Hydrochar from Carbon Quantum Dots (CQDs) Synthesis for Photocatalytic and Decontamination Applications in Presence of TiO2
Abstract
1. Introduction
2. Results and Discussion
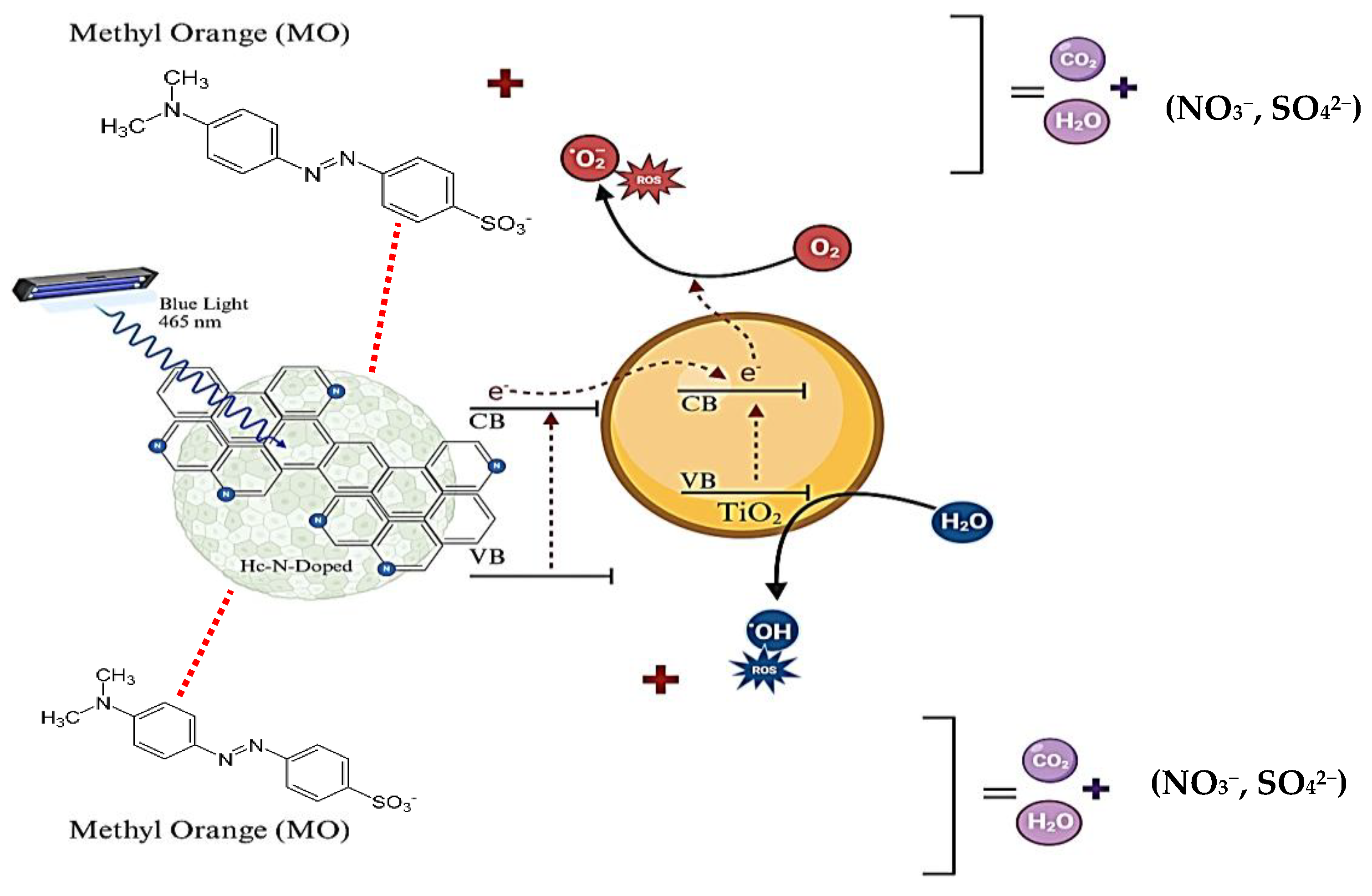
2.1. Photodegradation of MO Using Hc-TiO2 Composites
2.1.1. Effect of the Hydrochar and Temperature Synthesis
2.1.2. Effect of the Heteroatom Donor During Hydrochar Synthesis
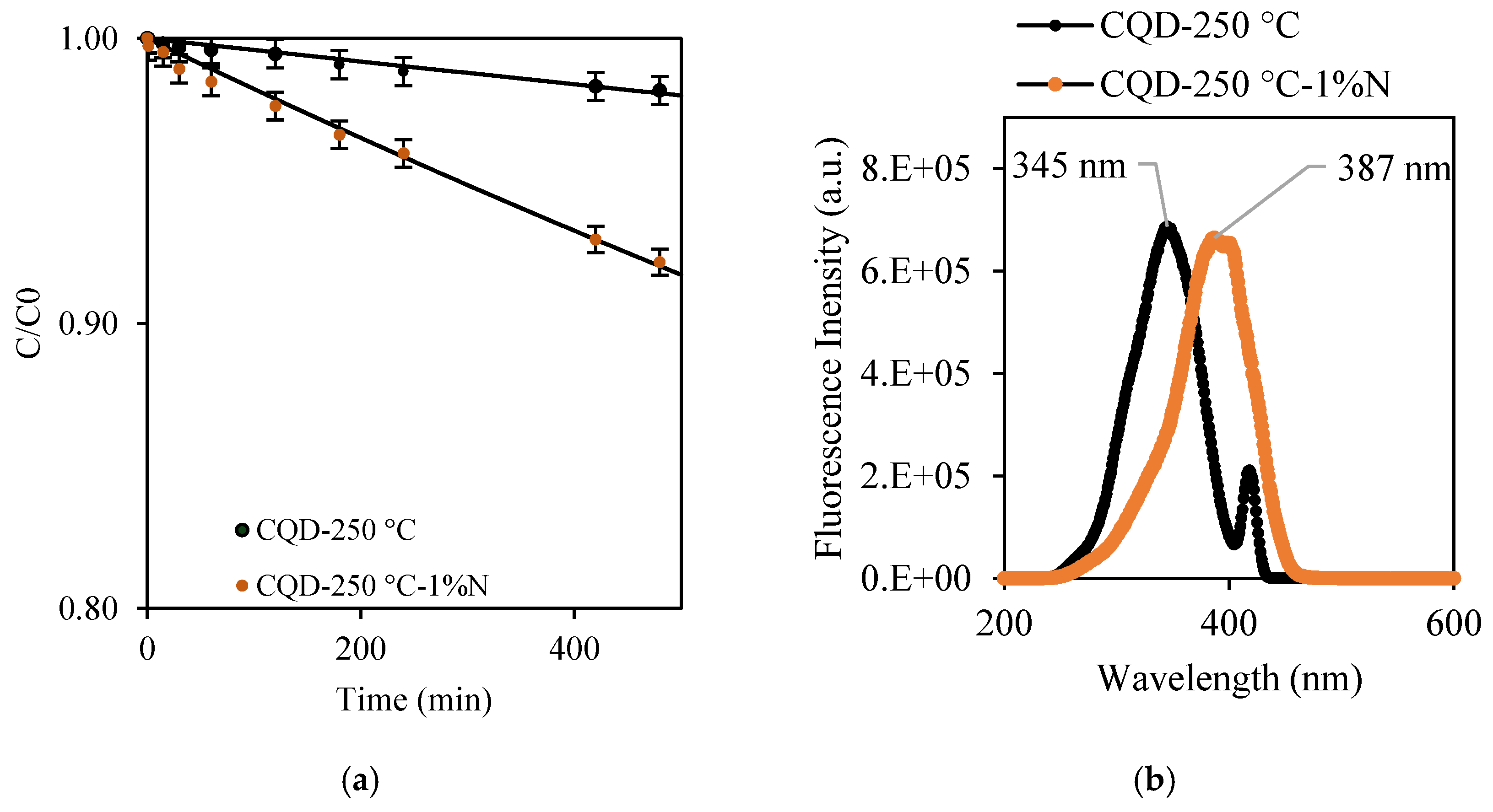
2.2. Photodegradation of MO Using Co-Produced CQDs
3. Materials and Methods
3.1. Chemicals
3.2. Co-Production of Hydrochar and CQDs Nanoparticles
3.3. Characterization of Carbon Structures
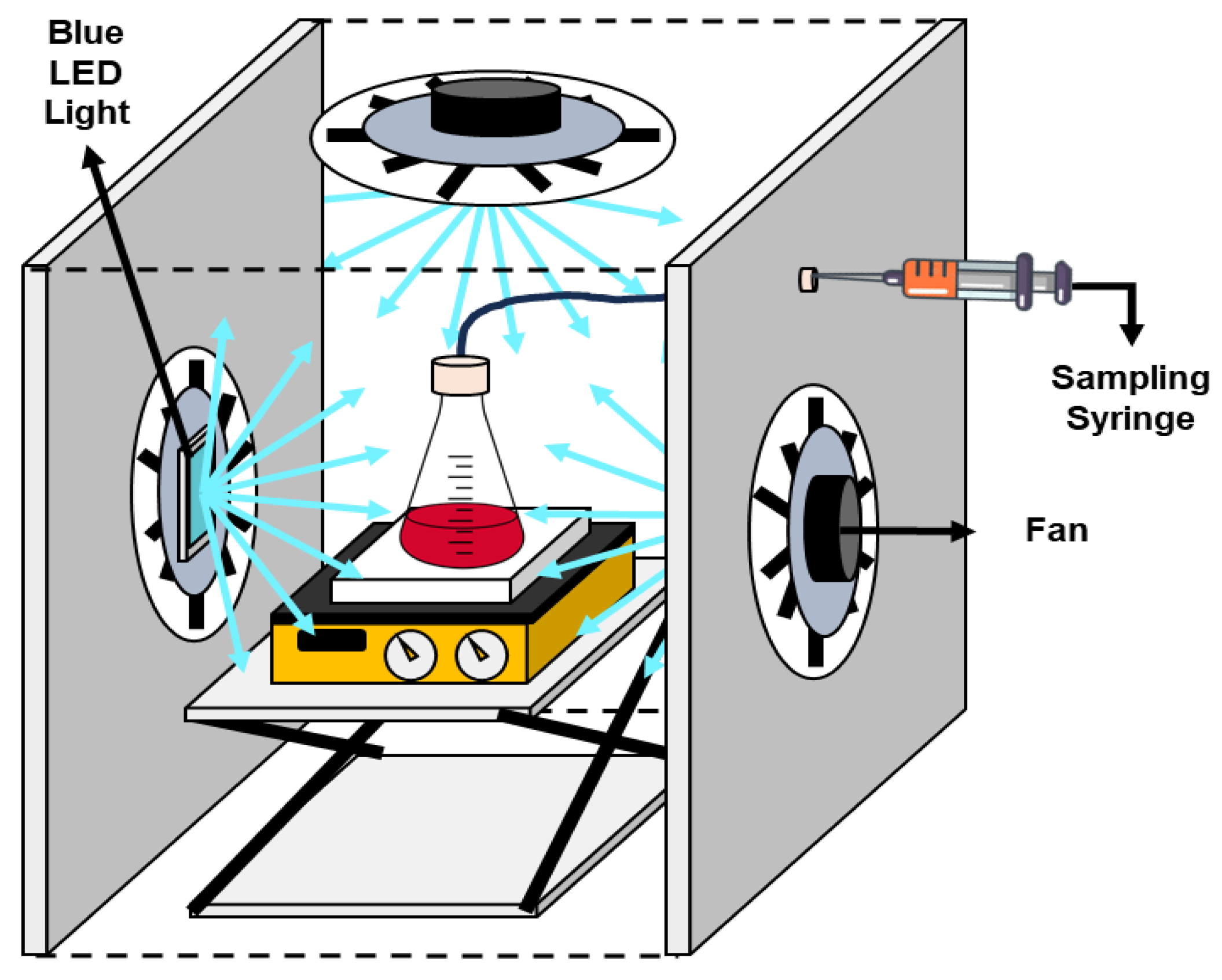
3.4. Photocatalytic Trials
3.5. Electrical Energy per Order (EEO)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hc | Hydrochar |
| MO | Methyl Orange |
| UV | Ultraviolet |
| VIS | Visible |
| ROS | Reactive oxygen species |
| TiO2 | Titanium dioxide |
| OH● | Hydroxyl radicals |
| H2O2 | Hydrogen peroxide |
| CQDs | Carbon quantum dots |
| PL | Photoluminescence |
| EBT | Eriochrome black t |
| N-CQDs | Nitrogen-doped carbon quantum dots |
| C2H8N2 | Ethylenediamine |
| H3PO4 | Orthophosphoric acid |
| N-donor | Nitrogen donor |
| P-donor | Phosphorous donor |
| HETAM | Heteroatom |
| FE-SEM | Field emission scanning electron microscopy |
| HR-TEM | High-resolution transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| LJ | Juice extract |
| TGA | Thermogravimetric analysis |
| %MO | % of MO photodegradation |
| D | Average crystal size |
| nm | Nanometer |
References
- Bensmaine, Z.; El Korso, S.; Moral-Rodríguez, A.I.; Bedrane, S.; Ziani-Cherif, C.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Bailón-García, E. Enhanced Photodegradation of Sulfamethoxazole Through Cutting-Edge Titania-Zirconia-Based Materials. Catalysts 2024, 14, 784. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-based nanostructures as a promising material for diverse environmental applications: A review. Reactions 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Prakash, J.; Cho, J.; Mishra, Y.K. Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: Scope against blocking the SARS-CoV-2 spread. Micro Nano Eng. 2022, 14, 100100. [Google Scholar] [CrossRef]
- Peiris, S.; de Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent development and future prospects of TiO2 photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Rajabzadeh-Khosroshahi, M.; Samadi, A.; Behzadmehr, R.; Rahdar, A.; Ferreira, L.F.R. Properties and application of carbon quantum dots (CQDs) in biosensors for disease detection: A comprehensive review. J. Drug Deliv. Sci. Technol. 2023, 80, 104156. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; Zuo, M.; Liang, Y.; Qin, P.; Wang, H.; Wu, Z.; Luo, L.; Liu, C.; Leng, L. Preparation of photocatalysts decorated by carbon quantum dots (CQDs) and their applications: A review. J. Environ. Chem. Eng. 2023, 11, 109487. [Google Scholar] [CrossRef]
- Mozdbar, A.; Nouralishahi, A.; Fatemi, S.; Talatori, F.S. The impact of Carbon Quantum Dots (CQDs) on the photocatalytic activity of TiO2 under UV and visible light. J. Water Process Eng. 2023, 51, 103465. [Google Scholar] [CrossRef]
- Gulati, S.; Baul, A.; Amar, A.; Wadhwa, R.; Kumar, S.; Varma, R.S. Eco-friendly and sustainable pathways to photoluminescent carbon quantum dots (CQDs). Nanomaterials 2023, 13, 554. [Google Scholar] [CrossRef]
- Najjar, M.; Nasseri, M.A.; Allahresani, A.; Darroudi, M. Green and efficient synthesis of carbon quantum dots from Cordia myxa L. and their application in photocatalytic degradation of organic dyes. J. Mol. Struct. 2022, 1266, 133456. [Google Scholar] [CrossRef]
- Rani, U.A.; Ng, L.Y.; Ng, Y.S.; Ng, C.Y.; Ong, Y.H.; Lim, Y.P. Photocatalytic degradation of methyl green dye by nitrogen-doped carbon quantum dots: Optimisation study by Taguchi approach. Mater. Sci. Eng. B 2022, 283, 115820. [Google Scholar] [CrossRef]
- Remli, U.; Aziz, A. Photocatalytic degradation of methyl orange using Carbon Quantum Dots (CQDs) derived from watermelon rinds. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 042038. [Google Scholar]
- Mindivan, F.; Göktaş, M. The green synthesis of carbon quantum dots (CQDs) and characterization of polycaprolactone (PCL/CQDs) films. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132446. [Google Scholar] [CrossRef]
- Khan, M.E.; Mohammad, A.; Yoon, T. State-of-the-art developments in carbon quantum dots (CQDs): Photo-catalysis, bio-imaging, and bio-sensing applications. Chemosphere 2022, 302, 134815. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal treatment of biomass feedstocks for sustainable production of chemicals, fuels, and materials: Progress and perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef]
- González-Arias, J.; Sánchez, M.E.; Cara-Jiménez, J.; Baena-Moreno, F.M.; Zhang, Z. Hydrothermal carbonization of biomass and waste: A review. Environ. Chem. Lett. 2022, 20, 211–221. [Google Scholar] [CrossRef]
- Ischia, G.; Cutillo, M.; Guella, G.; Bazzanella, N.; Cazzanelli, M.; Orlandi, M.; Miotello, A.; Fiori, L. Hydrothermal carbonization of glucose: Secondary char properties, reaction pathways, and kinetics. Chem. Eng. J. 2022, 449, 137827. [Google Scholar] [CrossRef]
- Hasan, M.R.; Saha, N.; Quaid, T.; Reza, M.T. Formation of carbon quantum dots via hydrothermal carbonization: Investigate the effect of precursors. Energies 2021, 14, 986. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Chen, G. Hydrochar production by hydrothermal carbonization: Microwave versus supercritical water treatment. Biomass 2024, 4, 574–598. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, C.; Qin, S.; Wang, B.; Zhao, P.; Cui, X. Effects of process water recirculation on yields and quality of hydrochar from hydrothermal carbonization process of rice husk. J. Anal. Appl. Pyrolysis 2022, 166, 105618. [Google Scholar] [CrossRef]
- Cavali, M.; Junior, N.L.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Filho, P.B.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar derived from municipal sludge through hydrothermal processing: A critical review on its formation, characterization, and valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef]
- Zunger, A.; Malyi, O.I. Understanding doping of quantum materials. Chem. Rev. 2021, 121, 3031–3060. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.; Desoky, W.M.; Hanafy, M.F.; Guirguis, O.W. Effect of TiO2 nanoparticles on the structural configurations and thermal, mechanical, and optical properties of chitosan/TiO2 nanoparticle composites. J. Phys. Chem. Solids 2021, 152, 109983. [Google Scholar] [CrossRef]
- Moustafa, H.; Karmalawi, A.M.; Youssef, A.M. Development of dapsone-capped TiO2 hybrid nanocomposites and their effects on the UV radiation, mechanical, thermal properties and antibacterial activity of PVA bionanocomposites. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100482. [Google Scholar] [CrossRef]
- Alluqmani, S.M.; Loulou, M.; Ouerfelli, J.; Alshahrie, A.; Salah, N. Annealing effect on structural and optical properties of nanostructured carbon of oil fly ash modified titania thin-film. Results Phys. 2021, 25, 104335. [Google Scholar] [CrossRef]
- Xia, S.; Huang, W.; Yan, W.; Yuan, X.; Chen, X.; Liu, L.; Fu, L.; Zhu, Y.; Huang, Q.; Wu, Y. A separator modified with rutile titania and three-dimensional interconnected graphene-like carbon for advanced Li–S batteries. ChemElectroChem 2022, 9, e202200301. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- García, E.B.; Barranco-López, A.; Moral-Rodríguez, A.I.; Fajardo-Puerto, E.; Elmouwahidi, A. Highly graphitic Fe-doped carbon xerogels as dual-functional electro-Fenton catalysts for the degradation of tetracycline in wastewater. Environ. Res. 2023, 228, 115757. [Google Scholar]
- Hamad, H.; Bailón-García, E.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Morales-Torres, S. Synthesis of TixOy nanocrystals in mild synthesis conditions for the degradation of pollutants under solar light. Appl. Catal. B Environ. 2019, 241, 385–392. [Google Scholar] [CrossRef]
- Munir, S.; Shah, S.M.; Hussain, H. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater. Des. 2016, 92, 64–72. [Google Scholar] [CrossRef]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the band gap energy and flat band potential for application of modified TiO2 photoanodes in water photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Guan, S.; Cheng, Y.; Hao, L.; Yoshida, H.; Tarashima, C.; Zhan, T.; Itoi, T.; Qiu, T.; Lu, Y. Oxygen vacancies induced band gap narrowing for efficient visible-light response in carbon-doped TiO2. Sci. Rep. 2023, 13, 14105. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, C.; Yang, Y.; Su, T.; Yi, G.; Zhang, X. Facile synthesis of molecularly imprinted black TiO2−x/carbon dots nanocomposite and its recognizable photocatalytic performance under visible-light. Appl. Surf. Sci. 2021, 551, 149476. [Google Scholar] [CrossRef]
- Bailón-García, E.; Elmouwahidi, A.; Álvarez, M.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. New carbon xerogel-TiO2 composites with high performance as visible-light photocatalysts for dye mineralization. Appl. Catal. B Environ. 2017, 201, 29–40. [Google Scholar] [CrossRef]
- Naik, V.M.; Bhosale, S.V.; Kolekar, G.B. A brief review on the synthesis, characterisation and analytical applications of nitrogen doped carbon dots. Anal. Methods 2022, 14, 877–891. [Google Scholar] [CrossRef]
- Yi, Z.; Li, X.; Zhang, H.; Ji, X.; Sun, W.; Yu, Y.; Liu, Y.; Huang, J.; Sarshar, Z.; Sain, M. High quantum yield photoluminescent N-doped carbon dots for switch sensing and imaging. Talanta 2021, 222, 121663. [Google Scholar] [CrossRef]
- Li, Y.; Miao, P.; Zhou, W.; Gong, X.; Zhao, X. N-doped carbon-dots for luminescent solar concentrators. J. Mater. Chem. A 2017, 5, 21452–21459. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, H.; Won, S.; Kim, H.; Kwon, W. Biocompatible nitrogen-doped carbon dots: Synthesis, characterization, and application. J. Mater. Chem. B 2020, 8, 8935–8951. [Google Scholar] [CrossRef]
- Cui, P.; Xue, Y. The role of center-N-doping in non-radiative recombination loss of nitrogen-doped graphene quantum dots. Mater. Sci. Semicond. Process. 2022, 139, 106323. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, Y.; Fu, Y.; Zheng, Y.; Shao, Y.; Bai, Y. Understanding and minimizing non-radiative recombination losses in perovskite light-emitting diodes. J. Mater. Chem. C 2022, 10, 13590–13610. [Google Scholar] [CrossRef]
- Borbolla-Gaxiola, J.E.; Ross, A.B.; Dupont, V. Multi-variate and multi-response analysis of hydrothermal carbonization of food waste: Hydrochar composition and solid fuel characteristics. Energies 2022, 15, 5342. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Sharif, N.F.M.; Shafie, S.; Ab Kadir, M.Z.A.; Md Din, M.F.; Yusuf, Y.; Shaban, S. TiO2 photoelectrode band gap modification using carbon quantum dots (CQDs) for dye-sensitised solar cells (DSSCs). Key Eng. Mater. 2022, 908, 265–270. [Google Scholar] [CrossRef]
- Alasri, T.M.; Ali, S.L.; Salama, R.S.; Alshorifi, F.T. Band-structure engineering of TiO2 photocatalyst by AuSe quantum dots for efficient degradation of malachite green and phenol. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1729–1740. [Google Scholar] [CrossRef]
- Khan, J.A.; Sayed, M.; Shah, N.S.; Khan, S.; Khan, A.A.; Sultan, M.; Tighezza, A.M.; Iqbal, J.; Boczkaj, G. Synthesis of N-doped TiO2 nanoparticles with enhanced photocatalytic activity for 2, 4-dichlorophenol degradation and H2 production. J. Environ. Chem. Eng. 2023, 11, 111308. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Patidar, A.; Dugyala, V.; Chakma, S.; Galodiya, M.; Giri, A. Reactive oxygen species aided photocatalytic degradation of tetracycline using non-metal activated carbon doped TiO2 nanocomposite under UV-light irradiation. Res. Chem. Intermed. 2024, 50, 1035–1063. [Google Scholar] [CrossRef]
- Yang, C.-C.; Tsai, M.-H.; Li, K.-Y.; Hou, C.-H.; Lin, F.-H. Carbon-doped TiO2 activated by x-ray irradiation for the generation of reactive oxygen species to enhance photodynamic therapy in tumor treatment. Int. J. Mol. Sci. 2019, 20, 2072. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Du, W.; Xu, X.; Hao, H.; Liu, R.; Zhang, D.; Gao, F.; Lu, Q. Green synthesis of fluorescent carbon quantum dots and carbon spheres from pericarp. Sci. China Chem. 2015, 58, 863–870. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, D.; Li, Q.; Zhou, X.; Wu, H.; Bao, Y.; Lu, H.; Luo, T.; Wang, J. Changes in organic acids, phenolic compounds, and antioxidant activities of lemon juice fermented by Issatchenkia terricola. Molecules 2021, 26, 6712. [Google Scholar] [CrossRef]
- Franco, C.A.; Candela, C.H.; Gallego, J.; Marin, J.; Patiño, L.E.; Ospina, N.; Patiño, E.; Molano, M.; Villamil, F.; Bernal, K.M. Easy and rapid synthesis of carbon quantum dots from Mortino (Vaccinium Meridionale Swartz) extract for use as green tracers in the oil and gas industry: Lab-to-field trial development in Colombia. Ind. Eng. Chem. Res. 2020, 59, 11359–11369. [Google Scholar] [CrossRef]
- Yashwanth, H.; Rondiya, S.R.; Dzade, N.Y.; Hoye, R.L.; Choudhary, R.J.; Phase, D.M.; Dhole, S.D.; Hareesh, K. Improved photocatalytic activity of TiO2 nanoparticles through nitrogen and phosphorus co-doped carbon quantum dots: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2022, 24, 15271–15279. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Cortes, J.M.; Moral-Rodríguez, A.I.; Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. BiVO4-Based Photocatalysts for the Degradation of Antibiotics in Wastewater: Calcination Role after Solvothermal Synthesis. Catalysts 2024, 14, 474. [Google Scholar] [CrossRef]
- Moral-Rodríguez, A.I.; Ramírez-Valencia, L.D.; Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Green synthesis of BiVO4/Eco-graphene nanostructures for the elimination of sulfamethoxazole by adsorption and photo-degradation using blue LED light. Environ. Res. 2024, 247, 118120. [Google Scholar] [CrossRef]
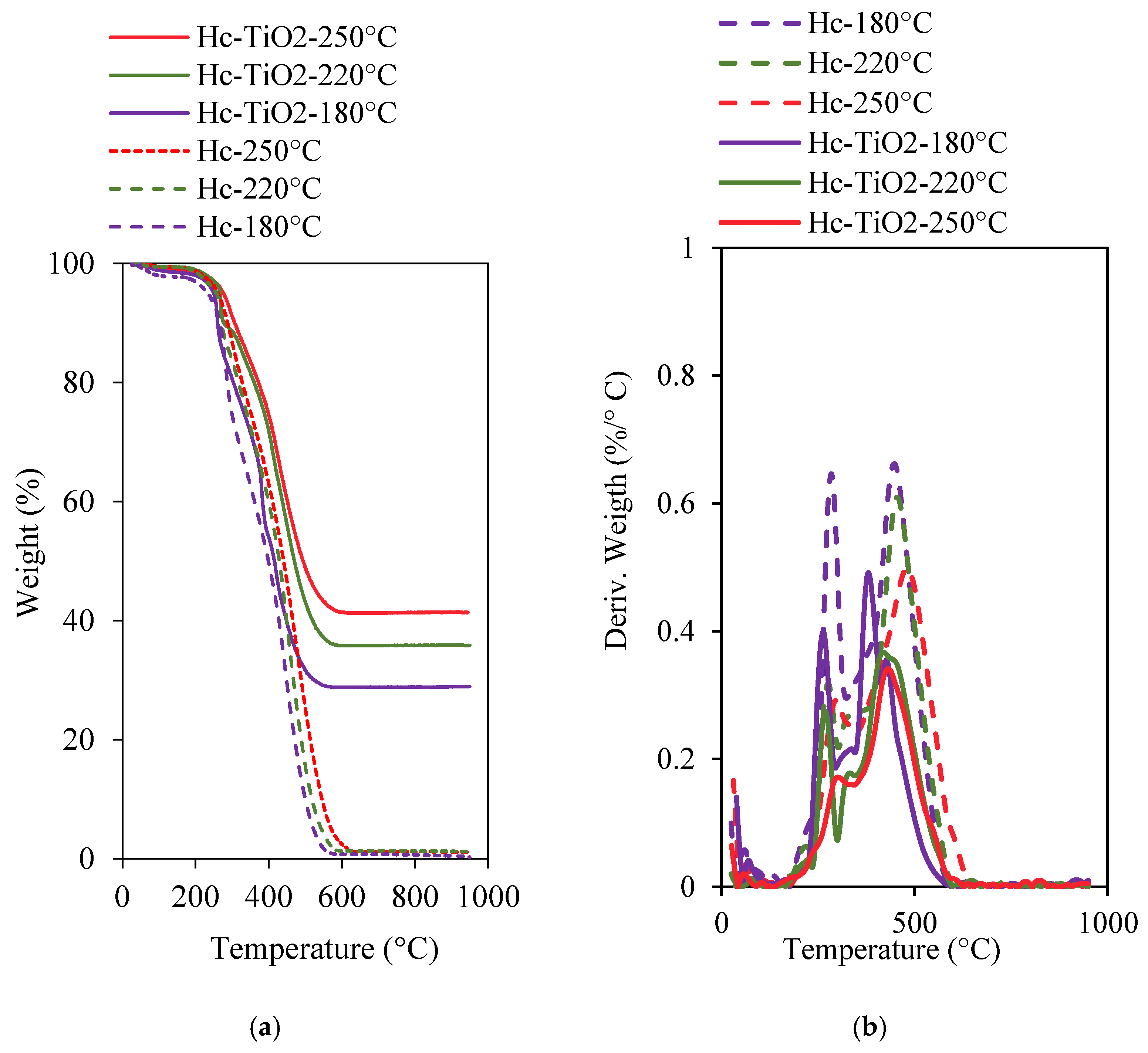
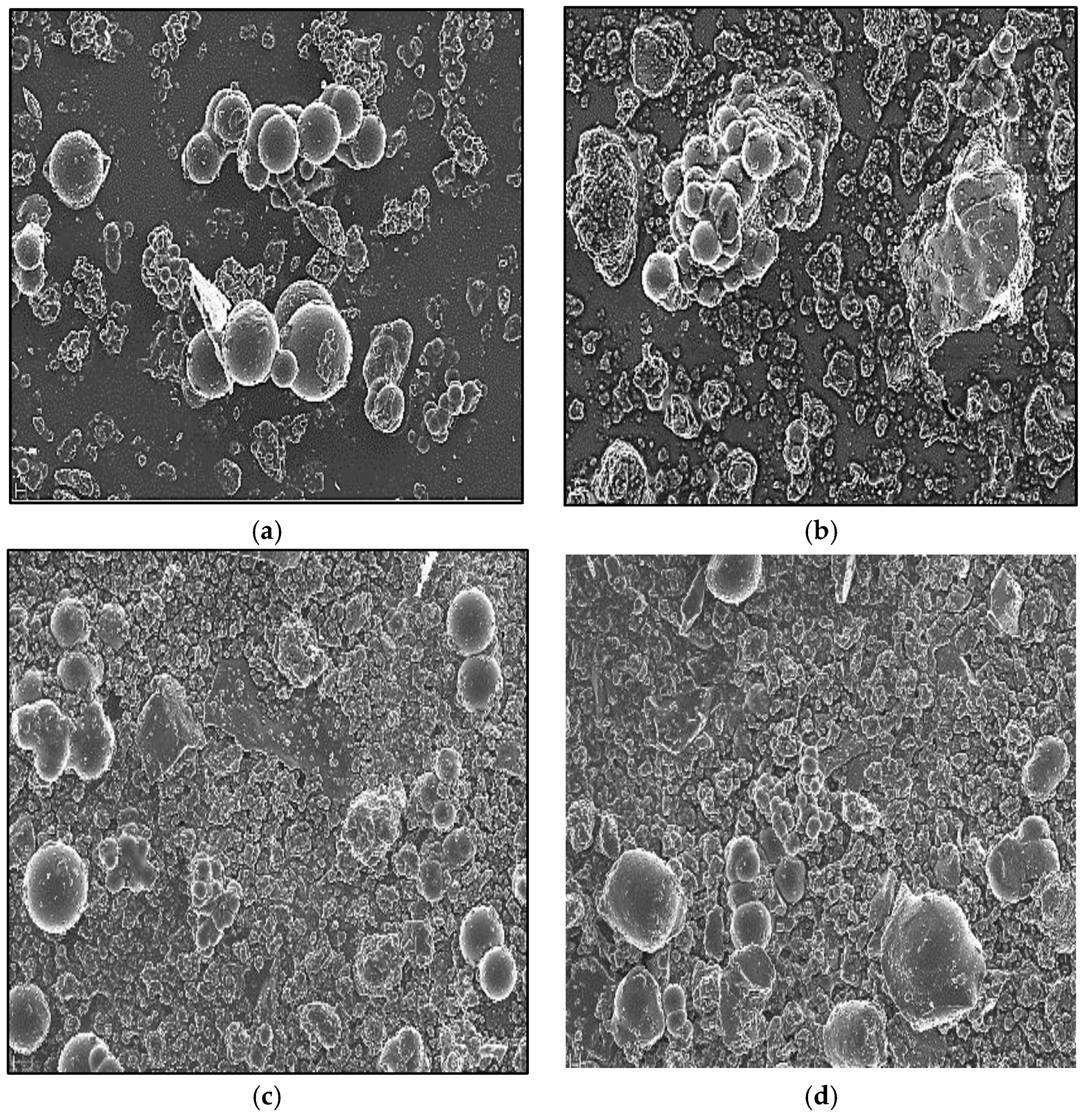
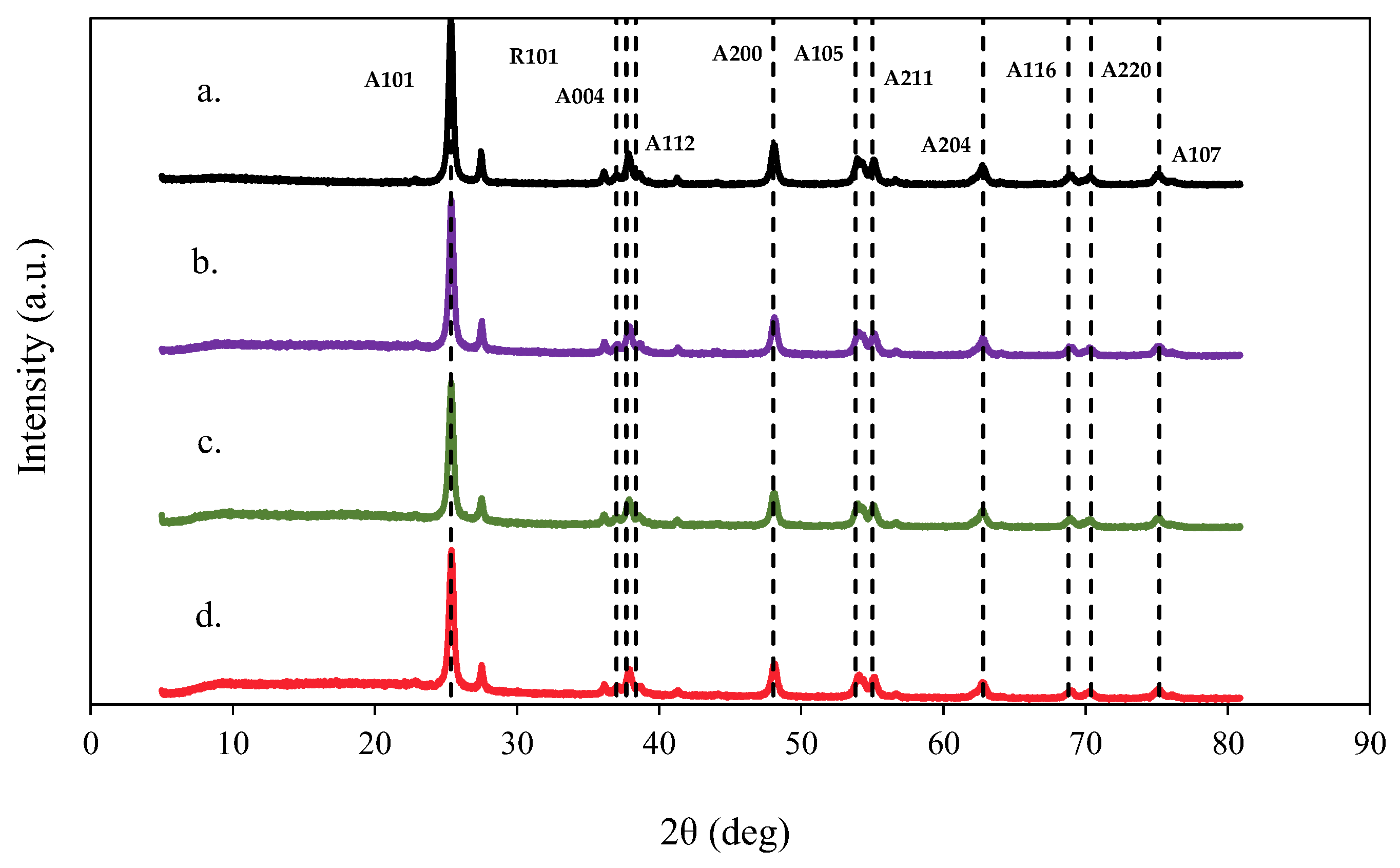
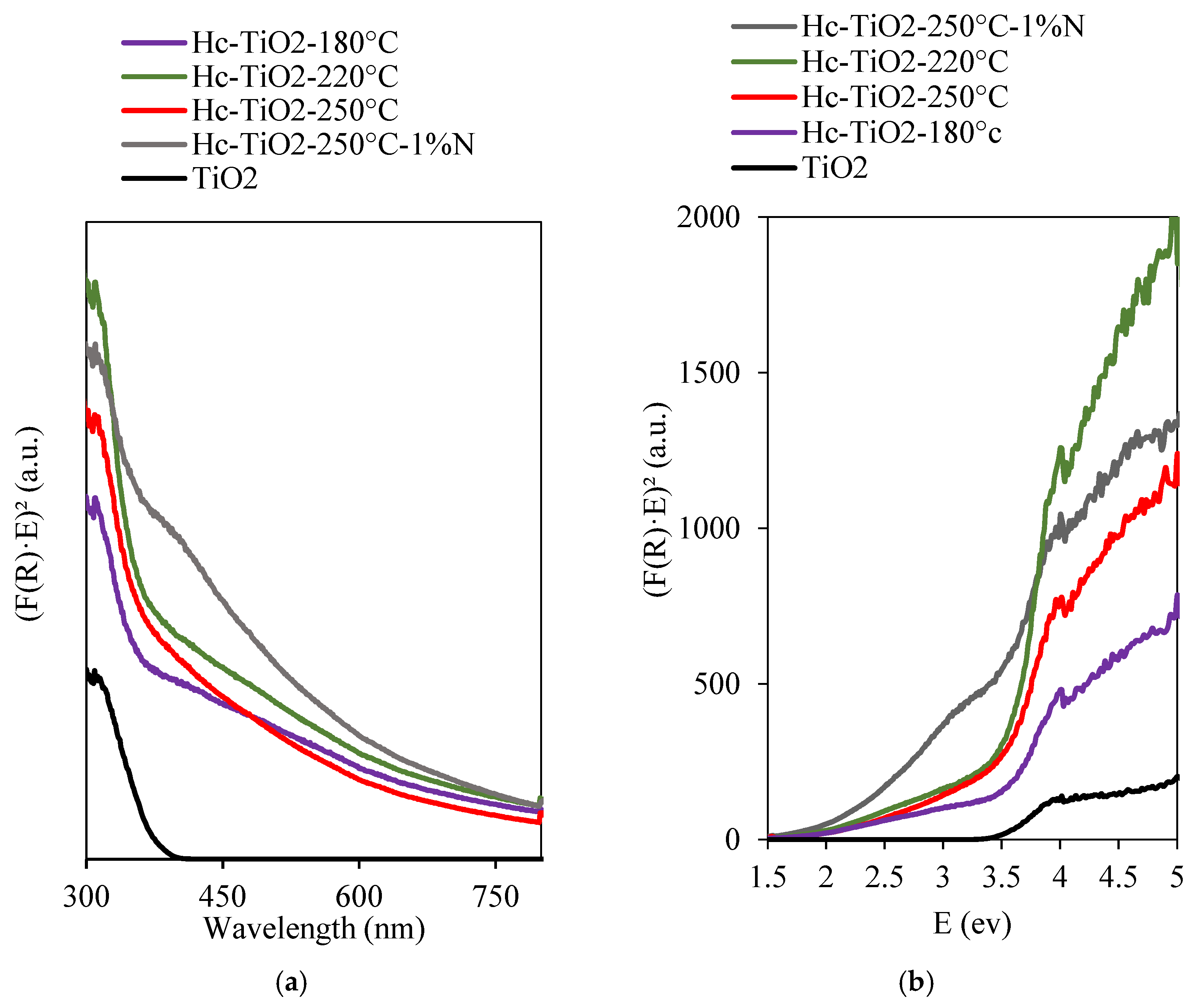




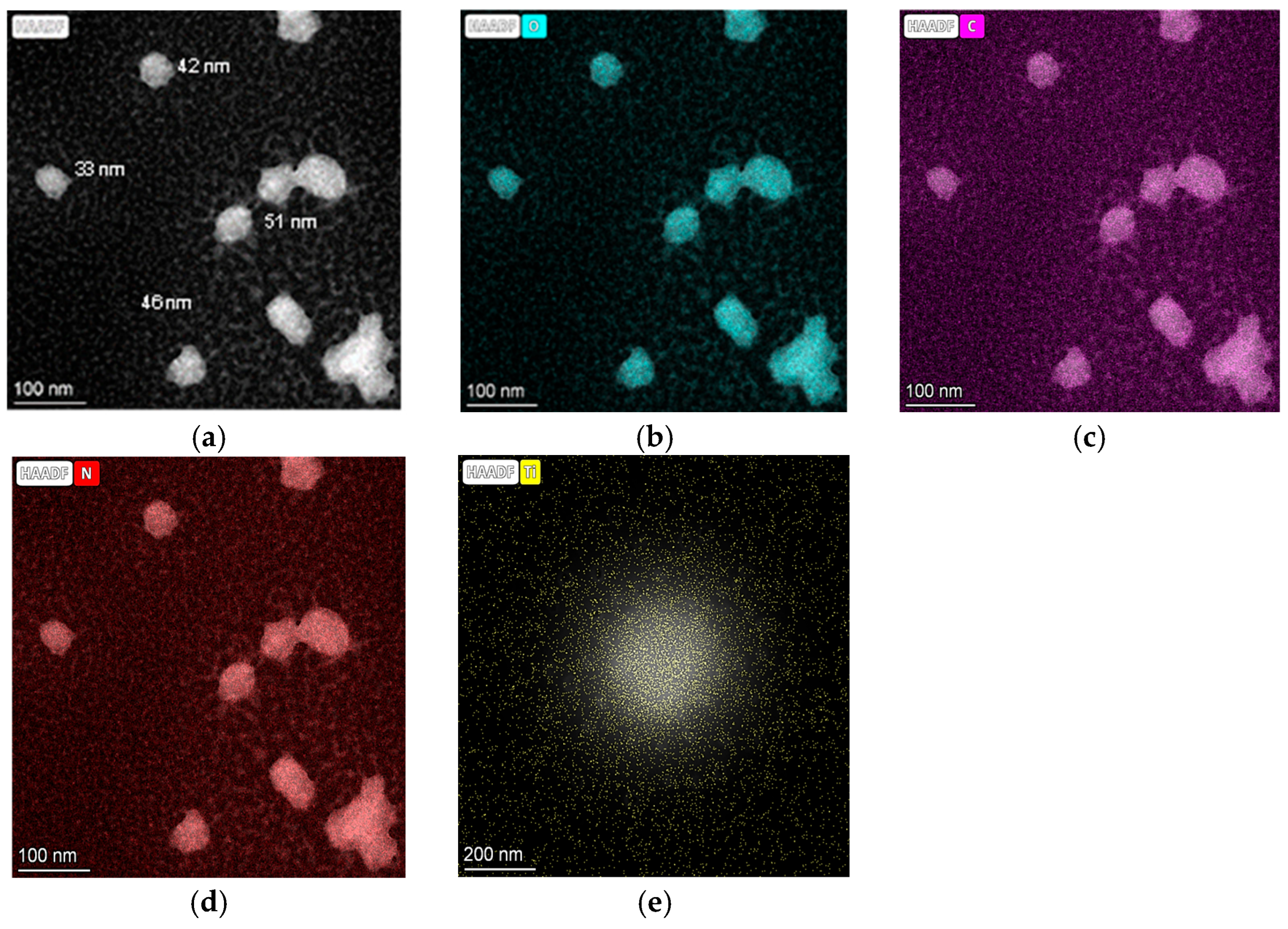
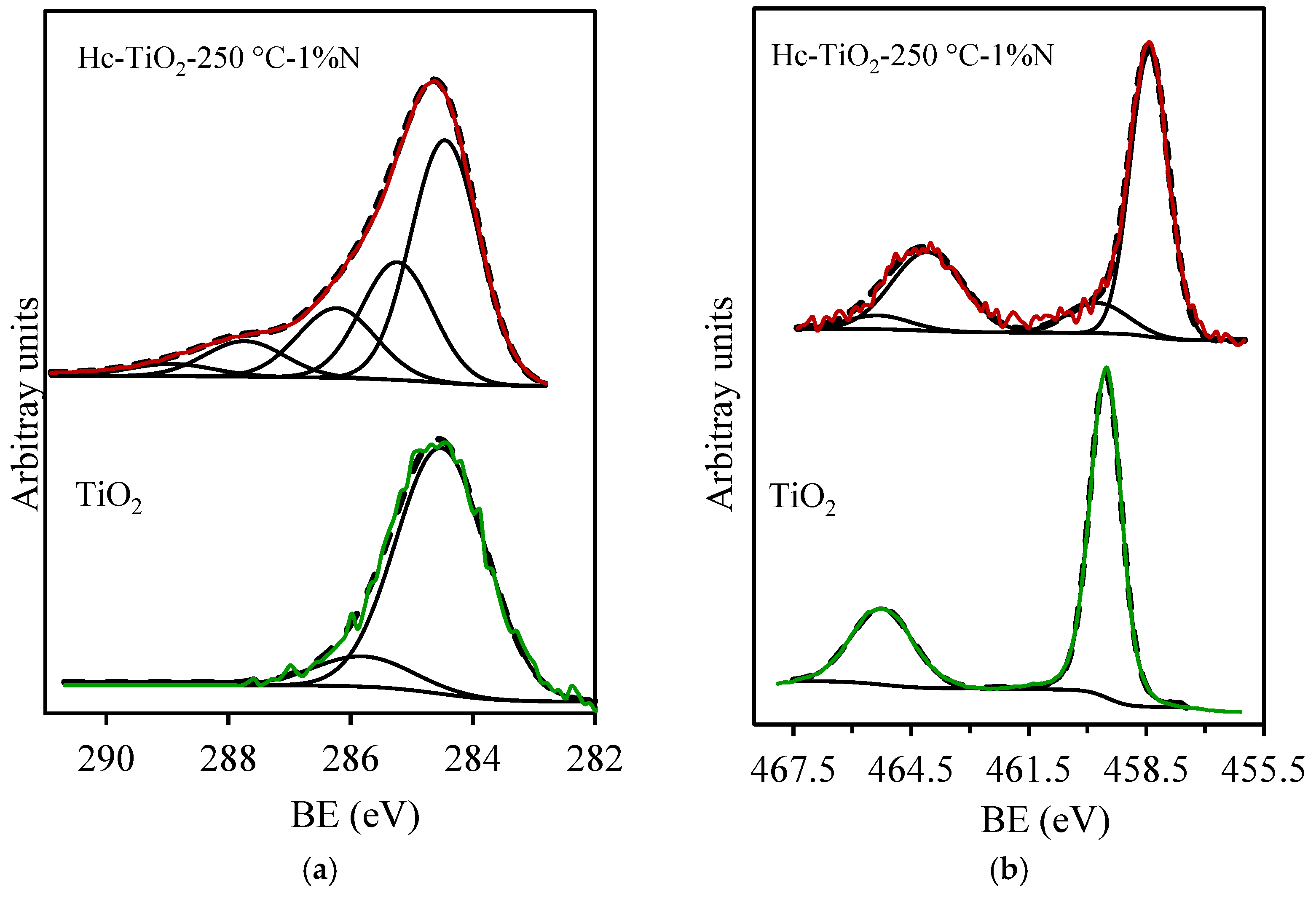
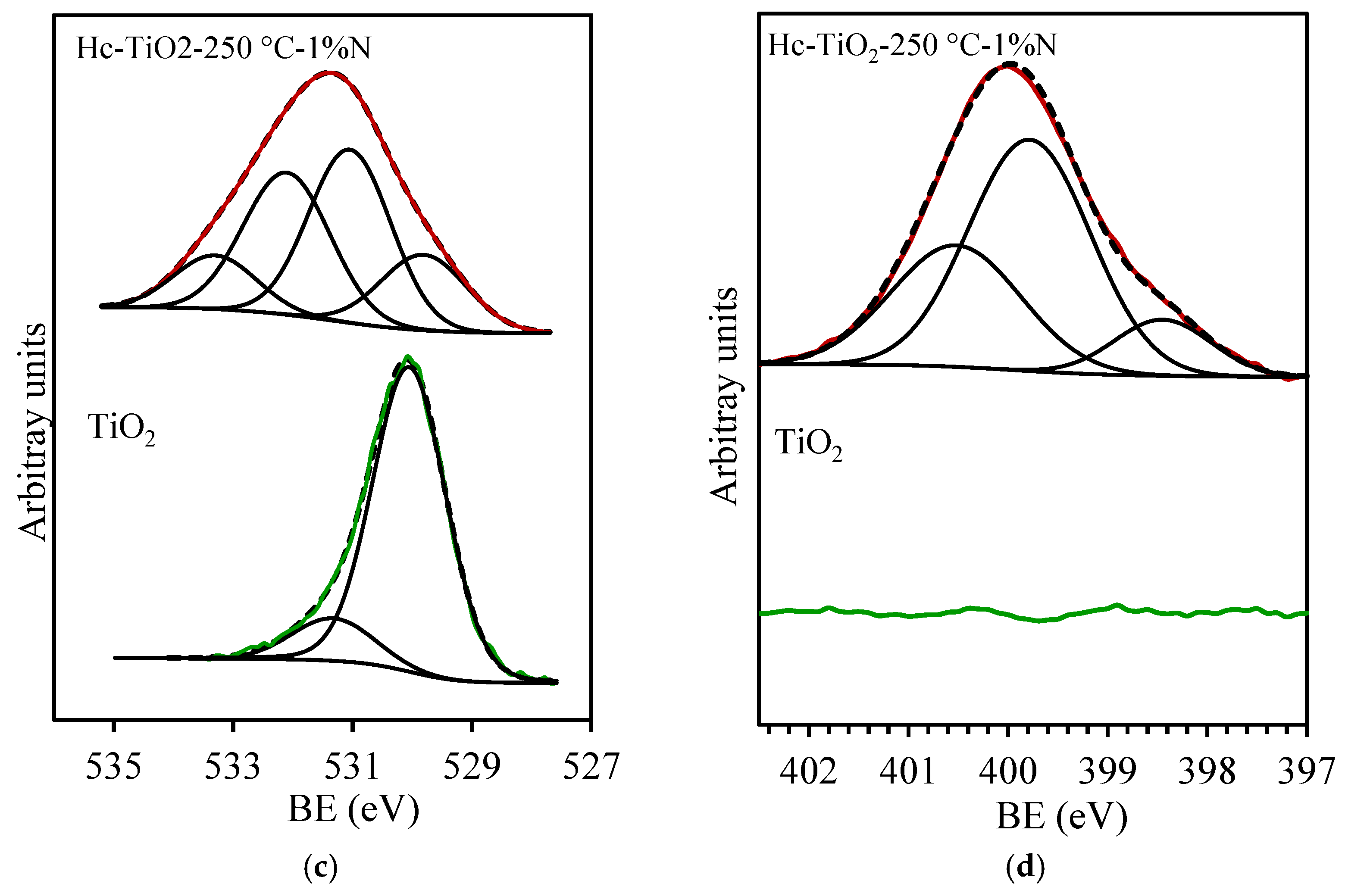
| Photocatalysts | D (nm) |
|---|---|
| TiO2 | 20.7 |
| Hc-TiO2-180 °C | 20.1 |
| Hc-TiO2-220 °C | 18.7 |
| Hc-TiO2-250 °C | 18.7 |
| Photocatalysts | k × 105 (L·mg−1·min−1) | %D | R2 |
|---|---|---|---|
| TiO2 | 0.54 | 0.24 | 0.99 |
| Hc-TiO2-180 °C | 0.56 | 0.13 | 0.99 |
| Hc-TiO2-220 °C | 1.29 | 1.20 | 0.99 |
| Hc-TiO2-250 °C | 2.74 | 0.51 | 0.98 |
| Photocatalysts | k × 105 (L·mg−1·min−1) | %D | R2 |
|---|---|---|---|
| TiO2 | 0.54 | 0.24 | 0.99 |
| Hc-TiO2-250 °C | 2.74 | 0.51 | 0.99 |
| Hc-TiO2-250 °C-1%P | 3.21 | 0.84 | 0.99 |
| Hc-TiO2-250 °C-1%N | 15.57 | 1.40 | 0.98 |
| Photocatalysts | k × 105 (L·mg−1·min−1) | %D |
|---|---|---|
| TiO2 | 0.54 | 0.24 |
| CQD-250 °C-1%N | 3.53 | 0.17 |
| Hc-TiO2-250 °C-1%N | 15.57 | 1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, D.; Zapata, K.; Ramírez-Valencia, L.D.; Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Franco, C.A.; Cortés, F.B. Hydrochar from Carbon Quantum Dots (CQDs) Synthesis for Photocatalytic and Decontamination Applications in Presence of TiO2. Int. J. Mol. Sci. 2025, 26, 4958. https://doi.org/10.3390/ijms26104958
López D, Zapata K, Ramírez-Valencia LD, Bailón-García E, Carrasco-Marín F, Pérez-Cadenas AF, Franco CA, Cortés FB. Hydrochar from Carbon Quantum Dots (CQDs) Synthesis for Photocatalytic and Decontamination Applications in Presence of TiO2. International Journal of Molecular Sciences. 2025; 26(10):4958. https://doi.org/10.3390/ijms26104958
Chicago/Turabian StyleLópez, Daniel, Karol Zapata, Lilian D. Ramírez-Valencia, Esther Bailón-García, Francisco Carrasco-Marín, Agustín F. Pérez-Cadenas, Camilo A. Franco, and Farid B. Cortés. 2025. "Hydrochar from Carbon Quantum Dots (CQDs) Synthesis for Photocatalytic and Decontamination Applications in Presence of TiO2" International Journal of Molecular Sciences 26, no. 10: 4958. https://doi.org/10.3390/ijms26104958
APA StyleLópez, D., Zapata, K., Ramírez-Valencia, L. D., Bailón-García, E., Carrasco-Marín, F., Pérez-Cadenas, A. F., Franco, C. A., & Cortés, F. B. (2025). Hydrochar from Carbon Quantum Dots (CQDs) Synthesis for Photocatalytic and Decontamination Applications in Presence of TiO2. International Journal of Molecular Sciences, 26(10), 4958. https://doi.org/10.3390/ijms26104958












