Abstract
Plants are exposed to various stressors, including pathogens, requiring specific environmental conditions to provoke/induce plant disease. This phenomenon is called the “disease triangle” and is directly connected with a particular plant–pathogen interaction. Only a virulent pathogen interacting with a susceptible plant cultivar will lead to disease under specific environmental conditions. This may seem difficult to accomplish, but soft rot Pectobacteriaceae (SRPs) is a group virulent of pathogenic bacteria with a broad host range. Additionally, waterlogging (and, resulting from it, hypoxia), which is becoming a frequent problem in farming, is a favoring condition for this group of pathogens. Waterlogging by itself is an important source of abiotic stress for plants due to lowered gas exchange. Therefore, plants have evolved an ethylene-based system for hypoxia sensing. Plant response is coordinated by hormonal changes which induce metabolic and physiological adjustment to the environmental conditions. Wetland species such as rice (Oryza sativa L.), and bittersweet nightshade (Solanum dulcamara L.) have developed adaptations enabling them to withstand prolonged periods of decreased oxygen availability. On the other hand, potato (Solanum tuberosum L.), although able to sense and response to hypoxia, is sensitive to this environmental stress. This situation is exploited by SRPs which in response to hypoxia induce the production of virulence factors with the use of cyclic diguanylate (c-di-GMP). Potato tubers in turn reduce their defenses to preserve energy to prevent the negative effects of reactive oxygen species and acidification, making them prone to soft rot disease. To reduce the losses caused by the soft rot disease we need sensitive and reliable methods for the detection of the pathogens, to isolate infected plant material. However, due to the high prevalence of SRPs in the environment, we also need to create new potato varieties more resistant to the disease. To reach that goal, we can look to wild potatoes and other Solanum species for mechanisms of resistance to waterlogging. Potato resistance can also be aided by beneficial microorganisms which can induce the plant’s natural defenses to bacterial infections but also waterlogging. However, most of the known plant-beneficial microorganisms suffer from hypoxia and can be outcompeted by plant pathogens. Therefore, it is important to look for microorganisms that can withstand hypoxia or alleviate its effects on the plant, e.g., by improving soil structure. Therefore, this review aims to present crucial elements of potato response to hypoxia and SRP infection and future outlooks for the prevention of soft rot disease considering the influence of environmental conditions.
1. Introduction
Throughout their lives, plants are exposed to various kinds of stress, which can be divided into biotic and abiotic. Biotic stress is caused by living organisms (such as animals, fungi, bacteria, and other plants), viruses, and viroids. Meanwhile, physical or chemical environmental factors cause abiotic stress [1]. The influence of these factors on plant health is usually analyzed independently. However, they frequently co-occur and, currently, we observe a growing number of studies dedicated to the influence of joint stress factors on plants and their response to them [2]. Abiotic stress affects plants and their pathogens, forcing plants to adjust their response to both factors [3]. Plant pathogens often cannot induce disease symptoms in conditions favorable for their host. Therefore, developing the disease requires a so-called “disease triangle” composed of three elements: virulent pathogens, susceptible plants, and appropriate environmental conditions [4].
Environmental stress, such as hypoxia (a state of lowered oxygen availability (1–5%)), can favor plant susceptibility to certain diseases [5]. Hypoxia inhibits plant aerobic respiration, leading to decreased metabolism, slowed growth, and lowered resistance to other stress factors, such as necrotrophic pathogens [6] or herbivorous animal invasion [7]. Hypoxia, as a stressor, is mostly induced by waterlogging [8]. The temporal rise in the water level leads to the submerging of the main part of the roots and/or aerial plant parts [9]. The soil air pockets are filled with water, additionally oxygen, as a nonpolar gas, poorly dissolves in water, leading to decreased oxygen mobility and availability for the submerged parts of the plant [10]. The result is a drastic change in plant metabolism, making the plants more susceptible to other environmental factors [9]. Above that, waterlogging causes soil structural rearrangement due to the high density of water leading to soil compaction. In the compact and deprived-of-oxygen soil, toxic microbial metabolites accumulate, and decreased redox potential leads to a drop in the soil pH and an increased amount of toxic metal ions [11]. Therefore, waterlogging can cause huge economic losses in agriculture and it is estimated that on average it reduces yield by 33%, which is expected to largely increase due to climate change and the deterioration of soil structure [12].
However, hypoxia can also be a more local physiological state for certain tissues or cells. For example, low oxygen content in meristematic tissues prevents cells from differentiation [13]. Decreased oxygen penetration into nodules, structures housing plant-beneficial Rhizobia and Sinorhizobia, causes plants to protects their nitrogenase from oxidization [14]. Also, thick and water-loaded structures such as tubers [15] or fruits [16] need to adapt to lower oxygen availability by decreasing the primary metabolism rate in the central part of the structure [17].
Due to low oxygen penetration to plant tissues, only the external part of the plants have a high metabolic activity [18]. The epidermis and subepidermal parts of plants’ organs have the best access to atmospheric oxygen and, therefore, the proper energetic potential to induce plant defenses, including secondary metabolism, and defend themselves against pathogen invasion [19]. But when the epidermis barrier is broken [20] or when oxygen diffusion is blocked, epidermal cells decrease their metabolism rate [17] and then the pathogens have access to less defended internal parts [21].
Waterlogging, however, does not only directly influence plant metabolism but also its inhabiting microorganisms, including pathogens. For some pathogens, the decreased oxygen availability can restrain their survival (e.g., Collybia fusipes [22]) and pathogenicity (e.g., Fusarium graminearum [23]), but others can benefit from it [6]. One of the most important bacterial plant pathogens, soft rot Pectobacteriaceae (SRP) [24], benefits from hypoxia using its adaptations [25] and the lowered defenses of the plant [26]. These bacteria cause soft rot disease in fruits and vegetables. Soft rot is one of the most economically important diseases affecting potatoes (Solanum tuberosum L.)—the utmost widely consumed vegetable [27]. SRPs enter the tubers through wounds or lenticels and, depending on the environmental conditions, either form latent infection or multiply and penetrate deeper into the plant tissues leading to the development of the disease [28]. Latently infected tubers (without any symptoms) are the most common source of these pathogens [29]. Latent infections lead to disease development under disease-favoring conditions: elevated temperature, high humidity, and low oxygen availability [30]. Summarizing according to the “disease triangle” concept [4], soft rot Pectobacteriace leads to the development of the soft rot disease in high-temperature and -humidity conditions in susceptible hosts such as potatoes by the production of virulence factors which are mainly plant cell wall degrading enzymes but also toxins [31], such as necrosis-inducing virulence protein (Nip) [32], but also type 4 secretion system (T4SS) [33], and type 6 secretion system (T6SS) [34] (Figure 1).
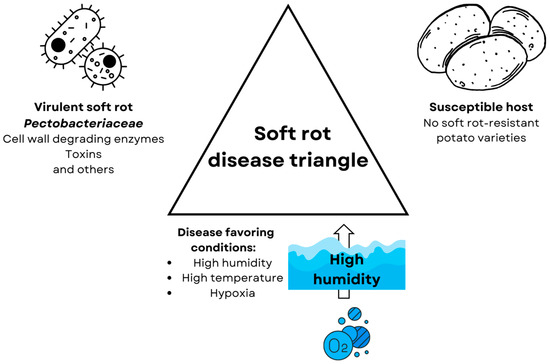
Figure 1.
The disease triangle for soft rot disease. All three components are necessary for the induction of soft rot disease. (1) Virulent strain of soft rot Pectobacteriaceae, whose virulence depends greatly on the production of cell wall degrading enzymes, but also other virulence factors such as the production of toxins [31], like necrose inducing protein (Nip) [32] and the type 4 [33] and 6 secretion systems [34]. (2) Susceptible hosts such as potatoes, currently there are no potato varieties resistant to soft rot disease [35,36]. (3) Disease-favoring conditions which are high humidity and temperature and, resulting from high humidity, hypoxia [37].
So far, attempts to create soft rot-resistant potato varieties have not succeeded [35,36]. It is proposed that the climate catastrophe will further increase the losses caused by SRP [38]. Therefore, it is imperative to quickly develop new strategies to protect potatoes from these pathogens. Solanum L. plant species that are better adapted to waterlogging can be a good source of soft rot disease resistance [39,40,41].
2. Plant Response to Hypoxia
2.1. Hypoxia Sensing
To survive short-term hypoxia, plants developed a signaling system to adapt to lower oxygen availability [42]. The system comprises hypoxia detection mechanisms and signaling systems cooperating with plant phytohormones, which can coordinate plant metabolic and morphological adjustments to lower oxygen availability. The presented signaling pathways are established mainly on the Arabidopsis (Arabidopsis thaliana (L.) Heynh.) model unless stated otherwise. The key element for hypoxia detection and signaling is ethylene, which is produced by all plant tissues but quickly escapes into the atmosphere [43]. Waterlogging disrupts the diffusion of nonpolar ethylene, accumulating in the plant’s oxygen-deprived parts (Figure 2) [43]. Ethylene is then bound by its receptors, inhibiting its ability to deactivate (phosphorylate) ethylene-insensitive 2 (EIN2) by constitutive triple response 1 (CTR1). Unphosphorylated EIN2 activates hypoxia transcription factors such as EIN3 and EIN3-like 1 and 2 (EIL1, EIL2) in the canonical pathway [44]. It has been proven that the ethylene signaling pathway is activated during hypoxia in Solanaceae but it does not lead to the activation of hypoxia response in waterlogging-susceptible potato varieties nor does it in tomato (Solanum lycopersicum L.). This implies that the differences responsible for different tolerance to waterlogging lie downstream from the explained signaling pathway [39]. The presence of an as yet not fully described non-canonical pathway connecting ethylene signaling with abscisic acid (ABA), a phytohormone, enables tighter regulation of plant response to hypoxia [45]. ABA, downregulated in soybean (Glycine max (L.) Merr.) during waterlogging, impairs the formation of adventitious roots and aerenchyma, positively affecting hypoxia tolerance [46].
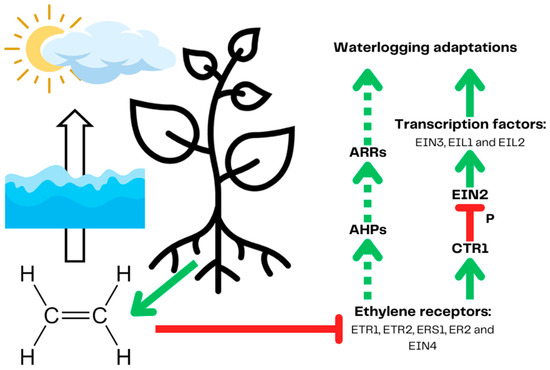
Figure 2.
Plant ethylene signaling leads to the expression of genes, leading to waterlogging adaptations. Constantly produced ethylene is trapped in submerged tissues due to the low diffusion of nonpolar gases in water and cannot escape into the atmosphere. Ethylene is then bound by ethylene receptors, inhibiting their activity. In the canonical pathway, ethylene receptors bind ethylene preventing them from activating constitutive triple response 1 (CTR1). Inactive CTR1 kinase no longer phosphorylates ethylene-insensitive 2 (EIN2), leading to its activation and following the activation of hypoxia transcription factors. The non-canonical pathway (marked by a dotted line) starts with a suppressed ETR1 receptor, which no longer activates CTR1 phosphorylates Arabidopsis histidine-containing phosphotransfer proteins (AHPs) (linking the ethylene sensing with cytokine signaling), which in turn activate Arabidopsis response regulators (ARR) by phosphotransfer. ARRs regulate the expression of waterlogging response genes. Prepared based on [43,45]. The red arrows demonstrate inhibition and the green arrows represent induction.
On the other hand, ABA promotes stomata closure and decreases hypoxia-caused senescence [47]. Another type of phytohormone, auxins, are upregulated and act antagonistically on ABA, they also promote aerenchyma and adventitious root formation in bittersweet nightshade (Solanum dulcamara L.) [48]. The involvement of auxins and ABA in the glucose–TOR signaling pathway [49] and ABA’s role in the non-canonical pathway of ethylene signaling [45] integrates nutrient status sensing with oxygen availability. The involvement of ABA in the non-canonical pathway of ethylene signaling integrates an energetic status to respond to waterlogging [45]. Meanwhile, an increased gibberellin concentration significantly causes plant internode elongation in deepwater rice (Oryza sativa L.), helping plants’ shoots and/or roots extend beyond the water level to reach oxygen [50]. Waterlogging can also lead to the accumulation of salicylic acid (SA), which is a major plant hormone responsible for resistance to abiotic stress and biotic stress caused by biotrophic pathogens [51]. SA promotes apoptosis, which is necessary for aerenchyma formation and the development of adventitious roots in Soybean [52]. Jasmonic acid (JA)—an SA antagonist, another major plant hormone, is responsible for plant resistance against herbivorous animals (mainly insects and arachnids) and necrotrophic pathogens [53]. For example, in citruses (Citrus), hypoxia leads to elevated JA in leaves but decreased JA levels in roots [54]. In waterlogging-sensitive cucumber (Cucumis sativus L.), the concentration of JA in hypocotyl was upregulated but downregulated in waterlogging-resistant lines [55]. This may result from the formation of adventitious root from hypocotyl. JA can positively influence waterlogging tolerance by increasing the concentration of ethylene in conifers [56] but, on the other hand, suppressing root growth and SA activity in soybeans [57]. Therefore, the concentration gradient of JA from shoots to the roots positively affects waterlogging tolerance. Also, brassinosteroids can positively resist waterlogging by promoting adventitious root development, hypocotyl expansion, and loosening in cucumber [58]. Relatively limited studies compared to other plant hormones are dedicated to melatonin’s effects on waterlogging [9,59]. It has been reported that melatonin can increase waterlogging tolerance by alleviating the negative impact of reactive oxygen species in Siberian crab apples (Malus baccata Borkh.) [60]. Alternatively, Zheng et al., 2017 [60] reported that melatonin reduces ethylene concentration, suggesting an antagonistic interaction, but this requires further study.
2.2. Adaptation to Hypoxia
While ethylene and energetic status are hypoxia sensors, ethylene, and plant hormones allow plants to integrate the response between different plant parts and tissues. To adapt their cellular metabolism to hypoxia, plants rely on ethylene response factors VII (ERF VII), which activate the transcription of hypoxia response genes. Plant cysteine oxidases oxidize the N-terminus cysteine residue of this protein, leading to ERF VII proteasomal degradation. During hypoxia, stabilized ERF VIIs activate the transcription of hypoxia-responsive genes (HRGs) [61]. ERF VIIs can integrate ethylene sensing and energetic status to induce the expression of the hypoxia response genes [62]. ERF VIIs signaling allows plants to induce anaerobic respiration before the tricarboxylic acid cycle deprives the tissues of remaining oxygen in the pea (Pisum sativum L.) [63]. However, alcohol fermentation is much less energetically efficient and leads to root carbon deprivation. In response to the high demand for organic carbon in flooded roots, carbohydrates are transported to the roots of ash (Fraxinus) [64]. The increased demand for organic carbon can never be met by photosynthesis, since its rate is downregulated during hypoxia. Due to stomata closure and lowered gas exchange, photosynthesis is less efficient in cotton (Gossypium hirsutum L.) [65].
Hypoxia also leads to the accumulation of reactive oxygen species by the mitochondrial electron transport chain, causing chlorophyll degradation and further decreasing the plant’s photosynthetic potential [66,67]. To tackle that problem, plants can recruit stored lipids for energy, signaling, and protection from toxic products of anaerobic respiration [68]. Due to the multiple ways environmental hypoxia can negatively influence plant development, the best-adapted waterlogging plants such as rice minimize their metabolic rate during stressful periods [69]. Wetland plants, such as Carex L., however, need anatomic adaptations for more efficient oxygen transportation [70].
Although lowered metabolism can help to withstand short-time hypoxia, wetland crops including rice need to maintain an efficient metabolism inside their flooded roots. This can only be achieved by root architectural changes [71] and metabolic adaptation [72]. One of the water plants’ adaptations for decreased oxygen availability is the development of internal ventilation [73]. Cortex cells deprived of oxygen die and degrade, forming natural cavities—resulting in aerenchyma formation. Aerenchyma can foster oxygen transportation and increase the adsorption area. Depending on the species, high water levels can lead to aerenchyma formation (e.g., wheat (Triticum aestivum L.) and corn (Zea mays subsp. mays L.) and an increase in its volume (e.g., rice) [71]. Rice exposed to waterlogging on its aerial (green) parts develops thin layers of gas film on its leaf surface, enabling gas exchange during submerging [74].
Another important adaptation is the development of a radial oxygen loss (ROL) barrier in many plant species. ROL limits the gas exchange to the root tips, decreasing oxygen loss in the upper part of the roots while still enabling the uptake of nutrients [75] In addition, cereals increase their roots’ cortex-to-stale ratio and produce fewer and shorter lateral roots to decrease oxygen consumption and increase soil penetration [71]. To penetrate the topsoil levels richer in oxygen, hypoxia induces root bending in Arabidopsis [76]. During prolonged waterlogging, low oxygen availability leads to the death of primary roots, whose function is overtaken by adventitious roots newly formed at the base of the stem closer to the surface in many plant species including tomatoes and bittersweet [77]. Low oxygen availability causes apical meristem elongation in rice induced by increased gibberellin production in a mechanism called low oxygen escape syndrome [78].
3. Hypoxia Influence on Soft Rot Disease
Hypoxia also influences plants-inhabiting beneficial and pathogenic microorganisms [79]. Although the effect of hypoxia can be beneficial, as in symbiotic Rhizobia and Sinorhizobia [14], likewise, the plant pathogenic Plasmodiophora brassicae [80] and Agrobacterium tumefaciens [81] benefit from hypoxia induced by the plant’s tumorous outgrowth. In the case of more hypoxia-sensitive organisms, such as fungi, the limited availability of oxygen may decrease the disease symptoms [6] by reducing the survival rate of hypoxia-sensitive species such as Collybia fusipes [22] or Phytophthora cryptogea [82]. Hypoxia not only influences survival but can also directly decrease the pathogenicity of some pathogen species. In Fusarium graminearum, hypoxia reduces the production of its main toxin, deoxynivalenol. However, it has no negative effect on its growth [23]. Also, plant susceptibility to biotic stress, such as pectinolytic bacteria (e.g., Pectobacterium carotovorum), can be induced by changes in plant gene expression, e.g., phenylalanine ammonia-lyase and extensions [83]. Since microbial metabolic activity in the soil can lead to root local hypoxia [84], plant pathogenic microorganisms can utilize this and compete for oxygen with their host, facilitating symptom development [80]. In this context, SRP, not only uses the decreased plant defenses [26,83,85,86] but also adapts their gene expression to hypoxic conditions to more effectively induce soft rot disease [25] (Figure 3). SRPs mainly comprise Dickeya and Pectobacterium genera and cause soft rot disease in many important vegetables and fruits [87]. D. dadantii [88] and P. atrosepticum [89] upregulate the expression of virulence factors, including pectinases and cellulases, under limited oxygen availability. These enzymes’ activity is one of the most important virulence factors for plant necrotrophic pathogens [90]. The limited oxygen environment on the rotting site of the tubers allows P. atrosepticum for nitrate respiration, enhancing its growth rate and invasion [91]. Pectinolytic bacteria utilize the global regulator c-di-GMP to adapt their metabolism to anaerobic or aerobic conditions [92]. A high level of c-di-GMP induced by low oxygen leads to increased virulence, biofilm formation, and decreased motility [93,94,95].
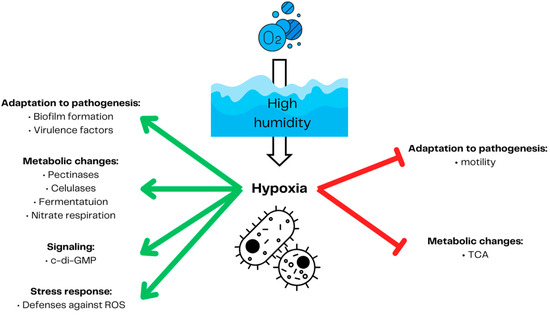
Figure 3.
Soft rot Pectobacteriaceae response to hypoxia. High humidity in soil leads to decreased oxygen penetration into deeper soil. This, in turn, forces the bacteria to switch from aerobic metabolism to fermentation. Low oxygen conditions lead to the increased production of master regulator c-di-GMP. As in many other bacteria groups, hypoxia in pectinolytic bacteria leads to the activation of reactive oxygen species defenses. Pectynolytic bacteria also increase nitrate respiration production of lytic enzymes and virulence factors. Global regulator c-di-GMP favors biofilm formation versus motility, triggering soft rot Pectobacteriaceae’s pathogenicity. Prepared based on [89,91,92,93,94,95]. The red arrows demonstrate inhibition and the green arrows represent induction.
It has been reported that hypoxia suppresses potato response to wounding (Figure 4) [26,85,86], a major route of SRP infection [96]. Potato tubers reduce anaerobic respiration to preserve oxygen. The energetic demand has to be met by induced anaerobic respiration [63]. The less effective anaerobic respiration leads to the accumulation of reactive oxygen species (ROS) and therefore requires plant upregulation of ROS defenses and the production of heat shock proteins [97]. While ROS scavengers reduce the concentration of ROS [98], heat shock proteins protect plants from harmful protein aggregates [99] emerging due to reactive oxygen species protein carbonylation [97]. Anaerobic respiration also leads to acidification, which causes elongation factor EF-1α binding to ribosomes, causing an arrest in protein production [100]. A low energetic status forces potato tubers to reduce the production of secondary metabolites and their defenses against wounding, including the production of extensines and phenylalanine ammonia lyase (PAL) [83]. Hypoxic conditions trigger plant hormonal changes, increasing the production of abscisic acid, auxins, and salicylic acid and inhibiting the production of jasmonate 12-oxo-phytodienoic acid (OPDA), which induces cell elongation to escape hypoxia and reach oxygen [101]. This, however, favors necrotrophic pathogens such as SRPs [102]. Furthermore, SRPs can cause latent infections in susceptible hosts [28] suggesting that SRPs are hemibiotrophic pathogens [31].
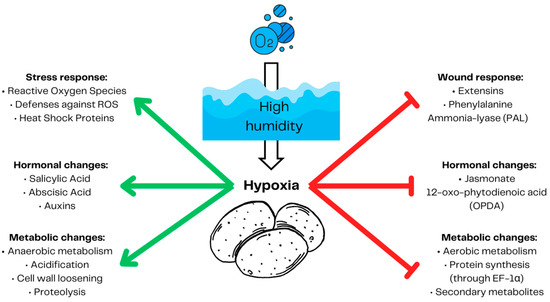
Figure 4.
Potato tuber response to hypoxia. High humidity leads to decreased gas exchange and hypoxia. During hypoxia, potato tubers decrease aerobic respiration to preserve oxygen and increase anaerobic respiration to provide energy. Increased anaerobic respiration produces acidification and reactive oxygen species (ROS). To protect the cells from the damaging effect of ROS, plants need to activate their defenses against ROS and produce heat shock proteins. A low energetic status abolishes plant defenses, including wound response factors such as extensins, phenylalanine ammonia lyase (PAL), and secondary metabolites such as expansin (EXP), early nodulin 93 (ENOD), 4-coumarate CoA ligase-like 2 (4CL), and HMG-CoA reductase (HMG). The production of proteins is arrested by EF-1α binding to ribosomes in acidic conditions. Plant hormonal response tailored for cell elongation to escape local hypoxic conditions makes tubers prone to the attack of necrotic pathogens. Prepared based on [26,83,85,86,100,101]. The red arrows demonstrate inhibition and the green arrows represent induction.
Even though latent infection by SRP is common and has been reported for many years [103], soft rot bacteria are considered classical necrotrophic pathogens [31]. This strange phenomenon is caused by the fact that the term necrotrophic, in contrast to biotrophic pathogens, was transferred from plant pathogenic fungi and, as such, does not describe the bacteria’s pathogenicity [104]. It has been proven that potato tubers inoculated with Dickeya solani initially respond with increased levels of SA, a response to the biotrophic stage, but later switch to the production of JA to target their response against the necrotrophic stage of pathogenesis [105]. This seemingly well-fitted response can easily fail when a plant is subjected to abiotic stress such as hypoxia [106]. The decreased wound response caused by hypoxia [26,86] can cause the rapid spread of pathogens and quick disease development when the plant is mechanically disrupted [107]. Even though it was proven that temperature, humidity, and inoculum are important for developing soft rot disease [37], there is still a lack of data on the transition from the latent infection of potatoes to the development of disease symptoms from both host and pathogen biology. Currently, we observe a growing number of studies dedicated to the detection of asymptomatic latent infections of potatoes with soft rot Pectobacteriaceae [108]. However, the subject of latent infections and the transition to symptomatic infection is still largely understudied [109,110] and, at present, the research concerning soft rot Pectobacteriaceae infection almost exclusively concerns symptomatic infection in disease-provocative conditions [111].
4. Prevention of Soft Rot Disease
4.1. Soft Rot Pectobacteriaceae Detection
Using certified and pathogen-free source material is still the best method of disease prevention [112]. Despite that, many farmers, especially from developing countries, use uncertified source material [113]. In Europe, where the temperature is lower and the usage of certified seed tubers is higher, the losses caused by these pathogens are estimated to be 46 M Euro yearly [27]. To reduce the huge losses caused by SRPs we can operate on the three sites of the disease triangle: the prevalence of SRPs in agricultural systems, the resistance of the potato varieties, and the influence of the environmental conditions.
Therefore, implementing modern and efficient pathogen detection methods is imperative to minimize the losses caused by plant pathogens, including SRPs [114]. The new techniques based on nucleic acids or specific antigen detection are much faster, more sensitive, and more accurate than traditional microbiology [115]. Due to developments in molecular biology, the number of available PCR-based methods for detecting SRPs is constantly increasing [108,116,117] including quantitative ones [118,119]. TaqMan PCR assays allow for specific detection of Pectobacterium brasiliense starting from 1000 colony forming units CFU/mL [120]. In loop-mediated isothermal amplification, it is possible to detect P. parmentieri with a detection limit of about 20 genomes (from gDNA isolation) or 10 CFU/mL of heat-killed bacterial cells [121]. ELISA-based methods, which could be good candidates for fast and reliable screening due to their simplicity, do not yet provide sufficient sensitivity for detecting SRPs in plant material [115]. Therefore, new methods based, for example, on immunosensors [122] or biosensors targeted for usage in the field have been created [123]. More sophisticated methods like metagenomic sequencing can be applied in seed production [124]. The early detection of SRPs can be assisted by automated image detection techniques [125,126].
Therefore, the sophisticated and sensitive methods for pathogen detection can assist seed potato producers in the production of high-quality seed material, while biosensors and visual observation-based methods can help farmers to quickly identify disease outbreaks, reducing the prevalence of SRPs in the environment.
4.2. Breeding for Resistance
Although early detection of pathogens can help to discard infected tubers, drastically reducing the possible losses, SRPs can overwinter in the soil, water [127], plant debris [128], or weeds [129]. Additionally, soil amendments may further decrease the chance of disease development [130]. Although, without at least partial plant resistance to the pathogens, this can lead to the rapid spread of the disease [28]. However, recent advancements in plant breeding and selection can facilitate the creation of new resistant plant cultivars [131]. The new or enhanced plant features can be obtained by cross-pollination, protoplast fusion, mutagenesis-assisted or non-assisted, agro-infection using viruses or bacteria, or transformation by foreign DNA [132]. The most time-consuming part of creating new plant varieties is selection [133]. Several generations must be developed to confirm that the new variety represents a stable and desired phenotype [131]. This process can be largely accelerated by speed breeding. Using this method, it is possible to obtain up to seven generations per year [134]. It relies, among others, on cultivating the plant in tailored photoperiod and temperature conditions to reduce the time to flowering [135]. The process can be further accelerated if we combine speed breading with genomic selection [136]. Utilizing advancements in plant physiology and genetics, high-throughput plant genomic sequencing makes an expedited process of selection possible [137]. Knowing which molecular markers are associated with resistance can facilitate the creation of new varieties with genomic selection [138]. Also, advanced phenotyping can shorten waiting for a visual phenotype to appear [139]. Firstly, we need to confirm the association between certain agronomic traits, such as resistance to pathogens with known metabolite concentration. Then, using chromatography coupled with mass spectrometry, we can verify which newly generated plant varieties possess a targeted feature before regenerating the whole plant [140]. This approach can effectively facilitate the process of selection of promising new varieties.
Although the already existing plant varieties can serve as a source of resistance against soft rot, it is suggested that wild species from the Solanaceae family can be a promising source of new resistance genes [141]. It has been proven that anthocyanins in colored potato varieties, that have gained popularity on the market, positively affect soft rot resistance [142]. Also, calcium has a positive influence on potato SRP resistance [143], either by soil amendment application [130] or calcium content in tubers [144,145]. It has been reported that features such as starch content [146], cell wall pectin esterification [147], and high levels of polyphenol oxidase and peroxidases [148] are positively correlated with SRP resistance. There are also indications that the genes responsible for better SRP resistance are located on the 2nd and 4th chromosomes [149]. Despite the multiple attempts to select and create new potato varieties resistant to soft rot disease [40], the prevention of this disease relies mainly on sanitation and certified seed material [30]. This is caused by the fact that the resistance to soft rot is multilocus and heavily relies on plant response to hypoxia [111]. The fact that plants belonging to the same genera differ in their response to hypoxia gives hope that new varieties more resistant to SRP can be generated [65,150]. It has been proven that, although waterlogging-susceptible potato (Solanum tuberosum L.) cv. Servesta and tomato (S. lycopersicum L. Moneymaker) activate the same ethylene signaling pathway in response to hypoxia as Bittersweet nightshade (Solanum dulcamara L.) (a wetland species of the Solanum genus) and Arabidopsis thaliana (L.) Heynh., this does not lead to the same hypoxia adaptations [39]. Therefore, it can be possible to transfer the waterlogging-responsive genes from wetland Solanaceae species to create new more resistant potato varieties. Additionally, the screening of wild native potato species can be a good source of resistance for creating new varieties [151]. The mechanisms of Solanum microdontum Bitter (a wild species of potato native to Bolivia) resistance are unknown, but the elevated calcium levels detected in the plant might play their part [40]. The resistance to soft rot of another wild potato species, Solanum chacoense Bitter, is associated with a novel mechanism of rapid wound healing [41]. Luckily, glycoalkaloid concentrations, which are toxic to humans, do not correlate with the wild potato species’ resistance to soft rot [152]. It has been shown that the offspring from the crossbreeding of two Solanum tuberosum Phureja Group clones can be used to breed new potato varieties with increased soft rot resistance [153]. Also, Solanum palustre Poepp. ex Schltdl, previously known as Solanum brevidens Phil., has proven to be a promising source of soft rot resistance [154,155]. The Solanum genus represents many species from tropical and subtropical regions adapted to high water levels and high humidity. Therefore, wild Solanum species can be a good source of metabolic and anatomical adaptations to waterlogging [156,157].
4.3. Pathogen Control
Natural plant defenses can also be stimulated by the application of chemicals [158] but also plant-beneficial microorganisms in the mechanism called induced systemic resistance (ISR) [159]. Bacteria from the species Bacillus amyloliquefaciens [160], B. pumilus, B. subtilis, B. thurigiensis [161], B. vallismortis [162], Klebsiella oxytoca [163], Ochrobactrum lupini [164], and Pseudomonas chlorapis [165] can induce plant systemic resistance against soft rot Pectrobacteriacae. Biological control agents (BCAs) induce plant systemic resistance by activation of Salicylic acid (SA)- or Jasmonic acid (JA)-dependent pathways [159]. It has been shown that Salicylic acid (SA) induces plant resistance to soft rot [166,167,168]. It seems counterintuitive that SA induces resistance against necrotrophic pathogens, as the defenses against those pathogens rely mainly on the Jasmonic acid (JA) pathway [102]. Indeed, the inoculation of potatoes with Pectobacterium carotovorum induces the JA pathway [85], but also SA in the early stages of infection [105], suggesting that SRPs are hemibiotrophic pathogens [31,104]. BCAs can also induce the natural defenses of plants against pathogens by expressing, e.g., phenylalanine ammonia-lyase (PAL) [161]. PAL is a key element of the plant’s stress response [169] to biotic and abiotic stress. Moreover, many microbial strains, including Bacillus spp. [170], Pseudomonas spp. [171], and Trichoderma spp. [172], produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase. This enzyme, produced by many BCA targets, such as 1-Aminocyclopropane-1-carboxylic acid (ACC), is a direct ethylene precursor, alleviating the negative impact of waterlogging [173].
Moreover, most of the abovementioned bacterial strains can suppress soft rot disease by other modes of action, such as direct growth inhibition of the pathogen [160,161,162]. And indeed, it has been proven that microbial communities play a major role in potato resistance against soft rot disease [174]. The natural interactions between soil microorganisms, which may impact both pathogens and their hosts, can be considered to protect plants from soft rot [175]. There is an increasing emphasis on research dedicated to the discovery of new strains of bacteria active against soft rot disease, including, but not limited to, the genera Agrobacterium [176], Bacillus [160,176,177,178,179,180,181,182,183,184,185,186,187], Bdellovibrio [188], Brevibacillus [185], Lactobacillus [177], Lellilottia [189], Ochrobactrum [190,191], Paenibacillus [192], Pseudomonas [178,179,183,184,186,193,194,195,196], Rahnella [189], Rhodococcus [197,198,199], Serratia [110,189,200], Streptomyces [178,201,202], and Variovorax [176]. There are several reports of biocontrol strains against SRP of fungi: Aspergillus [203], Penicillium [193], and Trichoderma [179,181,184]; and bacteriophages: Axomammavirus PP1 [204]; Cbunavirus CB1, CB3 and CB4 [205]; Corticovirus PM2 [206]; Kotilavirus PP16 [207]; Limestonevirus LIMEstone1, LIMEstone2 [208]; ϕD3, ϕD5 [209]; Myunavirus My1 [210]; Pemunavirus PM1 [211]; Phimunavirus peat1 [212]; Unyawovirus DUPPII phiPccP-1 [213]; ϕPD10.3, ϕPD23.1 [214], vB_PcaM-D1, vB_PcaM-J3, vB_PcaP-A3 [215]; ZF40 [216], and others [217]. More information about bacteriophages infecting SRP has been summarized in [217,218].
The abovementioned biocontrol agents can protect plants from disease through different modes of action, such as antagonism toward pathogens, suppressing their pathogenesis, and inducing plant defenses [219]. The best-studied mechanism of action of biological control agents is the production of antibiotics [220], due to the success of antibiotics in medicine [221]. Because antibiotics have their limitations [221], other modes of action are gaining interest [219]. The fact that SRPs tightly regulate their pathogenicity and induce virulence factors in disease-favoring conditions gives them an advantage in the field but can also be used against them [222]. SRPs rely on quorum sensing to coordinate the production of virulence factors [223]. SRPs produce a range of quorum sensing N-Acyl-Homoserine Lactones: N-hexanoyl-homoserine lactone (C6-HSL), N-3-oxohexanoyl-homoserine lactone (3OC6-HSL), N-octanoyl-homoserine lactone (C8-HSL), N-3-oxo-octanoyl-homoserine lactone (3OC8-HSL), N-decanoyl-homoserine lactone (C10-HSL), and N-3-oxo-decanoyl-homoserine lactone (3OC10-HSL) [223]. While quorum sensing based on AHLs in SRPs can help these pathogens turn on their virulence when the number of bacteria is sufficient, SRPs rely on c-di-GMP hypoxia sensing [224,225]. During hypoxia, Dickeya dadantii activates the c-di-GMP effector VfmE, which is a quorum sensing master regulator [226]. This allows SRPs to regulate their virulence responding to environmental conditions and the pathogen population. Plants can, however, sense quorum-sensing molecules and adjust their metabolism, depending on the type of molecule, by inducing appropriate defense genes. Generally, shorter-chain AHLs induce plant resistance against necrotrophic pathogens with the use of SA signaling while longer-chain AHL induces JA signaling and resistance to biotrophic pathogens [227]. For example, 3OC8-HSL increases Arabidopsis resistance to Pectobacterium carotovorum by JA [228]. Moreover, it has been suggested that the mixture of different types of AHLs is the most prominent method of induction of plant defenses [229]. Additionally, many biological control agents use quorum quenching (disruption of quorum sensing) as their mode of action against soft rot disease, e.g., Agrobacterium [176], Bacillus [176], Ochrobactrum [190,191], Rhodococcus [197,198,199], and Variovorax [176].
Therefore, successful inhibition of soft rot can be achieved by employing various modes of action of biocontrol agents to prevent infection during the biotrophic stage and reduce pathogenicity during the necrotrophic stage [30]. Since microorganisms shape the soil structure, influencing its ability to store minerals and water [230] by producing strong osmolytes such as trehalose [231], it is worth considering if they can shape the environment to decrease the negative influence of waterlogging on oxygen availability [79]. For example, with the extended hyphal net, fungi can connect environments with different oxygen availability and perform aerobic respiration in oxygen-depleted soils and anaerobic respiration in the upper parts of the soil, shaping the bacterial soil community [232]. Filamentous fungi can alleviate waterlogging and drought stress due to their ability to transport oxygen and store water in local niches [233].
4.4. Shaping the Microenvironment
Moreover, protozoan predation has been proven to increase oxygen penetration in flooded rice fields [234]. The potential of microorganisms relies not only on the production of antimicrobials but on multiple co-occurring interactions between plants, microorganisms, and the environment [175]. Hence, to fully utilize their potential, a close examination of those interactions is necessary [235]. However, while microorganisms can shape the microenvironment, they not only influence the plant and pathogens but also each other. Unfortunately, not much information has been published on the subject of the environmental impact on the activity of BCAs [79]. Waterlogging harms oxygen availability for microorganisms due to lower oxygen diffusion and competition with the host [236]. Plants decrease oxygen loss through the roots by their adaptations to flooding [5]. Reduced oxygen availability influences the microbial composition of soil favoring anaerobic over aerobic microorganisms [237]. This can harm the plant because of the negative influence on the abundance of microorganisms from plant-beneficial taxa (such as Streptomyces), which depend on oxygen supplementation by the plant roots. Also, the population of microbial endophytes and arbuscular mycorrhiza is significantly reduced by flooding [238]. However, there are some plant-beneficial endophytes including bacteria from the phyla: Deltaproteobacteria, Firmicutes [237], and Aquapirillium (Proteobacteria) [239], and fungi, mainly dark septate endophytes (DSE) [240], arbuscular mycorrhizal fungi (AMF) [241], and yeast: Cryptococcus, Exophiala, Sporobolomyces, and Rhodorotorula [242], which can adapt to anaerobic conditions. Microbial communities and their metabolism may also be influenced indirectly by waterlogging due to changes in plant exudation. In some cases, plants can implement mechanisms called “cry for help” which involve increased root exudation to attract plant-beneficial microorganisms [243]. On the other hand, the plant’s anaerobic metabolism creates products that are exudated to minimize their toxicity for the plant [244]. However, these toxic products (e.g., ethanol) can act as chemoattractants for pathogens sensing the host in a poor metabolic state [245]. Although we have some knowledge of how hypoxia influences the plant microbial communities [79], and its influence on the plant pathogen metabolism [6], very little is known about its influence on plant-beneficial microorganisms. It is suggested that the application of beneficial PGPR can only be successful when the soil is properly aerated [246]. Fighting against pathogens well adapted to hypoxic conditions with bacteriophages that do not affect their metabolism could be considered a method of choice in these circumstances. Unfortunately, the influence of hypoxia on the activity of bacteriophages is largely understudied [247,248]. For example, Pseudomonas aeruginosa tends to develop resistance to the myovirus PAK_P1 infection [249]. However, in the case of Salmonella s25pp, hypoxia reduces the infection efficiency and the burst size of the ϕSan23 bacteriophage but also decreases the number of resistant mutants [248]. Unfortunately, the data on the influence of hypoxia on plant pathogens and bacteriophages are lacking.
5. Future Perspectives
Due to climate change [38] and global vegetable transport, we observe rising losses caused by soft rot disease [250]. Knowledge on the pathogenicity of soft rot Pectobacteriace is constantly growing, especially owing to new advanced molecular methods [31]. Our understanding of plant responses to hypoxia has also recently been furthered significantly [251]. However, there remains a gap in the research encompassing environment, pathogens, and plant responses [31]. A comprehensive approach, including various strategies, such as good agricultural practices, sanitation, usage of disease-free seed material, early pathogen detection, breeding resistant varieties, and targeted disease control, is required to protect crops from bacterial diseases [252]. However, we need to put great emphasis on the involvement of environmental conditions which are crucial for the development of the disease. Potatoes and SRPs respond differently to waterlogging and the resulting hypoxia, which has a strong influence on all approaches for the mitigation of soft rot disease (Table 1).

Due to the high prevalence of SRPs in the environment and not fully established sources of infection, the chance of infection during potato production is high [31]. This calls for the development of sensitive and reliable methods for SRP detection during seed tuber production. Additionally, the population dynamics of different SRP species require adjustment of the used methods to the currently most threatening species from this group [87]. While sophisticated and sensitive methods are best suited for the process of seed tuber certification, the development of new, fast, and simple methods of detection (e.g., immunoenzymatic techniques) are crucial for the rapid identification and isolation of SRP foci of infection [122].
However, the usage of pathogen-free seed material and rapid disease identification in the field can help to reduce the pathogen load. The high incidence of soft rot disease during years with high precipitation requires the creation of new more resistant potato varieties. Breeding new resistant varieties and biological plant protection are considered the most promising approaches to fighting soft rot disease [112]. The use of the CRISPR-Cas system enables the easy and fast creation of new varieties of plants and has been gaining social acceptance for its application in agriculture [253]. Using wild Solanum species as a source of new genes in potato breeding seems promising for obtaining new varieties that are more resistant to diseases [254]. The majority of Solanum plants are native to tropical regions and possess multiple metabolic and physiological adaptations to high humidity [156,157]. The Solanum genus encompasses many wild potato species that possess promising strategies to mitigate waterlogging stress [151]. Therefore, the creation of new potato varieties less prone to hypoxia during waterlogging can help to alleviate the environmental factor favorable for the development of soft rot disease.
The resistance of crops to hypoxia and soft rot can be further increased by favorable microbiota. Biological plant protection can be a valuable source of resistance against SRPs [255] and mitigation [159] of waterlogging stress, which favors SRP pathogenesis [83]. Many new biocontrol bacteria strains and bacteriophages [256] against SRPs have been isolated recently [112]. Combining those isolates in multispecies consortia can be a promising approach to disease protection in changing environmental conditions [257]. However, it is important to consider the influence of hypoxia on their activity and how they can alleviate this abiotic stress, to decrease the chance of disease development [4]. Biological plant protection agents against soft rot disease should remain active in hypoxic conditions and not be outcompeted by the pathogens when the oxygen availability is lowered. Additionally, new biological control stains should be tested for their ability to decrease potato resistance to waterlogging, e.g., by inducing their systemic resistance [166,167,168] or via production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase [170,171,172]. The ability of microorganisms to shape the soil structure can also be considered a promising method for the alleviation of waterlogging stress, which decreases the chance of the development of soft rot disease [230].
To thoroughly plan an integrated approach of soft rot control, we must combine current knowledge in molecular biology, plant physiology, and pathogen biology, especially in the presence of coexisting abiotic stress [2]. This task requires tighter cooperation between scientists representing those fields [252].
Author Contributions
Conceptualization: T.M.; writing—original draft: T.M.; investigation: T.M.; supervision: E.K. and K.O.-K.; writing—review and editing; T.M., E.K., K.O.-K., S.J. and R.C.; resources, E.K. and K.O.-K.; project administration, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted during the realization of a project financed by the National Science Center, Poland; NCN, via a research grant MINIATURA 7 project decision number: DEC-2023/07/X/NZ9/00834.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A Review of Plants Strategies to Resist Biotic and Abiotic Environmental Stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Leisner, C.P.; Potnis, N.; Sanz-Saez, A. Crosstalk and Trade-Offs: Plant Responses to Climate Change-Associated Abiotic and Biotic Stresses. Plant Cell Environ. 2023, 46, 2946–2963. [Google Scholar] [CrossRef] [PubMed]
- Francl, L.J. The Disease Triangle: A Plant Pathological Paradigm Revisited. Plant Health Instr. 2001, 10. [Google Scholar] [CrossRef]
- Loreti, E.; Perata, P. The Many Facets of Hypoxia in Plants. Plants 2020, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Lee, Y.H. Hypoxia: A Double-Edged Sword During Fungal Pathogenesis? Front. Microbiol. 2020, 11, 551990. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.S.; Shin, S.Y.; Kim, S.G.; Lee, G.; Kim, H.S.; Jeon, J.H.; Cho, H.S. Submergence Deactivates Wound-Induced Plant Defence against Herbivores. Commun. Biol. 2020, 3, 651. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.A. Waterlogging Stress in Plants: A Review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Tromans, D. Temperature and Pressure Dependent Solubility of Oxygen in Water: A Thermodynamic Analysis. Hydrometallurgy 1998, 48, 327–342. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and Crop Management Practices to Minimize the Impact of Waterlogging on Crop Productivity. Front. Plant Sci. 2019, 10, 433079. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.X.; Zhang, Y.C.; Chen, P.L.; Zhang, F.F.; Li, J.; Yan, F.; Dong, Y.; Feng, B.L. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- Weits, D.A.; Kunkowska, A.B.; Kamps, N.C.W.; Portz, K.M.S.; Packbier, N.K.; Nemec Venza, Z.; Gaillochet, C.; Lohmann, J.U.; Pedersen, O.; van Dongen, J.T.; et al. An Apical Hypoxic Niche Sets the Pace of Shoot Meristem Activity. Nature 2019, 569, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Boscari, A.; Frendo, P.; Brouquisse, R. Nitric Oxide Signaling, Metabolism and Toxicity in Nitrogen-Fixing Symbiosis. J. Exp. Bot. 2019, 70, 4505–4520. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Fernie, A.R.; Gibon, Y.; Christ, M.; Stitt, M. Metabolic Activity Decreases as an Adaptive Response to Low Internal Oxygen in Growing Potato Tubers. Biol. Chem. 2000, 381, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Beauvoit, B.P.; Biais, B.; Chabane, M.; Allwood, J.W.; Deborde, C.; Maucourt, M.; Goodacre, R.; Cabasson, C.; Moing, A.; et al. Central Metabolism Is Tuned to the Availability of Oxygen in Developing Melon Fruit. Front. Plant Sci. 2019, 10, 454202. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P. Response of Plant Metabolism to Too Little Oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.T.; Schurr, U.; Pfister, M.; Geigenberger, P. Phloem Metabolism and Function Have to Cope with Low Internal Oxygen. Plant Physiol. 2003, 131, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The Formation and Functions of a Fundamental Plant Tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound Signalling in Plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef]
- Lake, J.A.; Wade, R.N. Plant-Pathogen Interactions and Elevated CO2: Morphological Changes in Favour of Pathogens. J. Exp. Bot. 2009, 60, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Camy, C.; Dreyer, E.; Delatour, C.; Marçais, B. Responses of the Root Rot Fungus Collybia Fusipes to Soil Waterlogging and Oxygen Availability. Mycol. Res. 2003, 107, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, C.; Ju, Z.; Zheng, S.; Wen, Z.; Xu, S.; Chen, Y.; Ma, Z. The Mitochondrial Membrane Protein FgLetm1 Regulates Mitochondrial Integrity, Production of Endogenous Reactive Oxygen Species and Mycotoxin Biosynthesis in Fusarium graminearum. Mol. Plant Pathol. 2018, 19, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Lisicka, W.; Fikowicz-Krosko, J.; Jafra, S.; Narajczyk, M.; Czaplewska, P.; Czajkowski, R. Oxygen Availability Influences Expression of Dickeya Solani Genes Associated with Virulence in Potato (Solanum tuberosum L.) and Chicory (Cichorium intybus L.). Front. Plant Sci. 2018, 9, 355412. [Google Scholar] [CrossRef] [PubMed]
- Vayda, M.E.; Schaeffer, H.J. Hypoxic Stress Inhibits the Appearance of Wound-Response Proteins in Potato Tubers. Plant Physiol. 1988, 88, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, B.; Nkuriyingoma, P.; Gijsegem, F. Economic Impact of Pectobacterium and Dickeya Species on Potato Crops: A Review and Case Study. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Pérombelon, M.C.M. Potato Diseases Caused by Soft Rot Erwinias: An Overview of Pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Pérombelon, M.C.M. The Role of the Seed Tuber in the Contamination by Erwinia Carotovora of Potato Crops in Scotland. Potato Res. 1974, 17, 187–199. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.C.M.; Van Veen, J.A.; Van der Wolf, J.M. Control of Blackleg and Tuber Soft Rot of Potato Caused by Pectobacterium and Dickeya Species: A Review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Davidsson, P.R.; Kariola, T.; Niemi, O.; Tapio Palva, E. Pathogenicity of and Plant Immunity to Soft Rot Pectobacteria. Front. Plant Sci. 2013, 4, 48399. [Google Scholar] [CrossRef]
- Mattinen, L.; Tshuikina, M.; Mäe, A.; Pirhonen, M. Identification and Characterization of Nip, Necrosis-Inducing Virulence Protein of Erwinia Carotovora Subsp. Carotovora. Mol. Plant-Microbe Interact. 2004, 17, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Glasner, J.D.; Marquez-Villavicencio, M.; Kim, H.S.; Jahn, C.E.; Ma, B.; Biehl, B.S.; Rissman, A.I.; Mole, B.; Yi, X.; Yang, C.H.; et al. Niche-Specificity and the Variable Fraction of the Pectobacterium Pan-Genome. Mol. Plant-Microbe Interact. 2008, 21, 549–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Coulthurst, S.J.; Pritchard, L.; Hedley, P.E.; Ravensdale, M.; Humphris, S.; Burr, T.; Takle, G.; Brurberg, M.B.; Birch, P.R.J.; et al. Quorum Sensing Coordinates Brute Force and Stealth Modes of Infection in the Plant Pathogen Pectobacterium Atrosepticum. PLoS Pathog. 2008, 4, e1000093. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, K.C.; McGuire, R.G.; Kelman, A. Resistance of Tubers from Different Potato Cultivars to Soft Rot Caused by Erwinia carotovora Subsp. Atroseptica. Am. Potato J. 1990, 67, 287–305. [Google Scholar] [CrossRef]
- Lebecka, R.; Zimnoch-Guzowska, E. The Inheritance of Resistance to Soft Rot (Erwinia carotovora Subsp. Atroseptica) in Diploid Potato Families. Am. J. Potato Res. 2004, 81, 395–401. [Google Scholar] [CrossRef]
- Moh, A.A.; Massart, S.; Jijakli, M.H.; Lepoivre, P. Models to Predict the Combined Effects of Temperature and Relative Humidity on Pectobacterium atrosepticum and Pectobacterium carotovorum Subsp. Carotovorum Population Density and Soft Rot Disease Development at the Surface of Wounded Potato Tubers. J. Plant Pathol. 2012, 94, 181–191. [Google Scholar]
- Skelsey, P.; Humphris, S.N.; Campbell, E.J.; Toth, I.K. Threat of Establishment of Non-Indigenous Potato Blackleg and Tuber Soft Rot Pathogens in Great Britain under Climate Change. PLoS ONE 2018, 13, e0205711. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; van Dongen, N.; Renneberg, D.M.H.J.; Welschen-Evertman, R.A.M.; Kociemba, J.; Sasidharan, R.; Voesenek, L.A.C.J. Ethylene Differentially Modulates Hypoxia Responses and Tolerance across Solanum Species. Plants 2020, 9, 1022. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lofton, L.; Bamberg, J.; Swingle, B. Identification of Resistance to Dickeya dianthicola Soft Rot in Solanum microdontum. Am. J. Potato Res. 2022, 99, 58–68. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, C.; Jansky, S. New Source of Bacterial Soft Rot Resistance in Wild Potato (Solanum chacoense) Tubers. Genet. Resour. Crop Evol. 2017, 64, 1963–1969. [Google Scholar] [CrossRef]
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An Overview of Plant Responses to Soil Waterlogging. Plant Stress 2008, 2, 20–27. [Google Scholar]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The Role of Ethylene in Metabolic Acclimations to Low Oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.N.; Wang, X.; Binder, B.M.; Schaller, G.E. Mechanisms of Signal Transduction by Ethylene: Overlapping and Non-Overlapping Signalling Roles in a Receptor Family. AoB Plants 2013, 5, plt010. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M. Ethylene Signaling in Plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, S.; Nishimura, T.; Koshiba, T.; Yamamoto, R.; Hiraga, S.; Nakamura, T.; Komatsu, S. Effects of Anti-Auxins on Secondary Aerenchyma Formation in Flooded Soybean Hypocotyls. Plant Prod. Sci. 2016, 19, 154–160. [Google Scholar] [CrossRef]
- Liu, P.; Sun, F.; Gao, R.; Dong, H. RAP2.6L Overexpression Delays Waterlogging Induced Premature Senescence by Increasing Stomatal Closure More than Antioxidant Enzyme Activity. Plant Mol. Biol. 2012, 79, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Dawood, T.; Yang, X.; Visser, E.J.W.; Te Beek, T.A.H.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C.; et al. A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; McCormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose-TOR Signalling Reprograms the Transcriptome and Activates Meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ayano, M.; Kani, T.; Kojima, M.; Sakakibara, H.; Kitaoka, T.; Kuroha, T.; Angeles-Shim, R.B.; Kitano, H.; Nagai, K.; Ashikari, M. Gibberellin Biosynthesis and Signal Transduction Is Essential for Internode Elongation in Deepwater Rice. Plant Cell Environ. 2014, 37, 2313–2324. [Google Scholar] [CrossRef]
- Zhong, Q.; Hu, H.; Fan, B.; Zhu, C.; Chen, Z. Biosynthesis and Roles of Salicylic Acid in Balancing Stress Response and Growth in Plants. Int. J. Mol. Sci. 2021, 22, 11672. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, S.J.; Waqas, M.; Khan, A.L.; Lee, J.H.; Lee, J.D.; Nguyen, H.T.; Lee, I.J. Comparative Analysis of Endogenous Hormones Level in Two Soybean (Glycine max L.) Lines Differing in Waterlogging Tolerance. Front. Plant Sci. 2015, 6, 141434. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 134647. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Gómez-Cadenas, A. Hormonal Modulation of Citrus Responses to Flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Ma, X.; Xu, Q.; Qi, X.; Chen, X. Comparative Proteomic Analysis Provides Insight into the Key Proteins Involved in Cucumber (Cucumis sativus L.) Adventitious Root Emergence under Waterlogging Stress. Front. Plant Sci. 2016, 7, 225242. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Franceschi, V.R. Methyl Jasmonate-Induced Ethylene Production Is Responsible for Conifer Phloem Defense Responses and Reprogramming of Stem Cambial Zone for Traumatic Resin Duct Formation. Plant Physiol. 2004, 135, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.H.M.; Komatsu, S. Jasmonic Acid Induced Protein Response to Biophoton Emissions and Flooding Stress in Soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Guo, S.R. 24-Epibrassinolide Improves Cucumber Photosynthesis under Hypoxia by Increasing CO2 Assimilation and Photosystem II Efficiency. Photosynthetica 2014, 52, 96–104. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, J.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin Improves Waterlogging Tolerance of Malus Baccata (Linn.) Borkh. Seedlings by Maintaining Aerobic Respiration, Photosynthesis and ROS Migration. Front. Plant Sci. 2017, 8, 250970. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Kosmacz, M.; Weits, D.A.; Giuntoli, B.; Giorgi, F.M.; Voesenek, L.A.C.J.; Perata, P.; Van Dongen, J.T. Oxygen Sensing in Plants Is Mediated by an N-End Rule Pathway for Protein Destabilization. Nature 2011, 479, 419–422. [Google Scholar] [CrossRef]
- Cho, H.Y.; Loreti, E.; Shih, M.C.; Perata, P. Energy and Sugar Signaling during Hypoxia. New Phytol. 2021, 229, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Van Dongen, J.T.; Froehlich, A.; Oliver, S.N.; Faix, B.; Gupta, K.J.; Schmälzlin, E.; Igal, M.; Orcaray, L.; Royuela, M.; et al. Regulation of Respiration and Fermentation to Control the Plant Internal Oxygen Concentration. Plant Physiol. 2009, 149, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, C.; Gessler, A.; Biller, S.; Rennenberg, H.; Kreuzwieser, J. Differences in C Metabolism of Ash Species and Provenances as a Consequence of Root Oxygen Deprivation by Waterlogging. J. Exp. Bot. 2009, 60, 4335–4345. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Jiang, W.; Wang, Q.; Xu, L.; Shabala, S.; Zhang, W.Y. Differential Response of Growth and Photosynthesis in Diverse Cotton Genotypes under Hypoxia Stress. Photosynthetica 2019, 57, 772–779. [Google Scholar] [CrossRef]
- Blokhina, O.; Fagerstedt, K.V. Oxidative Metabolism, ROS and NO under Oxygen Deprivation. Plant Physiol. Biochem. 2010, 48, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Zhou, Y.; Chen, Q.F.; Xiao, S. New Insights into the Role of Lipids in Plant Hypoxia Responses. Prog. Lipid Res. 2021, 81, 101072. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Albrecht, G. Tolerance of Crop Plants to Oxygen Deficiency Stress: Fermentative Activity and Photosynthetic Capacity of Entire Seedlings under Hypoxia and Anoxia. Physiol. Plant 2003, 117, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakamura, M. Root Respiratory Costs of Ion Uptake, Root Growth, and Root Maintenance in Wetland Plants: Efficiency and Strategy of O2 Use for Adaptation to Hypoxia. Oecologia 2016, 182, 667–678. [Google Scholar] [CrossRef]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of Root Adaptive Anatomical and Morphological Traits during Low Soil Oxygen. New Phytol. 2021, 229, 42–49. [Google Scholar] [CrossRef]
- Armstrong, W.; Beckett, P.M.; Colmer, T.D.; Setter, T.L.; Greenway, H. Tolerance of Roots to Low Oxygen: ‘Anoxic’ Cores, the Phytoglobin-Nitric Oxide Cycle, and Energy or Oxygen Sensing. J. Plant Physiol. 2019, 239, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Björn, L.O.; Middleton, B.A.; Germ, M.; Gaberščik, A. Ventilation Systems in Wetland Plant Species. Diversity 2022, 14, 517. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Nagai, K.; Huan, P.D.; Shimazaki, K.; Qu, H.; Mori, Y.; Toda, Y.; Kuroha, T.; Hayashi, N.; Aiga, S.; et al. Rice Leaf Hydrophobicity and Gas Films Are Conferred by a Wax Synthesis Gene (LGF1) and Contribute to Flood Tolerance. New Phytol. 2018, 218, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, M.; Fukao, T.; Miyashita, T.; Shiono, K. A Barrier to Radial Oxygen Loss Helps the Root System Cope with Waterlogging-Induced Hypoxia. Breed. Sci. 2021, 71, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Sauter, M. Root Bending Is Antagonistically Affected by Hypoxia and ERF-Mediated Transcription via Auxin Signaling. Plant Physiol. 2017, 175, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-Gibberellin Signaling Underlies Adaptation of Rice to Periodic Flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and Pathogenic Plant-Microbe Interactions during Flooding Stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef] [PubMed]
- Gravot, A.; Richard, G.; Lime, T.; Lemarié, S.; Jubault, M.; Lariagon, C.; Lemoine, J.; Vicente, J.; Robert-Seilaniantz, A.; Holdsworth, M.J.; et al. Hypoxia Response in Arabidopsis Roots Infected by Plasmodiophora Brassicae Supports the Development of Clubroot. BMC Plant Biol. 2016, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Kerpen, L.; Niccolini, L.; Licausi, F.; van Dongen, J.T.; Weits, D.A. Hypoxic Conditions in Crown Galls Induce Plant Anaerobic Responses That Support Tumor Proliferation. Front. Plant Sci. 2019, 10, 427736. [Google Scholar] [CrossRef]
- Atwell, B.J.; Heritage, A.D. Reduced Susceptibility of Roots of Safflower to Phytophthora Cryptogea after Prior Adaptation of Roots to Hypoxic Conditions. Aust. J. Bot. 1994, 42, 29–36. [Google Scholar] [CrossRef]
- Rumeau, D.; Maher, E.A.; Kelman, A.; Showalter, A.M. Extensin and Phenylalanine Ammonia-Lyase Gene-Expression Altered in Potato-Tubers in Response to Wounding, Hypoxia, and Erwinia Carotovora Infection. Plant Physiol. 1990, 93, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Siedt, M.; Teggers, E.M.; Linnemann, V.; Schäffer, A.; van Dongen, J.T. Microbial Degradation of Plant Residues Rapidly Causes Long-Lasting Hypoxia in Soil upon Irrigation and Affects Leaching of Nitrogen and Metals. Soil Syst. 2023, 7, 62. [Google Scholar] [CrossRef]
- Butler, W.; Cook, L.; Vayda, M.E. Hypoxic Stress Inhibits Multiple Aspects of the Potato Tuber Wound Response. Plant Physiol. 1990, 93, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Vayda, M.E.; Antonov, L.S.; Yang, Z.; Butler, W.O.; Lacy, G.H. Hypoxic Stress Inhibits Aerobic Wound-Induced Resistance and Activates Hypoxic Resistance to Bacterial Soft Rot. Am. Potato. J. 1992, 69, 239–253. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Toth, I.K.; van der Wolf, J.M. Soft Rot Pectobacteriaceae: A Brief Overview. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Hugouvieux-Cotte-Pattat, N.; Dominguez, H.; Robert-Baudouy, J. Environmental Conditions Affect Transcription of the Pectinase Genes of Erwinia Chrysanthemi 3937. J. Bacteriol. 1992, 174, 7807–7818. [Google Scholar] [CrossRef] [PubMed]
- Babujee, L.; Apodaca, J.; Balakrishnan, V.; Liss, P.; Kiley, P.J.; Charkowski, A.O.; Glasner, J.D.; Perna, N.T. Evolution of the Metabolic and Regulatory Networks Associated with Oxygen Availability in Two Phytopathogenic Enterobacteria. BMC Genom. 2012, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The Role of the Cell Wall in Plant Immunity. Front. Plant Sci. 2014, 5, 86833. [Google Scholar] [CrossRef] [PubMed]
- Smid, E.J.; Jansen, A.H.J.; Tuijn, C.J. Anaerobic Nitrate Respiration by Erwinia carotovora Subsp. Atroseptica during Potato Tuber Invasion. Appl. Environ. Microbiol. 1993, 59, 3648–3653. [Google Scholar] [CrossRef] [PubMed]
- Fekete, F.J.; Marotta, N.J.; Liu, X.; Weinert, E.E. An O2-Sensing Diguanylate Cyclase Broadly Affects the Aerobic Transcriptome in the Phytopathogen Pectobacterium Carotovorum. Front. Microbiol. 2023, 14, 1134742. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Barragán, D.A.; de Sandozequi, A.; Rodríguez, M.; Estrada, K.; Tovar-Herrera, O.E.; Martínez-Anaya, C. Analysis of Two Mexican Pectobacterium Brasiliense Strains Reveals an Inverted Relationship between C-Di-GMP Levels with Exopolysaccharide Production and Swarming Motility. Microbiol. Res. 2020, 235, 126427. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; West, J.A.; Ramsay, J.P.; Monson, R.E.; Griffin, J.L.; Toth, I.K.; Salmond, G.P.C. Comprehensive Overexpression Analysis of Cyclic-Di-GMP Signalling Proteins in the Phytopathogen Pectobacterium Atrosepticum Reveals Diverse Effects on Motility and Virulence Phenotypes. Microbiology 2014, 160, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mendoza, D.; Coulthurst, S.J.; Sanjuán, J.; Salmond, G.P.C. N-Acetylglucosamine-Dependent Biofilm Formation in Pectobacterium Atrosepticum Is Cryptic and Activated by Elevated c-Di-GMP Levels. Microbiology 2011, 157, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Pérombelon, M.C.M. Potato Blackleg: Epidemiology, Host-Pathogen Interaction and Control. Neth. J. Plant Pathol. 1992, 98, 135–146. [Google Scholar] [CrossRef]
- Hebelstrup, K.H.; Møller, I.M. Mitochondrial Signaling in Plants Under Hypoxia: Use of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Springer: Cham, Switzerland, 2015; Volume 23, pp. 63–77. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- McLoughlin, F.; Kim, M.; Marshall, R.S.; Vierstra, R.D.; Vierling, E. HSP101 Interacts with the Proteasome and Promotes the Clearance of Ubiquitylated Protein Aggregates. Plant Physiol. 2019, 180, 1829–1847. [Google Scholar] [CrossRef] [PubMed]
- Vayda, M.E.; Shewmaker, C.K.; Morelli, J.K. Translational Arrest in Hypoxic Potato Tubers Is Correlated with the Aberrant Association of Elongation Factor EF-1α with Polysomes. Plant Mol. Biol. 1995, 28, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Peivastegan, B.; Hadizadeh, I.; Nykyri, J.; Nielsen, K.L.; Somervuo, P.; Sipari, N.; Tran, C.; Pirhonen, M. Effect of Wet Storage Conditions on Potato Tuber Transcriptome, Phytohormones and Growth. BMC Plant Biol. 2019, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Ghozlan, M.H.; EL-Argawy, E.; Tokgöz, S.; Lakshman, D.K.; Mitra, A.; Ghozlan, M.H.; EL-Argawy, E.; Tokgöz, S.; Lakshman, D.K.; Mitra, A. Plant Defense against Necrotrophic Pathogens. Am. J. Plant Sci. 2020, 11, 2122–2138. [Google Scholar] [CrossRef]
- Hayward, A.C. Latent Infections by Bacteria. Annu. Rev. Phytopathol. 1974, 12, 87–97. [Google Scholar] [CrossRef]
- Kraepiel, Y.; Barny, M.A. Gram-Negative Phytopathogenic Bacteria, All Hemibiotrophs after All? Mol. Plant Pathol. 2016, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, I.; Peivastegan, B.; Wang, J.; Sipari, N.; Nielsen, K.L.; Pirhonen, M. Gene Expression and Phytohormone Levels in the Asymptomatic and Symptomatic Phases of Infection in Potato Tubers Inoculated with Dickeya Solani. PLoS ONE 2022, 17, e0273481. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Bailey-Serres, J. Plant Responses to Hypoxia—Is Survival a Balancing Act? Trends Plant Sci. 2004, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, P.; Förch, M.G.; Krijger, M.C.; van der Zouwen, P.S.; van den Berg, W.; van der Wolf, J.M. Systemic Colonization of Potato Plants Resulting from Potato Haulm Inoculation with Dickeya Solani or Pectobacterium Parmentieri. Can. J. Plant Pathol. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Wolf, J.M.; Cahill, G.; Gijsegem, F.; Helias, V.; Humphris, S.; Li, X.; Lojkowska, E.; Pritchard, L. Isolation, Detection and Characterization of Pectobacterium and Dickeya Species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tsers, I.; Parfirova, O.; Moruzhenkova, V.; Petrova, O.; Gogoleva, N.; Vorob’ev, V.; Gogolev, Y.; Gorshkov, V. A Switch from Latent to Typical Infection during Pectobacterium Atrosepticum—Tobacco Interactions: Predicted and True Molecular Players. Int. J. Mol. Sci. 2023, 24, 13283. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, I.; Peivastegan, B.; Hannukkala, A.; van der Wolf, J.M.; Nissinen, R.; Pirhonen, M. Biological Control of Potato Soft Rot Caused by Dickeya Solani and the Survival of Bacterial Antagonists under Cold Storage Conditions. Plant Pathol. 2019, 68, 297–311. [Google Scholar] [CrossRef]
- Charkowski, A.O. The Changing Face of Bacterial Soft-Rot Diseases. Annu. Rev. Phytopathol. 2018, 56, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.M.; Boer, S.H.; Czajkowski, R.; Cahill, G.; Gijsegem, F.; Davey, T.; Dupuis, B.; Ellicott, J.; Jafra, S.; Kooman, M.; et al. Management of Diseases Caused by Pectobacterium and Dickeya Species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Wasilewska-Nascimento, B.; Boguszewska-Mańkowska, D.; Zarzyńska, K. Challenges in the Production of High-Quality Seed Potatoes (Solanum tuberosum L.) in the Tropics and Subtropics. Agronomy 2020, 10, 260. [Google Scholar] [CrossRef]
- Tiwari, D.; Ashish, M.; Gangwar, N.; Sharma, A.; Patel, S.; Bhardwaj, S. Potato Leaf Diseases Detection Using Deep Learning. In Proceedings of the International Conference on Intelligent Computing and Control Systems, ICICCS 2020, Madurai, India, 13–15 May 2020. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. Plant Pathogenicity and Associated/Related Detection Systems. A Review. Talanta 2023, 251, 123808. [Google Scholar] [CrossRef]
- Rossmann, S.; Dees, M.W.; Torp, T.; Le, V.H.; Skogen, M.; Glorvigen, B.; van der Wolf, J.; Brurberg, M.B. Field-Scale Molecular Testing of Virulent Potato Soft Rot Pectobacteriaceae in Norway. Eur. J. Plant Pathol. 2020, 156, 501–517. [Google Scholar] [CrossRef]
- Kabir, M.N.; Taheri, A.; Korsi Dumenyo, C. Development of Pcr-Based Detection System for Soft Rot Pectobacteriaceae Pathogens Using Molecular Signatures. Microorganisms 2020, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Lukianova, A.A.; Evseev, P.V.; Stakheev, A.A.; Kotova, I.B.; Zavriev, S.K.; Ignatov, A.N.; Miroshnikov, K.A. Quantitative Real-time Pcr Assay for the Detection of Pectobacterium Parmentieri, a Causal Agent of Potato Soft Rot. Plants 2021, 10, 1880. [Google Scholar] [CrossRef] [PubMed]
- Dobhal, S.; Boluk, G.; Babler, B.; Stulberg, M.J.; Rascoe, J.; Nakhla, M.K.; Chapman, T.A.; Crockford, A.B.; Melzer, M.J.; Alvarez, A.M.; et al. Comparative Genomics Reveals Signature Regions Used to Develop a Robust and Sensitive Multiplex TaqMan Real-Time QPCR Assay to Detect the Genus Dickeya and Dickeya Dianthicola. J. Appl. Microbiol 2020, 128, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Muzhinji, N.; Dube, J.P.; de Haan, E.G.; Woodhall, J.W.; van der Waals, J.E. Development of a TaqMan PCR Assay for Specific Detection and Quantification of Pectobacterium Brasiliense in Potato Tubers and Soil. Eur. J. Plant Pathol. 2020, 158, 521–532. [Google Scholar] [CrossRef]
- Domingo, R.; Perez, C.; Klair, D.; Vu, H.; Candelario-Tochiki, A.; Wang, X.; Camson, A.; Uy, J.N.; Salameh, M.; Arizala, D.; et al. Genome-Informed Loop-Mediated Isothermal Amplification Assay for Specific Detection of Pectobacterium parmentieri in Infected Potato Tissues and Soil. Sci. Rep. 2021, 11, 21948. [Google Scholar] [CrossRef] [PubMed]
- Tameh, M.H.; Primiceri, E.; Chiriacò, M.S.; Poltronieri, P.; Bahar, M.; Maruccio, G. Pectobacterium Atrosepticum Biosensor for Monitoring Blackleg and Soft Rot Disease of Potato. Biosensors 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Veltman, B.; Harpaz, D.; Melamed, S.; Tietel, Z.; Tsror, L.; Eltzov, E. Whole-Cell Bacterial Biosensor for Volatile Detection from Pectobacterium-Infected Potatoes Enables Early Identification of Potato Tuber Soft Rot Disease. Talanta 2022, 247, 123545. [Google Scholar] [CrossRef] [PubMed]
- Roman-Reyna, V.; Rioux, R.; Babler, B.; Klass, T.; Jacobs, J. Concept Note: Toward Metagenomic Sequencing for Rapid, Sensitive, and Accurate Detection of Bacterial Pathogens in Potato Seed Production. PhytoFrontiers 2023, 3, 82–90. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Talukder, K.H. Detection of Potato Disease Using Image Segmentation and Machine Learning. In Proceedings of the 2020 International Conference on Wireless Communications, Signal Processing and Networking, WiSPNET 2020, Chennai, India, 4–6 August 2020; pp. 43–47. [Google Scholar] [CrossRef]
- Oppenheim, D.; Shani, G.; Erlich, O.; Tsror, L. Using Deep Learning for Image-Based Potato Tuber Disease Detection. Phytopathology 2019, 109, 1083–1087. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Bartoli, C. Plant Pathogenic Bacteria in Open Irrigation Systems: What Risk for Crop Health? Plant Pathol. 2015, 64, 757–766. [Google Scholar] [CrossRef]
- Burr, T.J. Occurrence of Soft-Rot Erwinia Spp. in Soil and Plant Material. Phytopathology 1977, 77, 1382–1387. [Google Scholar] [CrossRef]
- Fikowicz-Krosko, J.; Wszalek-Rozek, K.; Smolarska, A.; Czajkowski, R. First Report on Isolation of Soft Rot Pectobacterium Carotovorum Subsp. Carotovorum from Symptomless Bittersweet Nightshade Occuring in Rural Area in Poland. J. Plant Pathol. 2017, 99, 294. [Google Scholar] [CrossRef]
- Ngadze, E.; Coutinho, T.A.; Icishahayo, D.; Van der Waals, J.E. Effect of Calcium Soil Amendments on Phenolic Compounds and Soft Rot Resistance in Potato Tubers. Crop Prot. 2014, 62, 40–45. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Enfissi, E.M.A.; Drapal, M.; Perez-Fons, L.; Nogueira, M.; Berry, H.M.; Almeida, J.; Fraser, P.D. New Plant Breeding Techniques and Their Regulatory Implications: An Opportunity to Advance Metabolomics Approaches. J. Plant Physiol. 2021, 258, 153378. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Review: Improving Global Food Security through Accelerated Plant Breeding. Plant Science 2019, 287, 110207. [Google Scholar] [CrossRef] [PubMed]
- Samineni, S.; Sen, M.; Sajja, S.B.; Gaur, P.M. Rapid Generation Advance (RGA) in Chickpea to Produce up to Seven Generations per Year and Enable Speed Breeding. Crop J. 2020, 8, 164–169. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed Breeding in Growth Chambers and Glasshouses for Crop Breeding and Model Plant Research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Bernardo, R. Reinventing Quantitative Genetics for Plant Breeding: Something Old, Something New, Something Borrowed, Something BLUE. Heredity 2020, 125, 375–385. [Google Scholar] [CrossRef]
- Caruana, B.M.; Pembleton, L.W.; Constable, F.; Rodoni, B.; Slater, A.T.; Cogan, N.O.I. Validation of Genotyping by Sequencing Using Transcriptomics for Diversity and Application of Genomic Selection in Tetraploid Potato. Front. Plant Sci. 2019, 10, 420807. [Google Scholar] [CrossRef] [PubMed]
- van Eeuwijk, F.A.; Bustos-Korts, D.; Millet, E.J.; Boer, M.P.; Kruijer, W.; Thompson, A.; Malosetti, M.; Iwata, H.; Quiroz, R.; Kuppe, C.; et al. Modelling Strategies for Assessing and Increasing the Effectiveness of New Phenotyping Techniques in Plant Breeding. Plant Sci. 2019, 282, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Willmitzer, L.; Fernie, A.R. High-Resolution Metabolic Phenotyping of Genetically and Environmentally Diverse Potato Tuber Systems. Identification of Phenocopies. Plant Physiol. 2001, 127, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R. Genomic-Led Potato Breeding for Increasing Genetic Gains: Achievements and Outlook. Crop Breed. Genet. Genom. 2020, 2, e200010. [Google Scholar] [CrossRef]
- Wegener, C.B.; Jansen, G. Soft-Rot Resistance of Coloured Potato Cultivars (Solanum tuberosum L.): The Role of Anthocyanins. Potato Res. 2007, 50, 31–44. [Google Scholar] [CrossRef]
- Colleta, C.M.; Upenyu, M.; Goss, M.; Elizabeth, N. The Epidemiology of Pectobacterium and Dickeya Species and the Role of Calcium in Postharvest Soft Rot Infection of Potato (Solanum tuberosum) Caused by the Pathogens: A Review. Afr. J. Agric. Res. 2014, 9, 1509–1515. [Google Scholar] [CrossRef][Green Version]
- Wolters, P.J.; Collins, W.W. Estimation of Genetic Parameters for Resistance to Erwinia Soft Rot, Specific Gravity, and Calcium Concentration in Diploid Potatoes. Crop Sci. 1995, 35, 1346–1352. [Google Scholar] [CrossRef]
- Cother, E.J.; Cullis, B.R. The Influence of Tuber Position on Periderm Calcium Content and Its Relationship to Soft Rot Susceptibility. Potato Res. 1992, 35, 271–277. [Google Scholar] [CrossRef]
- Zimnoch-Guzowska, E.; Łojkowska, E. Resistance to Erwinia Spp. in Diploid Potato with a High Starch Content. Potato Res. 1993, 36, 177–182. [Google Scholar] [CrossRef]
- McMillan, G.P.; Hedley, D.; Fyffe, L.; Pérombelon, M.C.M. Potato Resistance to Soft-Rot Erwinias Is Related to Cell Wall Pectin Esterification. Physiol. Mol. Plant Pathol. 1993, 42, 279–289. [Google Scholar] [CrossRef]
- Łojkowska, E.; Hołubowska, M. The Role of Polyphenol Oxidase and Peroxidase in Potato Tuber Resistance to Soft Rot Caused by Erwinia carotovora. J. Phytopathol. 1992, 136, 319–328. [Google Scholar] [CrossRef]
- Lebecka, R.; Śliwka, J.; Grupa-Urbańska, A.; Szajko, K.; Marczewski, W. QTLs for Potato Tuber Resistance to Dickeya solani Are Located on Chromosomes II and IV. Plant Pathol. 2021, 70, 1745–1756. [Google Scholar] [CrossRef]
- Narsai, R.; Rocha, M.; Geigenberger, P.; Whelan, J.; Van Dongen, J.T. Comparative Analysis between Plant Species of Transcriptional and Metabolic Responses to Hypoxia. New Phytol. 2011, 190, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Lojkowska, E.; Kelman, A. Screening of Seedlings of Wild Solanum Species for Resistance to Bacterial Stem Rot Caused by Soft Rot Erwinias. Am. Potato J. 1989, 66, 379–390. [Google Scholar] [CrossRef]
- Andrivon, D.; Corbière, R.; Lucas, J.M.; Pasco, C.; Gravoueille, J.M.; Pellé, R.; Dantec, J.P.; Ellissèche, D. Resistance to Late Blight and Soft Rot in Six Potato Progenies and Glycoalkaloid Contents in the Tubers. Am. J. Potato Res. 2003, 80, 125–134. [Google Scholar] [CrossRef]
- Demaine, M.J.; Lees, A.K.; Bradshaw, J.E. Soft-Rot Resistance Combined with Other Tuber Characters in Long Day-Adapted Solanum phureja. Potato Res. 1998, 41, 69–82. [Google Scholar] [CrossRef]
- Tek, A.L.; Stevenson, W.R.; Helgeson, J.P.; Jiang, J. Transfer of Tuber Soft Rot and Early Blight Resistances from Solanum Brevidens into Cultivated Potato. Theor. Appl. Genet. 2004, 109, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Lojkowska, E.; Ehlcnfeldt, M.K.; Kelman, A.; Helgeson, J.P. Fertile Interspecific Somatic Hybrids of Solatium: A Novel Source of Resistance to Erwinia Soft Rot. Phytopathology 2021, 78, 1216–1220. [Google Scholar] [CrossRef]
- Kaunda, J.S.; Zhang, Y.J. The Genus Solanum: An Ethnopharmacological, Phytochemical and Biological Properties Review. Nat. Prod. Bioprospect. 2019, 9, 77–137. [Google Scholar] [CrossRef]
- Weese, T.L.; Bohs, L. A Three-Gene Phylogeny of the Genus Solanum (Solanaceae). Syst. Bot. 2007, 32, 445–463. [Google Scholar] [CrossRef]
- Andreu, A.B.; Guevara, M.G.; Wolski, E.A.; Daleo, G.R.; Caldiz, D.O. Enhancement of Natural Disease Resistance in Potatoes by Chemicals. Pest Manag. Sci. 2006, 62, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, P.; Huang, K.; Wang, Y.; Hu, H.; Sun, Y. Control of Postharvest Soft Rot Caused by Erwinia carotovora of Vegetables by a Strain of Bacillus amyloliquefaciens and Its Potential Modes of Action. World J. Microbiol. Biotechnol. 2013, 29, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Gerayeli, N.; Baghaee-Ravari, S.; Tarighi, S. Evaluation of the Antagonistic Potential of Bacillus Strains against Pectobacterium carotovorum Subsp. Carotovorum and Their Role in the Induction of Resistance to Potato Soft Rot Infection. Eur. J. Plant Pathol. 2018, 150, 1049–1063. [Google Scholar] [CrossRef]
- Park, J.W.; Balaraju, K.; Kim, J.W.; Lee, S.W.; Park, K. Systemic Resistance and Growth Promotion of Chili Pepper Induced by an Antibiotic Producing Bacillus vallismortis Strain BS07. Biol. Control 2013, 65, 246–257. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, Y.C.; Lee, S.; Kim, I.S. Identification of an ISR-Related Metabolite Produced by Rhizobacterium Klebsiella oxytoca C1036 Active against Soft-Rot Disease Pathogen in Tobacco. Pest Manag. Sci. 2009, 65, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Sumayo, M.; Hahm, M.S.; Ghim, S.Y. Determinants of Plant Growth-Promoting Ochrobactrum lupini KUDC1013 Involved in Induction of Systemic Resistance against Pectobacterium carotovorum Subsp. Carotovorum in Tobacco Leaves. Plant Pathol. J. 2013, 29, 174. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Anderson, A.J.; Yang, K.Y.; Cho, B.H.; Kim, K.Y.; Lee, M.C.; Kim, Y.H.; Kim, Y.C. Multiple Determinants Influence Root Colonization and Induction of Induced Systemic Resistance by Pseudomonas chlororaphis O6. Mol. Plant Pathol. 2006, 7, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Merzah, N.R.; Kadhim, J.H. Efficiency of Salicylic Acid in Induce Systemic Resistance in Potato Plants against Pectobacterium carotovorum. Plant Arch. 2020, 20, 244–288. [Google Scholar]
- Sallam, M.A.A.; Abd El-Moneem, K.M.H.; Hassan, M.A.E.; Khalil, H.M.M. Resistance Induced in Potato Tubers to Soft Rot Caused by Erwinia carotovora Subsp. Carotovora by Treatments with Salicylic Acid and Acetylsalicylic Acid. Assiut J. Agric. Sci. 2010, 41, 81–92. [Google Scholar] [CrossRef]
- Lápez, M.M.; Lápez-Lápez, M.J.; Martá, R.; Zamora, J.; Lápez-Sanchez, J.; Beltra, R. Effect of Acetylsalicylic Acid on Soft Rot Produced by Erwinia carotovora Subsp, Carotovora in Potato Tubers under Greenhouse Conditions. Potato Res. 2001, 44, 197–206. [Google Scholar] [CrossRef]
- Mo, F.; Li, L.; Zhang, C.; Yang, C.; Chen, G.; Niu, Y.; Si, J.; Liu, T.; Sun, X.; Wang, S.; et al. Genome-Wide Analysis and Expression Profiling of the Phenylalanine Ammonia-Lyase Gene Family in Solanum Tuberosum. Int. J. Mol. Sci. 2022, 23, 6833. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC Deaminase-Producing Rhizosphere Competent Bacillus Spp. Mitigate Salt Stress and Promote Zea mays Growth by Modulating Ethylene Metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Nascimento, F.X. Pseudomonas 1-aminocyclopropane-1-carboxylate (Acc) Deaminase and Its Role in Beneficial Plant-microbe Interactions. Microorganisms 2021, 9, 2467. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular Mechanisms of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Deaminase Producing Trichoderma Asperellum MAP1 in Enhancing Wheat Tolerance to Waterlogging Stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the Plant Growth-Promoting Enzyme ACC Deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Kurm, V.; Mendes, O.; Gros, J.; van der Wolf, J. Potato Tuber Origin and Microbial Composition Determines Resistance against Soft Rot Pectobacteriaceae. Eur. J. Plant Pathol. 2023, 168, 383–399. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial Interactions and Biocontrol in the Rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Ha, N.T.; Minh, T.Q.; Hoi, P.X.; Thanh Thuy, N.T.; Furuya, N.; Long, H.H. Biological Control of Potato Tuber Soft Rot Using N-Acyl-L-Homoserine Lactone-Degrading Endophytic Bacteria. Curr. Sci. 2018, 115, 1921–1927. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ali, M.E.; Khan, A.A.; Akanda, A.M.; Uddin, M.K.; Hashim, U.; Abd Hamid, S.B. Isolation, Characterization, and Identification of Biological Control Agent for Potato Soft Rot in Bangladesh. Sci. World J. 2012, 2012, 723293. [Google Scholar] [CrossRef]
- Salem, E.A.; El-Shafea, Y.M.A. Biological Control of Potato Soft Rot Caused by Erwinia carotovora Subsp. Carotovora. Egypt. J. Biol. Pest Control 2018, 28, 94. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; El-Nasr, H.I.S. Biological Control of Pectobacterium carotovorum Subsp. Carotovorum, the Causal Agent of Bacterial Soft Rot in Vegetables, In Vitro and In Vivo Tests. Bull. Natl. Res. Cent. 2021, 45, 37. [Google Scholar] [CrossRef]
- Azaiez, S.; Ben Slimene, I.; Karkouch, I.; Essid, R.; Jallouli, S.; Djebali, N.; Elkahoui, S.; Limam, F.; Tabbene, O. Biological Control of the Soft Rot Bacterium Pectobacterium carotovorum by Bacillus Amyloliquefaciens Strain Ar10 Producing Glycolipid-like Compounds. Microbiol. Res. 2018, 217, 23–33. [Google Scholar] [CrossRef] [PubMed]
- El-Khair, A.; Haggag, H. Application of Some Bactericides and Bioagents for Controlling the Soft Rot Disease in Potato. Res. J. Agric. Biol. Sci. 2007, 3, 463–473. [Google Scholar]
- Rashid, M.; Chowdhury, M.; Sultana, N. In-Vitro Screening of Some Chemicals and Biocontrol Agents against Erwinia carotovora Subsp. Carotovora, the Causal Agent of Soft Rot of Potato (Solanum tuberosum). Agriculturists 2013, 11, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Hu, M.; Xue, Y.; Chen, X.; Lu, G.; Zhang, L.; Zhou, J. Screening, Identification and Efficacy Evaluation of Antagonistic Bacteria for Biocontrol of Soft Rot Disease Caused by Dickeya zeae. Microorganisms 2020, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, A.H. Investigation on the Effect of Chemical and Biological Control of Bacterial Soft Root Disease of Potato in Storage. J. Biol. 2014, 4, 31–44. [Google Scholar]
- Khoiri, S.; Damayanti, T.A.; Giyanto, G. Identification of Quorum Quenching Bacteria and Its Biocontrol Potential against Soft Rot Disease Bacteria, Dickeya dadantii. Agrivita 2017, 39, 45–55. [Google Scholar] [CrossRef]
- Sbai Idrissi, N.; Ouarzane, A.; Elouazni, L.; Hmyene, A.; Elantri, S.; Amine, A. Exploring Rhizosphere and Potato Microbiome as Potential Antagonist to Control Blackleg and Potato Soft Rot Diseases in Morocco. Egypt. J. Biol. Pest Control 2021, 31, 41. [Google Scholar] [CrossRef]
- Dadaşoğlu, F.; Fredy, J.; Coy, M.; Özyurt, G.; Kotan, R. In Vitro Effect of Bacterial Biocontrol Organisms against Pectobacterium carotovorum on Potato. Production 2020, 1, 8–11. [Google Scholar] [CrossRef]
- Sason, G.; Jurkevitch, E.; Nussinovitch, A. Biological Control of Soft Rot in Potato by κ-Carrageenan Carriers Encapsulated Microbial Predators. Appl. Microbiol. Biotechnol. 2023, 107, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, D.M.; Maciag, T.; Siwinska, J.; Krychowiak, M.; Jafra, S.; Czajkowski, R. Compatible Mixture of Bacterial Antagonists Developed to Protect Potato Tubers from Soft Rot Caused by Pectobacterium Spp. and Dickeya Spp. Plant Dis. 2019, 103, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ye, T.; Li, Q.; Bhatt, P.; Zhang, L.; Chen, S. Potential of a Quorum Quenching Bacteria Isolate Ochrobactrum Intermedium D-2 Against Soft Rot Pathogen Pectobacterium carotovorum Subsp. Carotovorum. Front. Microbiol. 2020, 11, 521250. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, D.M.; Maciąg, T.; Ossowicki, A.; Rajewska, M.; Kaczyński, Z.; Czerwicka, M.; Rąbalski, Ł.; Czaplewska, P.; Jafra, S. Ochrobactrum quorumnocens Sp. Nov., a Quorum Quenching Bacterium from the Potato Rhizosphere, and Comparative Genome Analysis with Related Type Strains. PLoS ONE 2019, 14, e0210874. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Ali, M.A.; Lin, L.; Luo, J.; You, Y.; Masum, M.M.I.; Jiang, Y.; Wang, Y.; Li, B.; An, Q. Biocontrol of Soft Rot Dickeya and Pectobacterium Pathogens by Broad-Spectrum Antagonistic Bacteria within Paenibacillus polymyxa Complex. Microorganisms 2023, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A. Management of Soft Rot Disease, Caused by Erwinia carotovora Subsp. Carotovora in Potato Tubers. Afr. J. Biol. Sci. 2019, 15, 211–218. [Google Scholar] [CrossRef]
- Cigna, J.; Robic, K.; Dewaegeneire, P.; Hélias, V.; Beury, A.; Faure, D. Efficacy of Soft-Rot Disease Biocontrol Agents in the Inhibition of Production Field Pathogen Isolates. Microorganisms 2023, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, D.M.; Potrykus, M.; Golanowska, M.; Polonis, K.; Gwizdek-Wisniewska, A.; Lojkowska, E.; Jafra, S. Rhizosphere Bacteria as Potential Biocontrol Agents against Soft Rot Caused by Various Pectobacterium and Dickeya Spp. Strains. J. Plant Pathol. 2012, 94, 367–378. [Google Scholar] [CrossRef]
- Cronin, D.; Moënne-Loccoz, Y.; Fenton, A.; Dunne, C.; Dowling, D.N.; O’Gara, F. Ecological Interaction of a Biocontrol Pseudomonas fluorescens Strain Producing 2,4-Diacetylphloroglucinol with the Soft Rot Potato Pathogen Erwinia carotovora Subsp. Atroseptica. FEMS Microbiol. Ecol. 1997, 23, 95–106. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Cirou, A.; Tannières, M.; Orange, N.; Feuilloley, M.; Dessaux, Y.; Burini, J.F.; Faure, D.; Latour, X. Biological Control of Pathogen Communication in the Rhizosphere: A Novel Approach Applied to Potato Soft Rot due to Pectobacterium atrosepticum. Plant Soil 2012, 358, 27–37. [Google Scholar] [CrossRef]
- Chane, A.; Barbey, C.; Robert, M.; Merieau, A.; Konto-Ghiorghi, Y.; Beury-Cirou, A.; Feuilloley, M.; Pátek, M.; Gobert, V.; Latour, X. Biocontrol of Soft Rot: Confocal Microscopy Highlights Virulent Pectobacterial Communication and Its Jamming by Rhodococcal Quorum-Quenching. Mol. Plant-Microbe Interact. 2019, 31, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, X.; Li, J.; Zhang, Y.; Huang, Y.; Zhang, W.; Shi, Y.; Wang, J.; Chen, S. A Novel Quorum Quencher, Rhodococcus Pyridinivorans XN-36, Is a Powerful Agent for the Biocontrol of Soft Rot Disease in Various Host Plants. Biol. Control 2022, 169, 104889. [Google Scholar] [CrossRef]
- Czajkowski, R.; van der Wolfa, J.M. Draft Genome Sequence of the Biocontrol Strain Serratia Plymuthica A30, Isolated from Rotting Potato Tuber Tissue. J. Bacteriol. 2012, 194, 6999–7000. [Google Scholar] [CrossRef] [PubMed]
- Doolotkeldieva, T.; Bobusheva, S.; Suleymankisi, A. Biological Control of Erwinia carotovora Ssp. Carotovora by Streptomyces Species. Adv. Microbiol. 2016, 6, 104. [Google Scholar] [CrossRef]
- Mansour, F.A.; Mohamedin, A.H.; Esmaeel, A.E.; Badr, H.H. Control of Potato Bacterial Soft Rot Disease Caused by Erwinia carotovora Subsp. Carotovora with Streptomyces Sioyaensis and Cinnamon Oil. Egypt. J. Microbiol. 2008, 43, 1–20. [Google Scholar] [CrossRef]
- Choi, H.W.; Ahsan, S.M. Biocontrol Activity of Aspergillus Terreus ANU-301 against Two Distinct Plant Diseases, Tomato Fusarium Wilt and Potato Soft Rot. Plant Pathol. J. 2022, 38, 33. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Jee, S.; Lee, D.H.; Roh, E.; Jung, K.; Oh, C.; Heu, S. Biocontrol of Pectobacterium carotovorum Subsp. Carotovorum Using Bacteriophage PP1. J. Microbiol. Biotechnol. 2013, 23, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Buttimer, C.; Hendrix, H.; Lucid, A.; Neve, H.; Noben, J.P.; Franz, C.; O’Mahony, J.; Lavigne, R.; Coffey, A. Novel N4-like Bacteriophages of Pectobacterium atrosepticum. Pharmaceuticals 2018, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Lee, D.H.; Heu, S. Isolation and Genomic Characterization of the T4-Like Bacteriophage PM2 Infecting Pectobacterium carotovorum Subsp. Carotovorum. Plant Pathol. J. 2015, 31, 83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voronina, M.V.; Bugaeva, E.N.; Vasiliev, D.M.; Kabanova, A.P.; Barannik, A.P.; Shneider, M.M.; Kulikov, E.E.; Korzhenkov, A.A.; Toschakov, S.V.; Ignatov, A.N.; et al. Characterization of Pectobacterium carotovorum Subsp. Carotovorum Bacteriophage PP16 Prospective for Biocontrol of Potato Soft Rot. Microbiology 2019, 88, 451–460. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by “Dickeya solani”. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; Lojkowska, E. Isolation and Characterization of Novel Soilborne Lytic Bacteriophages Infecting Dickeya Spp. Biovar 3 (‘D. solani’). Plant Pathol. 2014, 63, 758–772. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.-H.; Shin, H.; Ji, S.; Roh, E.; Jung, K.; Ryu, S.; Choi, J.; Heu, S. Complete Genome Sequence of Pectobacterium carotovorum Subsp. Carotovorum Bacteriophage My1. J. Virol. 2012, 86, 11410–11411. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Shin, H.; Lee, D.H.; Han, S.W.; Lee, J.H.; Ryu, S.; Heu, S. Complete Genome Sequence of the Pectobacterium carotovorum Subsp. Carotovorum Virulent Bacteriophage PM1. Arch. Virol. 2014, 159, 2185–2187. [Google Scholar] [CrossRef] [PubMed]
- Kalischuk, M.; Hachey, J.; Kawchuk, L. Complete Genome Sequence of Phytopathogenic Pectobacterium atrosepticum Bacteriophage Peat1. Genome Announc. 2015, 3, e00760-15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Vu, N.T.; Oh, E.J.; Rahimi-Midani, A.; Thi, T.N.; Song, Y.R.; Hwang, I.S.; Choi, T.J.; Oh, C.S. Biocontrol of Soft Rot Caused by Pectobacterium odoriferum with Bacteriophage Phipccp-1 in Kimchi Cabbage. Microorganisms 2021, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; De Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Narajczyk, M.; Lojkowska, E. Genomic, Proteomic and Morphological Characterization of Two Novel Broad Host Lytic Bacteriophages ΦPD10.3 and ΦPD23.1 Infecting Pectinolytic Pectobacterium Spp. and Dickeya Spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef] [PubMed]
- Parena, A.J.S.; Silva, B.B.I.; Mercado, R.M.L.; Sendon, A.A.A.; Signabon, F.B.; Balidion, J.F.; Encabo, J.R. Lytic Phages Display Protective Effects against Soft Rot-Causing Pectobacterium sp. Biocontrol Sci. Technol. 2022, 32, 1326–1345. [Google Scholar] [CrossRef]
- Korol, N.A.; Tovkach, F.I. Identification of the Major Proteins of the Virions of Bacteriophage ZF40 Pectobacterium carotovorum. Mikrobiol. Z. 2012, 74, 64–70. [Google Scholar]
- Miroshnikov, K.A.; Evseev, P.V.; Lukianova, A.A.; Ignatov, A.N. Tailed Lytic Bacteriophages of Soft Rot Pectobacteriaceae. Microorganisms 2021, 9, 1819. [Google Scholar] [CrossRef]
- Czajkowski, R. Bacteriophages of Soft Rot Enterobacteriaceae—A Minireview. FEMS Microbiol. Lett. 2015, 363, fnv230. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Ogaz, A.L.; Muñoz-Castellanos, L.N.; Nevárez-Moorillón, G.V. Biocontrol of Phytopathogens: Antibiotic Production as Mechanism of Control. Battle Against Microb. Pathog. Basic Sci. Technol. Adv. Educ. Programs 2015, 1, 305–309. [Google Scholar]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; Barny, M.A.; Brurberg, M.B.; Condemine, G.; Czajkowski, R.; Elphinstone, J.G.; Helias, V.; Johnson, S.B.; Moleleki, L.N.; Pirhonen, M.; et al. Pectobacterium and Dickeya: Environment to Disease Development. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Springer: Cham, Switzerland, 2021; pp. 39–84. [Google Scholar] [CrossRef]
- Liu, F.; Hu, M.; Zhang, Z.; Xue, Y.; Chen, S.; Hu, A.; Zhang, L.H.; Zhou, J. Dickeya Manipulates Multiple Quorum Sensing Systems to Control Virulence and Collective Behaviors. Front. Plant Sci. 2022, 13, 838125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lv, M.; Liao, L.; Gu, Y.; Liang, Z.; Shi, Z.; Liu, S.; Zhou, J.; Zhang, L. Genetic Modulation of C-Di-GMP Turnover Affects Multiple Virulence Traits and Bacterial Virulence in Rice Pathogen Dickeya zeae. PLoS ONE 2016, 11, e0165979. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.L.; Jariwala, P.B.; Rivera, S.; Fontaine, B.M.; Briggs, L.; Weinert, E.E. Oxygen-Dependent Globin Coupled Sensor Signaling Modulates Motility and Virulence of the Plant Pathogen Pectobacterium carotovorum. ACS Chem. Biol. 2017, 12, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Zeng, Q.; Yu, M.; Hsueh, B.Y.; Waters, C.M.; Yang, C.-H. Quorum-Sensing Master Regulator VfmE Is a c-Di-GMP Effector That Controls Pectate Lyase Production in the Phytopathogen Dickeya dadantii. Microbiol. Spectr. 2022, 10, e01805-21. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Schikora, A. AHL-Priming for Enhanced Resistance as a Tool in Sustainable Agriculture. FEMS Microbiol. Ecol. 2020, 96, fiaa226. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Q.; Jia, Z.; Zhang, S.; Wang, J.; Song, S.; Jia, Y. N-3-Oxo-Octanoyl Homoserine Lactone Primes Plant Resistance Against Necrotrophic Pathogen Pectobacterium carotovorum by Coordinating Jasmonic Acid and Auxin-Signaling Pathways. Front. Plant Sci. 2022, 13, 886268. [Google Scholar] [CrossRef]
- Duan, Y.; Han, M.; Grimm, M.; Ponath, J.; Reichelt, M.; Mithöfer, A.; Schikora, A. Combination of Bacterial N-Acyl Homoserine Lactones Primes Arabidopsis Defenses via Jasmonate Metabolism. Plant Physiol. 2023, 191, 2027–2044. [Google Scholar] [CrossRef] [PubMed]
- Chenu, C.; Cosentino, D. Microbial Regulation of Soil Structural Dynamics. In The Architecture and Biology of Soils: Life in Inner Space; CABI: Wallingford, UK, 2011. [Google Scholar]
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-Tolerant Plant Growth Promoting Bacillus Spp.: Effect on Growth, Osmol Ytes, and Antioxidant Status of Maize under Drought Stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Xiong, B.J.; Kleinsteuber, S.; Sträuber, H.; Dusny, C.; Harms, H.; Wick, L.Y. Impact of Fungal Hyphae on Growth and Dispersal of Obligate Anaerobic Bacteria in Aerated Habitats. mBio 2022, 13, e00769-22. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Xiong, B.; Wick, L.Y.; Duran, R. Marine Fungi Select and Transport Aerobic and Anaerobic Bacterial Populations from Polycyclic Aromatic Hydrocarbon-Contaminated Sediments. mBio 2023, 14, e02761-22. [Google Scholar] [CrossRef] [PubMed]
- Murase, J.; Noll, M.; Frenzel, P. Impact of Protists on the Activity and Structure of the Bacterial Community in a Rice Field Soil. Appl. Environ. Microbiol. 2006, 72, 5436–5444. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 2452. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Oxygen Deficiency and Root Metabolism: Injury and Acclimation under Hypoxia and Anoxia. Annu. Rev. Plant Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Cid, G.; Kanukollu, S.; Ulrich, A.; Hajirezaei, M.R.; Kolb, S. Flooding Causes Dramatic Compositional Shifts and Depletion of Putative Beneficial Bacteria on the Spring Wheat Microbiota. Front. Microbiol. 2021, 12, 773116. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.J.; van der Gast, C.J.; McNamara, N.P.; Rowe, R.; Bending, G.D. Extreme Rainfall Affects Assembly of the Root-Associated Fungal Community. New Phytol. 2018, 220, 1172–1184. [Google Scholar] [CrossRef]
- Graff, A.; Conrad, R. Impact of Flooding on Soil Bacterial Communities Associated with Poplar (Populus sp.) Trees. FEMS Microbiol. Ecol. 2005, 53, 401–415. [Google Scholar] [CrossRef]
- de Marins, J.F.; Carrenho, R. Arbuscular Mycorrhizal Fungi and Dark Septate Fungi in Plants Associated with Aquatic Environments. Acta Bot. Bras. 2017, 31, 295–308. [Google Scholar] [CrossRef]
- Sah, S.; Reed, S.; Jayachandran, K.; Dunn, C.; Fisher, J.B. The Effect of Repeated Short-Term Flooding on Mycorrhizal Survival in Snap Bean Roots. HortScience 2006, 41, 598–602. [Google Scholar] [CrossRef]
- Freed, G.; Schlatter, D.; Paulitz, T.; Dugan, F. Mycological Insights into Wetland Fungal Communities: The Mycobiome of Camassia in the Pacific Northwest. Phytobiomes J. 2019, 3, 286–299. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Prakash Verma, J.; Schenk, P.M.; Singh, B.K. Evidence for the Plant Recruitment of Beneficial Microbes to Suppress Soil-Borne Pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef] [PubMed]
- Blom, C.W.P.M. Adaptations to Flooding Stress: From Plant Community to Molecule. Plant Biol. 1999, 1, 261–273. [Google Scholar] [CrossRef]
- Cameron, J.N.; Carlile, M.J. Fatty Acids, Aldehydes and Alcohols as Attractants for Zoospores of Phytophthora palmivora. Nature 1978, 271, 448–449. [Google Scholar] [CrossRef]
- Strigul, N.S.; Kravchenko, L.V. Mathematical Modeling of PGPR Inoculation into the Rhizosphere. Environ. Model. Softw. 2006, 21, 1158–1171. [Google Scholar] [CrossRef]
- Hodges, F.E.; Sicheritz-Pontén, T.; Clokie, M.R.J. The Effect of Oxygen Availability on Bacteriophage Infection: A Review. PHAGE Ther. Appl. Res. 2021, 2, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Vives, M.J. Phages in Anaerobic Systems. Viruses 2020, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Schumann, A.R.; Sue, A.D.; Roach, D.R. Hypoxia Increases the Tempo of Phage Resistance and Mutational Bottlenecking of Pseudomonas Aeruginosa. Front. Microbiol. 2022, 13, 905343. [Google Scholar] [CrossRef]
- Bhat, K.A.; Masood, S.D.; Bhat, N.A.; Bhat, M.A.; Razvi, S.M.; Mir, M.R.; Akhtar, S.; Wani, N.; Habib, M. Current Status of Post Harvest Soft Rot in Vegetables: A Review. Asian J. Plant Sci. 2010, 9, 200–208. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Sharma, A.; Abrahamian, P.; Carvalho, R.; Choudhary, M.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Future of Bacterial Disease Management in Crop Production. Annu. Rev. Phytopathol. 2022, 60, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M. Plant Breeding Advancements with “CRISPR-Cas” Genome Editing Technologies Will Assist Future Food Security. Front. Plant Sci. 2023, 14, 1133036. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.E.; Bryan, G.J.; Ramsay, G. Genetic Resources (Including Wild and Cultivated Solanum Species) and Progress in Their Utilisation in Potato Breeding. Potato Res. 2006, 49, 49–65. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Holtappels, D.; Fortuna, K.; Lavigne, R.; Wagemans, J. The Future of Phage Biocontrol in Integrated Plant Protection for Sustainable Crop Production. Curr. Opin. Biotechnol. 2021, 68, 60–71. [Google Scholar] [CrossRef]
- Verma, H.; Kant, C.; Singh, S.K.; White, J.F.; Kumar, A.; Droby, S. Biocontrol Potential of Microbial Consortia: Approaches in Food Security and Disease Management. In Microbial Biocontrol: Sustainable Agriculture and Phytopathogen Management: Volume 1; Springer: Cham, Switzerland, 2022; pp. 187–203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).