Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics
Abstract
1. Introduction
2. Results
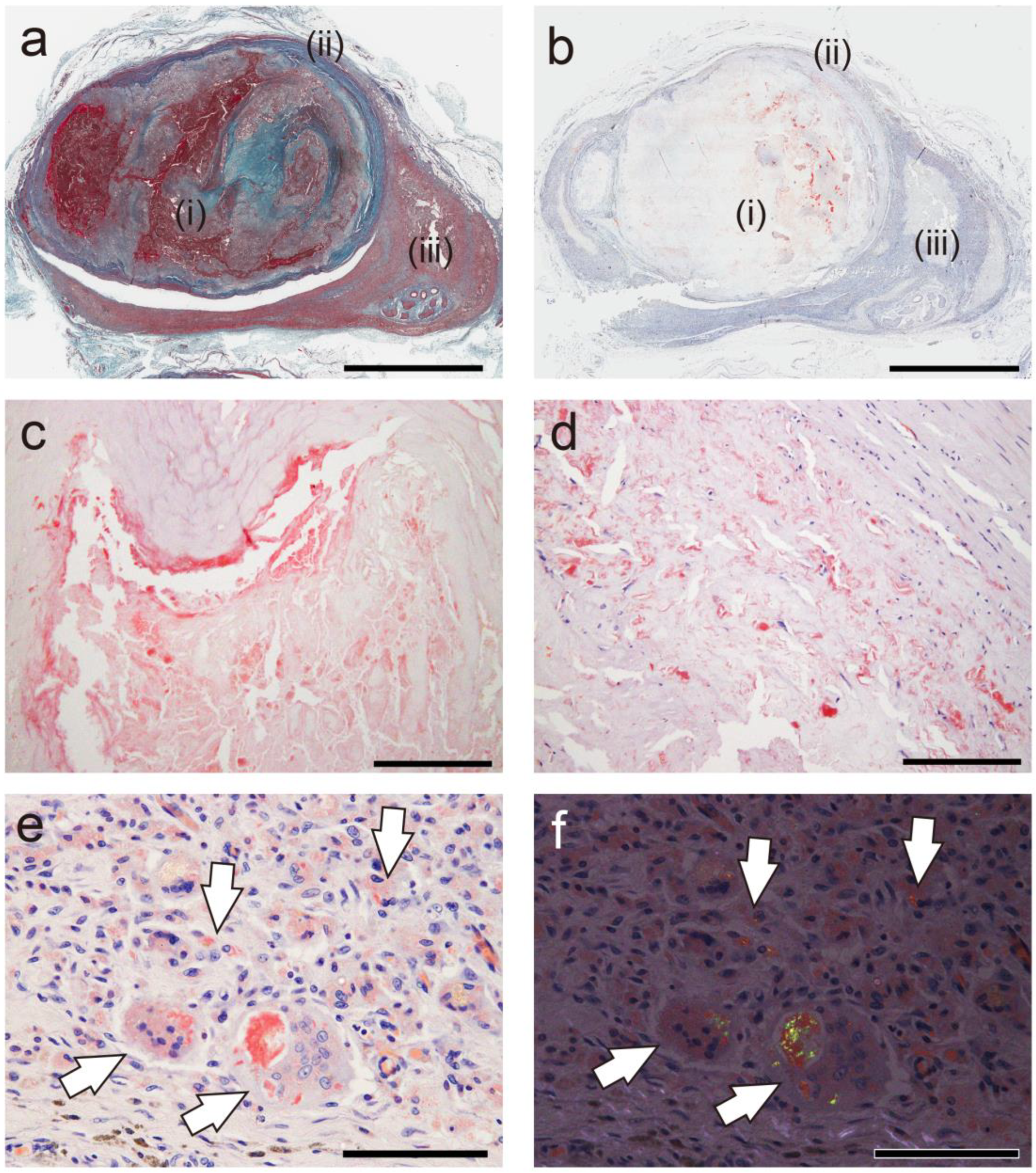
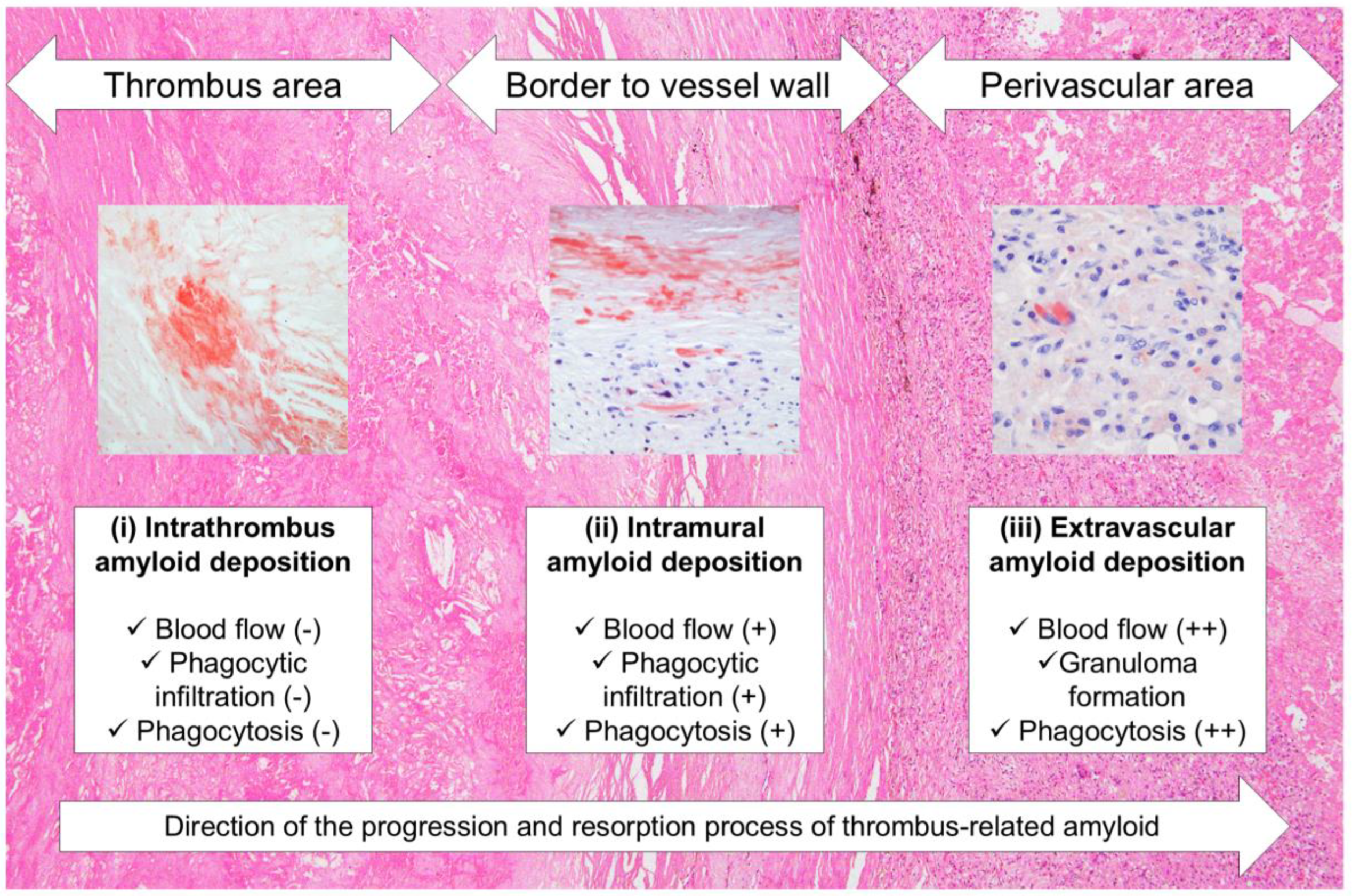
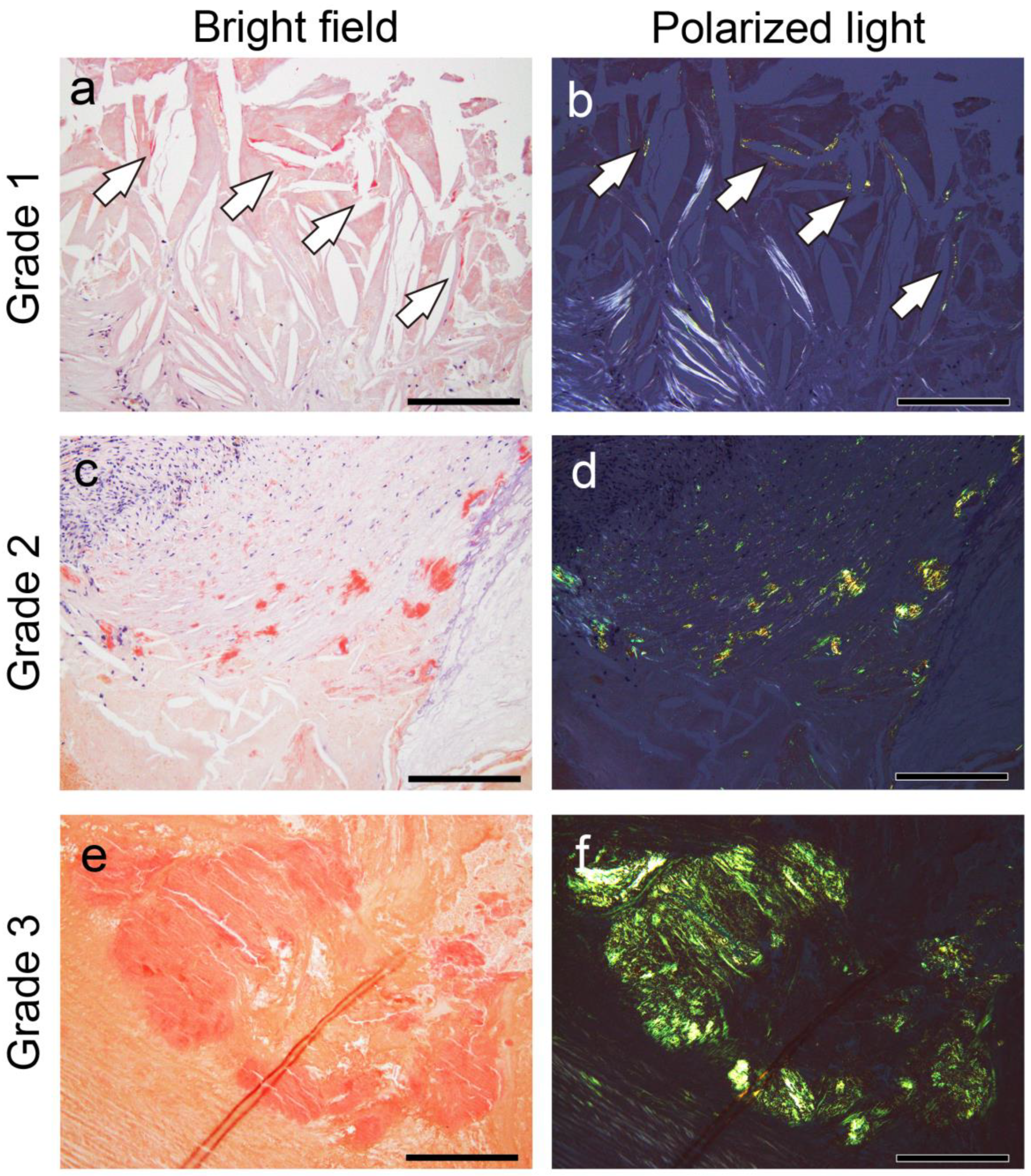
2.1. General Appearance of Thrombus-Associated Amyloid Deposition
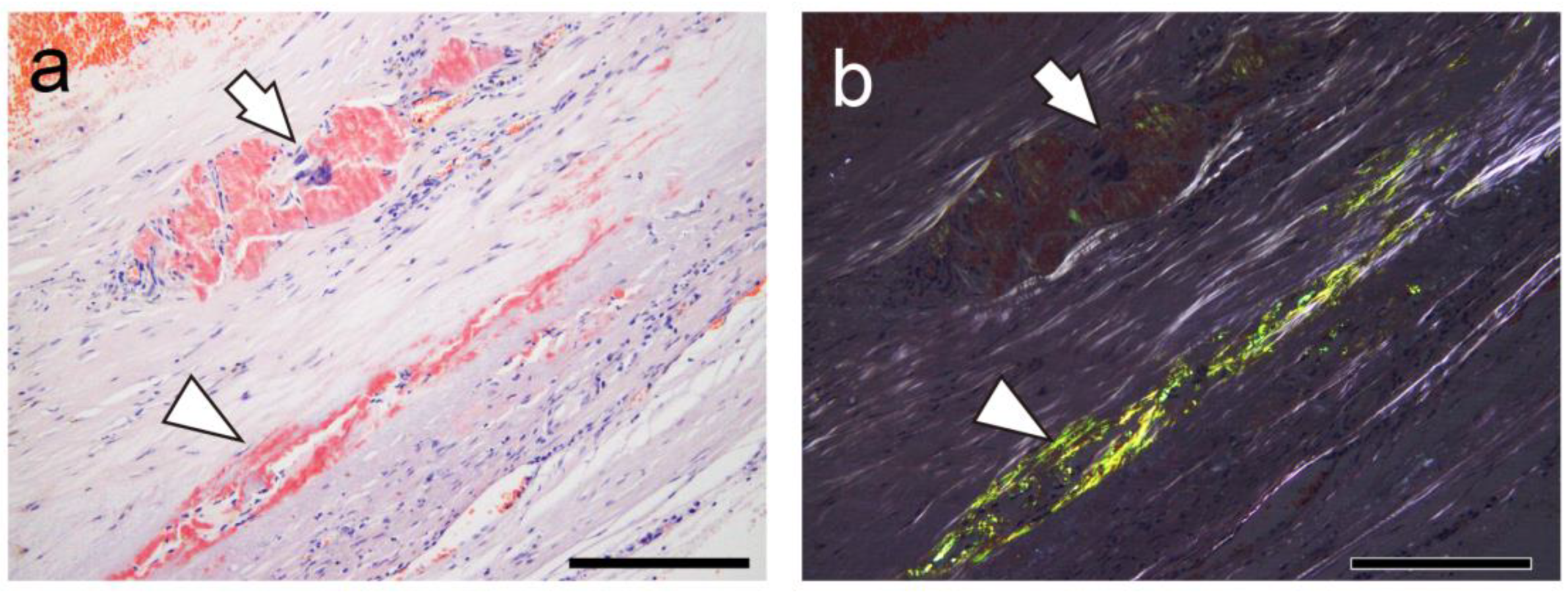
2.2. Relationship Between Thrombus-Associated Amyloid Deposition and Phagocytes
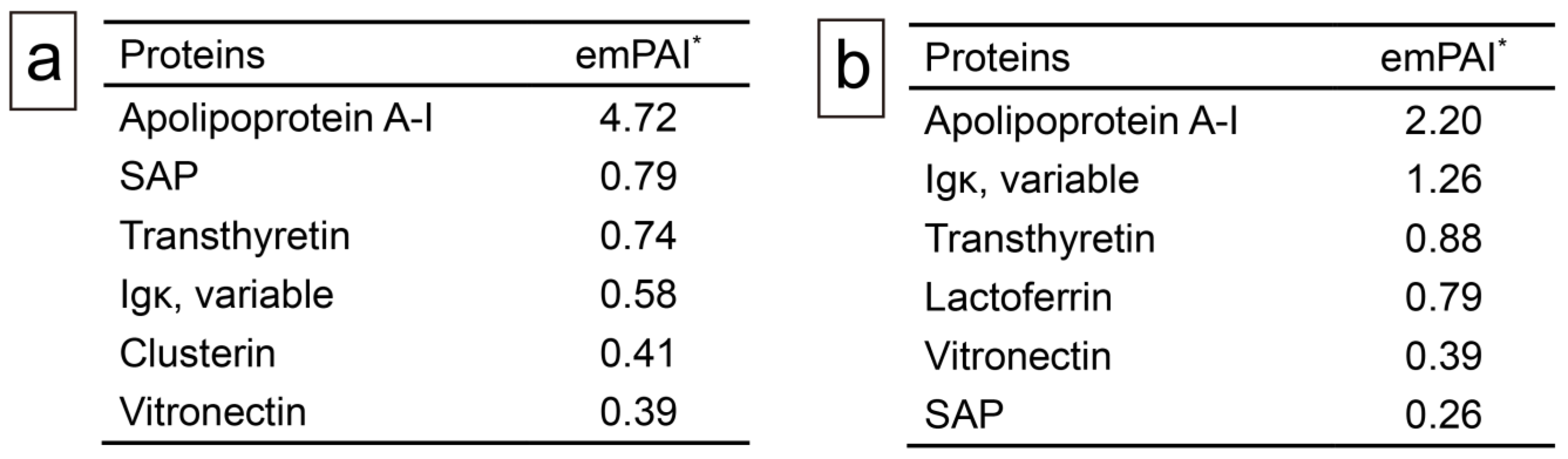
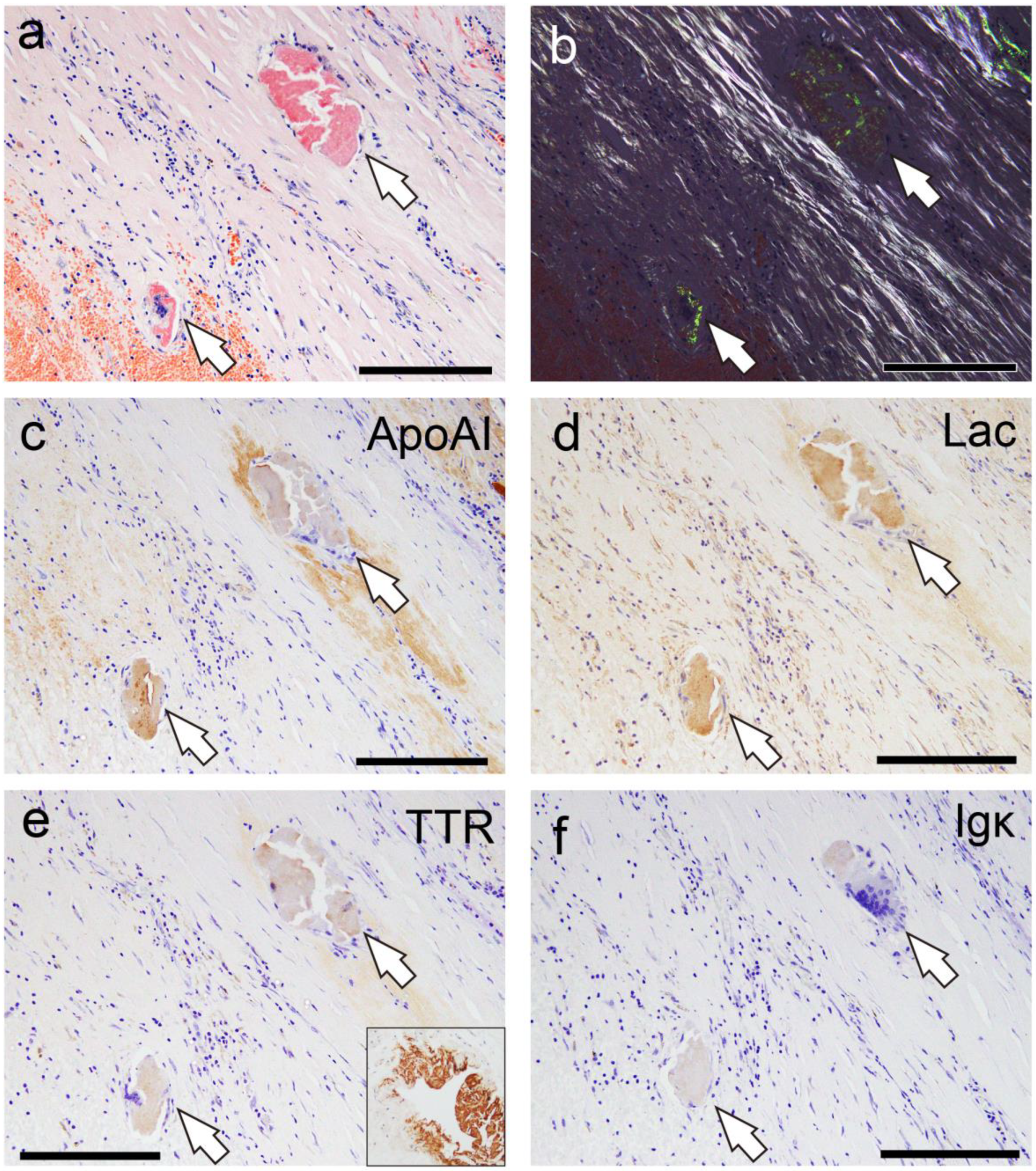
2.3. Proteomic and Immunohistochemical Features of Thrombus-Associated Amyloid Deposition
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Tissue Samples
4.3. Semiquantitative Grading System for Thrombus- and Atheromatous Plaque-Associated Amyloid Deposition
- Grade 0, none;
- Grade 1+, focal and tiny amyloid deposition;
- Grade 2+, multifocal deposition with nodular deposits; and
- Grade 3+, multifocal deposition with some massive deposits.
4.4. IHC
4.5. Proteomics Analysis Using Mass Spectrometry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Amyloid |
| ApoAI | Apolipoprotein A-I |
| CaA | Carotid artery |
| CoA | Coronary artery |
| CrA | Cranial artery |
| F | Female |
| FHT | Fibroatheroma with hemorrhage and thrombus |
| Igκ | immunoglobulin κ light chain |
| IHC | Immunohistochemistry |
| Lac | Lactoferrin |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LMD | Laser microdissection |
| M | Male |
| PA | Pulmonary artery |
| pCR | Phenol Congo red |
| SAP | Serum amyloid P-component |
| TTR | Transthyretin |
| VLE | Veins of the lower extremities |
References
- Buxbaum, J.N.; Eisenberg, D.S.; Fandrich, M.; McPhail, E.D.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2024: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2024, 31, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Westermark, P. Localized amyloids important in diseases outside the brain--lessons from the islets of Langerhans and the thoracic aorta. FEBS J. 2011, 278, 3918–3929. [Google Scholar] [CrossRef] [PubMed]
- Riba, M.; Del Valle, J.; Auge, E.; Vilaplana, J.; Pelegri, C. From corpora amylacea to wasteosomes: History and perspectives. Ageing Res. Rev. 2021, 72, 101484. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Hata, Y.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Sekijima, Y.; Nishida, N. Amyloid-forming corpora amylacea and spheroid-type amyloid deposition: Comprehensive analysis using immunohistochemistry, proteomics, and a literature review. Int. J. Mol. Sci. 2024, 25, 4040. [Google Scholar] [CrossRef]
- Castellani, R.J.; Shanes, E.D.; McCord, M.; Reish, N.J.; Flanagan, M.E.; Mesulam, M.M.; Jamshidi, P. Neuropathology of anti-amyloid-beta immunotherapy: A case report. J. Alzheimer’s Dis. 2023, 93, 803–813. [Google Scholar] [CrossRef]
- Westermark, P.; Mucchiano, G.; Marthin, T.; Johnson, K.H.; Sletten, K. Apolipoprotein A1-derived amyloid in human aortic atherosclerotic plaques. Am. J. Pathol. 1995, 147, 1186–1192. [Google Scholar]
- Mucchiano, G.I.; Häggqvist, B.; Sletten, K.; Westermark, P. Apolipoprotein A-1-derived amyloid in atherosclerotic plaques of the human aorta. J. Pathol. 2001, 193, 270–275. [Google Scholar] [CrossRef]
- Häggqvist, B.; Näslund, J.; Sletten, K.; Westermark, G.T.; Mucchiano, G.; Tjernberg, L.O.; Nordstedt, C.; Engström, U.; Westermark, P. Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc. Natl. Acad. Sci. USA 1999, 96, 8669–8674. [Google Scholar] [CrossRef]
- Tasaki, M.; Lavatelli, F.; Obici, L.; Obayashi, K.; Miyamoto, T.; Merlini, G.; Palladini, G.; Ando, Y.; Ueda, M. Age-related amyloidosis outside the brain: A state-of-the-art review. Ageing Res. Rev. 2021, 70, 101388. [Google Scholar] [CrossRef]
- Goffin, Y.A.; Rickaert, F. Histotopographic evidence that amyloid deposits in sclerocalcific heart valves and other chronic lesions of the cardiovascular system are related to old thrombotic material. Virchows Arch. A Pathol. Anat. Histopathol. 1986, 409, 61–77. [Google Scholar] [CrossRef]
- Abe, R.; Katoh, N.; Takahashi, Y.; Takasone, K.; Yoshinaga, T.; Yazaki, M.; Kametani, F.; Sekijima, Y. Distribution of amyloidosis subtypes based on tissue biopsy site–Consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol. Int. 2021, 71, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Haga, S. Accumulation of carboxy-terminal fragments of APP increases phosphodiesterase 8B. Neurobiol. Aging 2015, 36, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Yoshida, M.; Matsubara, T.; Murayama, S.; Saito, Y.; Kawakami, I.; Onaya, M.; Tanaka, H.; Kakita, A.; Robinson, A.C.; et al. Comparison of common and disease-specific post-translational modifications of pathological tau associated with a wide range of tauopathies. Front. Neurosci. 2020, 14, 581936. [Google Scholar] [CrossRef]
- Tasaki, M.; Okada, M.; Yanagisawa, A.; Nomura, T.; Matsushita, H.; Ueda, A.; Inoue, Y.; Masuda, T.; Misumi, Y.; Yamashita, T.; et al. Apolipoprotein AI amyloid deposits in the ligamentum flavum in patients with lumbar spinal canal stenosis. Amyloid 2021, 28, 107–112. [Google Scholar] [CrossRef]
- Hoshii, Y.; Nanbara, H.; Cui, D.; Takahashi, M.; Ikeda, E. Immunohistochemical examination of Akappa amyloidosis with antibody against adjacent portion of the carboxy terminus of immunoglobulin kappa light chain. Med. Mol. Morphol. 2012, 45, 124–128. [Google Scholar] [CrossRef]
- Ichimata, S.; Aoyagi, D.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Uehara, T.; Shiozawa, S. A case of spheroid-type localized lactoferrin amyloidosis in the bronchus. Pathol. Int. 2019, 69, 235–240. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Hirono, K.; Yamaguchi, Y.; Nishida, N. Clinicopathological features of clinically undiagnosed sporadic transthyretin cardiac amyloidosis: A forensic autopsy-based series. Amyloid 2021, 28, 125–133. [Google Scholar] [CrossRef]
- Cohen, O.C.; Blakeney, I.J.; Law, S.; Ravichandran, S.; Gilbertson, J.; Rowczenio, D.; Mahmood, S.; Sachchithanantham, S.; Wisniowski, B.; Lachmann, H.J.; et al. The experience of hereditary apolipoprotein A-I amyloidosis at the UK National Amyloidosis Centre. Amyloid 2022, 29, 237–244. [Google Scholar] [CrossRef]
- Rocken, C.; Tautenhahn, J.; Buhling, F.; Sachwitz, D.; Vockler, S.; Goette, A.; Burger, T. Prevalence and pathology of amyloid in atherosclerotic arteries. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 676–677. [Google Scholar] [CrossRef]
- Sekijima, Y. Transthyretin (ATTR) amyloidosis: Clinical spectrum, molecular pathogenesis and disease-modifying treatments. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1036–1043. [Google Scholar] [CrossRef]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Katoh, N.; Abe, R.; Yoshinaga, T.; Kametani, F.; Yazaki, M.; Uehara, T.; Sekijima, Y. A case of novel amyloidosis: Glucagon-derived amyloid deposition associated with pancreatic neuroendocrine tumour. Amyloid 2021, 28, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Katoh, N.; Abe, R.; Yoshinaga, T.; Kametani, F.; Yazaki, M.; Kusama, Y.; Sano, K.; Uehara, T.; Sekijima, Y. Somatostatin-derived amyloid deposition associated with duodenal neuroendocrine tumour (NET): A report of novel localised amyloidosis associated with NET. Amyloid 2022, 29, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, A.; Ueda, M.; Sueyoshi, T.; Nakamura, E.; Tasaki, M.; Suenaga, G.; Motokawa, H.; Toyoshima, R.; Kinoshita, Y.; Misumi, Y.; et al. Knee osteoarthritis associated with different kinds of amyloid deposits and the impact of aging on type of amyloid. Amyloid 2016, 23, 26–32. [Google Scholar] [CrossRef]
- Westermark, P. Localized AL amyloidosis: A suicidal neoplasm? Ups. J. Med. Sci. 2012, 117, 244–250. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Nomoto, K.; Nishida, N. Transthyretin-derived amyloid (ATTR) and sarcoidosis: Does ATTR deposition cause a granulomatous inflammatory response in older adults with sarcoidosis? Cardiovasc. Pathol. 2024, 70, 107624. [Google Scholar] [CrossRef]
- Fontana, M.; Gilbertson, J.; Verona, G.; Riefolo, M.; Slamova, I.; Leone, O.; Rowczenio, D.; Botcher, N.; Ioannou, A.; Patel, R.K.; et al. Antibody-associated reversal of ATTR amyloidosis-related cardiomyopathy. N. Engl. J. Med. 2023, 388, 2199–2201. [Google Scholar] [CrossRef]
- Ichimata, S.; Aikawa, A.; Sugishita, N.; Katoh, N.; Kametani, F.; Tagawa, H.; Handa, Y.; Yazaki, M.; Sekijima, Y.; Ehara, T.; et al. Enterocolic granulomatous phlebitis associated with epidermal growth factor-containing fibulin-like extracellular matrix protein 1 deposition and focal amyloid properties: A case report. Pathol. Int. 2024, 74, 146–153. [Google Scholar] [CrossRef]
- Ishii, W.; Matsuda, M.; Nakamura, N.; Katsumata, S.; Toriumi, H.; Suzuki, A.; Ikeda, S. Phenol congo red staining enhances the diagnostic value of abdominal fat aspiration biopsy in reactive aa amyloidosis secondary to rheumatoid arthritis. Intern. Med. 2003, 42, 400–405. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Yoshida, K.; Hirono, K.; Nishida, N. Distinctive deposition patterns of sporadic transthyretin-derived amyloidosis in the atria: A forensic autopsy-based study. Int. J. Mol. Sci. 2024, 25, 8176. [Google Scholar] [CrossRef]


| Aorta * | CaA | CoA ** | CrA ** | PA/VLE | Other *** | |
|---|---|---|---|---|---|---|
| Age (range) | 80.2 ± 8.8 (71–97) | 79.0 ± 4.9 (72–88) | 72.2 ± 12.8 (47–93) | 39.0 ± 8.0 (31–47) | 49.3 ± 20.4 (22–81) | 76.5 ± 17.8 (47–92) |
| Sex (F/M) | 3/3 | 1/6 | 1/10 | 0/2 | 4/2 | 0/4 |
| ATTR-CA-positive (%) | 2 (33) | 1 (14) | 1 (9) | 0 | 0 | 0 |
| Number of positive cases for amyloid deposition (%) | ||||||
| Thrombus/FHT | 3 (50) | 3 (43) | 6 (55) | 0 | 0 | 3 (75) |
| Border/vessel wall **** | 3 (50) | 4 (57) | 5 (45) | 0 | 0 | 3 (75) |
| Case # | Age | Sex | Anatomical Site | Location and Severity of Amyloid Deposition * | ||
|---|---|---|---|---|---|---|
| Thrombus/FHT | Border/Vessel Wall | Phagocytosis | ||||
| 1 | 97 | F | Abdominal aorta | 1+ | 1+ | Positive |
| 2 | 83 | M | Thoracic aorta | 3+ | 3+ | Positive |
| 3 | 81 | M | Abdominal aorta | 1+ | 1+ | Negative |
| 4 | 78 | M | Abdominal aorta | 1+ | 3+ | Positive |
| 5 | 78 | F | Internal carotid artery | 0 | 1+ | Negative |
| 6 | 75 | M | Internal carotid artery | 1+ | 1+ | Negative |
| 7 | 77 | M | Internal carotid artery | 1+ | 1+ | Negative |
| 8 | 83 | M | Internal carotid artery | 2+ | 3+ | Positive |
| 9 | 80 | M | Right coronary artery | 1+ | 2+ | Negative |
| 10 | 79 | F | Left coronary artery | 1+ | 0 | Negative |
| 11 | 66 | M | Left coronary artery | 1+ | 0 | Negative |
| 12 | 73 | M | Left coronary artery | 1+ | 2+ | Negative |
| 13 | 72 | M | Right coronary artery | 1+ | 0 | Negative |
| 14 | 93 | M | Left coronary artery | 1+ | 0 | Negative |
| 15 ** | 47 | M | Right coronary artery | 0 | 1+ | Negative |
| 16 | 65 | M | Right coronary artery | 0 | 1+ | Negative |
| 17 | 89 | M | Internal iliac artery | 1+ | 2+ | Negative |
| 18 ** | 47 | M | Arteriovenous fistula | 2+ | 3+ | Positive |
| 19 | 92 | M | Common iliac artery | 2+ | 3+ | Positive |
| Case # | Severity * | Intrathrombus/FHT Amyloid ** | Boder/Vessel Wall Amyloid ** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ApoAI | Lac | TTR *** | Igκ | ApoA1 | Lac | TTR *** | Igκ | ||
| 2 | 3+/3+ | 2+ | 1+ | 1+ | 0 | 2+ | 1+ | 1+ | 0 |
| 4 | 1+/3+ | NE | NE | NE | NE | 2+ | 2+ | 1+ | 0 |
| 8 | 2+/3+ | 2+ | 1+ | 0 | 0 | 2+ | 1+ | 0 | 0 |
| 9 | 1+/2+ | NE | NE | NE | NE | 2+ | 0 | 0 | 0 |
| 12 | 1+/2+ | NE | NE | NE | NE | 1+ | 1+ | 0 | 0 |
| 17 | 1+/2+ | NE | NE | NE | NE | 1+ | 1+ | 0 | 0 |
| 18 | 2+/3+ | 2+ | 1+ | 0 | 0 | 2+ | 1+ | 1+ | 0 |
| 19 | 2+/3+ | 1+ | 0 | 1+ | 0 | 2+ | 1+ | 1+ | 0 |
| Antibody | Source | Clone | Dilution | Antigen Retrieval | References |
|---|---|---|---|---|---|
| Apolipoprotein A-I | Abcam | EP1368Y | 1:4000 | 98% FA (1 min) | [12] |
| Immunoglobulin κ light chain | DB Biotech | H16-E | 1:500 | 98% FA (1 min) | [13] |
| Lactoferrin | Santa Cruz | B97 | 1:150 | 98% FA (1 min) | [4,14] |
| Prealbumin | Abcam | EPR3219 | 1:2000 | 98% FA (1 min) | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichimata, S.; Yoshinaga, T.; Sato, M.; Katoh, N.; Kametani, F.; Yazaki, M.; Sekijim, Y.; Hata, Y.; Nishida, N. Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics. Int. J. Mol. Sci. 2025, 26, 4505. https://doi.org/10.3390/ijms26104505
Ichimata S, Yoshinaga T, Sato M, Katoh N, Kametani F, Yazaki M, Sekijim Y, Hata Y, Nishida N. Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics. International Journal of Molecular Sciences. 2025; 26(10):4505. https://doi.org/10.3390/ijms26104505
Chicago/Turabian StyleIchimata, Shojiro, Tsuneaki Yoshinaga, Mitsuto Sato, Nagaaki Katoh, Fuyuki Kametani, Masahide Yazaki, Yoshiki Sekijim, Yukiko Hata, and Naoki Nishida. 2025. "Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics" International Journal of Molecular Sciences 26, no. 10: 4505. https://doi.org/10.3390/ijms26104505
APA StyleIchimata, S., Yoshinaga, T., Sato, M., Katoh, N., Kametani, F., Yazaki, M., Sekijim, Y., Hata, Y., & Nishida, N. (2025). Exploring the Histopathological Features of Thrombus-Associated Localized Amyloid Deposition: Comprehensive Analysis Employing Immunohistochemistry and Proteomics. International Journal of Molecular Sciences, 26(10), 4505. https://doi.org/10.3390/ijms26104505







