Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium
Abstract
1. Introduction
2. Results
2.1. Body and Reproductive Organ Weight
2.2. Concentration of Cd and Zn in Blood, Testes, and Epididymis
2.3. Serum Testosterone Concentration
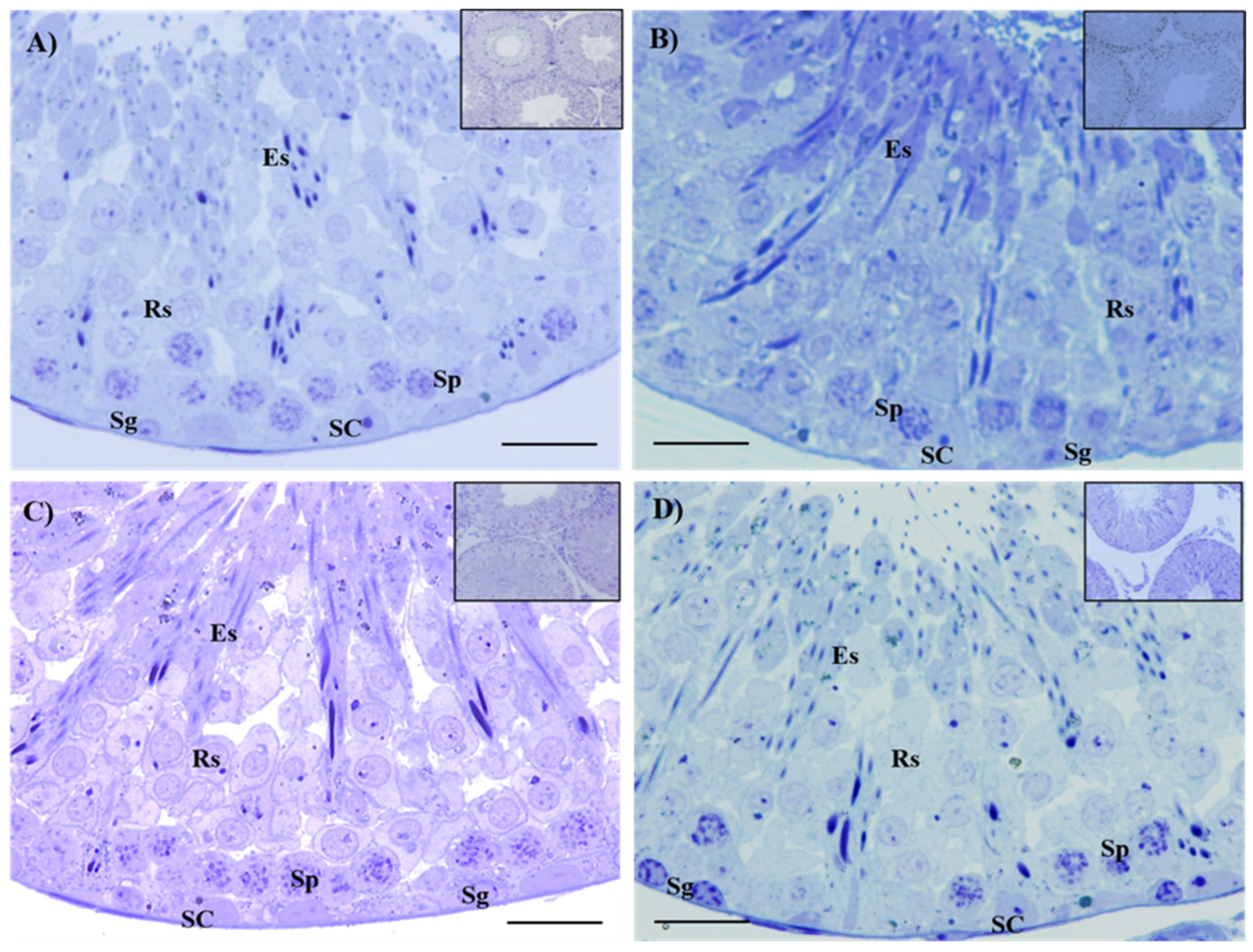
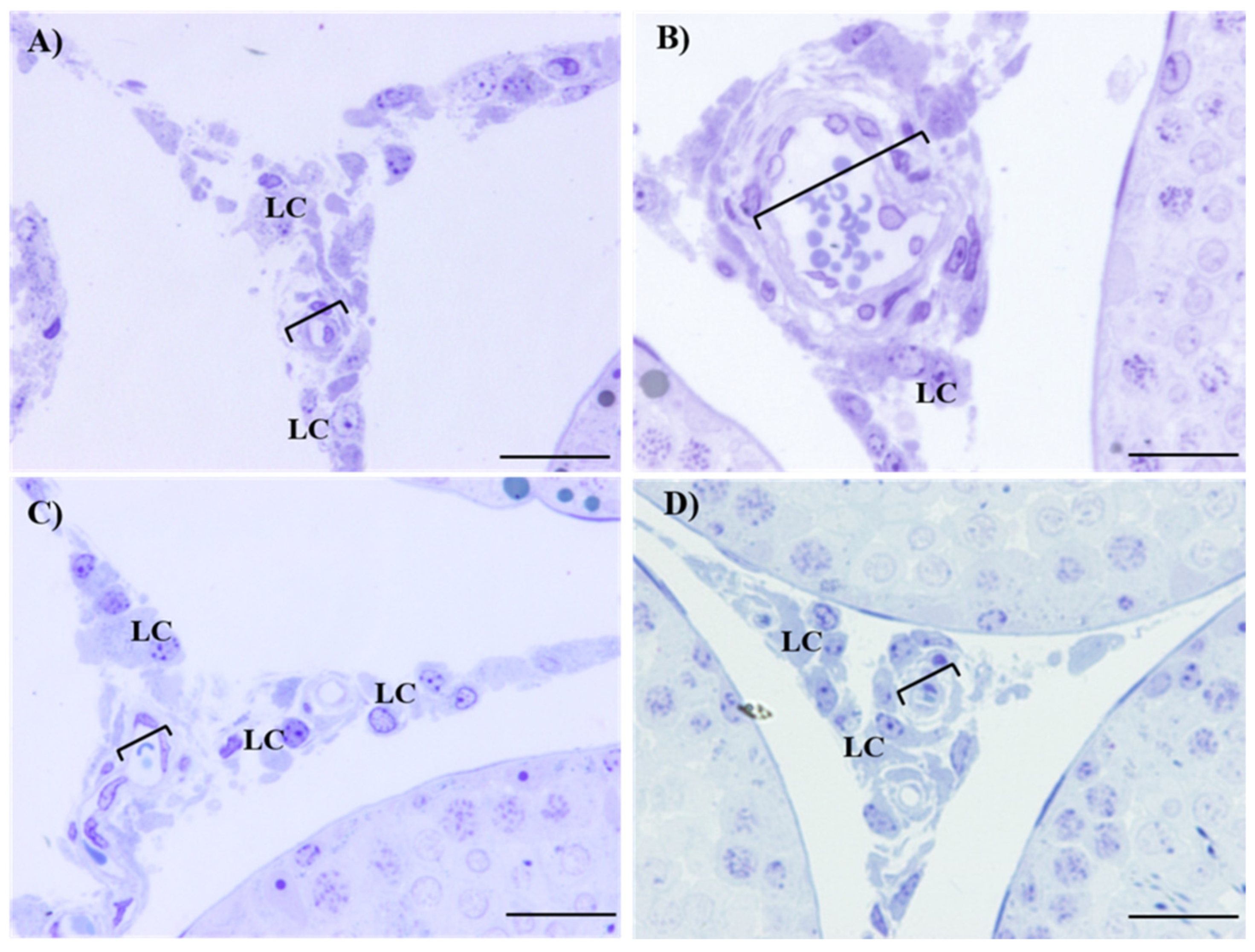
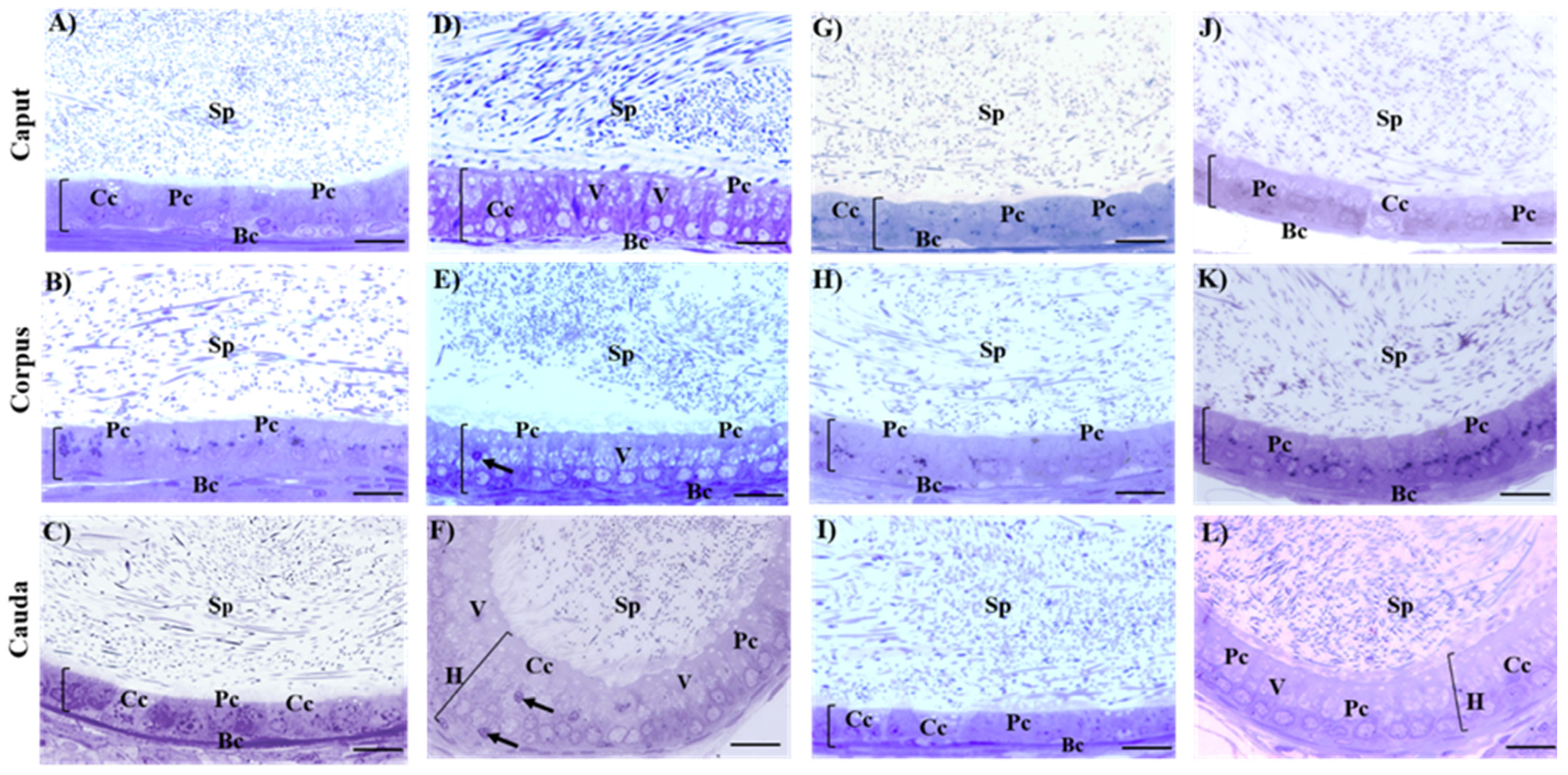
2.4. Histological Evaluation of Testes and Epididymis
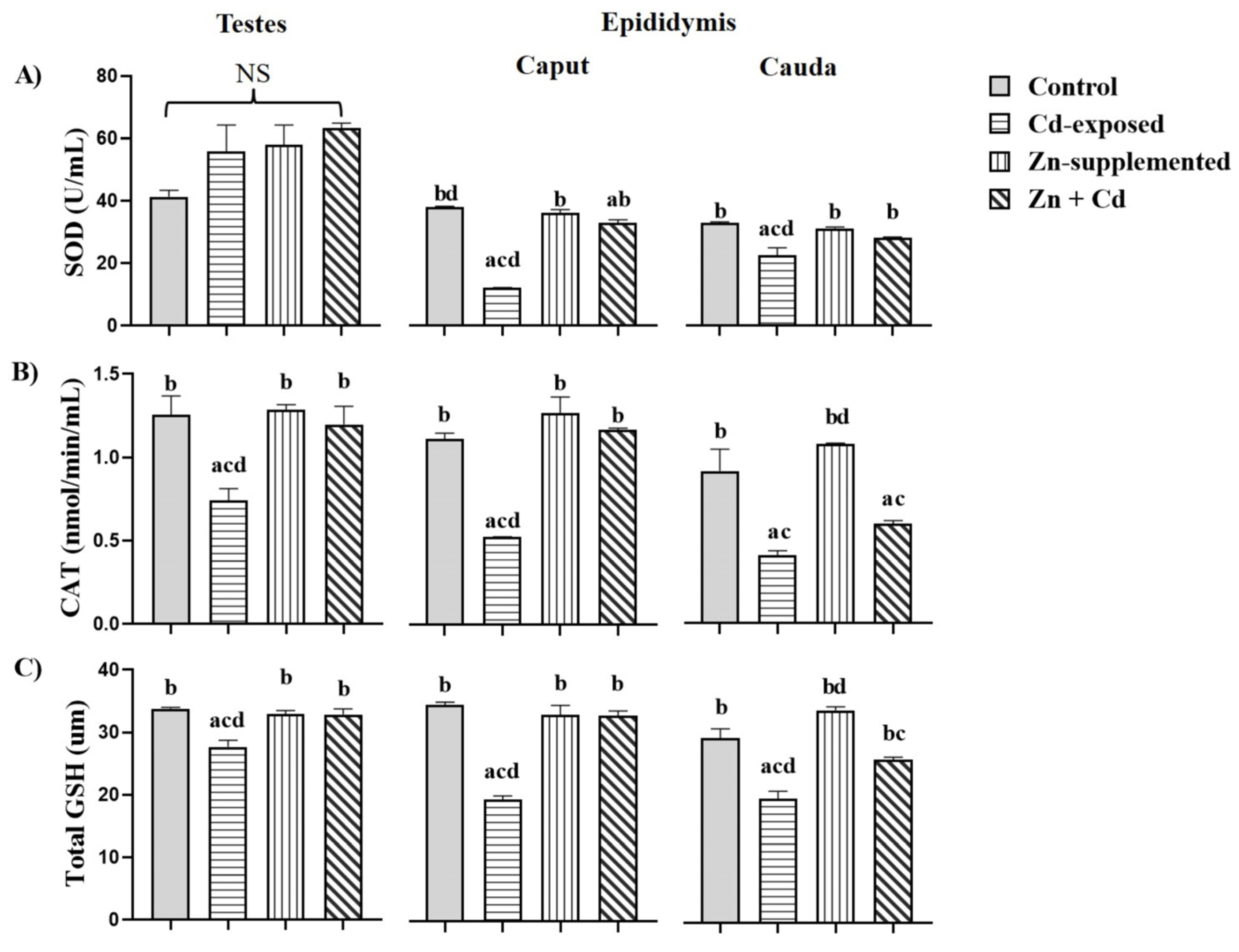
2.5. Antioxidant Activity in the Testes and Epididymis
2.6. Sperm Parameters
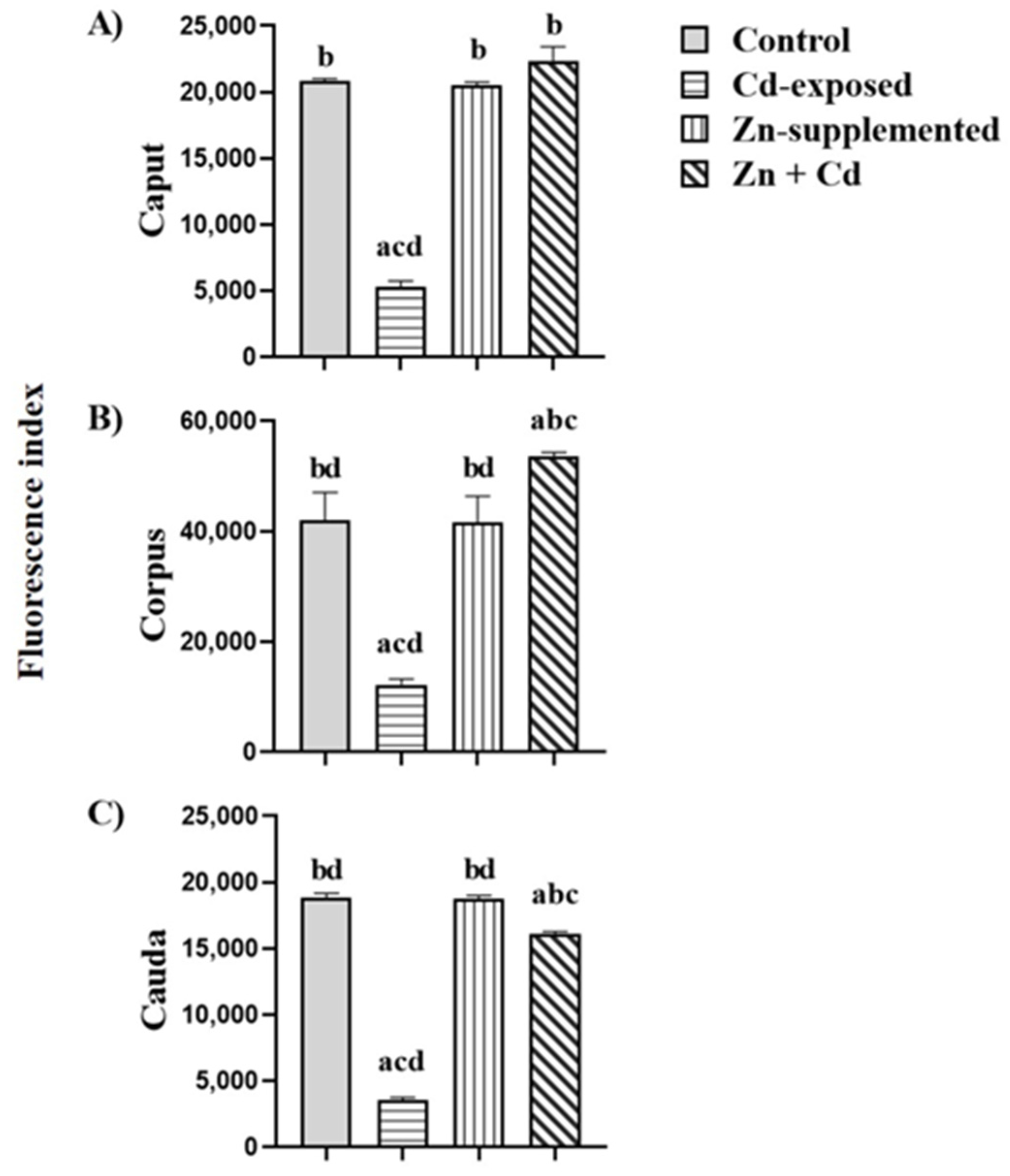
2.7. Carbohydrate Distribution and Fluorescence Index of N-Acetylglucosamine and/or Sialic Acid of the Sperm Membrane
2.8. Carbohydrate Distribution and Fluorescence Index of Mannose in the Sperm Membrane
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatments
4.3. Experimental Procedure
4.4. Biochemical Analysis
4.4.1. Quantification of Cd and Zn in Blood, Testes, and Epididymis
4.4.2. Quantification of Serum Testosterone
4.4.3. Processing and Histological Evaluation of Testes and Epididymis
4.4.4. Determination of Antioxidant Activity in the Testes and Epididymis
4.4.5. Collecting Sperm Samples from the Epididymis and Sperm Determination
Sperm Concentration
Sperm Viability
Sperm Morphology
4.4.6. Determination of Carbohydrate Distribution and Fluorescence Index in Sperm Membrane
4.4.7. Evaluation of Carbohydrate Distribution via Epifluorescence Microscopy in Membrane Sperm Cells
4.4.8. Determination of Fluorescence Index via Flow Cytometry in Sperm Cells
4.4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| Zn | Zn |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| MTs | Metallothioneins |
| PS | Postnatal day |
| BW | Body weight |
| ELISA | Enzyme-linked immunosorbent assay |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH | Glutathione |
| FITC | Fluorescein isothiocyanate |
| EPON | Epoxy resin |
| AAS | Atomic absorption spectrophotometry |
| IP | Intraperitoneal |
| WGA | Triticum vulgaris agglutinin |
| With A | Canavalia ensiformis agglutinin |
| StAR | Steroidogenic acute regulatory protein |
| P450SCC | Cytochrome P450 |
| P450c17 | Cytochrome P45017A1 |
| ZIP | Zinc-importing protein |
| BHE | Blood–epididymal barrier |
| GSR | Glutathione reductase |
| GPx | Glutathione peroxidase |
| See | Selenium |
| Mn | Manganese |
| Cu | Copper |
| Faith | Iron |
| MTF-1 | Metal regulatory transcription factor |
| MRE | Metal response elements |
| Nrf2 | Nuclear erythroid factor 2 |
| ARE | Antioxidant response elements |
References
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef]
- Dacheux, J.L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2013, 147, R27–R42. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Robaire, B.; Hinton, B.T. The Epididymis. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Elsevier: New York, NY, USA, 2015; pp. 619–677. [Google Scholar]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef]
- Breton, S.; Nair, A.V.; Battistone, M.A. Epithelial dynamics in the epididymis: Role in the maturation, protection, and storage of spermatozoa. Andrology 2019, 7, 631–643. [Google Scholar] [CrossRef]
- Tulsiani, D.R. Glycan-modifying enzymes in luminal fluid of the mammalian epididymis: An overview of their potential role in sperm maturation. Mol. Cell. Endocrinol. 2006, 250, 58–65. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Tecle, E.; Gagneux, P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015, 82, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Tulsiani, D.R. Glycan modifying enzymes in luminal fluid of rat epididymis: Are they involved in altering sperm surface glycoproteins during maturation? Microsc. Res. Tech. 2003, 61, 18–27. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef]
- Jalón Monzón, A.; Martín Benito, J.L.; Álvarez Múgica, M.; García Rodríguez, J.; Fernández Gómez, J.M.; Viña Alonso, L.; Jalón Monzón, M. Male infertility. Semer.—Med. De Fam. 2006, 32, 223–232. [Google Scholar]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of environmental toxin exposure on male fertility potential. Transl. Androl. Urol. 2020, 9, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preventing Disease Through Healthy Environments: Exposure to Cadmium: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Kim, N.H.; Hyun, Y.Y.; Lee, K.B.; Chang, Y.; Rhu, S.; Oh, K.H.; Ahn, C. Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci. 2015, 30, 272–277. [Google Scholar] [CrossRef]
- Torres-Sánchez, L.; Vázquez-Salas, R.A.; Vite, A.; Galván-Portillo, M.; Cebrián, M.E.; Macias-Jiménez, A.P.; Ríos, C.; Montes, S. Blood cadmium determinants among males over forty living in Mexico City. Sci. Total Environ. 2018, 637–638, 686–694. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Wang, R.; Sang, P.; Guo, Y.; Jin, P.; Cheng, Y.; Yu, H.; Xie, Y.; Yao, W.; Qian, H. Cadmium in food: Source, distribution and removal. Food Chem. 2023, 405, 134666. [Google Scholar] [CrossRef]
- Garner, R.; Levallois, P. Cadmium levels and sources of exposure among Canadian adults. Health Rep. 2016, 27, 10–18. [Google Scholar]
- Ikokide, E.J.; Oyagbemi, A.A.; Oyeyemi, M.O. Impacts of cadmium on male fertility: Lessons learned so far. Andrologia 2022, 54, e14516. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Ugwuja, E.I. Association of Blood and Seminal Plasma Cadmium and Lead Levels with Semen Quality in Non-Occupationally Exposed Infertile Men in Abakaliki, South East Nigeria. J. Fam. Reprod. Health 2017, 11, 97–103. [Google Scholar]
- Tuncay, G.; Karaer, A.; Tanrikut, E.; Ozgul, O. The effect of seminal plasma cadmium and lead levels on semen parameters in male subjects of infertile couples: A prospective cohort study. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2021, 41, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, B.; Liu, H.; Cai, L. Comprehensive Review of Cadmium Toxicity Mechanisms in Male Reproduction and Therapeutic Strategies. Rev. Environ. Contam. Toxicol. 2021, 258, 151–193. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Mendiola, J.; Roca, M.; López-Espín, J.J.; Guillén, J.J.; Moreno, J.M.; Moreno-Grau, S.; Martínez-García, M.J.; Vergara-Juárez, N.; Elvira-Rendueles, B.; et al. Correlations between Different Heavy Metals in Diverse Body Fluids: Studies of Human Semen Quality. Adv. Urol. 2012, 2012, 420893. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Arenas-Ríos, E.; Jiménez-Morales, I.; Cortés-Barberena, E.; Montes, S.; Vigueras-Villaseñor, R.M.; Arteaga-Silva, M. Postnatal cadmium administration affects the presence and distribution of carbohydrates in the sperm membrane during maturation in the epididymis in adult Wistar rats. Reprod. Fertil. Dev. 2021, 33, 349–362. [Google Scholar] [CrossRef]
- Batra, N.; Nehru, B.; Bansal, M.P. Influence of lead and zinc on rat male reproduction at ‘biochemical and histopathological levels’. J. Appl. Toxicol. JAT 2001, 21, 507–512. [Google Scholar] [CrossRef]
- Babaknejad, N.; Bahrami, S.; Moshtaghie, A.A.; Nayeri, H.; Rajabi, P.; Iranpour, F.G. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol. Trace Elem. Res. 2018, 185, 106–115. [Google Scholar] [CrossRef]
- Chemek, M.; Venditti, M.; Boughamoura, S.; Mimouna, S.B.; Messaoudi, I.; Minucci, S. Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J. Cell. Physiol. 2018, 233, 630–640. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Marín de Jesús, S.; Vigueras-Villaseñor, R.M.; Cortés-Barberena, E.; Hernández-Rodriguez, J.; Montes, S.; Arrieta-Cruz, I.; Pérez-Aguirre, S.G.; Bonilla-Jaime, H.; Limón-Morales, O.; Arteaga-Silva, M. Zinc and Its Impact on the Function of the Testicle and Epididymis. Int. J. Mol. Sci. 2024, 25, 8991. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Tinkov, A.A. Zinc. Adv. Food Nutr. Res. 2021, 96, 251–310. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Drobnis, E.Z.; Sutovsky, M.; Sutovsky, P. Zinc ion flux during mammalian sperm capacitation. Nat. Commun. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Polykretis, P.; Cencetti, F.; Donati, C.; Luchinat, E.; Banci, L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019, 21, 101102. [Google Scholar] [CrossRef]
- Kerns, K.; Zigo, M.; Sutovsky, P. Zinc: A Necessary Ion for Mammalian Sperm Fertilization Competency. Int. J. Mol. Sci. 2018, 19, 4097. [Google Scholar] [CrossRef]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef]
- Boldrini, G.G.; Martín Molinero, G.; Pérez Chaca, M.V.; Ciminari, M.E.; Moyano, F.; Córdoba, M.E.; Pennacchio, G.; Fanelli, M.; Álvarez, S.M.; Gómez, N.N. Glycine max (soy) based diet improves antioxidant defenses and prevents cell death in cadmium intoxicated lungs. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2022, 35, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2022, 13, 36. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Saïd, L.; Banni, M.; Kerkeni, A.; Saïd, K.; Messaoudi, I. Influence of combined treatment with zinc and selenium on cadmium induced testicular pathophysiology in rat. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Chabchoub, I.; Nouioui, M.A.; Araoud, M.; Mabrouk, M.; Amira, D.; Ben Aribia, M.H.; Mahmoud, K.; Zhioua, F.; Merdassi, G.; Hedhili, A. Effects of lead, cadmium, copper and zinc levels on the male reproductive function. Andrologia 2021, 53, e14181. [Google Scholar] [CrossRef]
- Yu, H.T.; Zhen, J.; Leng, J.Y.; Cai, L.; Ji, H.L.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Vance, T.M.; Chun, O.K. Zinc Intake Is Associated with Lower Cadmium Burden in U.S. Adults. J. Nutr. 2015, 145, 2741–2748. [Google Scholar] [CrossRef]
- Jahan, S.; Khan, M.; Ahmed, S.; Ullah, H. Comparative analysis of antioxidants against cadmium induced reproductive toxicity in adult male rats. Syst. Biol. Reprod. Med. 2014, 60, 28–34. [Google Scholar] [CrossRef]
- Shi, X.; Fu, L. Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Dev. Ther. 2019, 13, 2811–2824. [Google Scholar] [CrossRef]
- Yi, L.; Dai, J.; Chen, Y.; Tong, Y.; Li, Y.; Fu, G.; Teng, Z.; Huang, J.; Quan, C.; Zhang, Z.; et al. Reproductive toxicity of cadmium in pubertal male rats induced by cell apoptosis. Toxicol. Ind. Health 2021, 37, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef]
- Sen Gupta, R.; Sen Gupta, E.; Dhakal, B.K.; Thakur, A.R.; Ahnn, J. Vitamin C and vitamin E protect the rat testis from cadmium-induced reactive oxygen species. Mol. Cells 2004, 17, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Alkhedaide, A.; Alshehri, Z.S.; Sapry, A.; Ghaffar, T.A.; Soliman, M.M.; Attia, H. Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol. Med. Rep. 2016, 13, 3101–3109. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Wang, H.; Zhou, S.; Li, L.; Chen, X.; Wang, G.; Liu, J.; Ge, H.S.; Ge, R.S. A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis. Sci. Rep. 2017, 7, 6337. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.A.; Nassar, N.N. Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. J. Biochem. Mol. Toxicol. 2011, 25, 15–25. [Google Scholar] [CrossRef]
- Almeer, R.S.; Soliman, D.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Metwally, D.; Abdel Moneim, A.E. Royal jelly abrogates cadmium-induced oxidative challenge in mouse testes: Involvement of the Nrf2 pathway. Int. J. Mol. Scinces 2018, 19, 3979. [Google Scholar] [CrossRef]
- Amara, S.; Abdelmelek, H.; Garrel, C.; Guiraud, P.; Douki, T.; Ravanat, J.L.; Favier, A.; Sakly, M.; Ben Rhouma, K. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J. Reprod. Dev. 2008, 54, 129–134. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, T.; Yang, B.; Chi, Z.; Wang, Z.Y.; Gu, H.F. A novel role for zinc transporter 8 in the facilitation of zinc accumulation and regulation of testosterone synthesis in Leydig cells of human and mouse testis. Metab. Clin. Exp. 2018, 88, 40–50. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Oruitemeka, S.; Uwadileke, I.A.; Omeodu, S.I.; Okoye, N.F.; Mgbudom-Okah, C.J.; Ohanador, R. Oral administration of cadmium depletes intratesticular and epididymal iron levels and inhibits lipid peroxidation in the testis and epididymis of adult rats. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2018, 48, 213–223. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Azizi, A.; Abedini, S.; Hemayatkhah Jahromi, V.; Abidi, H.; Jafari Barmak, M. Green tea improves rat sperm quality and reduced cadmium chloride damage effect in spermatogenesis cycle. J. Med. Life 2018, 11, 371–380. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Changes in the peritubular tissue of rat testis after cadmium treatment. Biol. Trace Elem. Res. 2010, 134, 288–295. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Siwach, A.; Sachdeva, D.; Sachdeva, S.N. Revisiting cadmium-induced toxicity in the male reproductive system: An update. Arch. Toxicol. 2024, 98, 3619–3639. [Google Scholar] [CrossRef]
- Gunn, S.A.; Gould, T.C.; Anderson, W.A. The selective injurious response of testicular and epididymal blood vessels to cadmium and its prevention by zinc. Am. J. Pathol. 1963, 42, 685–702. [Google Scholar] [PubMed]
- Yang, S.H.; Chen, S.T.; Liang, C.; Shi, Y.H.; Chen, Q.S. Effects of Cadmium Exposure on Leydig Cells and Blood Vessels in Mouse Testis. Int. J. Environ. Res. Public Health 2022, 19, 2416. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R.; Woods, J.M. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006, 79, 1493–1506. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The vascular system as a target of metal toxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 102, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Machado-Neves, M. Effect of heavy metals on epididymal morphology and function: An integrative review. Chemosphere 2022, 291, 133020. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod. Biol. Endocrinol. RBE 2016, 14, 42. [Google Scholar] [CrossRef]
- Lamas, C.A.; Cuquetto-Leite, L.; do Nascimento da Silva, E.; Thomazini, B.F.; Cordeiro, G.D.S.; Predes, F.S.; Gollücke, A.P.B.; Dolder, H. Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats. Int. J. Exp. Pathol. 2017, 98, 86–99. [Google Scholar] [CrossRef]
- De Grava Kempinas, W.; Klinefelter, G.R. Interpreting histopathology in the epididymis. Spermatogenesis 2015, 4, e979114. [Google Scholar] [CrossRef] [PubMed]
- Sullivan R Epididymosomes: Role of extracellular microvesicles in sperm maturation. Front. Biosci. 2016, 8, 106–114. [CrossRef]
- Nemmiche, S. Oxidative Signaling Response to Cadmium Exposure. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 156, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, T.; Lei, W.W.; Liu, D.M.; Li, Y.J.; Xuan, R.J.; Ma, J.J. Correction: Cadmium-Induced Oxidative Stress and Apoptotic Changes in the Testis of Freshwater Crab, Sinopotamon henanense. PLoS ONE 2013, 8, 10–1371. [Google Scholar] [CrossRef]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019, 39, BSR20180515. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J.; López, A.L.; Montes, S.; Bonilla-Jaime, H.; Morales, I.; Limón-Morales, O.; Ríos, C.; Hernández-González, M.; Vigueras-Villaseñor, R.M.; Arteaga-Silva, M. Delay in puberty indices of Wistar rats caused by Cadmium. Focus on the redox system in reproductive organs. Reprod. Toxicol. 2021, 99, 71–79. [Google Scholar] [CrossRef]
- Iftikhar, A.; Akhtar, M.F.; Saleem, A.; Riaz, A.; Zehravi, M.; Rahman, M.H.; Md Ashraf, G. Comparative Potential of Zinc Sulfate, L-Carnitine, Lycopene, and Coenzyme Q10 on Cadmium-Induced Male Infertility. Int. J. Endocrinol. 2022, 2022, 6266613. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Wang, G.; Guan, T.; Zhu, C.; Li, J.; Wang, H. Effects of cadmium on antioxidant and non-specific immunity of Macrobrachium nipponense. Ecotoxicol. Environ. Saf. 2021, 224, 112651. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxidative Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Chu, C.; Yu, L.; Henry-Berger, J.; Ru, Y.F.; Kocer, A.; Champroux, A.; Li, Z.T.; He, M.; Xie, S.S.; Ma, W.B.; et al. Knockout of glutathione peroxidase 5 down-regulates the piRNAs in the caput epididymidis of aged mice. Asian J. Androl. 2020, 22, 590–601. [Google Scholar] [CrossRef]
- Mah, V.; Jalilehvand, F. Cadmium(II) complex formation with glutathione. J. Biol. Inorg. Chem. JBIC A Publ. Soc. Biol. Inorg. Chem. 2010, 15, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Ozoani, H.; Ezejiofor, A.N.; Okolo, K.O.; Orish, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Zinc and selenium attenuate quaternary heavy metal mixture-induced testicular damage via amplification of the antioxidant system, reduction in metal accumulation, inflammatory and apoptotic biomarkers. Toxicol. Res. 2023, 39, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Iannuzzi, C. The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase. Molecules 2017, 22, 1429. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell. Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef]
- Ha, K.N.; Chen, Y.; Cai, J.; Sternberg, P., Jr. Increased glutathione synthesis through an ARE-Nrf2-dependent pathway by zinc in the RPE: Implication for protection against oxidative stress. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2709–2715. [Google Scholar] [CrossRef]
- de Souza Predes, F.; Monteiro, J.C.; Matta, S.L.; Garcia, M.C.; Dolder, H. Testicular histomorphometry and ultrastructure of rats treated with cadmium and Ginkgo biloba. Biol. Trace Elem. Res. 2011, 140, 330–341. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef]
- Tourzani, D.A.; Paudel, B.; Miranda, P.V.; Visconti, P.E.; Gervasi, M.G. Changes in Protein O-GlcNAcylation During Mouse Epididymal Sperm Maturation. Front. Cell Dev. Biol. 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, J.G.; Canovas, S.; Barajas, P.; Marcos, J.; Jiménez-Movilla, M.; Gallego, R.G.; Ballesta, J.; Avilés, M.; Coy, P. Role of sialic acid in bovine sperm-zona pellucida binding. Mol. Reprod. Dev. 2007, 74, 617–628. [Google Scholar] [CrossRef]
- Tulsiani, D.R.; Abou-Haila, A. Biological Processes that Prepare Mammalian Spermatozoa to Interact with an Egg and Fertilize It. Scientifica 2012, 2012, 607427. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, M.J.; Robles-Gómez, L.; Huerta-Retamal, N.; Sáez-Espinosa, P.; Avilés, M.; Aizpurua, J.; Romero, A. FE-SEM Characterization of α-Mannose Density and Surface Mapping Changes in Human Sperm Head During In Vitro Capacitation. Microsc. Microanal. Off. J. Microsc. Soc. Am. Microbeam Anal. Soc. Microsc. Soc. Can. 2020, 26, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sampson, N.S. Fucose, mannose, and β-N-acetylglucosamine glycopolymers initiate the mouse sperm acrosome reaction through convergent signaling pathways. ACS Chem. Biol. 2014, 9, 468–475. [Google Scholar] [CrossRef]
- Hall, J.C.; Killian, G.J. Changes in rat sperm membrane glycosidase activities and carbohydrate and protein contents associated with epididymal transit. Biol. Reprod. 1987, 36, 709–718. [Google Scholar] [CrossRef]
- Benoff, S.; Hurley, I.; Cooper, G.W.; Mandel, F.S.; Rosenfeld, D.L.; Hershlag, A. Head-specific mannose-ligand receptor expression in human spermatozoa is dependent on capacitation-associated membrane cholesterol loss. Hum. Reprod. 1993, 8, 2141–2154. [Google Scholar] [CrossRef]
- Durin, Z.; Houdou, M.; Legrand, D.; Foulquier, F. Metalloglycobiology: The power of metals in regulating glycosylation. Biochim. Et Biophys. Acta-Gen. Subjects 2023, 1867, 130412. [Google Scholar] [CrossRef]
- NIST 1577b; Bovine Liver SRM 1577b. National Institute of Standards Technology: Gaithersburg, MD, USA, 1991.
- Johnsen, S.G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Vigueras-Villaseñor, R.M.; Molina-Ortiz, D.; Reyes-Torres, G.; del Angel, D.S.; Moreno-Mendoza, N.A.; Cruz, M.E.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Effect of allopurinol on damage caused by free radicals to cryptorchid testes. Acta Histochem. 2009, 111, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Ríos, E.; León-Galván, M.A.; Mercado, P.E.; Rosado, A. Superoxide dismutase, catalase, and glutathione peroxidase during epididymal maturation and prolonged storage of spermatozoa in the Mexican big-eared bat (Corynorhinus mexicanus). Can. J. Zool. 2005, 83, 1556–1565. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021.
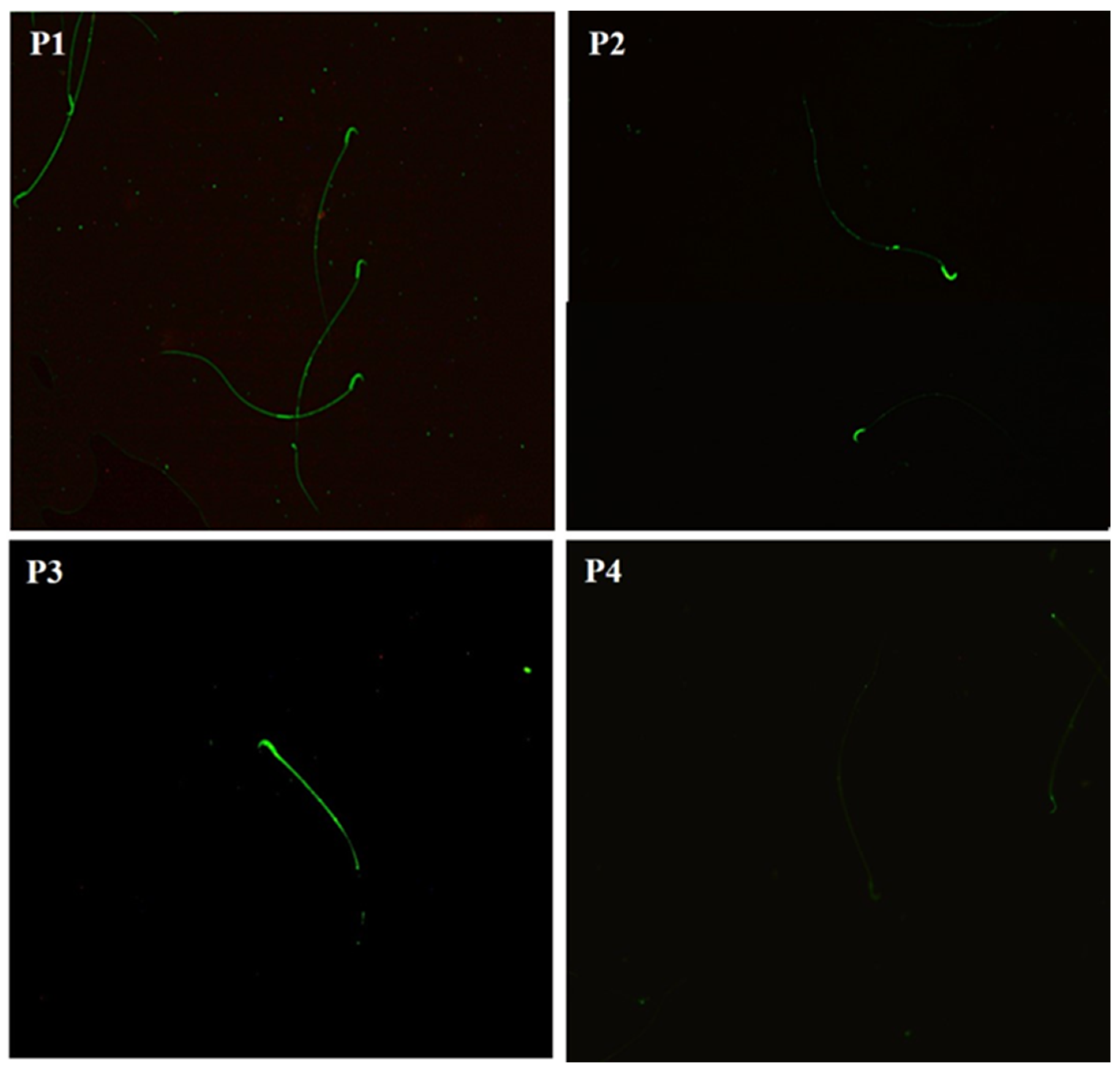
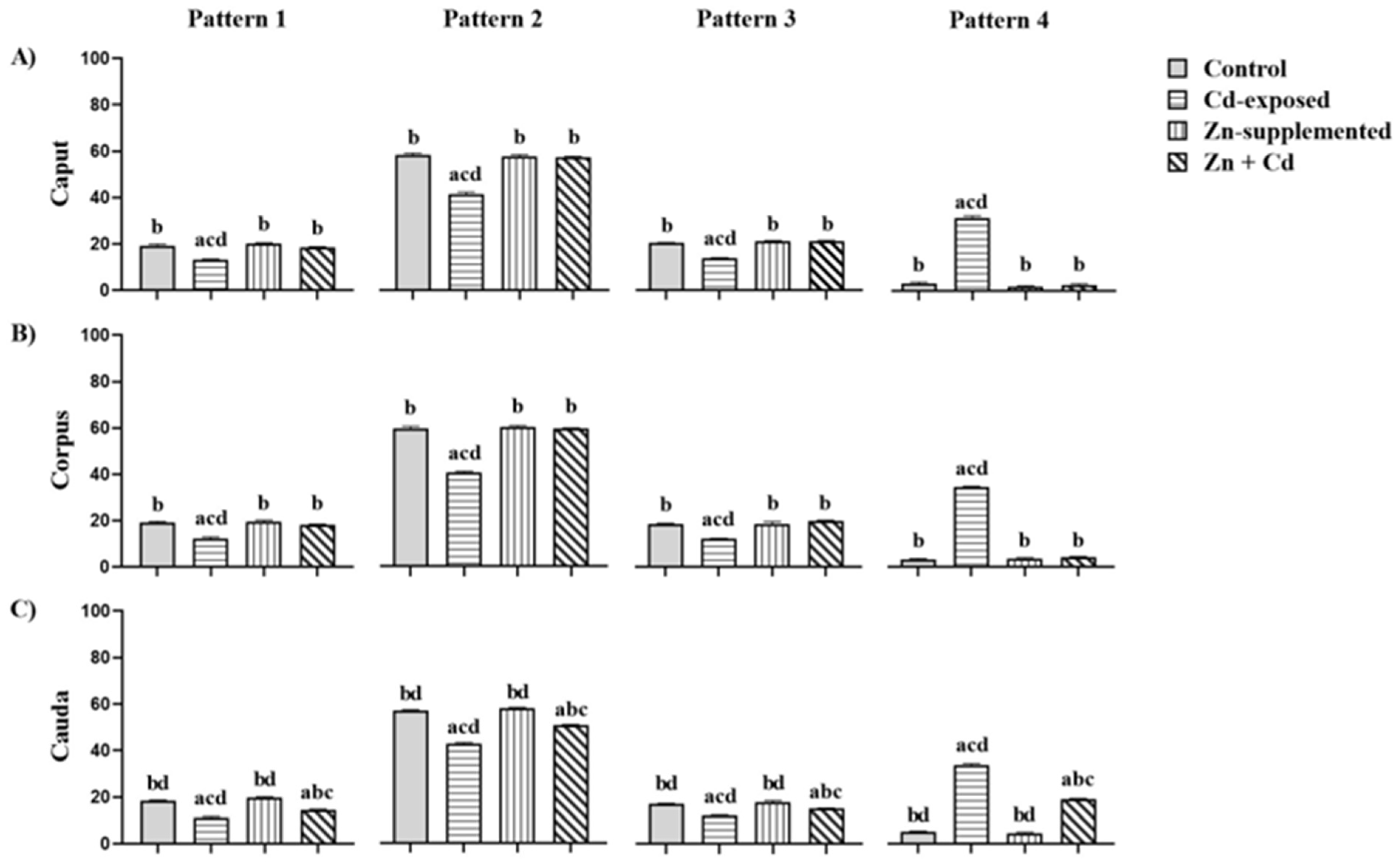

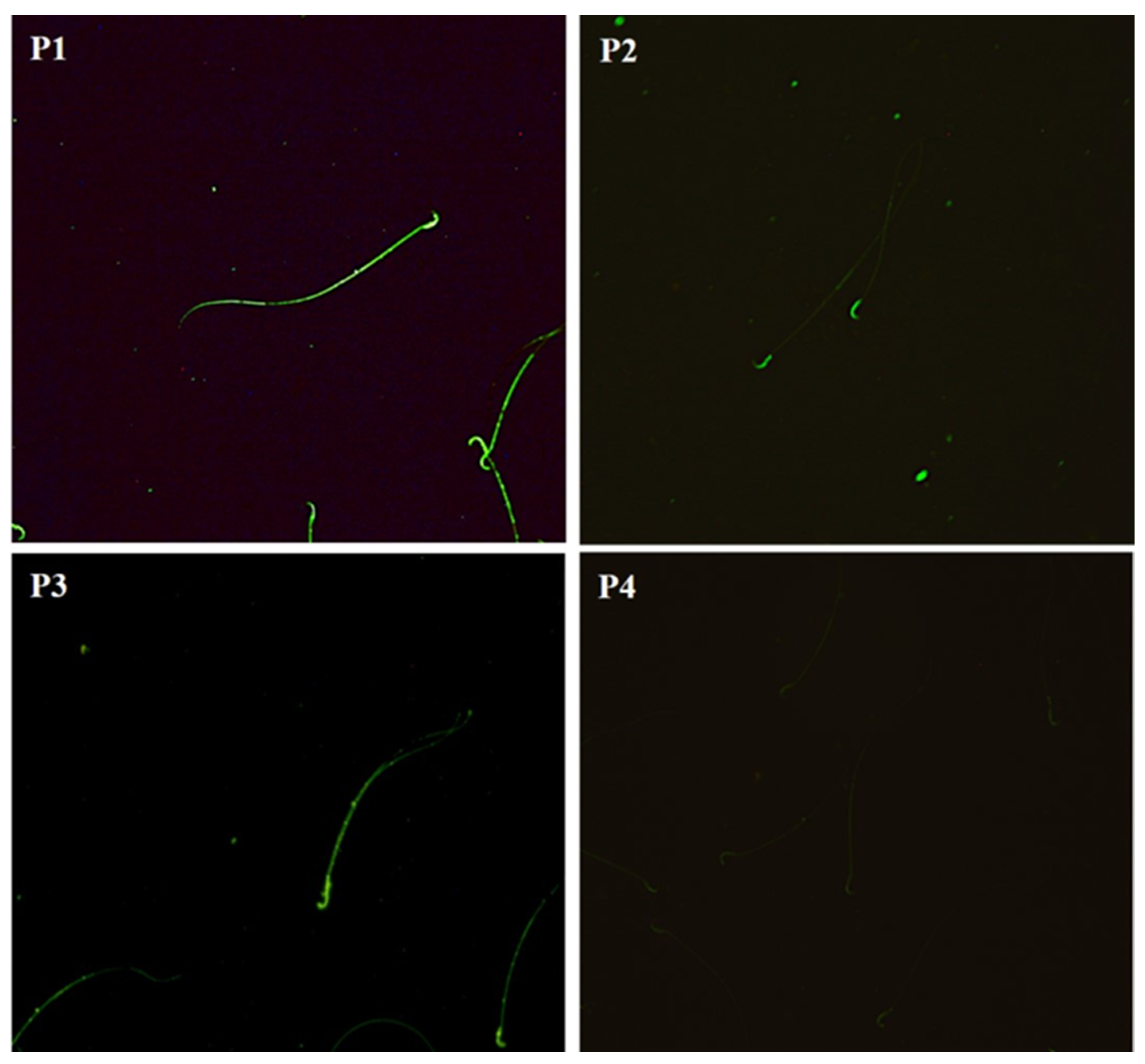
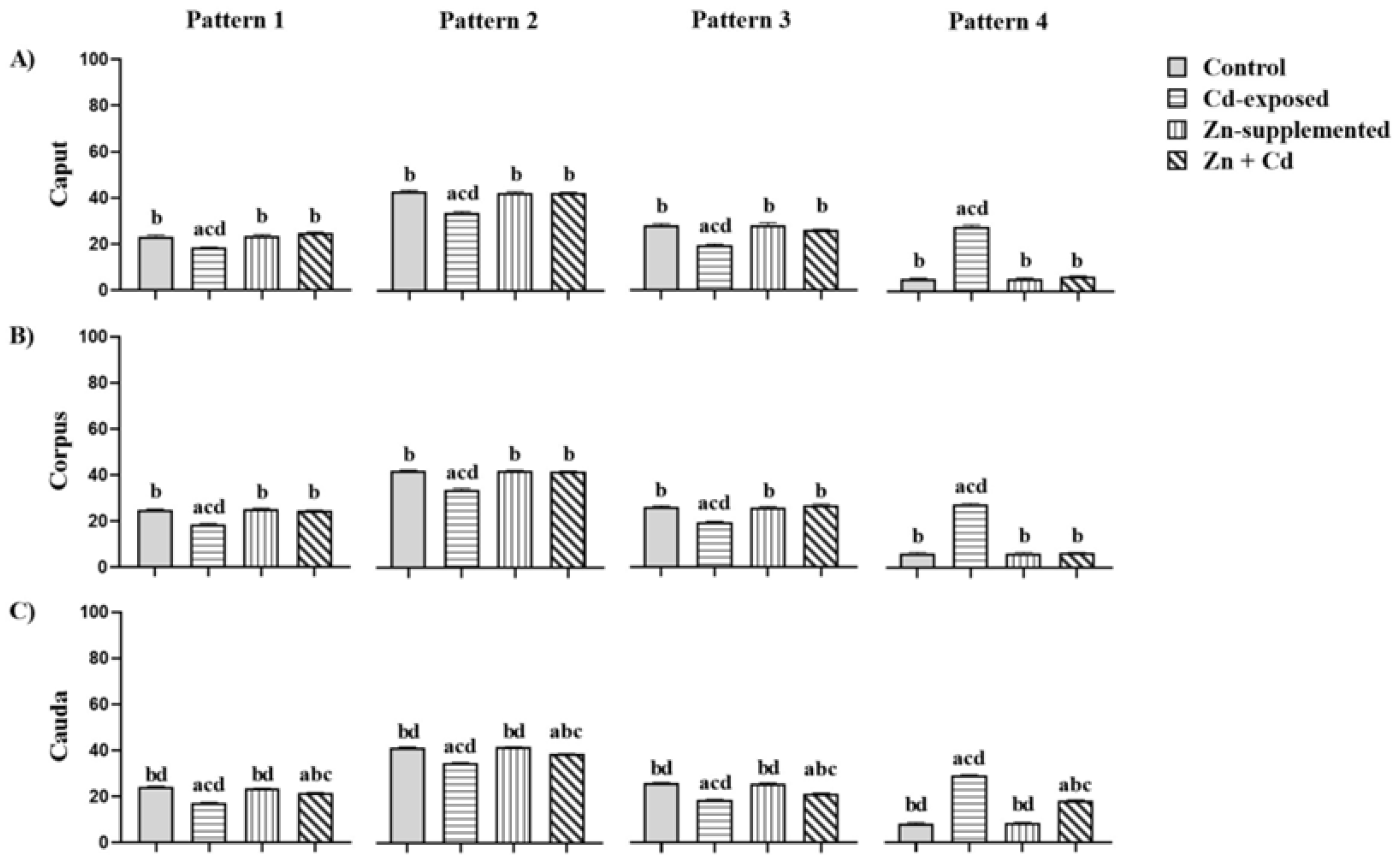

| Control | Cd-Exposed | Zn-Supplemented | Zn + Cd | |
|---|---|---|---|---|
| Blood (μg/mL) | ||||
| Cd | 0.0048 ± 0.00078 b | 0.075 ± 0.031 acd | 0.0034 ± 0.0008 b | 0.0079 ± 0.0016 b |
| Zn | 0.66 ± 0.052 b | 0.41 ± 0.023 acd | 0.83 ± 0.067 b | 0.71 ± 0.059 b |
| Testes (μg/g) | ||||
| Cd | 0.0061 ± 0.0011 bd | 1.2 ± 0.13 acd | 0.0028 ± 0.0047 bd | 0.62 ± 0.052 abc |
| Zn | 0.96 ± 0.016 bc | 0.30 ± 0.0052 acd | 1.7 ± 0.30 abd | 1.1 ± 0.11 bc |
| Epididymis (caput) | ||||
| Cd | 0.0064 ± 0.00094 bd | 0.97 ± 0.092 acd | 0.0045 ± 0.012 bd | 0.59 ± 0.061 abc |
| Zn | 1.3 ± 0.17 b | 0.44 ± 0.044 acd | 1.8 ± 0.22 b | 1.5 ± 0.26 b |
| Epididymis (cauda) | ||||
| Cd | 0.0050 ± 0.00070 bd | 1.2 ± 0.063 acd | 0.0041 ± 0.0081 bd | 0.44 ± 0.045 abc |
| Zn | 1.3 ± 0.29 b | 0.30 ± 0.0036 acd | 1.8 ± 0.15 bd | 1.0 ± 0.022 bc |
| Control | Cd-Exposed | Zn-Supplemented | Zn + Cd |
|---|---|---|---|
| 2.9 ± 0.098 b | 1.9 ± 0.28 acd | 3.1 ± 0.096 b | 2.6 ± 0.050 b |
| Control | Cd-Exposed | Zn-Supplemented | Zn + Cd | |
|---|---|---|---|---|
| Diameter (µm) | 331 ± 8.9 | 326 ± 6.2 | 336 ± 5.2 | 329 ± 7.6 |
| Area (µm2) | 63,212 ± 2330 | 61,124 ± 3300 | 71,766 ± 3064 | 67,190 ± 2344 |
| Maturation index | 9.6 ± 0.15 | 9.2 ± 0.21 | 9.7 ± 0.14 | 9.4 ± 0.19 |
| Histopathological index | 0.71 ± 0.18 | 0.86 ± 0.26 | 0.57 ± 0.20 | 0.71 ± 0.29 |
| Control | Cd-Exposed | Zn-Supplemented | Zn + Cd | ||
|---|---|---|---|---|---|
| Length (µm) | caput | 31 ± 0.30 b | 37 ± 0.62 acd | 30 ± 0.14 b | 29 ± 0.66 b |
| corpus | 32 ± 0.33 b | 37 ± 0.80 acd | 31 ± 0.47 b | 30 ± 0.26 b | |
| cauda | 14 ± 0.30 bd | 35 ± 1.2 acd | 16 ± 0.36 bd | 21 ± 0.46 abc | |
| Length (µm) | caput | 323 ± 16 b | 445 ± 12 acd | 306 ± 19 b | 330 ± 16 b |
| corpus | 329 ± 10 b | 451 ± 16 acd | 334 ± 12 b | 328 ± 14 b | |
| cauda | 126 ± 5.5 bd | 281 ± 6.5 acd | 120 ± 5.1 bd | 202 ± 9.3 abc |
| Control | Cd-Exposed | Zn-Supplemented | Zn + Cd | ||
|---|---|---|---|---|---|
| Concentration (×106/mL) | Caput | 69 ± 1.9 b | 43 ± 0.77 acd | 72 ± 2.2 bd | 64 ± 0.35 bc |
| Corpus | 53 ± 0.70 b | 35 ± 1.8 acd | 56 ± 1.5 bd | 50 ± 0.49 bc | |
| Cauda | 87 ± 1.1 bd | 51 ± 0.43 acd | 88 ± 1.0 bd | 82 ± 0.22 abc | |
| Viability (%) | Caput | 94± 0.42 b | 83 ± 1.0 acd | 94 ± 0.73 b | 92 ± 0.48 b |
| Corpus | 95 ± 0.86 b | 78 ± 1.5 acd | 96 ± 0.33 bd | 92 ± 0.37 bc | |
| Cauda | 94 ± 0.43 bd | 75 ± 1.8 acd | 93 ± 0.58 bd | 88 ± 0.84 abc | |
| Morphological Normality (%) | Caput | 95 ± 0.56 b | 72 ± 0.86 acd | 94 ± 0.49 b | 94 ± 0.42 b |
| Corpus | 97 ± 0.22 b | 73 ± 0.92 acd | 97 ± 0.40 b | 96 ± 0.40 b | |
| Cauda | 94 ± 0.26 bd | 70 ± 1.2 acd | 94 ± 0.54 bd | 90 ± 0.42 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín de Jesús, S.; Vigueras-Villaseñor, R.M.; Cortés-Barberena, E.; Hernández-Rodríguez, J.; Pérez-Aguirre, S.G.; Montes, S.; Carrizales-Yáñez, L.; Arrieta-Cruz, I.; Arteaga-Silva, M. Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium. Int. J. Mol. Sci. 2025, 26, 4589. https://doi.org/10.3390/ijms26104589
Marín de Jesús S, Vigueras-Villaseñor RM, Cortés-Barberena E, Hernández-Rodríguez J, Pérez-Aguirre SG, Montes S, Carrizales-Yáñez L, Arrieta-Cruz I, Arteaga-Silva M. Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium. International Journal of Molecular Sciences. 2025; 26(10):4589. https://doi.org/10.3390/ijms26104589
Chicago/Turabian StyleMarín de Jesús, Sergio, Rosa María Vigueras-Villaseñor, Edith Cortés-Barberena, Joel Hernández-Rodríguez, Sonia Guadalupe Pérez-Aguirre, Sergio Montes, Leticia Carrizales-Yáñez, Isabel Arrieta-Cruz, and Marcela Arteaga-Silva. 2025. "Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium" International Journal of Molecular Sciences 26, no. 10: 4589. https://doi.org/10.3390/ijms26104589
APA StyleMarín de Jesús, S., Vigueras-Villaseñor, R. M., Cortés-Barberena, E., Hernández-Rodríguez, J., Pérez-Aguirre, S. G., Montes, S., Carrizales-Yáñez, L., Arrieta-Cruz, I., & Arteaga-Silva, M. (2025). Early Zinc Supplementation Enhances Epididymal Sperm Glycosylation, Endocrine Activity, and Antioxidant Activity in Rats Exposed to Cadmium. International Journal of Molecular Sciences, 26(10), 4589. https://doi.org/10.3390/ijms26104589







