Yeast Culture Supplementation Improves Meat Quality by Enhancing Immune Response and Purine Metabolism of Small-Tail Han Sheep (Ovis aries)
Abstract
1. Introduction
2. Results
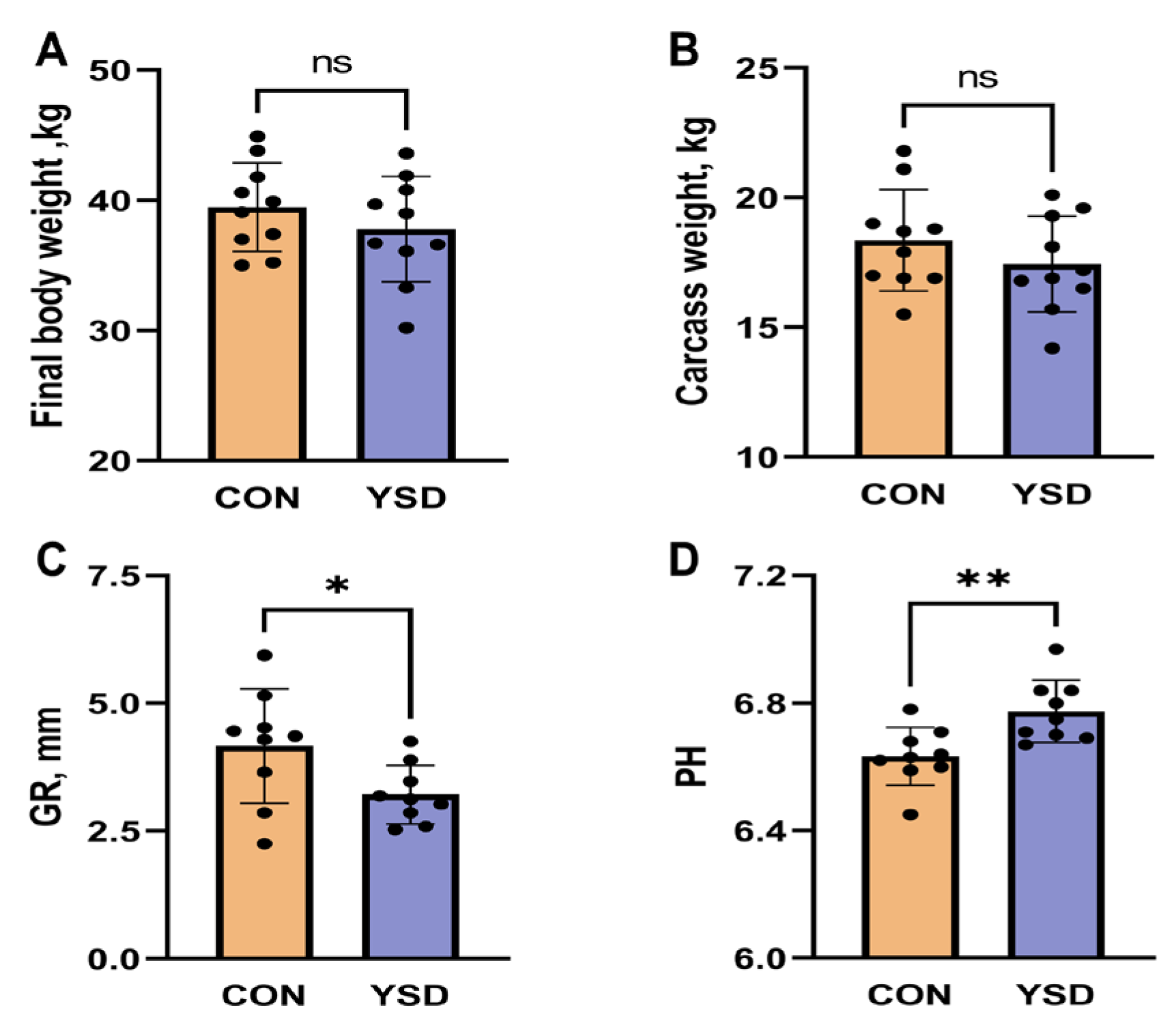
2.1. Slaughter Performance
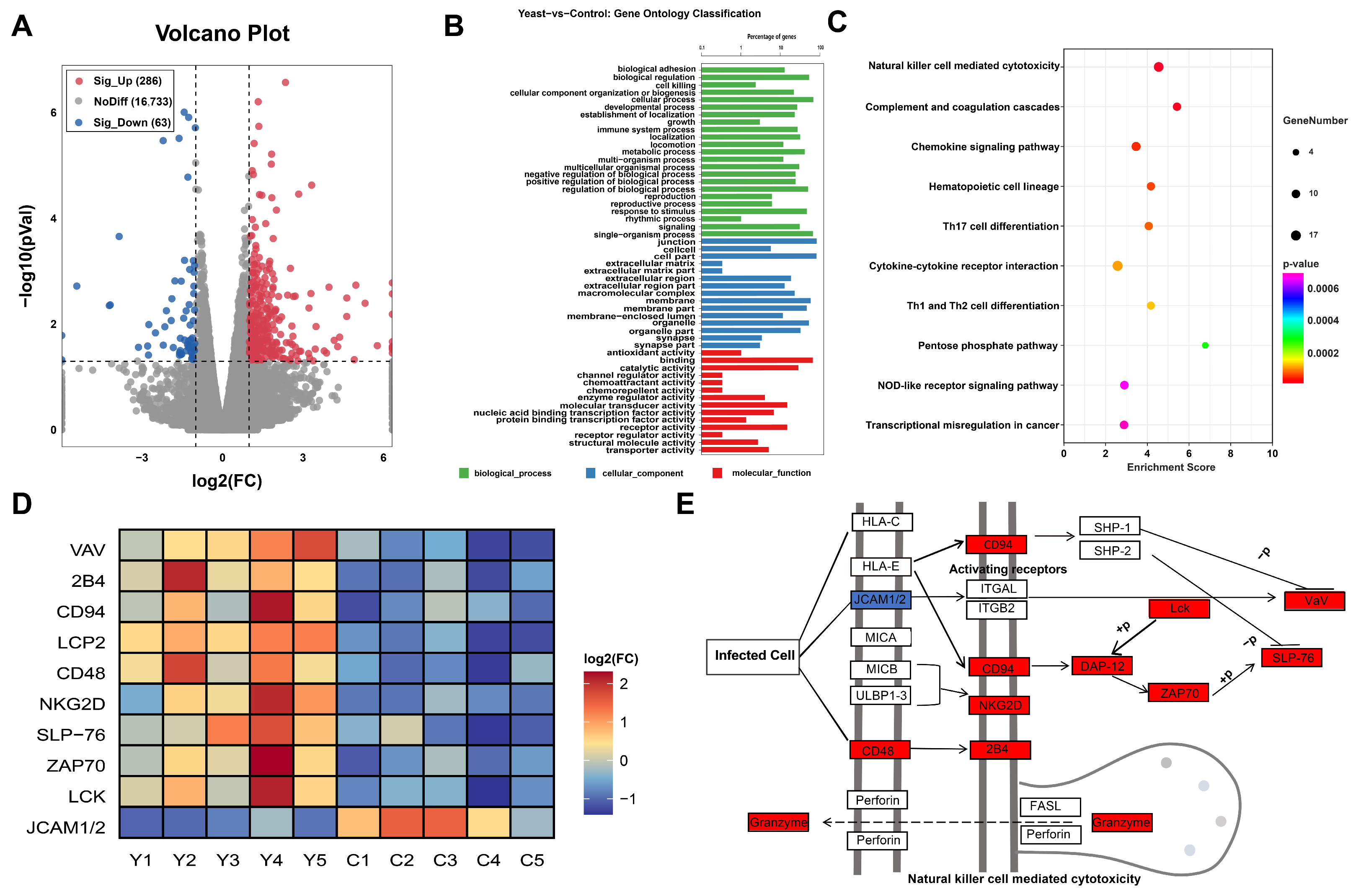
2.2. Transcriptomic Analysis in the LOD Muscle
2.3. Metabolomic Analysis in the LOD Muscle
2.4. Combined Transcriptomic and Metabolomic Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Sample Collection
4.2. Carcass Characteristics and Meat Quality Analysis
4.3. Total RNA Extraction and Transcriptomic Analysis
4.3.1. mRNA Enrichment and Sequencing
4.3.2. Quality Control and Sequence Mapping
4.3.3. Gene Expression Quantification and Differential Expression Analysis
4.4. Metabolite Extraction and Metabolomic Analysis
4.4.1. Metabolite Extraction
4.4.2. UHPLC-QE-MS Analysis and Data Preprocessing
4.4.3. Metabolomics Analysis
4.5. Comprehensive Analysis of Transcriptome and Metabolome Data
4.6. Statistical Analysis
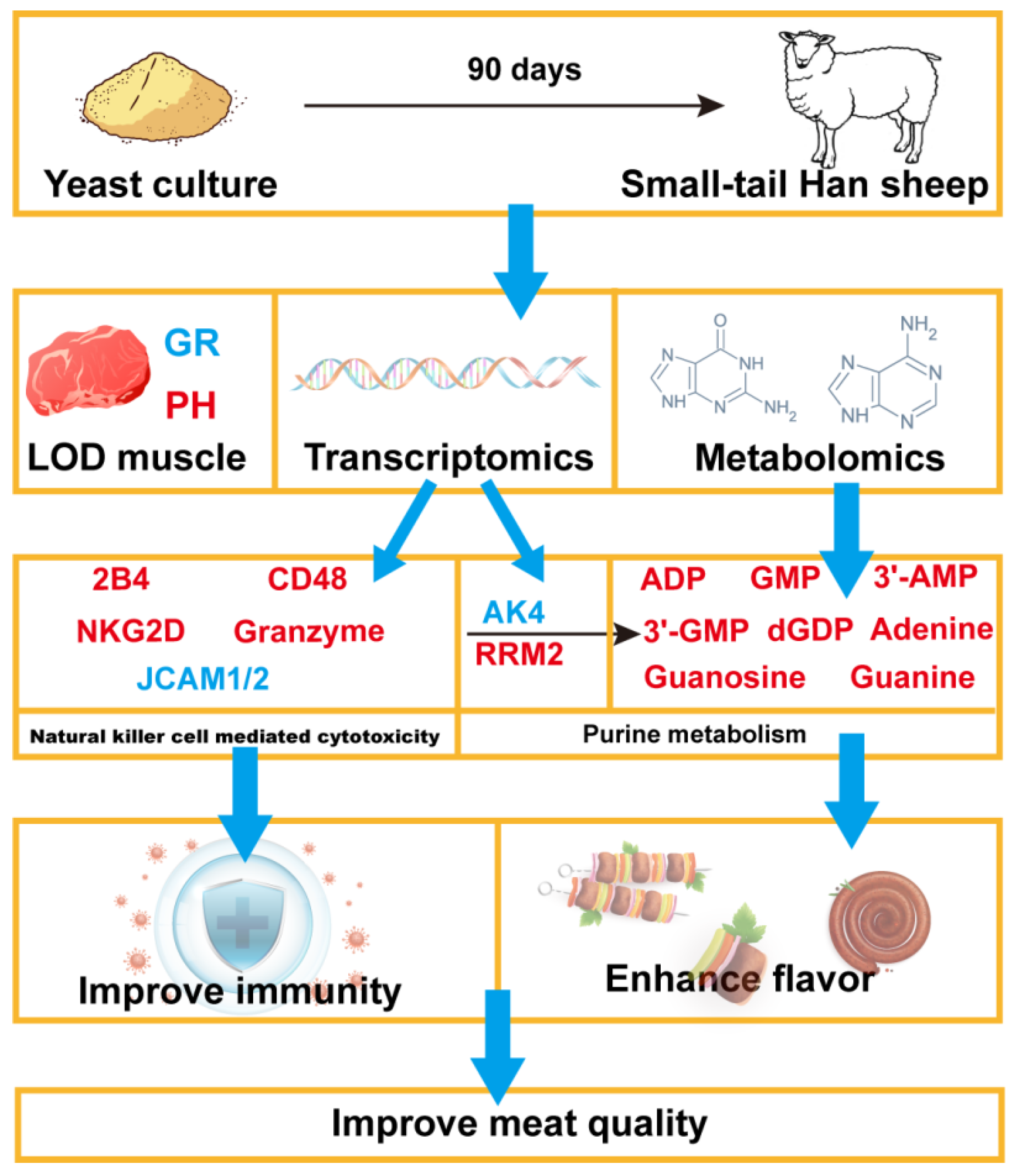
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CON | The Control group was fed a basic diet |
| YSD | The treatment group fed a basic diet supplemented with 1% yeast culture |
| LOD | Longissimus dorsi muscle |
| DEGs | Differentially expressed genes |
| DMs | Differential metabolites |
| GR | Girth rib value |
| PSE | Pale, Soft, and Exudative meat condition |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IMP | Inosine Monophosphate |
| AK4 | Adenylate Kinase 4 |
| RRM2 | Ribonucleotide Reductase M2 |
References
- Revell, B. Meat and Milk Consumption 2050: The Potential for Demand-Side Solutions to Greenhouse Gas Emissions Reduction. EuroChoices 2015, 14, 4–11. [Google Scholar] [CrossRef]
- Ding, W.; Lu, Y.; Xu, B.; Chen, P.; Li, A.; Jian, F.; Yu, G.; Huang, S. Meat of Sheep: Insights into Mutton Evaluation, Nutritive Value, Influential Factors, and Interventions. Agriculture 2024, 14, 1060. [Google Scholar] [CrossRef]
- Wang, X.; Shen, W.; Wu, P.; Wang, C.; Li, J.; Wang, D.; Yue, W. How Food Consumption Trends Change the Direction of Sheep Breeding in China. Animals 2024, 14, 3047. [Google Scholar] [CrossRef]
- Wang, L.; Qi, W.; Mao, S.; Zhu, W.; Liu, J. Effects of Whole Corn High-Grain Diet Feeding on Ruminal Bacterial Community and Epithelial Gene Expression Related to Vfa Absorption and Metabolism in Fattening Lambs. J. Anim. Sci. 2022, 100, skac056. [Google Scholar] [CrossRef]
- Kasapidou, E.; Basdagianni, Z.; Papadopoulos, V.; Karaiskou, C.; Kesidis, A.; Tsiotsias, A. Effects of Intensive and Semi-Intensive Production on Sheep Milk Chemical Composition, Physicochemical Characteristics, Fatty Acid Profile, and Nutritional Indices. Animals 2021, 11, 2578. [Google Scholar] [CrossRef]
- Wu, G. Management of Metabolic Disorders (Including Metabolic Diseases) in Ruminant and Nonruminant Animals. In Animal Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 471–491. [Google Scholar] [CrossRef]
- Cuervo, W.; Gomez, C.; Tarnonsky, F.; Marenchino, I.F.; Podversich, F.; Maderal, A.; Schulmeister, T.M.; de J Vargas, J.; DiLorenzo, N. Effects of Cashew Nutshell Extract Inclusion into a High-Grain Finishing Diet on Methane Emissions, Nutrient Digestibility, and Ruminal Fermentation in Beef Steers. J. Anim. Sci. 2025, 103, skae359. [Google Scholar] [CrossRef]
- Chang, G.; Zhang, K.; Xu, T.; Jin, D.; Seyfert, H.M.; Shen, X.; Zhuang, S. Feeding a High-Grain Diet Reduces the Percentage of Lps Clearance and Enhances Immune Gene Expression in Goat Liver. BMC Vet. Res. 2015, 11, 67. [Google Scholar] [CrossRef]
- Wang, H.; Su, M.; Wang, C.; Li, D.; Li, Q.; Liu, Z.; Qi, X.; Wu, Y.; Zhao, Y.; Li, T.; et al. Yeast Culture Repairs Rumen Epithelial Injury by Regulating Microbial Communities and Metabolites in Sheep. Front. Microbiol. 2023, 14, 1305772. [Google Scholar] [CrossRef]
- de Melo, L.; de Oliveira, P.E.P.; Plodoviski, D.C.; da Costa, L.; da Rosa, C.B.; Pereira, E.L.C.; de Souza, A.M.; Neumann, M. Supplementation of Yeast Culture Combined with an Enzyme Complex in the Diet for Confined Steers. Ciênc. Anim. Bras. 2023, 24, e-74470. [Google Scholar] [CrossRef]
- Shurson, G. Yeast and Yeast Derivatives in Feed Additives and Ingredients: Sources, Characteristics, Animal Responses, and Quantification Methods. Anim. Feed Sci. Technol. 2018, 235, 60–76. [Google Scholar] [CrossRef]
- Hansen, H.H.; El-Bordeny, N.E.; Ebeid, H.M. Response of Primiparous and Multiparous Buffaloes to Yeast Culture Supplementation During Early and Mid-Lactation. Anim. Nutr. 2017, 3, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wu, T.; You, P.; Wang, H.; Burke, J.L.; Kang, K.; Yu, W.; Wang, M.; Li, B.; He, Y.; et al. Dietary Supplementation of Yeast Culture into Pelleted Total Mixed Rations Improves the Growth Performance of Fattening Lambs. Front. Vet. Sci. 2021, 8, 657816. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, X.; Ma, Q.; Wang, Z.; Fang, F.; Zhang, D. Consumer Preference, Behaviour and Perception About Lamb Meat in China. Meat Sci. 2022, 192, 108878. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The Potential Role of Secondary Metabolites in Modulating the Flavor and Taste of the Meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef]
- Watkins, P.J.; Frank, D.; Singh, T.K.; Young, O.A.; Warner, R.D. Sheepmeat Flavor and the Effect of Different Feeding Systems: A Review. J. Agric. Food Chem. 2013, 61, 3561–3579. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Li, L.; Chen, W.; Zhao, Q.; Su, G.; Zhao, M. Application of Electronic Tongue in Umami Detection and Soy Sauce Refining Process. Food Chem. X 2023, 18, 100652. [Google Scholar] [CrossRef]
- Reis, L.F.; Sousa, R.S.; Oliveira, F.L.C.; Rodrigues, F.; Araújo, C.; Meira-Júnior, E.B.S.; Barrêto-Júnior, R.A.; Mori, C.S.; Minervino, A.H.H.; Ortolani, E.L. Comparative Assessment of Probiotics and Monensin in the Prophylaxis of Acute Ruminal Lactic Acidosis in Sheep. BMC Vet. Res. 2018, 14, 9. [Google Scholar] [CrossRef]
- Majewska, M.P.; Miltko, R.; Bełżecki, G.; Kowalik, B. Population of Protozoa and Carbohydrate-Digesting Enzymes in the Rumen of Sheep Fed a Diet Supplemented with Yeast Saccharomyces Cerevisiae. Small Rumin. Res. 2021, 205, 106544. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Li, S.; Li, D.; Li, X.; Xu, Z.; Liu, D. Effects of Yeast Culture on Growth Performance, Immune Function, Antioxidant Capacity and Hormonal Profile in Mongolian Ram Lambs. Front. Vet. Sci. 2024, 11, 1424073. [Google Scholar] [CrossRef]
- Qi, P.; Wang, L. Effect of Adding Yeast Cultures to High-Grain Conditions on Production Performance, Rumen Fermentation Profile, Microbial Abundance, and Immunity in Goats. Animals 2024, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Geng, Y.; Ling, Y.; Wang, D.; Hu, G. Yeast Culture Is Beneficial for Improving the Rumen Fermentation and Promoting the Growth Performance of Goats in Summer. Fermentation 2024, 10, 307. [Google Scholar] [CrossRef]
- Fu, R.; Liang, C.; Chen, D.; Yan, H.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of Dietary Bacillus Coagulans and Yeast Hydrolysate Supplementation on Growth Performance, Immune Response and Intestinal Barrier Function in Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, G.; Zhuang, Y.; Chai, J.; Zhang, N. Yeast (Saccharomyces Cerevisiae) Culture Promotes the Performance of Fattening Sheep by Enhancing Nutrients Digestibility and Rumen Development. Fermentation 2022, 8, 719. [Google Scholar] [CrossRef]
- Pascual, A.; Pauletto, M.; Giantin, M.; Radaelli, G.; Ballarin, C.; Birolo, M.; Zomeño, C.; Dacasto, M.; Bortoletti, M.; Vascellari, M.; et al. Effect of Dietary Supplementation with Yeast Cell Wall Extracts on Performance and Gut Response in Broiler Chickens. J. Anim. Sci. Biotechnol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Jia, L.; Wu, J.; Lei, Y.; Kong, F.; Zhang, R.; Sun, J.; Wang, L.; Li, Z.; Shi, J.; Wang, Y. Oregano Essential Oils Mediated Intestinal Microbiota and Metabolites and Improved Growth Performance and Intestinal Barrier Function in Sheep. Front. Immunol. 2022, 13, 908015. [Google Scholar] [CrossRef]
- De Smet, S.; Raes, K.; Demeyer, D. Meat Fatty Acid Composition as Affected by Fatness and Genetic Factors: A Review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- Kocak, O.; Ekiz, B.; Yalcintan, H.; Yakan, A.; Yilmaz, A. Carcass and Meat Quality of Organic Lambs Compared with Lambs Reared under Traditional and Intensive Production Systems. Anim. Prod. Sci. 2016, 56, 38–47. [Google Scholar] [CrossRef]
- Karunanayaka, D.S.; Jayasena, D.D.; Jo, C. Prevalence of Pale, Soft, and Exudative (PSE) Condition in Chicken Meat Used for Commercial Meat Processing and Its Effect on Roasted Chicken Breast. J. Anim. Sci. Technol. 2016, 58, 27. [Google Scholar] [CrossRef]
- Tian, K.; Jing, D.; Lan, J.; Lv, M.; Wang, T. Commensal Microbiome and Gastrointestinal Mucosal Immunity: Harmony and Conflict with Our Closest Neighbor. Immun. Inflamm. Dis. 2024, 12, e1316. [Google Scholar] [CrossRef]
- Claus, M.; Wingert, S.; Watzl, C. Modulation of Natural Killer Cell Functions by Interactions between 2b4 and Cd48 in Cis and in Trans. Open Biol. 2016, 6, 160010. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Nkg2d Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Gunturi, A.; Berg, R.E.; Forman, J. The Role of Cd94/Nkg2 in Innate and Adaptive Immunity. Immunol. Res. 2004, 30, 29–34. [Google Scholar] [CrossRef]
- Prager, I.; Liesche, C.; Van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. Nk Cells Switch from Granzyme B to Death Receptor–Mediated Cytotoxicity During Serial Killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, Y.; Ji, J.; Xiao, S.; Ma, J.; Huang, L. Effects of Breeds, Tissues and Genders on Purine Contents in Pork and the Relationships between Purine Content and Other Meat QualityTraits. Meat Sci. 2018, 143, 81–86. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, M.; Huang, Y.; Liu, X.; Zhong, L.; Ji, J.; Zhou, L.; Zeng, Q.; Ma, J.; Huang, L. The Effect of Purine Content on Sensory Quality of Pork. Meat Sci. 2021, 172, 108346. [Google Scholar] [CrossRef]
- Zhao, W.; Cai, Z.; Zhang, J.; Zhang, X.; Yu, B.; Fu, X.; Zhang, T.; Hu, J.; Shao, Y.; Gu, Y. Pkm2 Promotes Myoblast Growth and Inosine Monophosphate-Specific Deposition in Jingyuan Chicken. Res. Vet. Sci. 2024, 173, 105275. [Google Scholar] [CrossRef]
- Jiang, Q.; Lin, L.; Xie, F.; Jin, W.; Zhu, W.; Wang, M.; Qiu, Q.; Li, Z.; Liu, J.; Mao, S. Metagenomic Insights into the Microbe-Mediated B and K(2) Vitamin Biosynthesis in the Gastrointestinal Microbiome of Ruminants. Microbiome 2022, 10, 109. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhou, Z.; Chang, Y.; Liu, Y.; Shen, Y.; Li, Q.; Zhang, L. Ribonucleotide Reductase M2 (Rrm2): Regulation, Function and Targeting Strategy in Human Cancer. Genes Dis. 2024, 11, 218–233. [Google Scholar] [CrossRef]
- Fujisawa, K.; Terai, S.; Takami, T.; Yamamoto, N.; Yamasaki, T.; Matsumoto, T.; Yamaguchi, K.; Owada, Y.; Nishina, H.; Noma, T.; et al. Modulation of Anti-Cancer Drug Sensitivity through the Regulation of Mitochondrial Activity by Adenylate Kinase 4. J. Exp. Clin. Cancer Res. 2016, 35, 48. [Google Scholar] [CrossRef]
- Zhong, Y.; Jia, B.; Xie, C.; Hu, L.; Liao, Z.; Liu, W.; Zhang, Y.; Huang, G. Adenylate Kinase 4 Promotes Neuronal Energy Metabolism and Mitophagy in Early Cerebral Ischemia Via Parkin/Pkm2 Pathway. Exp. Neurol. 2024, 377, 114798. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Zhang, N.; Ungerfeld, E.; Guo, L.; Zhang, X.; Wang, M.; Ma, Z. Effects of Supplementing a Yeast Culture in a Pelleted Total Mixed Ration on Fiber Degradation, Fermentation Parameters, and the Bacterial Community in the Rumen of Sheep. Anim. Feed. Sci. Technol. 2023, 296, 115565. [Google Scholar] [CrossRef]


| Ingredients, % | Content |
|---|---|
| Corn | 56.00 |
| Corn stalk | 20.00 |
| Soybean meal | 5.60 |
| Distiller’s Dried Grain with Solubles (corn) | 5.20 |
| Cottonseed meal | 8.00 |
| NaHCO3 | 0.96 |
| Salt | 0.24 |
| Premix 1 | 4.00 |
| Total | 100 |
| Nutrient content, % | |
| Metabolizable energy 2, MJ/kg | 9.08 |
| Crude fat | 1.24 |
| Crude protein | 14.29 |
| Neutral detergent fiber | 48.73 |
| Acid detergent fiber | 21.84 |
| Calcium | 0.81 |
| Phosphorus | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Wang, L.; Sun, H.; Sun, L.; An, J.; Fu, S.; Zhao, M.; Liu, F.; Ren, X.; Liu, Z.; et al. Yeast Culture Supplementation Improves Meat Quality by Enhancing Immune Response and Purine Metabolism of Small-Tail Han Sheep (Ovis aries). Int. J. Mol. Sci. 2025, 26, 4512. https://doi.org/10.3390/ijms26104512
Bai X, Wang L, Sun H, Sun L, An J, Fu S, Zhao M, Liu F, Ren X, Liu Z, et al. Yeast Culture Supplementation Improves Meat Quality by Enhancing Immune Response and Purine Metabolism of Small-Tail Han Sheep (Ovis aries). International Journal of Molecular Sciences. 2025; 26(10):4512. https://doi.org/10.3390/ijms26104512
Chicago/Turabian StyleBai, Xiaobo, Liwei Wang, Hua Sun, Lvhui Sun, Jianghong An, Shaoyin Fu, Mengran Zhao, Fang Liu, Xiaoqi Ren, Zheng Liu, and et al. 2025. "Yeast Culture Supplementation Improves Meat Quality by Enhancing Immune Response and Purine Metabolism of Small-Tail Han Sheep (Ovis aries)" International Journal of Molecular Sciences 26, no. 10: 4512. https://doi.org/10.3390/ijms26104512
APA StyleBai, X., Wang, L., Sun, H., Sun, L., An, J., Fu, S., Zhao, M., Liu, F., Ren, X., Liu, Z., He, J., & Liu, Y. (2025). Yeast Culture Supplementation Improves Meat Quality by Enhancing Immune Response and Purine Metabolism of Small-Tail Han Sheep (Ovis aries). International Journal of Molecular Sciences, 26(10), 4512. https://doi.org/10.3390/ijms26104512







