Comparison of Swim-Up and Microfluidic Sperm Sorting Methods in Selection of Sperm for Intracytoplasmic Sperm Injection
Abstract
1. Introduction
2. Results
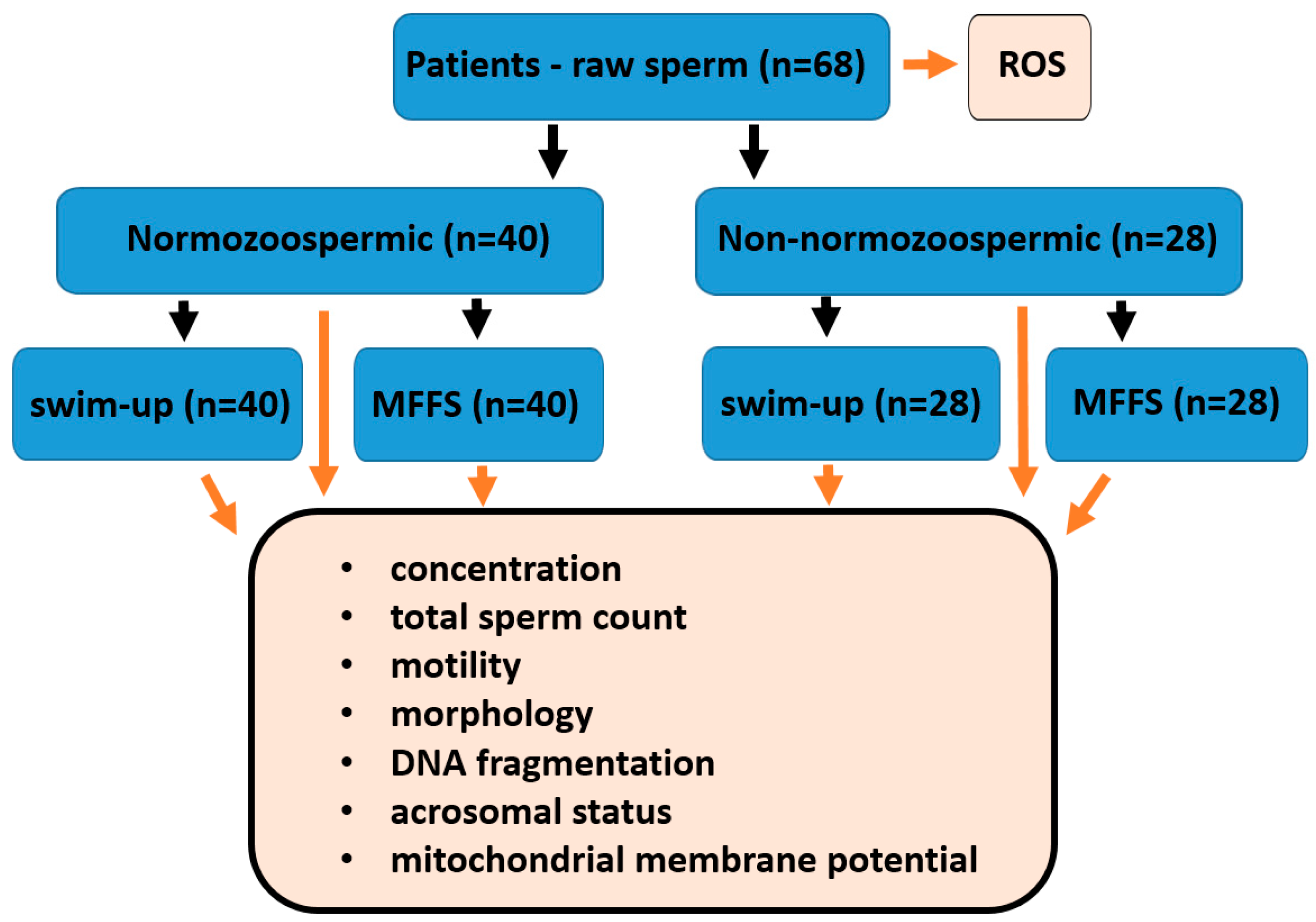
2.1. Patients
2.2. Results of Semen Analyses
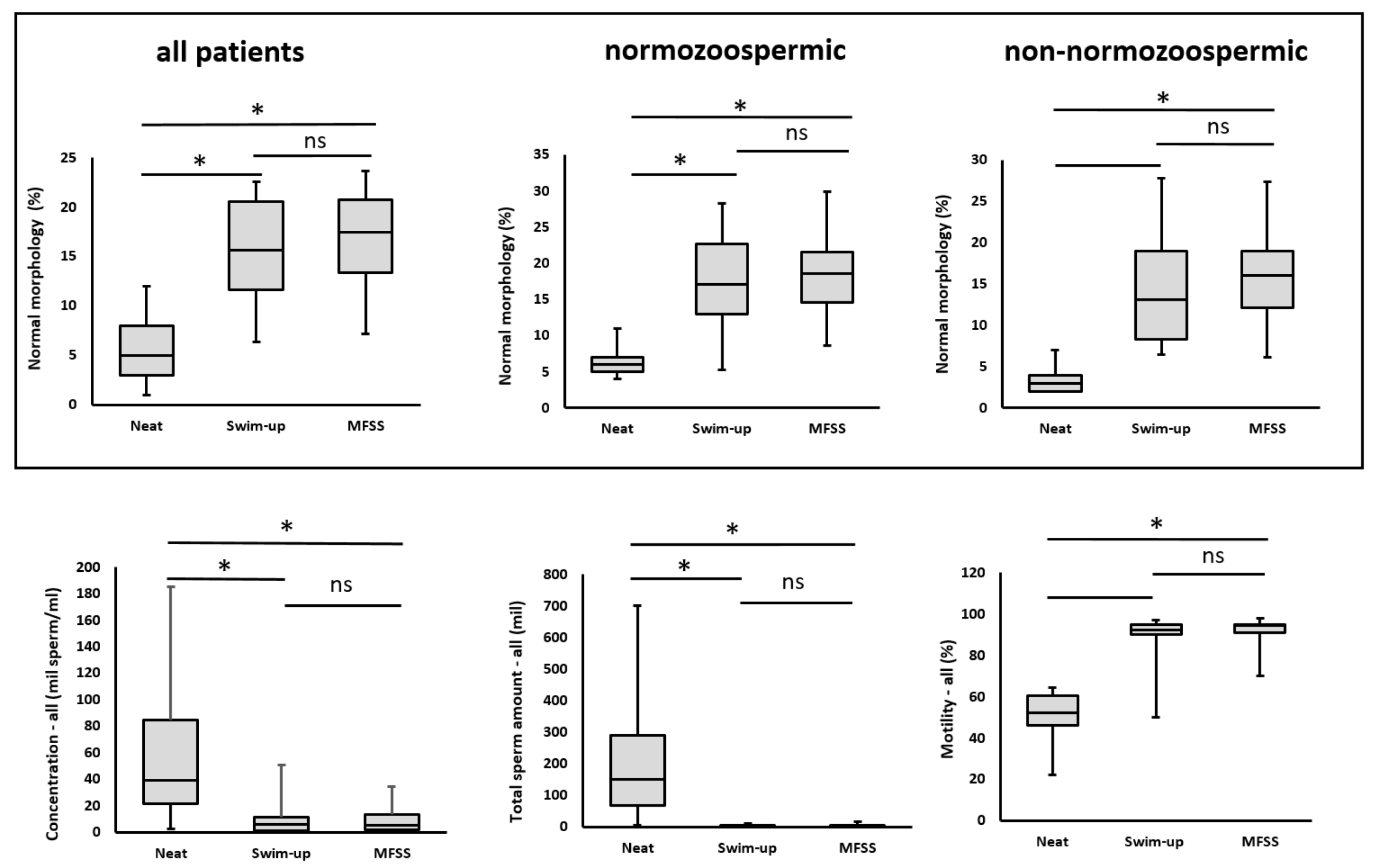
2.2.1. Sperm Concentration
2.2.2. Total Sperm Count
2.2.3. Sperm Motility
2.2.4. Sperm Morphology
2.2.5. DNA Integrity
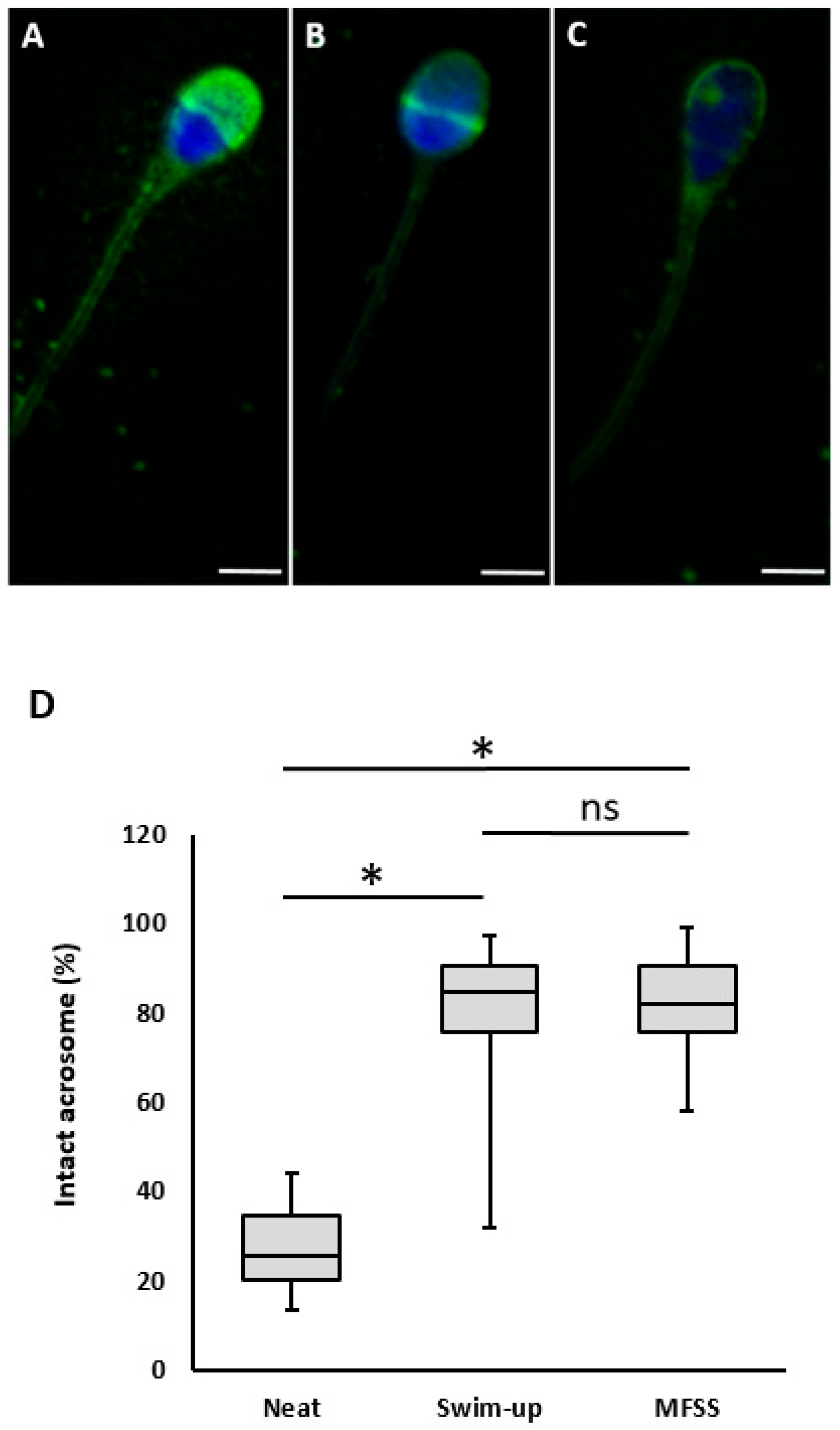
2.2.6. Acrosomal Status
2.2.7. Mitochondrial Activity
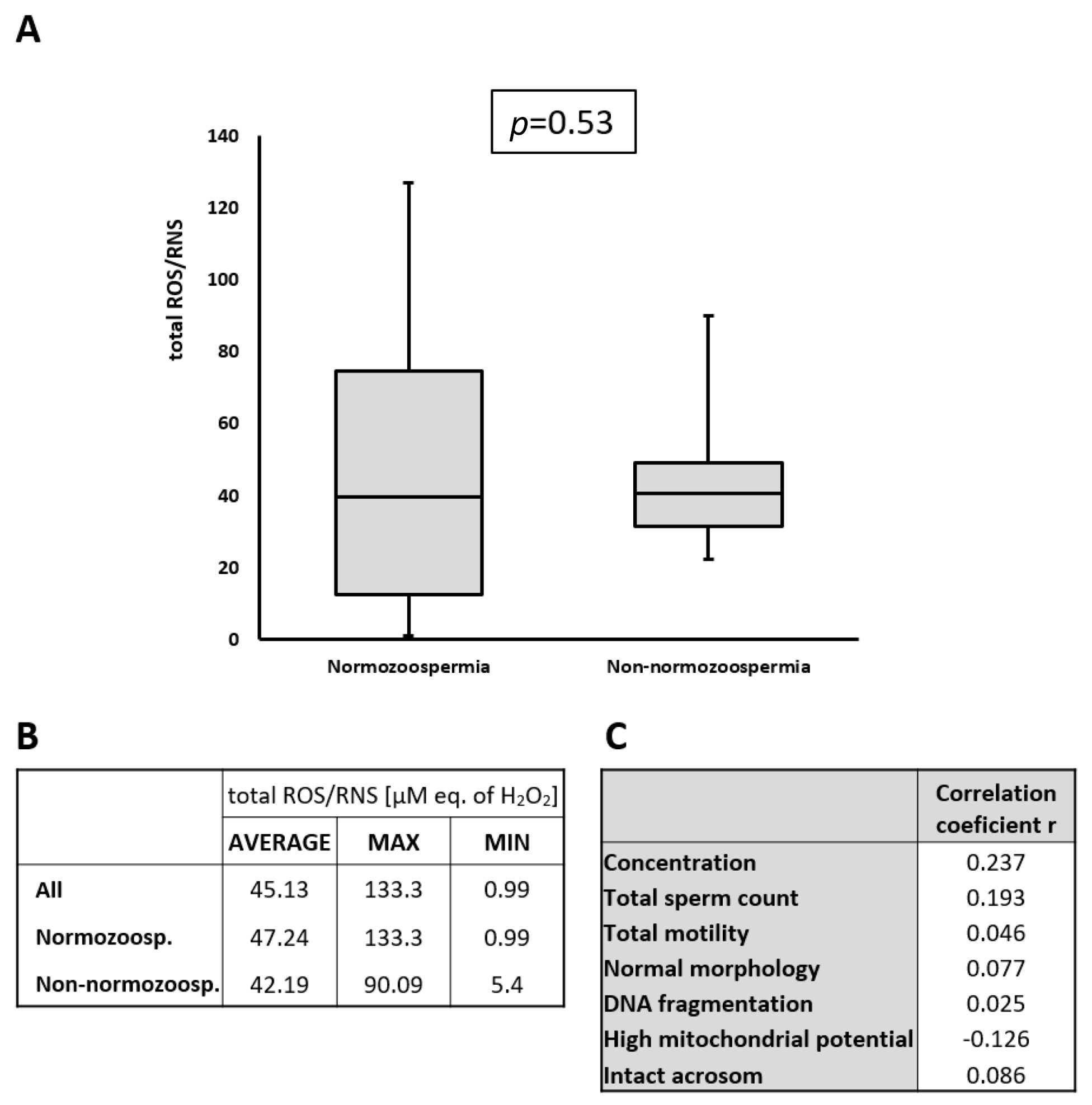
2.3. Detection of ROS/RNS in Seminal Plasma
2.4. Normozoospermic and Non-Normozoospermic Patients
3. Discussion
4. Materials and Methods
4.1. Study Design, Patient Eligibility Criteria and Sample Division
- The first portion (180 µL) was not separated, only analyzed.
- The second portion (1000 µL) was separated using MFSS (Ca0 microfluidic chip; LensHOOKE Bonraybio, Taichung, Taiwan).
- The third portion (≥400 µL) was processed using the swim-up (SU) method (Figure 4).
4.2. Sperm Selection
4.2.1. Microfluidic Sperm Sorting
4.2.2. Swim-Up Method
4.3. Semen Analyses
4.4. Assessment of DNA Fragmentation
4.5. Evaluation of Acrosomal Status
4.6. Mitochondrial Evaluation
4.7. ROS/RNS Assessment
4.8. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaughan, D.A.; Leung, A.; Resetkova, N.; Ruthazer, R.; Penzias, A.S.; Sakkas, D.; Alper, M.M. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil. Steril. 2017, 107, 397–404.e3. [Google Scholar] [CrossRef] [PubMed]
- Giojalas, L.C.; Guidobaldi, H.A. Getting to and away from the egg, an interplay between several sperm transport mechanisms and a complex oviduct physiology. Mol. Cell. Endocrinol. 2020, 518, 110954. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Thompson, L.A.; Li, T.C.; Mackenna, A.; Barratt, C.L.; Cooke, I.D. Uterine flushing: A method to recover spermatozoa and leukocytes. Hum. Reprod. 1993, 8, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D.; Leslie, E.E.; Kelly, R.W.; Templeton, A.A. Morphological selection of human spermatozoa in vivo and in vitro. J. Reprod. Fertil. 1982, 64, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; Gutiérrez-Adán, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L. Sperm selection in natural conception: What can we learn from mother nature to improve assisted reproduction outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm selection for ICSI: Do we have a winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef]
- Henkel, R. Sperm preparation: State-of-the-art-physiological aspects and application of advanced sperm preparation methods. Asian J. Androl. 2012, 14, 260–269. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, Z.; Shan, G.; Chu, L.T.; Huang, Z.; Moskovtsev, S.; Librach, C.; Jarvi, K.; Sun, Y. Advances in sperm analysis: Techniques, discoveries and applications. Nat. Rev. Urol. 2021, 18, 447–467. [Google Scholar] [CrossRef]
- Dehghanpour, F.; Khalili, M.A.; Mangoli, E.; Talebi, A.R.; Anbari, F.; Shamsi, F.; Woodward, B.; Doostabadi, M.R. Free centrifuge sorting method for high-count sperm preparation improves biological characteristics of human spermatozoa and clinical outcome: A sibling oocytes study. Andrologia 2022, 54, e14554. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Tomita, K.; Hashimoto, S.; Matsumoto, H.; Satoh, M.; Kato, H.; Hosoi, Y.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J. Reprod. Dev. 2016, 62, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Gandini, L.; Lombardo, F.; Paoli, D.; Caruso, F.; Eleuteri, P.; Leter, G.; Ciriminna, R.; Culasso, F.; Dondero, F.; Lenzi, A.; et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum. Reprod. 2004, 19, 1409–1417. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Gotsiridze, K.; Nana, M.; Mariam, M.; Tamar, J. Live motile sperm sorting device improves embryo aneuploidy: A Retrospective Cohort Study. Fertil. Reprod. 2024, 06, 117–122. [Google Scholar] [CrossRef]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef]
- Sheibak, N.; Amjadi, F.; Shamloo, A.; Zarei, F.; Zandieh, Z. Microfluidic sperm sorting selects a subpopulation of high-quality sperm with a higher potential for fertilization. Hum. Reprod. 2024, 39, 902–911. [Google Scholar] [CrossRef]
- Banti, M.; Van Zyl, E.; Kafetzis, D. Suprem preparation with microfluidic sperm sorting chip may improve intracytoplasmic sperm injection outcomes compared to density gradient centrifugation. Reprod. Sci. 2024, 31, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Anbari, F.; Khalili, M.A.; Sultan Ahamed, A.M.; Mangoli, E.; Nabi, A.; Dehghanpour, F.; Sabour, M. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: A pilot study. Syst. Biol. Reprod. Med. 2021, 67, 137–143. [Google Scholar] [CrossRef]
- Hsu, C.T.; Lee, C.I.; Lin, F.S.; Wang, F.Z.; Chang, H.C.; Wang, T.E.; Huang, C.C.; Tsao, H.M.; Lee, M.S.; Agarwal, A. Live motile sperm sorting device for enhanced sperm-fertilization competency: Comparative analysis with density-gradient centrifugation and microfluidic sperm sorting. J. Assist. Reprod. Genet. 2023, 40, 1855–1864. [Google Scholar] [CrossRef]
- Feyzioglu, B.S.; Avul, Z. Effects of sperm separation methods before intrauterine insemination on pregnancy outcomes and live birth rates: Differences between the swim-up and microfluidic chip techniques. Medicine 2023, 102, e36042. [Google Scholar] [CrossRef] [PubMed]
- Leisinger, C.A.; Adaniya, G.; Freeman, M.R.; Behnke, E.J.; Aguirre, M.; VerMilyea, M.D.; Schiewe, M.C. Effect of microfluidic sperm separation vs. standard sperm washing processes on laboratory outcomes and clinical pregnancy rates in an unselected patient population. Reprod. Med. 2021, 2, 125–130. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Oktarina, M.; Sharaswati, I.A. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J. Hum. Reprod. Sci. 2021, 14, 121–128. [Google Scholar] [CrossRef]
- Andrabi, S.W.; Ara, A.; Saharan, A.; Jaffar, M.; Gugnani, N.; Esteves, S.C. Sperm DNA fragmentation: Causes, evaluation and management in male infertility. JBRA Assist. Reprod. 2024, 28, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Vasilescu, S.A.; Ding, L.; Parast, F.Y.; Nosrati, R.; Warkiani, M.E. Sperm quality metrics were improved by a biomimetic microfluidic selection platform compared to swim-up methods. Microsyst. Nanoeng. 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Kocur, O.M.; Xie, P.; Cheung, S.; Souness, S.; McKnight, M.; Rosenwaks, Z.; Palermo, G.D. Can a sperm selection technique improve embryo ploidy? Andrology 2023, 11, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Keating, D.; Cheung, S.; Xie, P.; Stewart, J.D.; Rosenwaks, Z.; Palermo, G.D. A treatment approach for couples with disrupted sperm DNA integrity and recurrent ART failure. J. Assist. Reprod. Genet. 2019, 36, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Shirota, K.; Yotsumoto, F.; Itoh, H.; Obama, H.; Hidaka, N.; Nakajima, K.; Miyamoto, S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 2016, 105, 315–321.e1. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Cambi, M.; Boni, L.; Iorio, A.L.; Passaro, C.; Luppino, B.; Nadalini, M.; Marchiani, S.; Tamburrino, L.; et al. Variation of DNA fragmentation levels during density gradient sperm selection for assisted reproduction techniques: A possible new male predictive parameter of pregnancy? Medicine 2016, 95, e3624. [Google Scholar] [CrossRef]
- Takeshima, T.; Yumura, Y.; Kuroda, S.; Kawahara, T.; Uemura, H.; Iwasaki, A. Effect of density gradient centrifugation on reactive oxygen species in human semen. Syst. Biol. Reprod. Med. 2017, 63, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Raad, G.; Bakos, H.W.; Bazzi, M.; Mourad, Y.; Fakih, F.; Shayya, S.; Mchantaf, L.; Fakih, C. Differential impact of four sperm preparation techniques on sperm motility, morphology, DNA fragmentation, acrosome status, oxidative stress, and mitochondrial activity: A prospective study. Andrology 2021, 9, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Sharma, R.K.; Thomas, A.J.; Agarwal, A. Effect of swim-up sperm washing and subsequent capacitation on acrosome status and functional membrane integrity of normal sperm. Int. J. Fertil. Womens Med. 2000, 45, 335–341. [Google Scholar]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol. Clin. N. Am. 2002, 29, 817–827. [Google Scholar] [CrossRef]
- Ferreira Aderaldo, J.; da Silva Maranhão, K.; Ferreira Lanza, D.C. Does microfluidic sperm selection improve clinical pregnancy and miscarriage outcomes in assisted reproductive treatments? A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292891. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.R.; Ziarati, N.; Dadkhah, E.; Bucak, M.N.; Rahimizadeh, P.; Shahverdi, A.; Sadighi Gilani, M.A.; Topraggaleh, T.R. Microfluidics: The future of sperm selection in assisted reproduction. Andrology 2024, 12, 1236–1252. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010; pp. 1–271. [Google Scholar]
- Doostabadi, M.R.; Mangoli, E.; Marvast, L.D.; Dehghanpour, F.; Maleki, B.; Torkashvand, H.; Talebi, A.R. Microfluidic devices employing chemo- and thermotaxis for sperm selection can improve sperm parameters and function in patients with high DNA fragmentation. Andrologia 2022, 54, e14623. [Google Scholar] [CrossRef]
- Estevez, S.C.; Verza, S. Relationship of in vitro acrosome reacton to sperm function: An update. Open Rep. Sci. J. 2011, 3, 72–84. [Google Scholar] [CrossRef]
- Gangwar, C.; Kharche, S.D.; Mishra, A.K.; Saraswat, S.; Kumar, N.; Sikarwar, A.K. Effect of diluent sugars on capacitation status and acrosome reaction of spermatozoa in buck semen at refrigerated temperature. Trop. Anim. Health Prod. 2020, 52, 3409–3415. [Google Scholar] [CrossRef]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Mean ± SD |
|---|---|
| Number of patients | 68 |
| Age (years) | 32.14 ± 7.1 |
| Volume (mL) | 3.93 ± 1.83 |
| Concentration (×106 cell/mL) | 44.57 ± 31.12 |
| Progressive motility (%) | 43.91 ± 14.55 |
| Total motility (%) | 53.05 ± 13.81 |
| Normal morphology (%) | 5.3 ± 2.45 |
| High MMP (%) | 87.04 ± 3.92 |
| DFI (%) | 18.27 ± 8.36 |
| Intact acrosome (%) | 29.15 ± 8.77 |
| ROS (µM, H2O2) | 45.13 ± 32.19 |
| Normozoospermic patients | n = 40 |
| Non-normozoospermic patients | n = 28 |
| Parameters | Ejaculate | Separation Methods | Mean ± SD |
|---|---|---|---|
| Concentration (×106 sperm/mL) | 44.57 ± 31.12 | Swim-up | 7.68 ± 7.75 a |
| MFSS | 8.52 ±8.69 a | ||
| Total sperm count (×106) | 177.87 ± 141.6 | Swim-up | 3.72 ± 4.25 a |
| MFSS | 3.96 ± 4.01 a | ||
| Total motility (%) | 53.05 ± 13.8 | Swim-up | 91.29 ± 8.72 a |
| MFSS | 93.57 ± 4.24 a | ||
| Normal morphology (%) | 5.3 ± 2.45 | Swim-up | 16.01 ± 6.21 a |
| MFSS | 17.26 ± 5.34 a | ||
| DNA fragmentation (%) | 18.27 ± 8.36 | Swim-up | 9.36 ± 7.5 a |
| MFSS | 5.98 ± 5.95 b | ||
| High mitochondrial potential (%) | 87.04 ± 3.85 | Swim-up | 92.85 ± 2.43 a |
| MFSS | 92.79 ± 2.75 a | ||
| Intact acrosome (%) | 29.15 ± 8.77 | Swim-up | 81.09 ± 13.61 a |
| MFSS | 81.78 ± 10.6 a |
| Parameters | Swim-Up | MFSS | p-Value | Neat |
|---|---|---|---|---|
| Concentration (×106 sperm/mL) | 9.6 ± 6.84 | 11.53 ± 9.02 | 0,28 | 55.25 ± 29.32 |
| Total sperm count (×106) | 4.44 ± 3.21 | 5.36 ± 4.2 | 0,27 | 202.34 ± 107.46 |
| Total motility (%) | 92.5 ± 5.16 | 93.06 ± 4.48 | 0,39 | 58.95 ± 10.4 |
| Normal morphology (%) | 16.42 ± 5.99 | 16.50 ± 6.1 | 0,95 | 6.62 ± 1.99 |
| DNA fragmentation (DFI %) | 9.27 ± 7.02 | 6.69 ± 6.36 | 0,08 | 17.6 ± 8.76 |
| High mitochondrial potential (%) | 93.05 ± 2.85 | 93.62 ± 2.8 | 0,37 | 88.24 ± 4.22 |
| Intact acrosome (%) | 85.09 ± 12.27 | 85.94 ± 10.16 | 0,73 | 28.82 ± 8.07 |
| Parameters | Swim-Up | MFSS | p-Value | Neat |
|---|---|---|---|---|
| Concentration (×106 sperm/mL) | 3.98 ± 5.63 | 4.3 ± 5.59 | 0.83 | 28.89 ± 27.43 |
| Total sperm count (×106) | 1.87 ± 2.7 | 2.01 ± 2.6 | 0.84 | 113.08 ± 148.24 |
| Total motility (%) | 89.57 ± 12.05 | 93.78 ± 3.94 | 0.18 | 44.64 ± 13.86 |
| Normal morphology (%) | 13.22 ± 5.95 | 14.32 ± 5.7 | 0.48 | 3.42 ± 1.72 |
| DNA fragmentation (%) | 10.0 ± 7.83 | 5.69 ± 6.32 | 0.027 | 19.24 ± 7.81 |
| High mitochondrial potential (%) | 93.42 ± 2.56 | 92.67 ± 3.28 | 0.34 | 86.73 ± 4.09 |
| Intact acrosome (%) | 80.79 ± 14.13 | 81.25 ± 10.8 | 0.89 | 26.45 ± 7.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ješeta, M.; Doubravská, A.; Antalíková, J.; Mekiňová, L.; Franzová, K.; Remundová, K.; Hošek, J.; Kempisty, B.; Hudeček, R. Comparison of Swim-Up and Microfluidic Sperm Sorting Methods in Selection of Sperm for Intracytoplasmic Sperm Injection. Int. J. Mol. Sci. 2025, 26, 5374. https://doi.org/10.3390/ijms26115374
Ješeta M, Doubravská A, Antalíková J, Mekiňová L, Franzová K, Remundová K, Hošek J, Kempisty B, Hudeček R. Comparison of Swim-Up and Microfluidic Sperm Sorting Methods in Selection of Sperm for Intracytoplasmic Sperm Injection. International Journal of Molecular Sciences. 2025; 26(11):5374. https://doi.org/10.3390/ijms26115374
Chicago/Turabian StyleJešeta, Michal, Adéla Doubravská, Jana Antalíková, Lenka Mekiňová, Kateřina Franzová, Kateřina Remundová, Jan Hošek, Bartosz Kempisty, and Robert Hudeček. 2025. "Comparison of Swim-Up and Microfluidic Sperm Sorting Methods in Selection of Sperm for Intracytoplasmic Sperm Injection" International Journal of Molecular Sciences 26, no. 11: 5374. https://doi.org/10.3390/ijms26115374
APA StyleJešeta, M., Doubravská, A., Antalíková, J., Mekiňová, L., Franzová, K., Remundová, K., Hošek, J., Kempisty, B., & Hudeček, R. (2025). Comparison of Swim-Up and Microfluidic Sperm Sorting Methods in Selection of Sperm for Intracytoplasmic Sperm Injection. International Journal of Molecular Sciences, 26(11), 5374. https://doi.org/10.3390/ijms26115374









