Single Cell RNA Sequencing of Papillary Cancer Mesenchymal Stem/Stromal Cells Reveals a Transcriptional Profile That Supports a Role for These Cells in Cancer Progression
Abstract
1. Introduction
2. Results
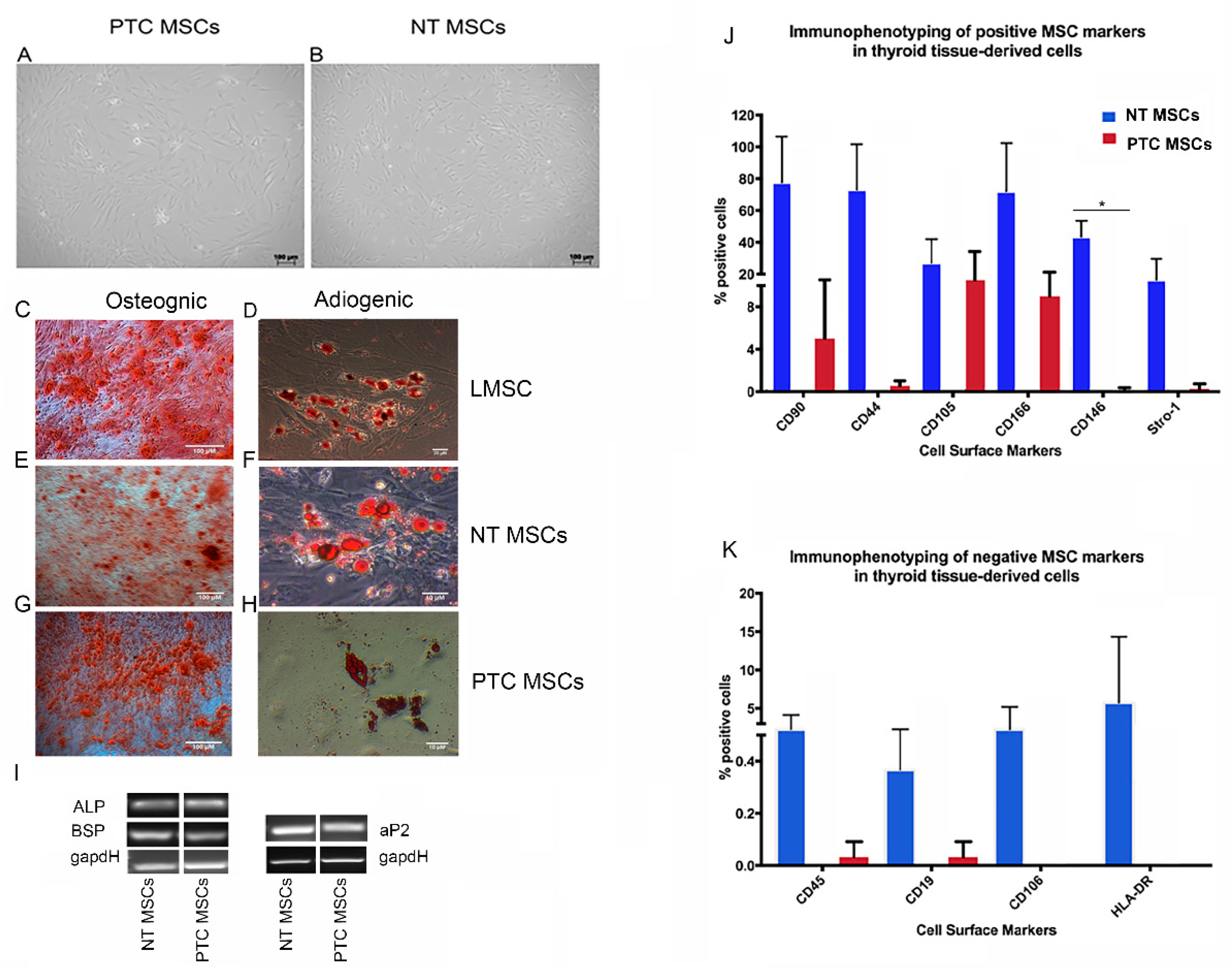
2.1. Cells with Mesenchymal Stem Cell Characteristics Can Be Isolated from Normal Thyroid Tissue and Papillary Thyroid Cancers
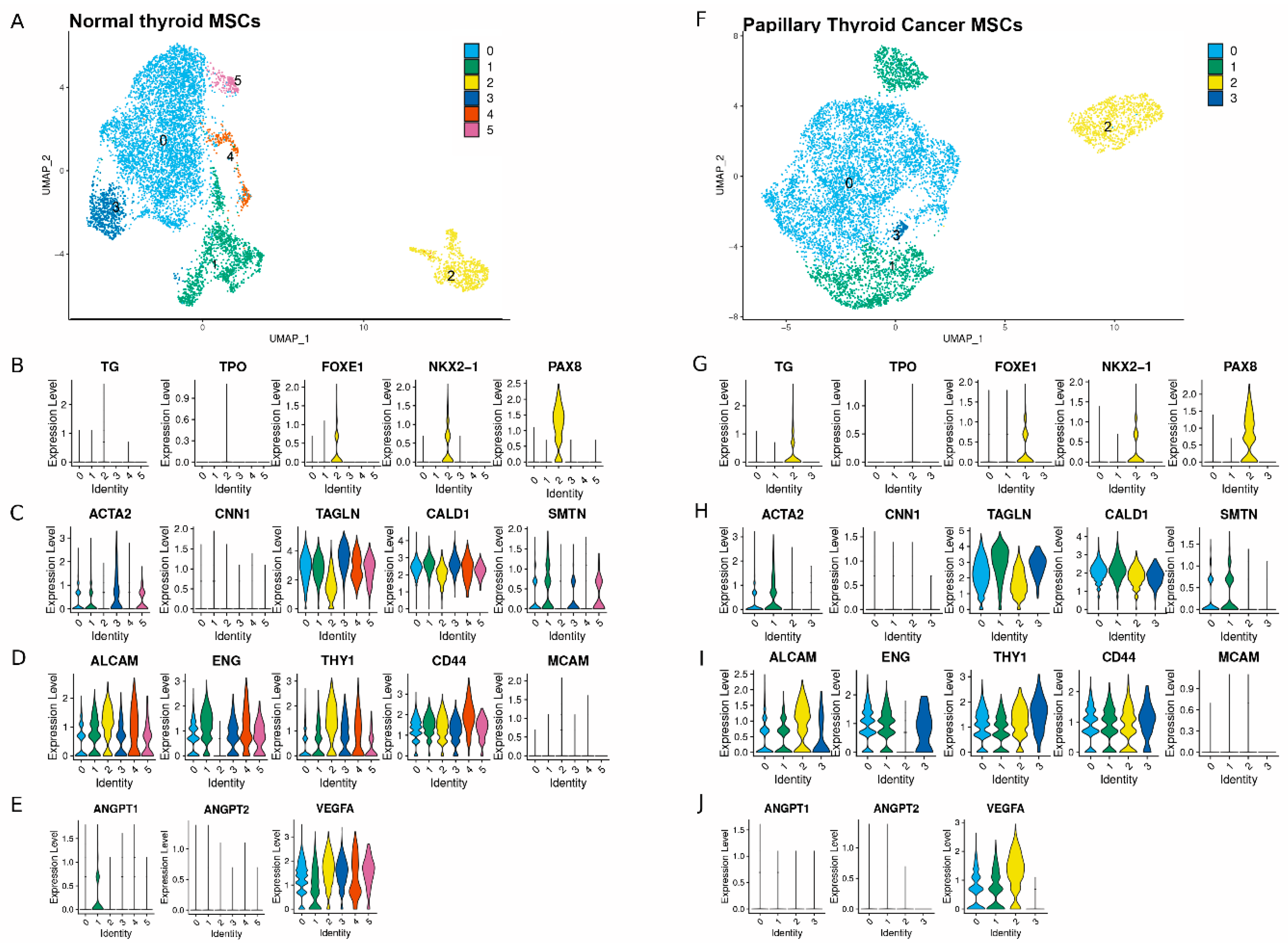
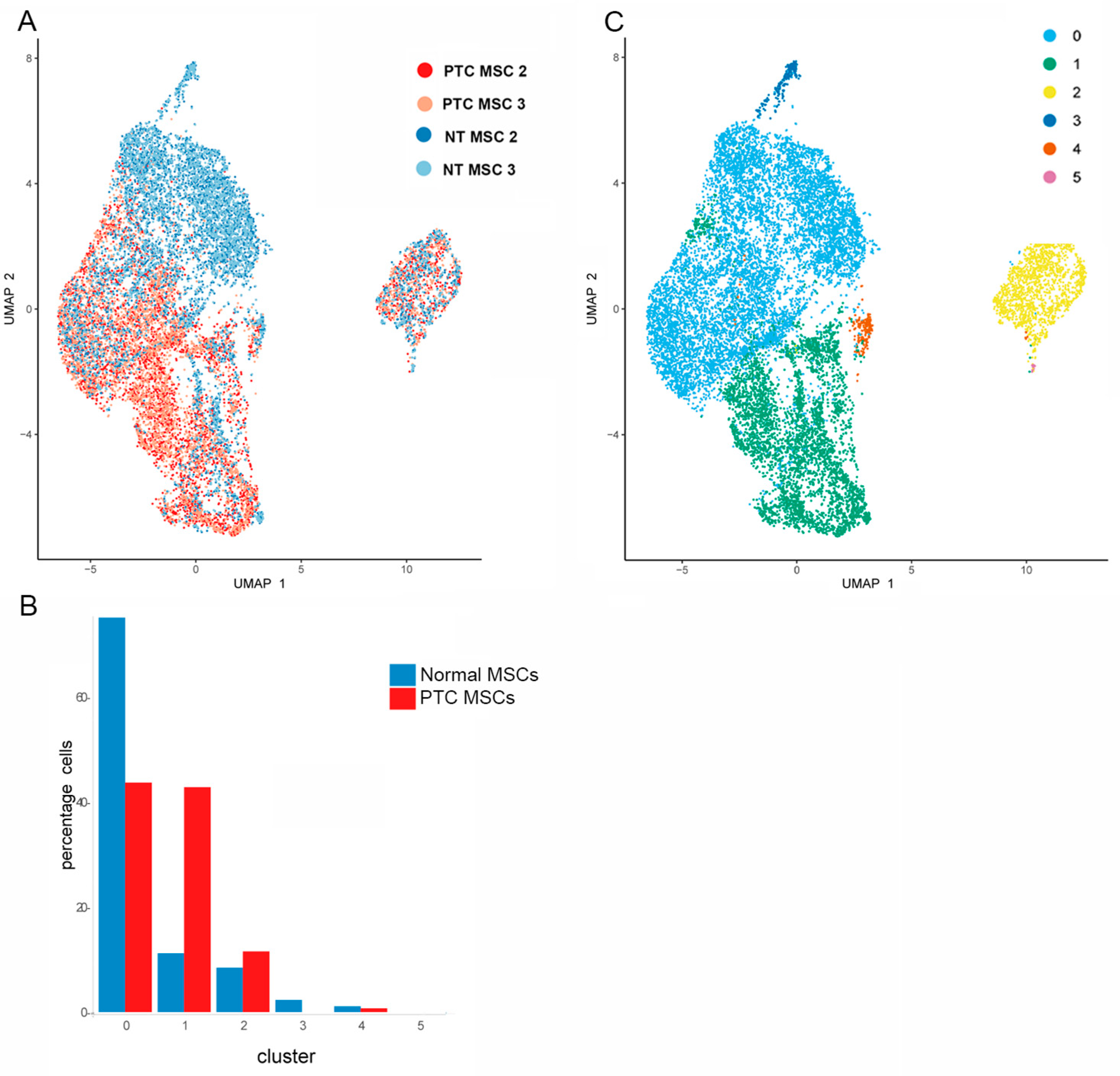
2.2. Single Cell RNAseq Cluster Analysis for Select Genes Used to Annotate the Subpopulations Within Both MSC Populations
2.3. Determination of Differentially Expressed Genes Upregulated in PTC MSCs
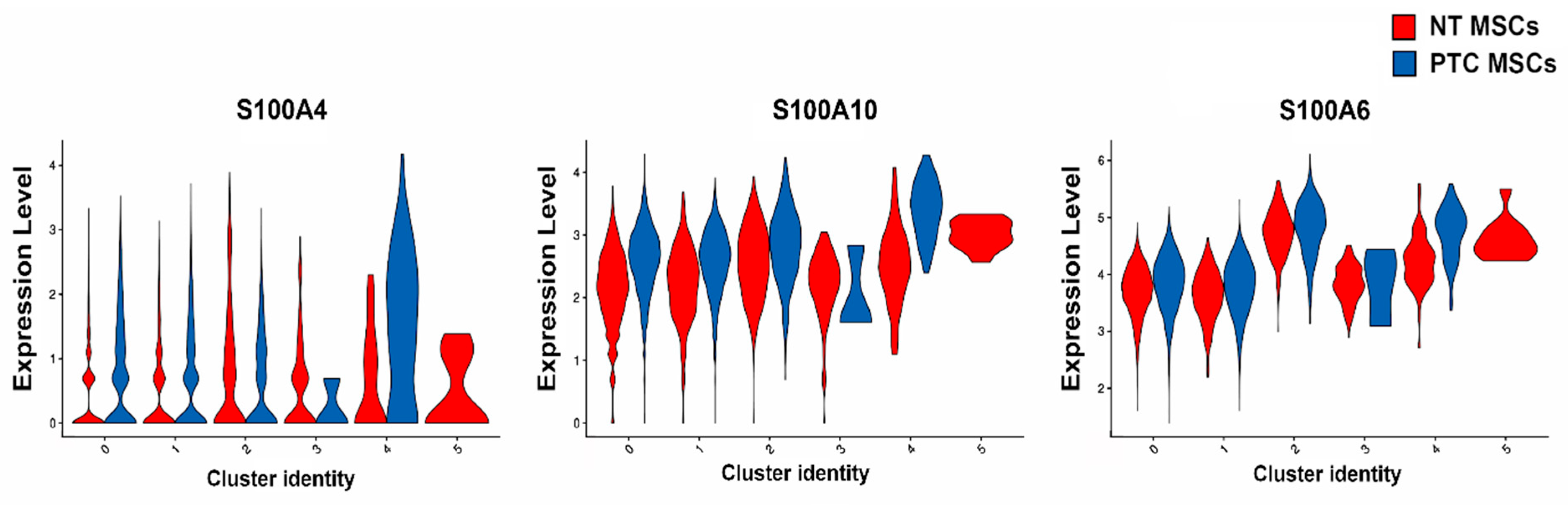
2.4. Analysis of S100A and IGFBP Gene Family Members Expressed by MSCs
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Isolation of MSCs
4.3. Immunophenotyping of MSCs
4.4. Osteogenic and Adipogenic Differentiation Potential of Mesenchymal Stem Cells
4.5. Semi–Quantitative PCR Analysis of Differentiated MSCs
4.6. Single Cell RNA Sequencing
4.7. Bioinformatics Analysis
4.8. Statistical Analysis for Single Cell RNA Sequencing
4.9. Statistical Analysis of 28 Genes Identified as Differentially Expressed Genes Upregulated in PTC MSCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Karnik, I.; Sutherland, R.; Elson, J.; Aspinall, S.; Meeson, A. TGF-β, to target or not to target; to prevent thyroid cancer progression? BBA-Rev. Cancer 2022, 1877, 4. [Google Scholar] [CrossRef]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: Stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Takigawa, H.; Kitadai, Y.; Shinagawa, K.; Yuge, R.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Mesenchymal Stem Cells Induce Epithelial to Mesenchymal Transition in Colon Cancer Cells through Direct Cell-to-Cell Contact. Neoplasia 2017, 19, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Parascandolo, A.; Rappa, F.; Cappello, F.; Kim, J.; Cantu, D.A.; Chen, H.; Mazzoccoli, G.; Hematti, P.; Castellone, M.D.; Salvatore, M.; et al. Extracellular Superoxide Dismutase Expression in Papillary Thyroid Cancer Mesenchymal Stem/Stromal Cells Modulates Cancer Cell Growth and Migration. Sci. Rep. 2017, 7, 41416. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Dominici, K.; LeBlanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar]
- Espagnolle, N.; Guilloton, F.; Deschaseaux, F.; Gadelorge, M.; Sensébé, L.; Bourin, P. CD146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J. Cell Mol. Med. 2014, 18, 104–114. [Google Scholar] [CrossRef]
- Maleki, M.; Ghanbarvand, F.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Anderegg, U. Thy-1: More than a marker for mesenchymal stromal cells. FASEB J. 2019, 33, 6689–6696. [Google Scholar] [CrossRef]
- García-Bernal, D.; Blanquer, M.; Martínez, C.M.; García-Guillén, A.I.; García-Hernández, A.M.; Carmen Algueró, M.; Yáñez, R.; Lamana, M.L.; Moraleda, J.M.; Sackstein, R. Enforced mesenchymal stem cell tissue colonization counteracts immunopathology. npj Regen. Med. 2022, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Brinkhof, B.; Zhang, B.; Cui, Z.; Ye, H.; Wang, H. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene 2020, 763, 100031. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, F.; Li, G.; Li, G.; Yang, X.; Liu, L.; Zhang, R.; Zhang, B.; Feng, Y. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.R.; Nair, V.A.; Malhab, L.J.B.; Abu-Gharbieh, E.; Ranade, A.V.; Pintus, G.; Hamad, M.; Busch, H.; Kirfel, J.; Hamoudi, R.; et al. Emerging role of caldesmon in cancer: A potential biomarker for colorectal cancer and other cancers. World J. Gastrointest. Oncol. 2022, 14, 1637–1653. [Google Scholar] [CrossRef]
- Brun, J.; Lutz, K.A.; Neumayer, K.M.H.; Klein, G.; Seeger, T.; Uynuk-Ool, T.; Wörgötter, K.; Schmid, S.; Kraushaar, U.; Guenther, E.; et al. Smooth Muscle Like Cells Generated from Human Mesenchymal Stromal Cells Display Marker Gene Expression and Electrophysiological Competence Comparable to Bladder Smooth Muscle Cells. PLoS ONE 2015, 10, e0145153. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Peck, K.; Chang, Y.L.; Pan, S.H.; Cheng, Y.F.; Lin, J.C.; Yang, R.B.; Hong, T.M.; Yang, P.C. SCUBE3 is an endogenous TGF-β receptor ligand and regulates the epithelial-mesenchymal transition in lung cancer. Oncogene 2011, 30, 3682–3693. [Google Scholar] [CrossRef]
- Liang, W.; Gao, R.; Yang, M.; Wang, X.; Cheng, K.; Shi, X.; He, C.; Li, Y.; Wu, Y.; Shi, L.; et al. MARCKSL1 promotes the proliferation, migration and invasion of lung adenocarcinoma cells. Oncol. Lett. 2020, 19, 2272–2280. [Google Scholar] [CrossRef]
- Tian, T.; Leng, Y.; Tang, B.; Dong, X.; Ren, Q.; Liang, J.; Liu, T.; Liu, Y.; Feng, W.; Liu, S.; et al. The oncogenic role and regulatory mechanism of PGK1 in human non-small cell lung cancer. Biol. Direct. 2024, 19, 1. [Google Scholar] [CrossRef]
- Song, G.D.; Sun, Y.; Shen, H.; Li, W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumour Biol. 2015, 36, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, C.; Feng, Z.; Gong, Y.; Sun, B.; Li, Z.; Lu, Y.; Fei, X.; Wu, W.; Sun, X.; et al. SOX4 promotes the growth and metastasis of breast cancer. Cancer Cell Int. 2020, 29, 468. [Google Scholar] [CrossRef]
- Tsai, C.N.; Yu, S.C.; Lee, C.W.; Pang, J.S.; Wu, C.H.; Lin, S.E.; Chung, Y.H.; Tsai, C.L.; Hsieh, S.Y.; Yu, M.C. SOX4 activates CXCL12 in hepatocellular carcinoma cells to modulate endothelial cell migration and angiogenesis in vivo. Oncogene 2020, 39, 4695–4710. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.; Cui, Z.; Tang, J.; Xie, M.; Ren, G. Elevated expression of GNAS promotes breast cancer cell proliferation and migration via the PI3K/AKT/Snail1/E-cadherin axis. Clin. Transl. Oncol. 2019, 21, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, X.; Su, Y.; Jia, C.; Dai, C. GNAS promotes inflammation-related hepatocellular carcinoma progression by promoting STAT3 activation. Cell Mol. Biol. Lett. 2020, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, B.; Hebbard, L. A novel function for HEG1 in promoting metastasis in hepatocellular carcinoma. Clin. Sci. 2019, 133, 2019–2022. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, G.H.; Wang, B.; Chen, Y.-J. Plastin-3 is a diagnostic and prognostic marker for pancreatic adenocarcinoma and distinguishes from diffuse large B-cell lymphoma. Cancer Cell Int. 2021, 21, 411. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Chen, Q.; Yuan, M.; Zeng, X.; Zeng, Y.; He, M.; Wang, B.; Han, B. Bioinformatics Identification of Therapeutic Gene Targets for Gastric Cancer. Adv. Ther. 2023, 40, 1456–1473. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Dong, C.; Chen, T.; Dong, A.; Ren, J.; Li, W.; Shu, G.; Yang, J.; Shen, W.; et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene 2023, 42, 83–98. [Google Scholar] [CrossRef]
- Dai, D.N.; Li, Y.; Chen, B.; Du, Y.; Li, S.B.; Lu, S.X.; Zhao, Z.P.; Zhou, A.J.; Xue, N.; Xia, T.L.; et al. Elevated expression of CST1 promotes breast cancer progression and predicts a poor prognosis. J. Mol. Med. 2017, 95, 873–886. [Google Scholar] [CrossRef]
- Gasca, J.; Flores, M.L.; Jiménez-Guerrero, R.; Sáez, M.E.; Barragán, I.; Ruíz-Borrego, M.; Tortolero, M.; Romero, F.; Sáez, C.; Japón, M.A. EDIL3 promotes epithelial-mesenchymal transition and paclitaxel resistance through its interaction with integrin αVβ3 in cancer cells. Cell Death Discov. 2020, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, S.P.; Creighton, C.J.; Dai, F.; Xie, X.; Cheng, C.M.; Frolov, A.; Ayala, G.; Lin, X.; Feng, X.H.; et al. COUP-TFII inhibits TGF-β-induced growth barrier to promote prostate tumorigenesis. Nature 2013, 493, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, P.; Zhao, X.; Lu, X.; Deng, R.; Wang, X.; Su, Z.; Hong, C.; Lin, J. Ltbp1 promotes esophageal squamous cell carcinoma progression through epithelial-mesenchymal transition and cancer-associated fibroblasts transformation. J. Transl. Med. 2020, 18, 139. [Google Scholar] [CrossRef]
- de Cristofaro, T.; Di Palma, T.; Ferraro, A.; Corrado, A.; Lucci, V.; Franco, R.; Fusco, A.; Zannini, M. TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur. J. Cancer 2011, 47, 926–933. [Google Scholar] [CrossRef]
- Gugnoni, M.; Sancisi, V.; Gandolfi, G.; Manzotti, G.; Ragazzi, M.; Giordano, D.; Tamagnini, I.; Tigano, M.; Frasoldati, A.; Piana, S.; et al. Cadherin-6 promotes EMT and cancer metastasis by restraining autophagy. Oncogene 2017, 36, 667–677. [Google Scholar] [CrossRef]
- Hardin, H.; Guo, Z.; Shan, W.; Montemayor-Garcia, C.; Asioli, S.; Yu, X.-M.; Harrison, A.D.; Chen, H.; Lloyd, R.V. The Roles of the Epithelial-Mesenchymal Transition Marker PRRX1 and miR-146b-5p in Papillary Thyroid Carcinoma Progression. Am. J. Pathol. 2014, 184, 2342–2354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, W.; Huang, R.; Ye, M.; Min, Z. SIRT6/HIF-1α axis promotes papillary thyroid cancer progression by inducing epithelial–mesenchymal transition. Cancer Cell Int. 2019, 19, 17. [Google Scholar] [CrossRef]
- Zou, M.; Al-Baradie, R.S.; Al-Hindi, H.; Farid, N.R.; Shi, Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br. J. Cancer 2005, 93, 1277–1284. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, H.; Zhang, S.; Tian, R.; Long, H.; Zhang, H.; Guo, X.; Li, D.; Tan, S.; Zhu, X. Myc-Associated Zinc Finger Protein Promotes Metastasis of Papillary Thyroid Cancer. Front. Biosci. 2023, 28, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Choy, M.; Guang, H.; Li, Y. MON-536 SPOCK1 Promotes the Progression of Papillary Thyroid Cancer ViaPI3K/Akt Signaling Activation. J. Endocr. Soc. 2020, 4, MON-536. [Google Scholar] [CrossRef]
- Wu, C.; Lin, J.; Chen, J.; Chang, C.; Weng, H.; Hsueh, C.; Chien, H.; Yu, J. Integrated analysis of fine-needle-aspiration cystic fluid proteome, cancer cell secretome, and public transcriptome datasets for papillary thyroid cancer biomarker discovery. Oncotarget 2018, 9, 12079–12100. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, R.; Ning, Z.; Fu, N.; Xie, M. Identification of a four-gene signature for determining the prognosis of papillary thyroid carcinoma by integrated bioinformatics analysis. Int. J. Gen. Med. 2022, 15, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Nipp, M.; Elsner, M.; Balluff, B.; Meding, S.; Sarioglu, H.; Ueffing, M.; Rauser, S.; Unger, K.; Höfler, H.; Walch, A.; et al. S100-A10, thioredoxin, and S100-A6 as biomarkers of papillary thyroid carcinoma with lymph node metastasis identified by MALDI imaging. J. Mol. Med. 2012, 90, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chang, A.; Zhou, W.; Zhao, H.; Zhuo, X. IGFBP3 as an indicator of lymph node metastasis and unfavorable prognosis for papillary thyroid carcinoma. Clin. Exp. Med. 2020, 20, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Stolf, B.S.; Carvalho, A.F.; Martins, W.K.; Runza, F.B.; Brun, M.; Hirata, R., Jr.; Jordão Neves, E.; Soares, F.A.; Postigo-Dias, J.; Kowalski, L.; et al. Differential expression of IGFBP-5 and two human ESTs in thyroid glands with goiter, adenoma and papillary or follicular carcinomas. Cancer Lett. 2003, 191, 193–202. [Google Scholar] [CrossRef]
- Bach, L.A. Recent insights into the actions of IGFBP-6. J. Cell Commun. Signal 2015, 9, 189–200. [Google Scholar] [CrossRef]
- Jeon, H.J.; Park, J.; Shin, J.H.; Chang, M.S. Insulin-like growth factor binding protein-6 released from human mesenchymal stem cells confers neuronal protection through IGF-1R-mediated signaling. Int. J. Mol. Med. 2017, 40, 1860–1868. [Google Scholar] [CrossRef][Green Version]
- Vizioli, M.; Sensi, M.; Miranda, C.; Cleris, L.; Formelli, F.; Anania, M.C.; Pierotti, M.A.; Greco, A. IGFBP7: An oncosuppressor gene in thyroid carcinogenesis. Oncogene 2010, 29, 3835–3844. [Google Scholar] [CrossRef]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 2023, 9, 17. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Secretome analysis of in vitro aged human mesenchymal stem cells reveals IGFBP7 as a putative factor for promoting osteogenesis. Sci. Rep. 2018, 8, 4632. [Google Scholar] [CrossRef]
- Li, X.; Feng, L.; Zhang, C.; Wang, J.; Wang, S.; Hu, L. Insulin-like growth factor binding proteins 7 prevents dental pulp-derived mesenchymal stem cell senescence via metabolic downregulation of p21. Sci. China Life Sci. 2022, 65, 2218–2232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.Y. From bulk, single-cell to spatial RNA sequencing. Int. J. Oral. Sci. 2021, 13, 36. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef]
- Shaharuddin, B.; Osei-Bempong, C.; Ahmad, S.; Rooney, P.; Ali, S.; Oldershaw, R.; Meeson, A. Human limbal mesenchymal stem cells express ABCB5 and can grow on amniotic membrane. Regen. Med. 2016, 11, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Choe, G.; Kim, S.W.; Park, Y.J.; Park, D.J.; Youn, Y.K.; Park, S.H.; Cho, B.Y.; Park, S.Y. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod. Pathol. 2008, 21, 748–755. [Google Scholar] [CrossRef] [PubMed][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 September 2023).
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 2, pl1. [Google Scholar] [CrossRef]
- de Bruijn, I.; Kundra, R.; Mastrogiacomo, B.; Tran, T.N.; Sikina, L.; Mazor, T.; Li, X.; Ochoa, A.; Zhao, G.; Lai, B.; et al. Analysis and Visualization of Longitudinal Genomic and Clinical Data from the AACR Project GENIE Biopharma Collaborative in cBioPortal. Cancer Res. 2023, 83, 3861–3867. [Google Scholar] [CrossRef] [PubMed]
- Parlato, R.; Rosica, A.; Rodriguez-Mallon, A.; Affuso, A.; Postiglione, M.P.; Arra, C.; Mansouri, M.; Kimura, S.; Di Lauro, R.; De Felice, M. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev. Biol. 2004, 276, 464–475. [Google Scholar] [CrossRef]

| Gene | p Value | Average Log2FC | PCT% | NT% | p Value Adjusted (Bonferroni Correction) | MSC Population |
|---|---|---|---|---|---|---|
| IGFBP7 | 3.0152 × 10−30 | 0.18022276 | 0.985 | 0.988 | 5.0513 × 10−26 | PTC |
| IGFBP3 | 0 | 1.568476 | 0.767 | 0.309 | 0 | PTC |
| IGFBP5 | 1.65 × 10−74 | 0.573006 | 0.775 | 0.647 | 2.77 × 10−70 | PTC |
| IGFBP6 | 1.67 × 10−218 | 0.586049 | 0.987 | 0.953 | 2.79 × 10−214 | PTC |
| S100A4 | 2.81 × 10−91 | 0.439812 | 0.572 | 0.441 | 4.71 × 10−87 | PTC |
| S100A6 | 4.73 × 10−62 | 0.255939 | 1 | 1 | 7.92 × 10−58 | PTC |
| S100A10 | 0 | 0.550230 | 1 | 0.992 | 0 | PTC |
| PRRX1 | 1.20 × 10−42 | 0.383299 | 0.75 | 0.64 | 2.01 × 10−138 | PTC |
| PCOLCE2 | 0 | 0.609609 | 0.42 | 0.14 | 0 | PTC |
| HIF1A | 0 | 0.496238 | 0.941 | 0.864 | 0 | PTC |
| SH3BGRL3 | 4.03 × 10−280 | 0.460636 | 0.999 | 0.999 | 6.75 × 10−276 | PTC |
| WWTR1 | 0 | 0.676019 | 0.718 | 0.512 | 0 | PTC |
| CDH6 | 0 | 0.724795 | 0.509 | 0.153 | 0 | PTC |
| FBN1 | 0 | 0.790647 | 0.986 | 0.893 | 0 | PTC |
| HEG1 | 4.92 × 10−147 | 0.389943 | 0.466 | 0.288 | 8.24 × 10−143 | PTC |
| CST1 | 6.17 × 10−147 | 0.438862 | 0.144 | 0.03 | 1.03 × 10−142 | PTC |
| MAZ | 2.67 × 10−169 | 0.392678 | 0.518 | 0.33 | 4.48 × 10−165 | PTC |
| PGK1 | 0 | 0.569080 | 0.954 | 0.867 | 0 | PTC |
| SPOCK1 | 1.30 × 10−186 | 0.399679 | 0.967 | 0.915 | 2.17 × 10−182 | PTC |
| MARCKSL1 | 1.12 × 10−303 | 0.541839 | 0.722 | 0.494 | 1.88 × 10−299 | PTC |
| LTBP1 | 2.12 × 10−216 | 0.457493 | 0.921 | 0.845 | 3.55 × 10−212 | PTC |
| GNAS | 0 | 0.793568 | 0.969 | 0.851 | 0 | PTC |
| NR2F2 | 2.55 × 10−170 | 0.448546 | 0.819 | 0.716 | 4.28 × 10−166 | PTC |
| PLS3 | 4.07 × 10−271 | 0.490727 | 0.755 | 0.577 | 6.81 × 10−267 | PTC |
| EDIL3 | 0 | 0.590966 | 0.463 | 0.15 | 0 | PTC |
| SPARC | 0 | 0.944666 | 0.994 | 0.881 | 0 | PTC |
| SCUBE3 | 1.51 × 10−267 | 0.474113 | 0.292 | 0.08 | 2.53 × 10−263 | PTC |
| SOX4 | 0 | 0.721343 | 0.843 | 0.624 | 0 | PTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jandu, D.; Latar, N.; Bajrami, A.; Queen, R.; Hasoon, M.; Teasdale, M.; Hussain, R.; Coxhead, J.; Aspinall, S.; Meeson, A. Single Cell RNA Sequencing of Papillary Cancer Mesenchymal Stem/Stromal Cells Reveals a Transcriptional Profile That Supports a Role for These Cells in Cancer Progression. Int. J. Mol. Sci. 2025, 26, 4957. https://doi.org/10.3390/ijms26104957
Jandu D, Latar N, Bajrami A, Queen R, Hasoon M, Teasdale M, Hussain R, Coxhead J, Aspinall S, Meeson A. Single Cell RNA Sequencing of Papillary Cancer Mesenchymal Stem/Stromal Cells Reveals a Transcriptional Profile That Supports a Role for These Cells in Cancer Progression. International Journal of Molecular Sciences. 2025; 26(10):4957. https://doi.org/10.3390/ijms26104957
Chicago/Turabian StyleJandu, Danny, Nani Latar, Artida Bajrami, Rachel Queen, Megan Hasoon, Matthew Teasdale, Rafiqul Hussain, Jonathan Coxhead, Sebastian Aspinall, and Annette Meeson. 2025. "Single Cell RNA Sequencing of Papillary Cancer Mesenchymal Stem/Stromal Cells Reveals a Transcriptional Profile That Supports a Role for These Cells in Cancer Progression" International Journal of Molecular Sciences 26, no. 10: 4957. https://doi.org/10.3390/ijms26104957
APA StyleJandu, D., Latar, N., Bajrami, A., Queen, R., Hasoon, M., Teasdale, M., Hussain, R., Coxhead, J., Aspinall, S., & Meeson, A. (2025). Single Cell RNA Sequencing of Papillary Cancer Mesenchymal Stem/Stromal Cells Reveals a Transcriptional Profile That Supports a Role for These Cells in Cancer Progression. International Journal of Molecular Sciences, 26(10), 4957. https://doi.org/10.3390/ijms26104957






