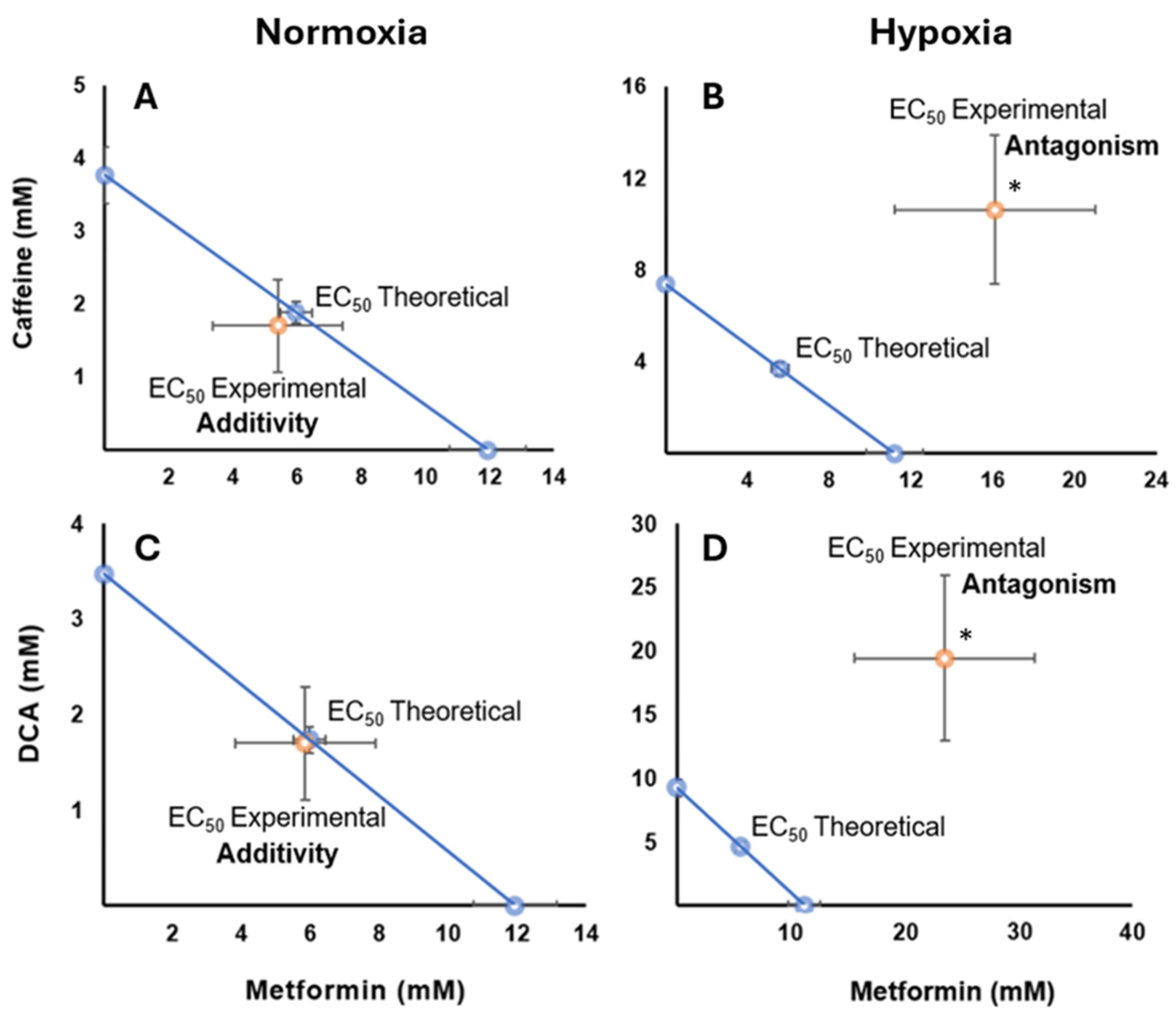
| Metformin | Caffeine | Metformin | Caffeine | Metformin | DCA | Metformin | DCA | |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.59 | 2.13 | 5.59 | 3.69 | 7.59 | 2.02 | 5.58 | 4.61 |
| 2 | 3.79 | 1.06 | 3.79 | 1.84 | 3.79 | 1.01 | 2.79 | 2.30 |
| 3 | 1.89 | 0.53 | 1.89 | 0.92 | 1.89 | 0.50 | 1.39 | 1.15 |
| 4 | 0.94 | 0.26 | 0.94 | 0.46 | 0.94 | 0.25 | 0.69 | 0.57 |
| Normoxia | Hypoxia | Normoxia | Hypoxia | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Martin, M.O.; Castillejos-Lopez, M.; Prado-Garcia, H.; Romero-Garcia, S.; Huerta-Cruz, J.C.; Choreño-Parra, J.A.; Gonzalez-Avila, G.; Colín-Godínez, L.A.; Paz-Gomez, D.; Carlos-Reyes, Á.; et al. Modulation of the Antitumor Response to Metformin, Caffeine, and Sodium Dichloroacetate by the Hypoxic Microenvironment in Lung Cancer Cells. Int. J. Mol. Sci. 2025, 26, 5014. https://doi.org/10.3390/ijms26115014
Garcia-Martin MO, Castillejos-Lopez M, Prado-Garcia H, Romero-Garcia S, Huerta-Cruz JC, Choreño-Parra JA, Gonzalez-Avila G, Colín-Godínez LA, Paz-Gomez D, Carlos-Reyes Á, et al. Modulation of the Antitumor Response to Metformin, Caffeine, and Sodium Dichloroacetate by the Hypoxic Microenvironment in Lung Cancer Cells. International Journal of Molecular Sciences. 2025; 26(11):5014. https://doi.org/10.3390/ijms26115014
Chicago/Turabian StyleGarcia-Martin, Misael Osmar, Manuel Castillejos-Lopez, Heriberto Prado-Garcia, Susana Romero-Garcia, Juan Carlos Huerta-Cruz, José Alberto Choreño-Parra, Georgina Gonzalez-Avila, Luz A. Colín-Godínez, Daniel Paz-Gomez, Ángeles Carlos-Reyes, and et al. 2025. "Modulation of the Antitumor Response to Metformin, Caffeine, and Sodium Dichloroacetate by the Hypoxic Microenvironment in Lung Cancer Cells" International Journal of Molecular Sciences 26, no. 11: 5014. https://doi.org/10.3390/ijms26115014
APA StyleGarcia-Martin, M. O., Castillejos-Lopez, M., Prado-Garcia, H., Romero-Garcia, S., Huerta-Cruz, J. C., Choreño-Parra, J. A., Gonzalez-Avila, G., Colín-Godínez, L. A., Paz-Gomez, D., Carlos-Reyes, Á., Ruiz, V., Romero, Y., Flores-Soto, E., Rodríguez-Silverio, J., Lara-Lemus, R., Velázquez-Cruz, R., Salinas-Lara, C., Torres-Espíndola, L. M., & Aquino-Gálvez, A. (2025). Modulation of the Antitumor Response to Metformin, Caffeine, and Sodium Dichloroacetate by the Hypoxic Microenvironment in Lung Cancer Cells. International Journal of Molecular Sciences, 26(11), 5014. https://doi.org/10.3390/ijms26115014







