Influence of Soluble Guanylate Cyclase on Cardiac, Vascular, and Renal Structure and Function: A Physiopathological Insight
Abstract
1. Introduction
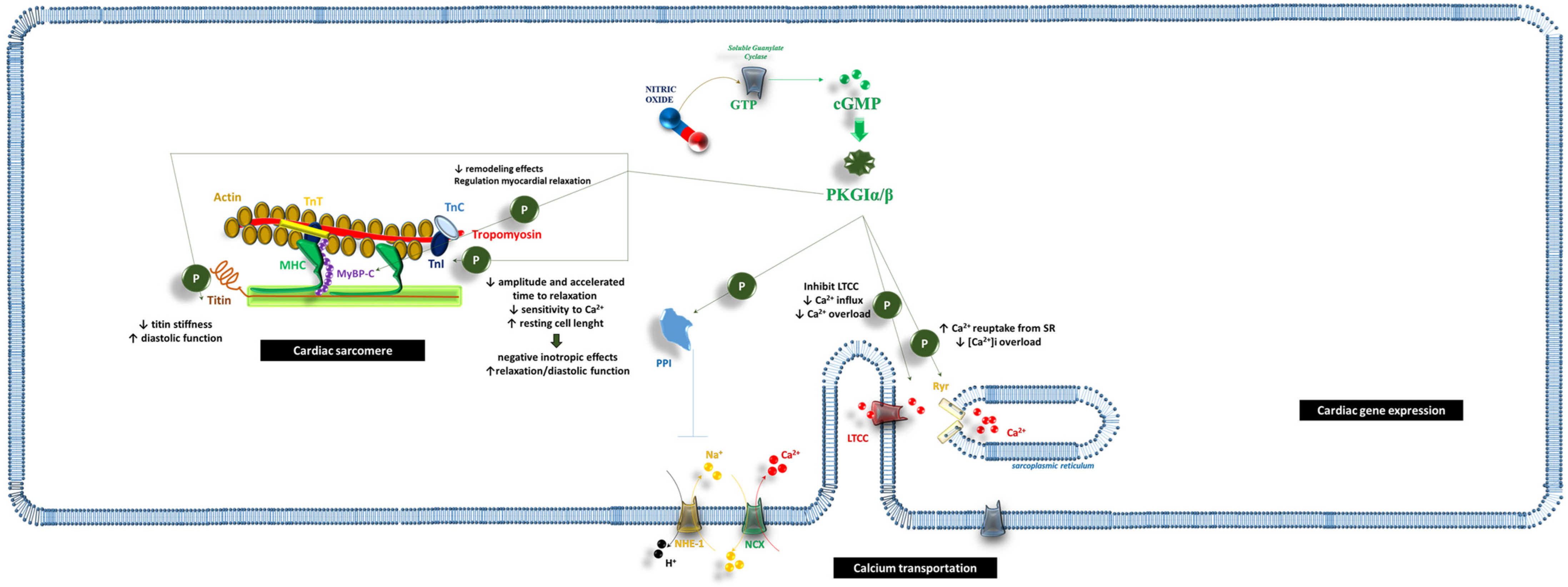
2. Cardiac Muscle Cells
2.1. cGMP/PKG and the Cardiac Sarcomere
2.2. cGMP and Ca2+ Transport
2.3. cGMP and Cardiac Gene Expression
2.4. Inflammatory Regulation
2.5. Future Perspective
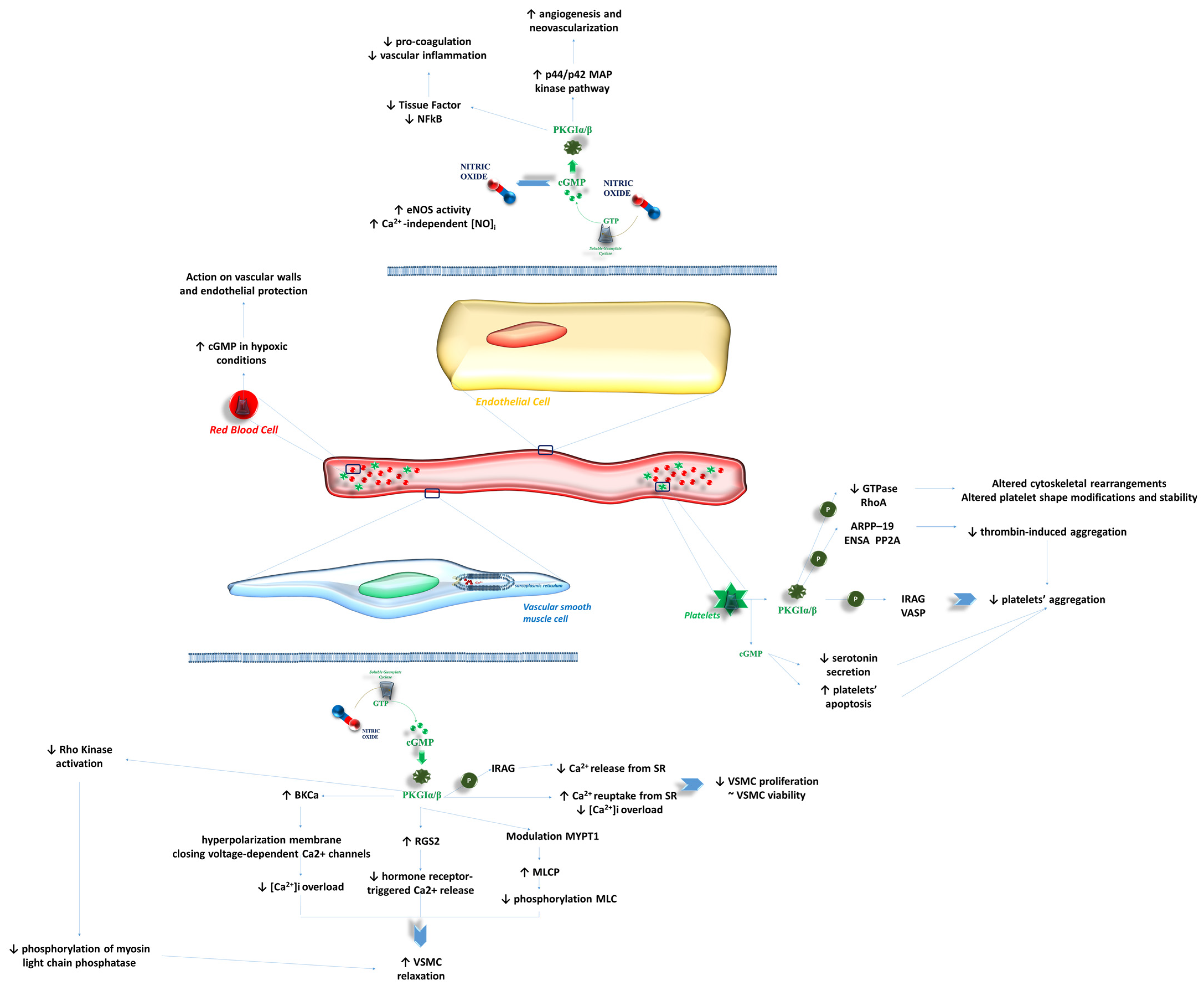
3. Peripheral Endothelial Function
3.1. Effects of NO-sGC-cGMP on VSMCs
3.2. Effects of NO-sGC-cGMP on Endothelial Cells
3.3. Effects of NO-sGC-cGMP on Bloodstream Cells
3.4. Future Perspectives
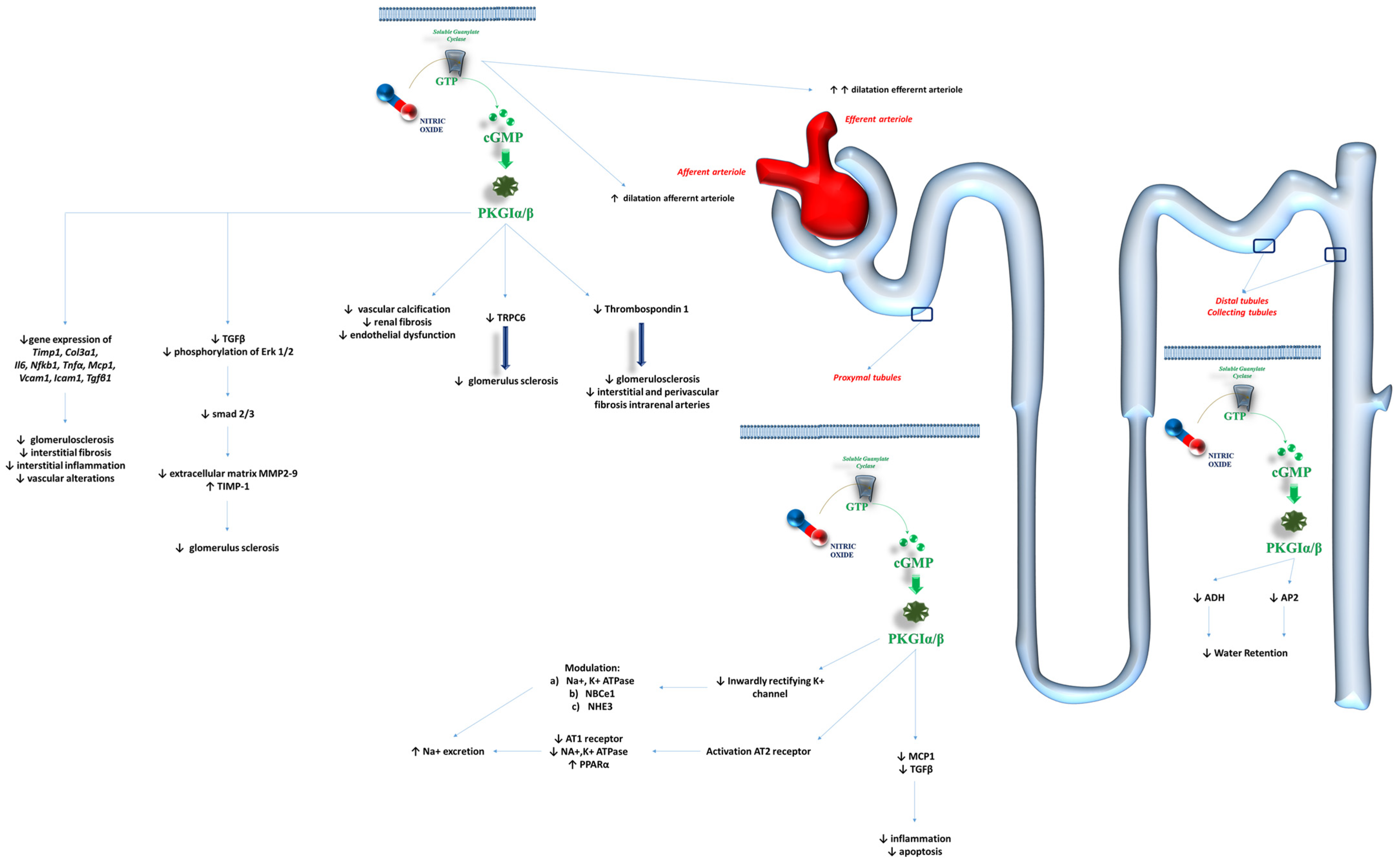
4. Kidneys and Renal Function
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- The Nobel Prize in Physiology or Medicine 1998. NobelPrize.org. Nobel Prize Outreach 2025. Available online: https://www.nobelprize.org/prizes/medicine/1998/summary/ (accessed on 9 December 2024).
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Costa, D.; Napoli, C. Novel Insights Regarding Nitric Oxide and Cardiovascular Diseases. Angiology 2021, 72, 411–425. [Google Scholar] [CrossRef]
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir. Med. 2017, 122, S1–S9. [Google Scholar] [CrossRef]
- Michalak, M.; Armstrong, P.W. Exploring New Cardiovascular Pathways: Are Soluble Guanylate Cyclase Stimulators the Right Direction? Circ. Heart Fail. 2018, 11, e004813. [Google Scholar] [CrossRef]
- Feil, R.; Lehners, M.; Stehle, D.; Feil, S. Visualising and understanding cGMP signals in the cardiovascular system. Br. J. Pharmacol. 2022, 179, 2394–2412. [Google Scholar] [CrossRef]
- Wakai, A.; McCabe, A.; Kidney, R.; Brooks, S.C.; Seupaul, R.A.; Diercks, D.B.; Salter, N.; Fermann, G.J.; Pospisil, C. Nitrates for acute heart failure syndromes. Cochrane Database Syst. Rev. 2013, 8, CD005151. [Google Scholar]
- Zhuang, X.D.; Long, M.; Li, F.; Hu, X.; Liao, X.X.; Du, Z.M. PDE5 inhibitor sildenafil in the treatment of heart failure: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014, 172, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, E.; Kasparian, C.; Haddour, G.; Boissel, J.P.; Nony, P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst. Rev. 2005, 1, CD002230. [Google Scholar] [CrossRef]
- Afsar, B.; Ortiz, A.; Covic, A.; Gaipov, A.; Esen, T.; Goldsmith, D.; Kanbay, M. Phosphodiesterase type 5 inhibitors and kidney disease. Int. Urol. Nephrol. 2015, 47, 1521–1528. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Wheeler, D.C.; Debén, F.M.; Speeckaert, M.; Thomas, D.; Berger, M.; Klein, S.; Friedrichs, F.; Paraschin, K.; Schmieder, R.E. The soluble guanylate cyclase activator runcaciguat significantly improves albuminuria in patients with chronic kidney disease: A randomized placebo-controlled clinical trial. Nephrol. Dial. Transplant, 2024; gfae261, (Online ahead of print). [Google Scholar]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef]
- Cerra, M.C.; Pellegrino, D. Cardiovascular cGMP-generating systems in physiological and pathological conditions. Curr. Med. Chem. 2007, 14, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.S.; Trochu, J.N.; Donal, E.; Galinier, M.; Logeart, D.; De Groote, P.; Juillière, Y. Vericiguat for the treatment of heart failure: Mechanism of action and pharmacological properties compared with other emerging therapeutic options. Expert Opin. Pharmacother. 2021, 22, 1847–1855. [Google Scholar] [CrossRef]
- Cuello, F.; Nikolaev, V.O. Cardiac cGMP Signaling in Health and Disease: Location, Location, Location. J. Cardiovasc. Pharmacol. 2020, 75, 399–409. [Google Scholar] [CrossRef]
- Hofmann, F.; Ammendola, A.; Schlossmann, J. Rising behind NO: cGMP-dependent protein kinases. J. Cell. Sci. 2000, 113, 1671–1676. [Google Scholar] [CrossRef]
- Adler, J.; Kuret, A.; Längst, N.; Lukowski, R. Targets of cGMP/cGKI in Cardiac Myocytes. J. Cardiovasc. Pharmacol. 2020, 75, 494–507. [Google Scholar] [CrossRef]
- Hofmann, F.; Feil, R.; Kleppisch, T.; Schlossmann, J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol. Rev. 2006, 86, 1–23. [Google Scholar] [CrossRef]
- Hofmann, F. A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic Res. Cardiol. 2018, 113, 31. [Google Scholar] [CrossRef]
- Yuasa, K.; Michibata, H.; Omori, K.; Yanaka, N. A novel interaction of cGMP-dependent protein kinase I with troponin T. J. Biol. Chem. 1999, 274, 37429–37434. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Kötter, S.; Grützner, A.; Lang, P.; Andresen, C.; Redfield, M.M.; Butt, E.; dos Remedios, C.G.; Linke, W.A. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 2009, 104, 87–94. [Google Scholar] [CrossRef]
- Layland, J.; Li, J.M.; Shah, A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002, 540, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Vila-Petroff, M.G.; Younes, A.; Egan, J.; Lakatta, E.G.; Sollott, S.J. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ. Res. 1999, 84, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Thoonen, R.; Giovanni, S.; Govindan, S.; Lee, D.I.; Wang, G.R.; Calamaras, T.D.; Takimoto, E.; Kass, D.A.; Sadayappan, S.; Blanton, R.M. Molecular Screen Identifies Cardiac Myosin-Binding Protein-C as a Protein Kinase G-Iα Substrate. Circ. Heart Fail. 2015, 8, 1115–1122. [Google Scholar] [CrossRef]
- Sadayappan, S.; de Tombe, P.P. Cardiac myosin binding protein-C as a central target of cardiac sarcomere signaling: A special mini review series. Pflug. Arch. 2014, 466, 195–200. [Google Scholar] [CrossRef]
- Barefield, D.; Sadayappan, S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J. Mol. Cell. Cardiol. 2010, 48, 866–875. [Google Scholar] [CrossRef]
- Copeland, O.; Sadayappan, S.; Messer, A.E.; Steinen, G.J.; van der Velden, J.; Marston, S.B. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J. Mol. Cell. Cardiol. 2010, 49, 1003–1011. [Google Scholar] [CrossRef]
- El-Armouche, A.; Pohlmann, L.; Schlossarek, S.; Starbatty, J.; Yeh, Y.H.; Nattel, S.; Dobrev, D.; Eschenhagen, T.; Carrier, L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J. Mol. Cell. Cardiol. 2007, 43, 223–229. [Google Scholar] [CrossRef]
- Pedriali, G.; Ramaccini, D.; Bouhamida, E.; Wieckowski, M.R.; Giorgi, C.; Tremoli, E.; Pinton, P. Perspectives on mitochondrial relevance in cardiac ischemia/reperfusion injury. Front. Cell. Dev. Biol. 2022, 10, 1082095. [Google Scholar] [CrossRef]
- Oldenburg, O.; Qin, Q.; Krieg, T.; Yang, X.M.; Philipp, S.; Critz, S.D.; Cohen, M.V.; Downey, J.M. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H468–H476. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.D.; Garlid, K.D. Intramitochondrial signaling: Interactions among mitoKATP, PKCepsilon, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H874–H882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fisher, P.W.; Xi, L.; Kukreja, R.C. Essential role of mitochondrial Ca2+-activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Zakharov, S.I.; Bellinger, A.M.; Mongillo, M.; Marx, S.O. Protein kinase G phosphorylates Cav1.2 α1c and β2 subunits. Circ. Res. 2007, 101, 465–474. [Google Scholar] [CrossRef]
- Schröder, F.; Klein, G.; Fiedler, B.; Bastein, M.; Schnasse, N.; Hillmer, A.; Ames, S.; Gambaryan, S.; Drexler, H.; Walter, U. Single L-type Ca2+ channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice. Cardiovasc. Res. 2003, 60, 268–277. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Greenstein, J.L.; Winslow, R.L. Mechanisms of the cyclic nucleotide cross-talk signaling network in cardiac L-type calcium channel regulation. J. Mol. Cell. Cardiol. 2017, 106, 29–44. [Google Scholar] [CrossRef]
- Gonano, L.A.; Aitken-Buck, H.M.; Chakraborty, A.D.; Worthington, L.P.I.; Cully, T.R.; Lamberts, R.R.; Vila-Petroff, M.G.; Jones, P.P. Regulation of cardiac ryanodine receptor function by the cyclic-GMP dependent protein kinase G. Curr. Res. Physiol. 2022, 5, 171–178. [Google Scholar] [CrossRef]
- Ullrich, N.D.; Valdivia, H.H.; Niggli, E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J. Mol. Cell. Cardiol. 2012, 53, 33–42. [Google Scholar] [CrossRef][Green Version]
- Xiao, B.; Tian, X.; Xie, W.; Jones, P.P.; Cai, S.; Wang, X.; Jiang, D.; Kong, H.; Zhang, L.; Chen, K. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: Sensitization of store overload-induced Ca2+ release. J. Biol. Chem. 2007, 282, 30256–30264. [Google Scholar] [CrossRef]
- Cornwell, T.L.; Pryzwansky, K.B.; Wyatt, T.A.; Lincoln, T.M. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol. Pharmacol. 1991, 40, 923–931. [Google Scholar]
- Sabine, B.; Willenbrock, R.; Haase, H.; Karczewski, P.; Wallukat, G.; Dietz, R.; Krause, E.G. Cyclic GMP-mediated phospholamban phosphorylation in intact cardiomyocytes. Biochem. Biophys. Res. Commun. 1995, 214, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.A.; Simon, J.N.; Ma, R.; Loonat, A.A.; Crabtree, M.J.; Paterson, D.J.; Fahlman, R.P.; Casadei, B.; Fliegel, L.; Swietach, P. Nitric oxide modulates cardiomyocyte pH control through a biphasic effect on sodium/hydrogen exchanger-1. Cardiovasc. Res. 2020, 116, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Inserte, J.; Barba, I.; Poncelas-Nozal, M.; Hernando, V.; Agulló, L.; Ruiz-Meana, M.; Garcia-Dorado, D. cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J. Mol. Cell. Cardiol. 2011, 50, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Yeves, A.M.; Garciarena, C.D.; Nolly, M.B.; Chiappe de Cingolani, G.E.; Cingolani, H.E.; Ennis, I.L. Decreased activity of the Na+/H+ exchanger by phosphodiesterase 5A inhibition is attributed to an increase in protein phosphatase activity. Hypertension 2010, 56, 690–695. [Google Scholar] [CrossRef]
- Toyoda, Y.; Khan, S.; Chen, W.; Parker, R.A.; Levitsky, S.; McCully, J.D. Effects of NHE-1 inhibition on cardioprotection and impact on protection by K/Mg cardioplegia. Ann. Thorac. Surg. 2001, 72, 836–843. [Google Scholar] [CrossRef]
- Domes, K.; Patrucco, E.; Loga, F.; Dietrich, A.; Birnbaumer, L.; Wegener, J.W.; Hofmann, F. Murine cardiac growth, TRPC channels, and cGMP kinase I. Pflug. Arch. 2015, 467, 2229–2234. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Iribe, G.; Nishida, M.; Naruse, K. Role of TRPC3 and TRPC6 channels in the myocardial response to stretch: Linking physiology and pathophysiology. Prog. Biophys. Mol. Biol. 2017, 130, 264–272. [Google Scholar] [CrossRef]
- Fiedler, B.; Lohmann, S.M.; Smolenski, A.; Linnemuller, S.; Pieske, B.; Schroder, F.; Molkentin, J.D.; Drexler, H.; Wollert, K.C. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 11363–11368. [Google Scholar] [CrossRef]
- Lukowski, R.; Rybalkin, S.D.; Loga, F.; Leiss, V.; Beavo, J.A.; Hofmann, F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 5646–5651. [Google Scholar] [CrossRef]
- Fiedler, B.; Feil, R.; Hofmann, F.; Willenbockel, C.; Drexler, H.; Smolenski, A.; Lohmann, S.M.; Wollert, K.C. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J. Biol. Chem. 2006, 281, 32831–32840. [Google Scholar] [CrossRef]
- Ranek, M.J.; Kokkonen-Simon, K.M.; Chen, A.; Dunkerly-Eyring, B.L.; Vera, M.P.; Oeing, C.U.; Patel, C.H.; Nakamura, T.; Zhu, G.; Bedja, D. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 2019, 566, 264–269. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Yu, Y.; Schwartz, R.J. PKG-1α mediates GATA4 transcriptional activity. Cell Signal. 2016, 28, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kalra, D.; Baumgarten, G.; Dibbs, Z.; Seta, Y.; Sivasubramanian, N.; Mann, D.L. Nitric oxide provokes tumor necrosis factor-alpha expression in adult feline myocardium through a cGMP-dependent pathway. Circulation 2000, 102, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Weber, G.F. Phosphorylation of NF-kappaB proteins by cyclic GMP-dependent kinase. A noncanonical pathway to NF-kappaB activation. Eur. J. Biochem. 2003, 270, 2174–2185. [Google Scholar] [CrossRef]
- Dhingra, R.; Shaw, J.A.; Aviv, Y.; Kirshenbaum, L.A. Dichotomous actions of NF-kappaB signaling pathways in heart. J. Cardiovasc. Transl. Res. 2010, 3, 344–354. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Iacoviello, M.; Puzzovivo, A.; Scicchitano, P.; Monitillo, F.; De Crescenzo, F.; Caragnano, V.; Sassara, M.; Quistelli, G.; Guida, P. Clinical correlates of endothelial function in chronic heart failure. Clin. Res. Cardiol. 2011, 100, 515–521. [Google Scholar] [CrossRef]
- Areas, G.P.T.; Mazzuco, A.; Caruso, F.R.; Jaenisch, R.B.; Cabiddu, R.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Flow-mediated dilation and heart failure: A review with implications to physical rehabilitation. Heart Fail. Rev. 2019, 24, 69–80. [Google Scholar] [CrossRef]
- Kemp-Harper, B.; Schmidt, H.H. cGMP in the vasculature. Handb. Exp. Pharmacol. 2009, 191, 447–467. [Google Scholar]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Suzuki, E.; Nishimatsu, H.; Satonaka, H.; Walsh, K.; Goto, A.; Omata, M.; Fujita, T.; Nagai, R.; Hirata, Y. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T cell-dependent transcriptional activation in vascular myocytes. Circ. Res. 2002, 90, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.S.; Prakash, Y.S.; Johnson, D.E.; Sieck, G.C. Nitric oxide inhibits calcium release from sarcoplasmic reticulum of porcine tracheal smooth muscle cells. Am. J. Physiol. 1997, 272, L1–L7. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, M.; Martinotti, G.; Azzena, G.B.; Grassi, C. cGMP/protein kinase G-dependent inhibition of N-type Ca2+ channels induced by nitric oxide in human neuroblastoma IMR32 cells. J. Neurosci. 2002, 22, 7485–7492. [Google Scholar] [CrossRef]
- Satoh, S.; Makino, N. Intracellular mechanisms of cGMP-mediated regulation of myocardial contraction. Basic Res. Cardiol. 2001, 96, 652–658. [Google Scholar] [CrossRef]
- Li, S.J.; Sun, N.L. Regulation of intracellular Ca2+ and calcineurin by NO/PKG in proliferation of vascular smooth muscle cells. Acta Pharmacol. Sin. 2005, 26, 323–328. [Google Scholar] [CrossRef]
- Sun, X.; Kaltenbronn, K.M.; Steinberg, T.H.; Blumer, K.J. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol. Pharmacol. 2005, 67, 631–639. [Google Scholar] [CrossRef]
- Tang, K.M.; Wang, G.R.; Lu, P.; Karas, R.H.; Aronovitz, M.; Heximer, S.P.; Kaltenbronn, K.M.; Blumer, K.J.; Siderovski, D.P.; Zhu, Y. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat. Med. 2003, 9, 1506–1512. [Google Scholar] [CrossRef]
- Surks, H.K.; Mochizuki, N.; Kasai, Y.; Georgescu, S.P.; Tang, K.M.; Ito, M.; Lincoln, T.M.; Mendelsohn, M.E. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 1999, 286, 1583–1587. [Google Scholar] [CrossRef]
- Wooldridge, A.A.; MacDonald, J.A.; Erdodi, F.; Ma, C.; Borman, M.A.; Hartshorne, D.J.; Haystead, T.A. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J. Biol. Chem. 2004, 279, 34496–34504. [Google Scholar] [CrossRef]
- Schmid, J.; Müller, B.; Heppeler, D.; Gaynullina, D.; Kassmann, M.; Gagov, H.; Mladenov, M.; Gollasch, M.; Schubert, R. The Unexpected Role of Calcium-Activated Potassium Channels: Limitation of NO-Induced Arterial Relaxation. J. Am. Heart Assoc. 2018, 7, e007808. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar] [PubMed]
- Fukao, M.; Mason, H.S.; Britton, F.C.; Kenyon, J.L.; Horowitz, B.; Keef, K.D. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J. Biol. Chem. 1999, 274, 10927–10935. [Google Scholar] [CrossRef] [PubMed]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef]
- Mistry, D.K.; Garland, C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998, 124, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Ataei Ataabadi, E.; Golshiri, K.; Jüttner, A.A.; de Vries, R.; Van den Berg-Garrelds, I.; Nagtzaam, N.M.A.; Khan, H.N.; Leijten, F.P.J.; Brandt, R.M.C.; Dik, W.A. Soluble guanylate cyclase activator BAY 54-6544 improves vasomotor function and survival in an accelerated ageing mouse model. Aging Cell. 2022, 21, e13683. [Google Scholar] [CrossRef]
- Bykov, V.V.; Bykova, A.V.; Dzyuman, A.N.; Ivanov, V.V.; Khazanov, V.A.; Vengerovskii, A.I.; Udut, V.V. Anti-Atherosclerotic Action of a New Stimulator of Soluble Guanylate Cyclase in an Experiment. Bull. Exp. Biol. Med. 2023, 174, 333–336. [Google Scholar] [CrossRef]
- da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Lima Silva, E.M.; Gouvêa, F.F.F.; de França Lopes, L.G.; Araújo, A.V.; Ferraz Pereira, K.N.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef]
- Martinelli, A.M.; Rodrigues, C.N.D.S.; Moraes, T.F.; Rodrigues, G.J. In Endothelial Cells, the Activation or Stimulation of Soluble Guanylyl Cyclase Induces the Nitric Oxide Production by a Mechanism Dependent of Nitric Oxide Synthase Activation. J. Pharm. Pharm. Sci. 2018, 21, 38–45. [Google Scholar] [CrossRef]
- Dhahri, W.; Dussault, S.; Raguema, N.; Desjarlais, M.; Rivard, A. Stimulation of soluble guanylate cyclase activity with riociguat promotes angiogenesis and improves neovascularization after limb ischemia. Atherosclerosis 2023, 372, 32–40. [Google Scholar] [CrossRef]
- Priviero, F.B.; Webb, R.C. Heme-dependent and independent soluble guanylate cyclase activators and vasodilation. J. Cardiovasc. Pharmacol. 2010, 56, 229–233. [Google Scholar] [CrossRef]
- Sovershaev, M.A.; Egorina, E.M.; Hansen, J.B.; Østerud, B.; Pacher, P.; Stasch, J.P.; Evgenov, O.V. Soluble guanylate cyclase agonists inhibit expression and procoagulant activity of tissue factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.H.; Kimlinger, M.J.; Billings, F.T., IV; Lopez, M.G. Targeting Soluble Guanylyl Cyclase during Ischemia and Reperfusion. Cells 2023, 12, 1903. [Google Scholar] [CrossRef] [PubMed]
- Nickel, K.F.; Laux, V.; Heumann, R.; von Degenfeld, G. Thrombin has biphasic effects on the nitric oxide-cGMP pathway in endothelial cells and contributes to experimental pulmonary hypertension. PLoS ONE 2013, 8, e63504. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Mergia, E.; Kramer, C.M.; Lückstädt, W.; Yang, J.; Wolff, G.; Panknin, C.; Bracht, T.; Sitek, B.; Pernow, J. Identification of a soluble guanylate cyclase in RBCs: Preserved activity in patients with coronary artery disease. Redox Biol. 2018, 14, 328–337. [Google Scholar] [CrossRef]
- Jiao, T.; Collado, A.; Mahdi, A.; Tengbom, J.; Tratsiakovich, Y.; Milne, G.T.; Alvarsson, M.; Lundberg, J.O.; Zhou, Z.; Yang, J. Stimulation of Erythrocyte Soluble Guanylyl Cyclase Induces cGMP Export and Cardioprotection in Type 2 Diabetes. JACC Basic Transl. Sci. 2023, 8, 907–918. [Google Scholar] [CrossRef]
- Yang, J.; Sundqvist, M.L.; Zheng, X.; Jiao, T.; Collado, A.; Tratsiakovich, Y.; Mahdi, A.; Tengbom, J.; Mergia, E.; Catrina, S.B.; et al. Hypoxic erythrocytes mediate cardioprotection through activation of soluble guanylate cyclase and release of cyclic GMP. J. Clin. Investig. 2023, 133, e167693. [Google Scholar] [CrossRef]
- Vuorinen, P.; Laustiola, K.E. Exogenous GTP increases cyclic GMP and inhibits thrombin-induced aggregation of washed human platelets: Comparison with ATP, adenosine and guanosine. Pharmacol. Toxicol. 1992, 71, 289–293. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Walter, U.; Friebe, A.; Gambaryan, S. Differentiation of cGMP-dependent and -independent nitric oxide effects on platelet apoptosis and reactive oxygen species production using platelets lacking soluble guanylyl cyclase. Thromb. Haemost. 2011, 106, 922–933. [Google Scholar] [CrossRef]
- Mauersberger, C.; Sager, H.B.; Wobst, J.; Dang, T.A.; Lambrecht, L.; Koplev, S.; Stroth, M.; Bettaga, N.; Schlossmann, J.; Wunder, F. Loss of soluble guanylyl cyclase in platelets contributes to atherosclerotic plaque formation and vascular inflammation. Nat. Cardiovasc. Res. 2022, 1, 1174–1186. [Google Scholar] [CrossRef]
- Grande, D.; Gioia, M.I.; Terlizzese, P.; Iacoviello, M. Heart Failure and Kidney Disease. Adv. Exp. Med. Biol. 2018, 1067, 219–238. [Google Scholar]
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.M.; Kraehling, J.R.; Eitner, F.; Bénardeau, A.; Sandner, P. The Impact of the Nitric Oxide (NO)/Soluble Guanylyl Cyclase (sGC) Signaling Cascade on Kidney Health and Disease: A Preclinical Perspective. Int. J. Mol. Sci. 2018, 19, 1712. [Google Scholar] [CrossRef] [PubMed]
- Theilig, F.; Bostanjoglo, M.; Pavenstädt, H.; Grupp, C.; Holland, G.; Slosarek, I.; Gressner, A.M.; Russwurm, M.; Koesling, D.; Bachmann, S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J. Am. Soc. Nephrol. 2001, 12, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Stehle, D.; Xu, M.Z.; Schomber, T.; Hahn, M.G.; Schweda, F.; Feil, S.; Kraehling, J.R.; Eitner, F.; Patzak, A.; Sandner, P. Novel soluble guanylyl cyclase activators increase glomerular cGMP, induce vasodilation and improve blood flow in the murine kidney. Br. J. Pharmacol. 2022, 179, 2476–2489. [Google Scholar] [CrossRef]
- Wennysia, I.C.; Zhao, L.; Schomber, T.; Braun, D.; Golz, S.; Summer, H.; Benardeau, A.; Lai, E.Y.; Lichtenberger, F.B.; Schubert, R. Role of soluble guanylyl cyclase in renal afferent and efferent arterioles. Am. J. Physiol. Ren. Physiol. 2021, 320, F193–F202. [Google Scholar] [CrossRef]
- Dautzenberg, M.; Kahnert, A.; Stasch, J.P.; Just, A. Role of soluble guanylate cyclase in renal hemodynamics and autoregulation in the rat. Am. J. Physiol. Ren. Physiol. 2014, 307, F1003–F1012. [Google Scholar] [CrossRef][Green Version]
- ’t Hart, D.; Li, J.; van der Vlag, J.; Nijenhuis, T. Repurposing Riociguat to Target a Novel Paracrine Nitric Oxide-TRPC6 Pathway to Prevent Podocyte Injury. Int. J. Mol. Sci. 2021, 22, 12485. [Google Scholar] [CrossRef]
- Atteia, H.H.; Alamri, E.S.; Sirag, N.; Zidan, N.S.; Aljohani, R.H.; Alzahrani, S.; Arafa, M.H.; Mohammad, N.S.; Asker, M.E.; Zaitone, S.A. Soluble guanylate cyclase agonist, isoliquiritigenin attenuates renal damage and aortic calcification in a rat model of chronic kidney failure. Life Sci. 2023, 317, 121460. [Google Scholar] [CrossRef]
- Braun, D.; Zollbrecht, C.; Dietze, S.; Schubert, R.; Golz, S.; Summer, H.; Persson, P.B.; Carlström, M.; Ludwig, M.; Patzak, A. Hypoxia/Reoxygenation of Rat Renal Arteries Impairs Vasorelaxation via Modulation of Endothelium-Independent sGC/cGMP/PKG Signaling. Front. Physiol. 2018, 9, 480. [Google Scholar] [CrossRef]
- Harloff, M.; Prüschenk, S.; Seifert, R.; Schlossmann, J. Activation of soluble guanylyl cyclase signalling with cinaciguat improves impaired kidney function in diabetic mice. Br. J. Pharmacol. 2022, 179, 2460–2475. [Google Scholar] [CrossRef]
- Zeisberg, M.; Tampe, B.; LeBleu, V.; Tampe, D.; Zeisberg, E.M.; Kalluri, R. Thrombospondin-1 deficiency causes a shift from fibroproliferative to inflammatory kidney disease and delays onset of renal failure. Am. J. Pathol. 2014, 184, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Kalk, P.; Godes, M.; Relle, K.; Rothkegel, C.; Hucke, A.; Stasch, J.P.; Hocher, B. NO-independent activation of soluble guanylate cyclase prevents disease progression in rats with 5/6 nephrectomy. Br. J. Pharmacol. 2006, 148, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Schinner, E.; Wetzl, V.; Schramm, A.; Kees, F.; Sandner, P.; Stasch, J.P.; Hofmann, F.; Schlossmann, J. Inhibition of the TGFβ signalling pathway by cGMP and cGMP-dependent kinase I in renal fibrosis. FEBS Open Bio 2017, 7, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.M.; Price, G.M.; Liu, G.; Sarno, R.; Buys, E.S.; Currie, M.G.; Masferrer, J.L. Soluble guanylate cyclase stimulator praliciguat attenuates inflammation, fibrosis, and end-organ damage in the Dahl model of cardiorenal failure. Am. J. Physiol. Ren. Physiol. 2020, 318, F148–F159. [Google Scholar] [CrossRef]
- Zhang, C.; Mayeux, P.R. NO/cGMP signaling modulates regulation of Na+-K+-ATPase activity by angiotensin II in rat proximal tubules. Am. J. Physiol. Ren. Physiol. 2001, 280, F474–F479. [Google Scholar] [CrossRef]
- Shirai, A.; Yamazaki, O.; Horita, S.; Nakamura, M.; Satoh, N.; Yamada, H.; Suzuki, M.; Kudo, A.; Kawakami, H.; Hofmann, F. Angiotensin II dose-dependently stimulates human renal proximal tubule transport by the nitric oxide/guanosine 3′,5′-cyclic monophosphate pathway. J. Am. Soc. Nephrol. 2014, 25, 1523–1532. [Google Scholar] [CrossRef]
- Nakamura, K.; Hirano, J.; Kubokawa, M. Regulation of an inwardly rectifying K+ channel by nitric oxide in cultured human proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2004, 287, F411–F417. [Google Scholar] [CrossRef]
- Lin, D.H.; Duan, X.P.; Zheng, J.Y.; Wang, W.H. Role of inwardly rectifying K+ channel 5.1 (Kir5.1) in the regulation of renal membrane transport. Curr. Opin. Nephrol. Hypertens. 2022, 31, 479–485. [Google Scholar] [CrossRef]
- da Silva, L.L.; Cavalcante, F.; Axelband, F.; De Souza, A.M.; Lopes, A.G.; Caruso-Neves, C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7). Biochem. J. 2006, 395, 183–190. [Google Scholar]
- Yang, J.; Chen, C.; Ren, H.; Han, Y.; He, D.; Zhou, L.; Hopfer, U.; Jose, P.A.; Zeng, C. Angiotensin II AT2 receptor decreases AT1 receptor expression and function via nitric oxide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J. Hypertens. 2012, 30, 1176–1184. [Google Scholar] [CrossRef]
- Newaz, M.A.; Ranganna, K.; Oyekan, A.O. Relationship between PPARalpha activation and NO on proximal tubular Na+ transport in the rat. BMC Pharmacol. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shea, C.M.; Jones, J.E.; Price, G.M.; Warren, W.; Lonie, E.; Yan, S.; Currie, M.G.; Profy, A.T.; Masferrer, J.L. Praliciguat inhibits progression of diabetic nephropathy in ZSF1 rats and suppresses inflammation and apoptosis in human renal proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2020, 319, F697–F711. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.H.; Stoos, B.A.; Carretero, O.A.; Garvin, J.L. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension 1996, 27, 679–683. [Google Scholar] [CrossRef]
- Wagner, K.; Unger, L.; Salman, M.M.; Kitchen, P.; Bill, R.M.; Yool, A.J. Signaling Mechanisms and Pharmacological Modulators Governing Diverse Aquaporin Functions in Human Health and Disease. Int. J. Mol. Sci. 2022, 23, 1388. [Google Scholar] [CrossRef]
- Noda, Y.; Sasaki, S. Updates and Perspectives on Aquaporin-2 and Water Balance Disorders. Int. J. Mol. Sci. 2021, 22, 12950. [Google Scholar] [CrossRef]
- Klokkers, J.; Langehanenberg, P.; Kemper, B.; Kosmeier, S.; Bally, G.V.; Riethmüller, C.; Wunder, F.; Sindic, A.; Pavenstädt, H.; Schlatter, E. Atrial natriuretic peptide and nitric oxide signaling antagonizes vasopressin-mediated water permeability in inner medullary collecting duct cells. Am. J. Physiol. Ren. Physiol. 2009, 297, F693–F703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Feo, D.; Massari, F.; Campanella, C.; Livrieri, A.; Ciccone, M.M.; Caldarola, P.; De Palo, M.; Scicchitano, P. Influence of Soluble Guanylate Cyclase on Cardiac, Vascular, and Renal Structure and Function: A Physiopathological Insight. Int. J. Mol. Sci. 2025, 26, 4550. https://doi.org/10.3390/ijms26104550
De Feo D, Massari F, Campanella C, Livrieri A, Ciccone MM, Caldarola P, De Palo M, Scicchitano P. Influence of Soluble Guanylate Cyclase on Cardiac, Vascular, and Renal Structure and Function: A Physiopathological Insight. International Journal of Molecular Sciences. 2025; 26(10):4550. https://doi.org/10.3390/ijms26104550
Chicago/Turabian StyleDe Feo, Daniele, Francesco Massari, Cosimo Campanella, Anna Livrieri, Marco Matteo Ciccone, Pasquale Caldarola, Micaela De Palo, and Pietro Scicchitano. 2025. "Influence of Soluble Guanylate Cyclase on Cardiac, Vascular, and Renal Structure and Function: A Physiopathological Insight" International Journal of Molecular Sciences 26, no. 10: 4550. https://doi.org/10.3390/ijms26104550
APA StyleDe Feo, D., Massari, F., Campanella, C., Livrieri, A., Ciccone, M. M., Caldarola, P., De Palo, M., & Scicchitano, P. (2025). Influence of Soluble Guanylate Cyclase on Cardiac, Vascular, and Renal Structure and Function: A Physiopathological Insight. International Journal of Molecular Sciences, 26(10), 4550. https://doi.org/10.3390/ijms26104550







