Anatomical Anal Stenosis after PPH: Insights from a Retrospective Study and Rat Model
Abstract
1. Introduction
2. Results
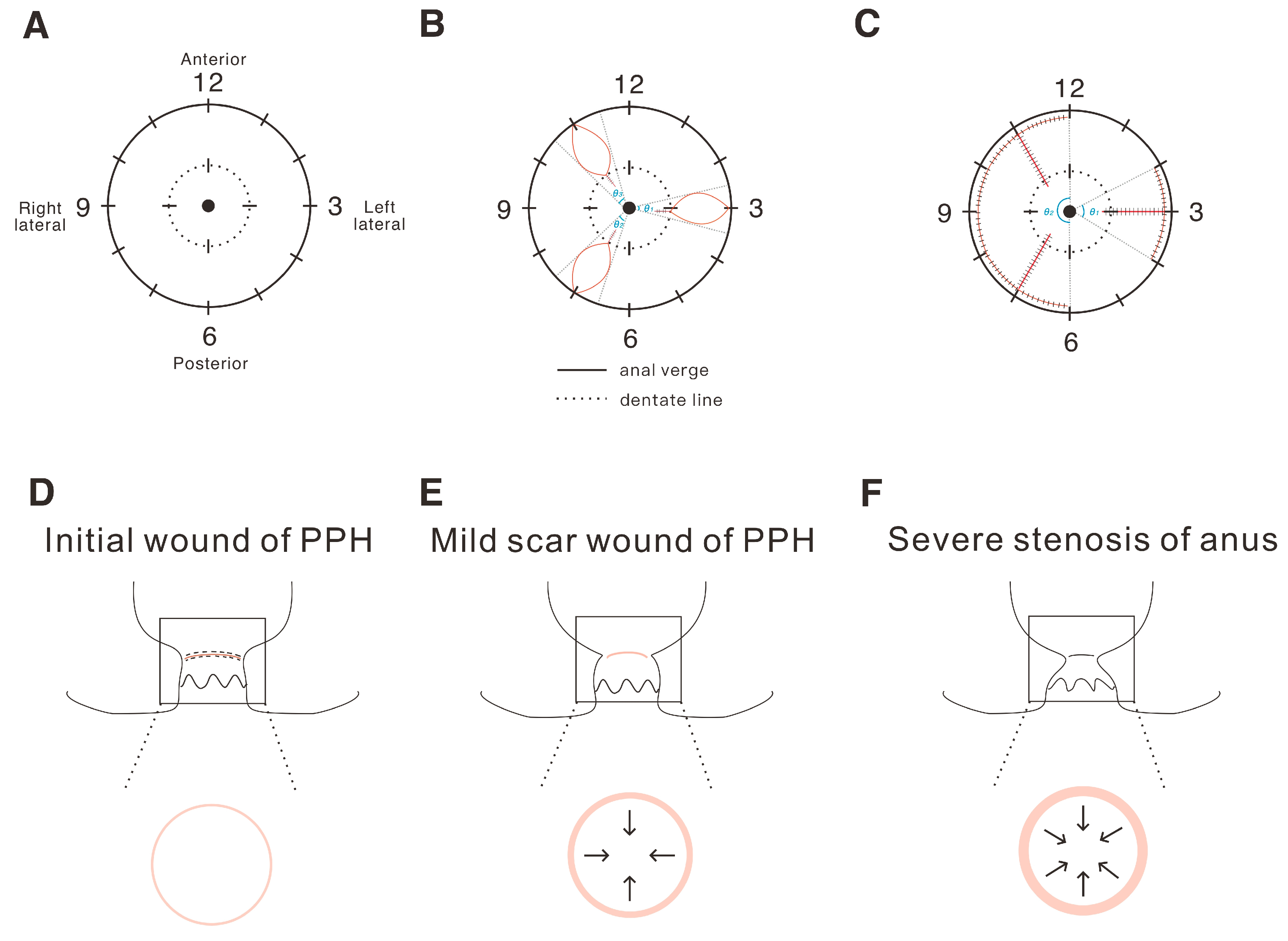
2.1. The Definition and Analysis of the Tension of the Operation Wound in Hemorrhoids
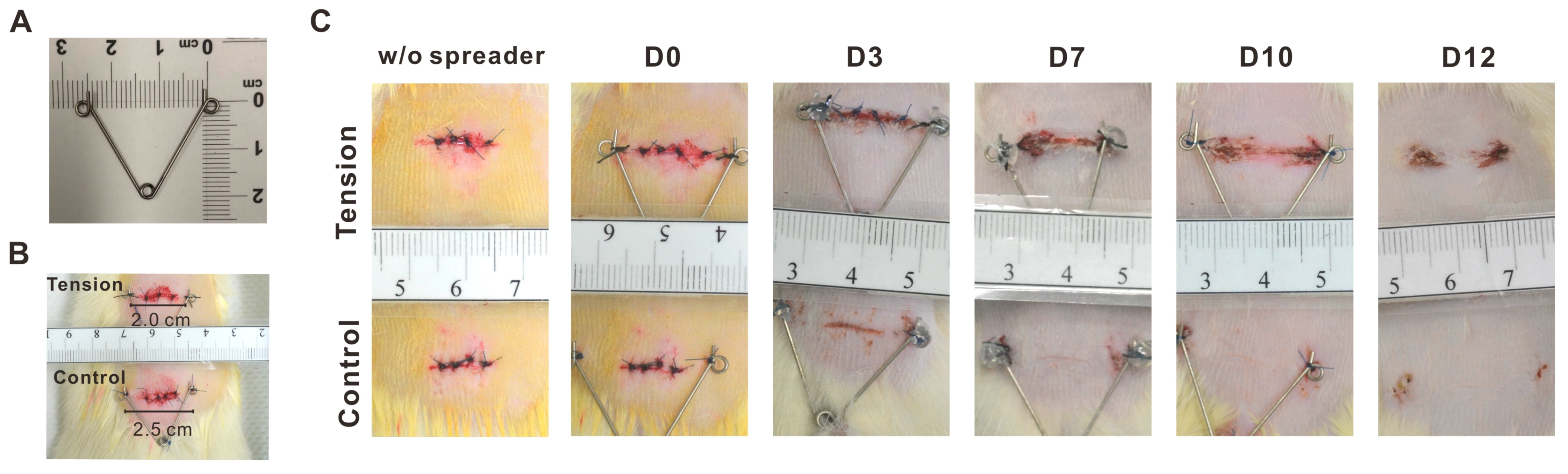
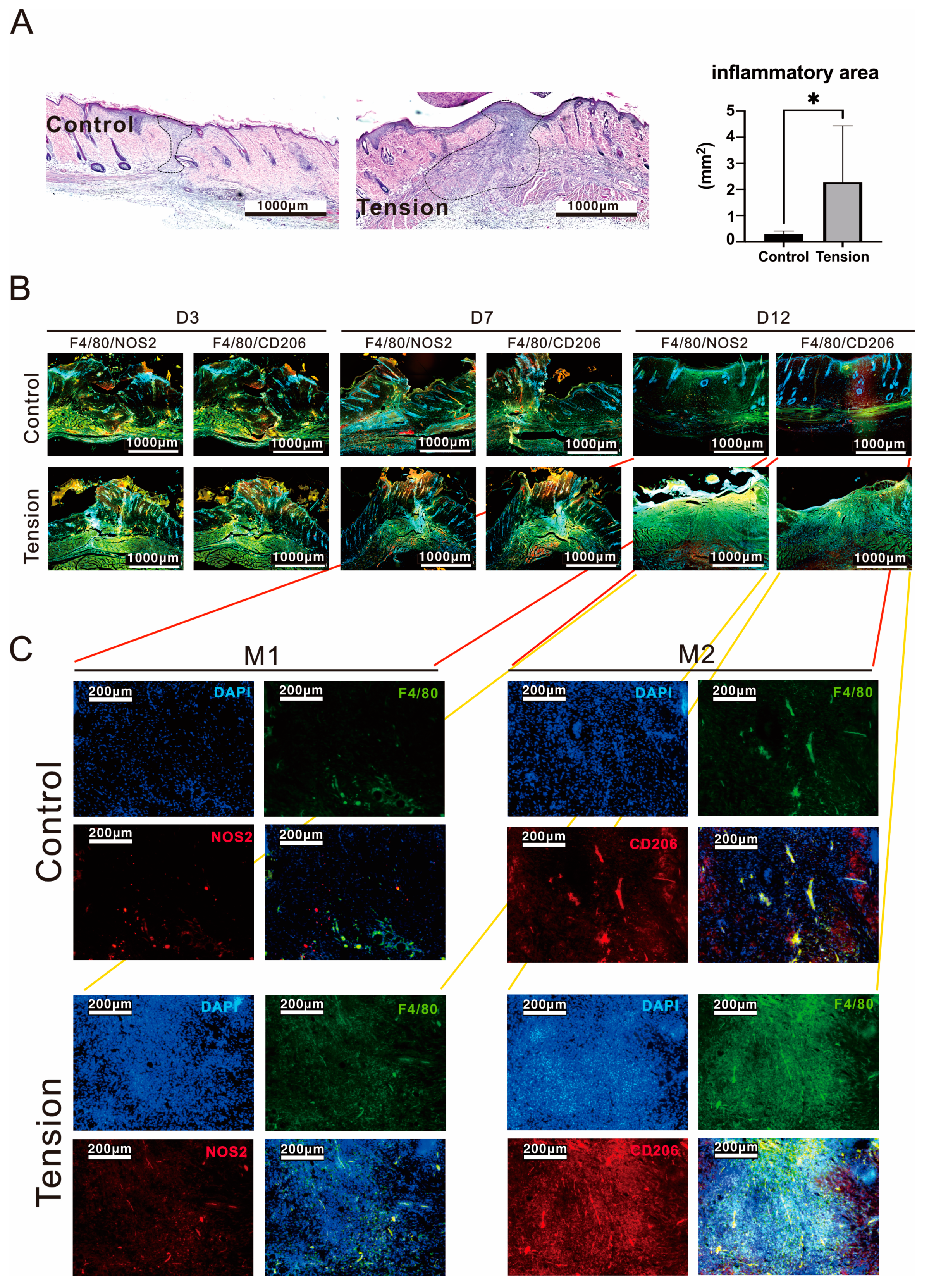
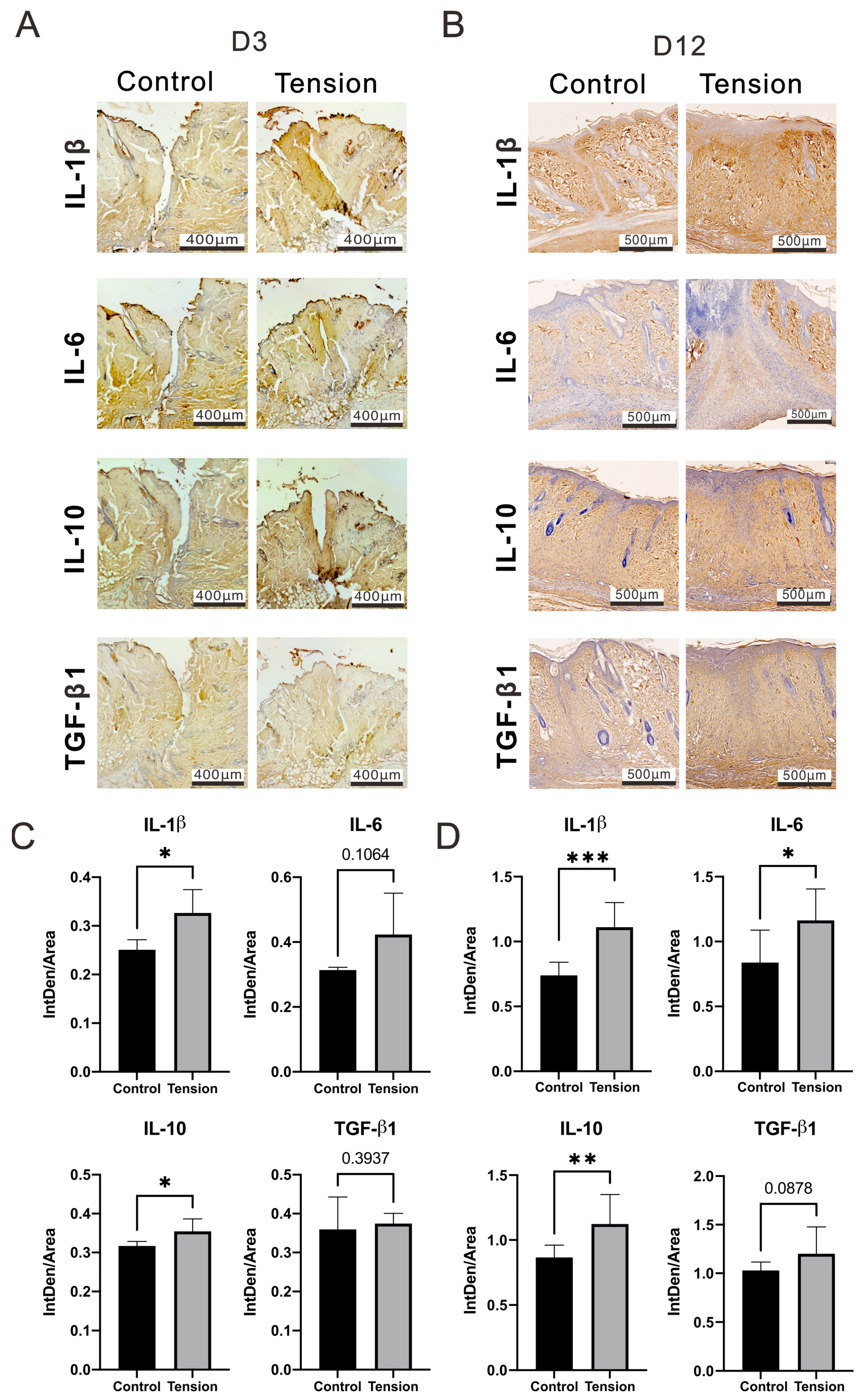
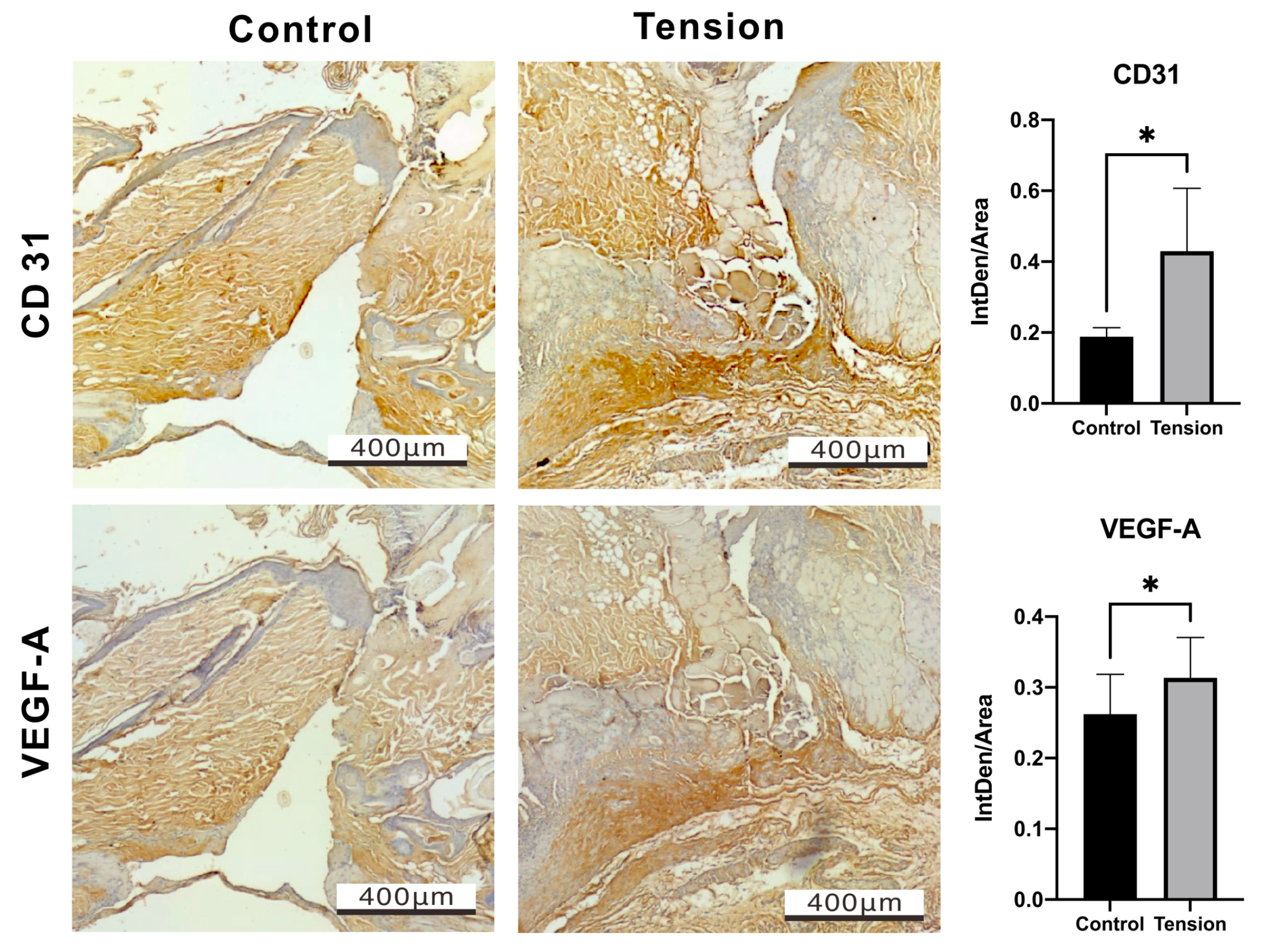
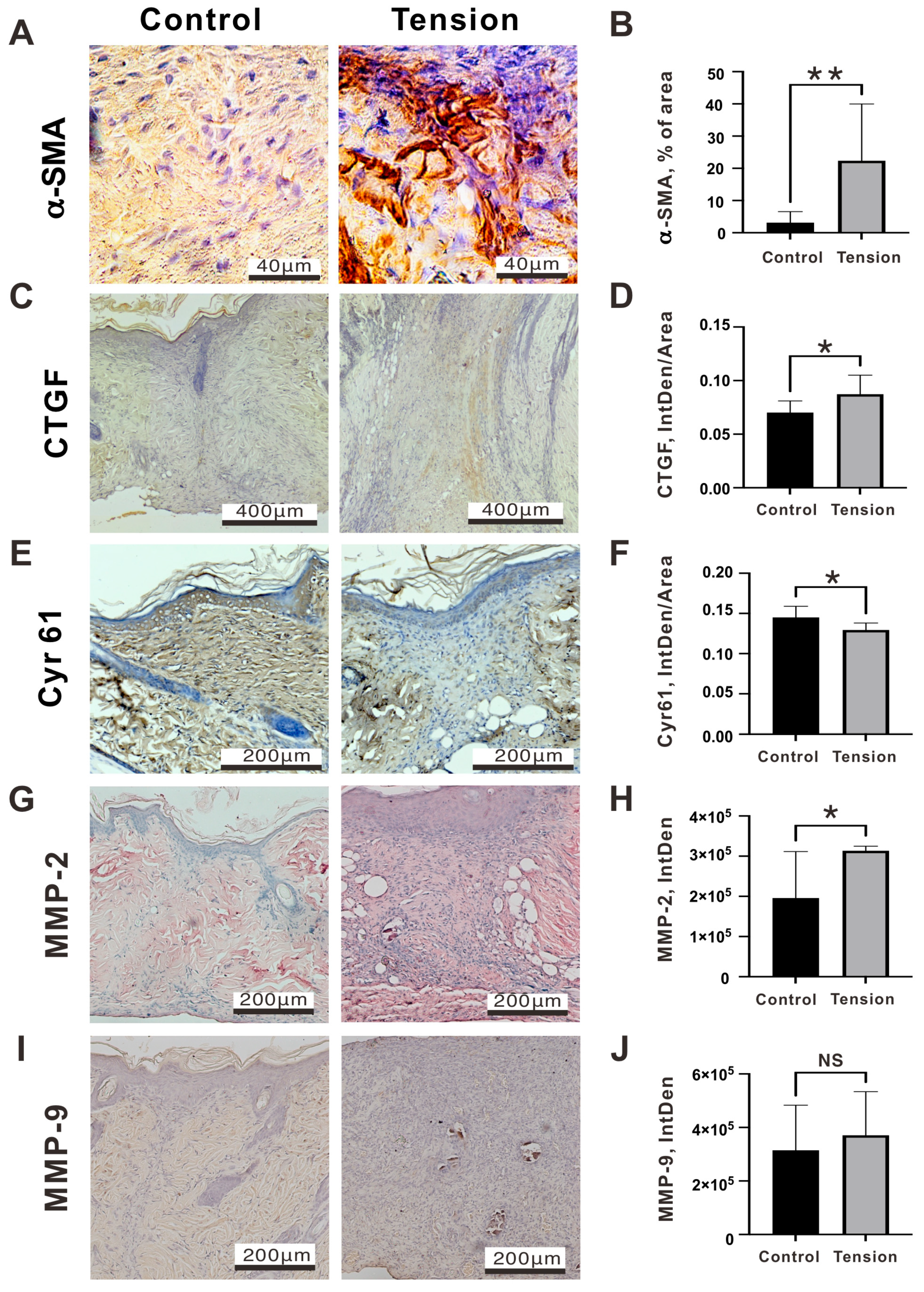
2.2. The Verification and Effects of Tensile Force on Wounds in Our Animal Model
3. Discussion
4. Materials and Methods
4.1. Study Participants and Patient Flow
4.2. An Animal Model for the Study of Tensile Force
4.3. Hematoxylin-Eosin (H&E) Staining
4.4. Masson’s Trichrome Staining
4.5. Immunohistochemistry Staining
4.6. Immunofluorescence Staining
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zollner, A.M.; Buganza Tepole, A.; Kuhl, E. On the biomechanics and mechanobiology of growing skin. J. Theor. Biol. 2012, 297, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Carver, W.; Goldsmith, E.C. Regulation of tissue fibrosis by the biomechanical environment. Biomed. Res. Int. 2013, 2013, 101979. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.G.; da Silva, L.P.; Cerqueira, M.T.; Ibanez, R.; Murphy, C.M.; Reis, R.L.; FJ, O.B.; Marques, A.P. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022, 150, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.M.; Mentz, J.; Collier, I.; Davis, M.J.; Abu-Ghname, A.; Colchado, D.; Short, W.D.; King, A.; Buchanan, E.P.; Balaji, S. Strategies to Minimize Surgical Scarring: Translation of Lessons Learned from Bedside to Bench and Back. Adv Wound Care 2022, 11, 311–329. [Google Scholar] [CrossRef]
- Kollmannsberger, P.; Bidan, C.M.; Dunlop, J.W.C.; Fratzl, P.; Vogel, V. Tensile forces drive a reversible fibroblast-to-myofibroblast transition during tissue growth in engineered clefts. Sci. Adv. 2018, 4, eaao4881. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Gemmati, D.; Kanase, A.; Pandey, I.; Misra, V.; Kishore, V.; Jahnke, T.; Bill, J. Nanobiomaterials for vascular biology and wound management: A review. Veins Lymphat. 2018, 7, 7196. [Google Scholar] [CrossRef]
- Lohsiriwat, V. Treatment of hemorrhoids: A coloproctologist’s view. World J. Gastroenterol. 2015, 21, 9245–9252. [Google Scholar] [CrossRef]
- Thomson, W.H. The nature of haemorrhoids. Br. J. Surg. 1975, 62, 542–552. [Google Scholar] [CrossRef]
- Sardinha, T.C.; Corman, M.L. Hemorrhoids. Surg. Clin. N. Am. 2002, 82, 1153–1167. [Google Scholar] [CrossRef]
- Hughes, E.S. Surgical anatomy of the anal canal. Aust. N. Z. J. Surg. 1956, 26, 48–55. [Google Scholar] [CrossRef]
- Katdare, M.V.; Ricciardi, R. Anal stenosis. Surg. Clin. N. Am. 2010, 90, 137–145. [Google Scholar] [CrossRef]
- Jayaraman, S.; Colquhoun, P.H.; Malthaner, R.A. Stapled versus conventional surgery for hemorrhoids. Cochrane Database Syst. Rev. 2006, 4, CD005393. [Google Scholar]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Colquhoun, P.H.; Malthaner, R.A. Stapled hemorrhoidopexy is associated with a higher long-term recurrence rate of internal hemorrhoids compared with conventional excisional hemorrhoid surgery. Dis. Colon. Rectum 2007, 50, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liang, R.; Wang, J.; Ke, M.; Chen, Z.; Huang, J.; Shi, R. Network meta-analysis of randomized controlled trials comparing the procedure for prolapse and hemorrhoids, Milligan-Morgan hemorrhoidectomy and tissue-selecting therapy stapler in the treatment of grade III and IV internal hemorrhoids(Meta-analysis). Int. J. Surg. 2020, 74, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Quan, S.; Dong, T.; Meng, Q. Comparison of surgical procedures implemented in recent years for patients with grade III and IV hemorrhoids: A network meta-analysis. Int. J. Color. Dis. 2019, 34, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.G.; Wu, J.; Yin, H.M.; Ma, C.M.; Cheng, S.J. Comparison of the efficacy and safety of different surgical procedures for patients with hemorrhoids: A network meta-analysis. Tech. Coloproctol. 2023, 27, 799–811. [Google Scholar] [CrossRef] [PubMed]
- George, B.D.; Shetty, D.; Lindsey, I.; Mortensen, N.J.; Warren, B.F. Histopathology of stapled haemorrhoidectomy specimens: A cautionary note. Color. Dis. 2002, 4, 473–476. [Google Scholar] [CrossRef]
- Hong, Y.K.; Choi, Y.J.; Kang, J.G. Correlation of histopathology with anorectal manometry following stapled hemorrhoidopexy. Ann. Coloproctol. 2013, 29, 198–204. [Google Scholar] [CrossRef]
- Vyslouzil, K.; Zboril, P.; Skalicky, P.; Vomackova, K. Effect of hemorrhoidectomy on anorectal physiology. Int. J. Color. Dis. 2010, 25, 259–265. [Google Scholar] [CrossRef]
- Brisinda, G.; Vanella, S.; Cadeddu, F.; Marniga, G.; Mazzeo, P.; Brandara, F.; Maria, G. Surgical treatment of anal stenosis. World J. Gastroenterol. 2009, 15, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFbeta Signaling in Wound Epithelialization. Adv Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Xu, J.; Xie, J.; Harris, D.C.H.; Zheng, G. The Role of Macrophages in Kidney Fibrosis. Front. Physiol. 2021, 12, 705838. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Chen, X.; Han, X.; Dong, P. M2 macrophages induce EMT through the TGF-beta/Smad2 signaling pathway. Cell Biol. Int. 2017, 41, 960–968. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.M.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward understanding scarless skin wound healing and pathological scarring. F1000Res 2019, 8, 787. [Google Scholar] [CrossRef]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast-Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Deaton, R.A.; Su, C.; Valencia, T.G.; Grant, S.R. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J. Biol. Chem. 2005, 280, 31172–31181. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, N.; Chu, H.Y.; Yu, Y.; Zhang, Z.K.; Zhang, G.; Zhang, B.T. Connective Tissue Growth Factor: From Molecular Understandings to Drug Discovery. Front. Cell Dev. Biol. 2020, 8, 593269. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, N.; Lau, L.F. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J. Biol. Chem. 2001, 276, 10443–10452. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef]
- Jun, J.I.; Lau, L.F. Cellular senescence controls fibrosis in wound healing. Aging 2010, 2, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Lohsiriwat, V. Hemorrhoids: From basic pathophysiology to clinical management. World J. Gastroenterol. 2012, 18, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Festen, S.; Molthof, H.; van Geloven, A.A.; Luchters, S.; Gerhards, M.F. Predictors of recurrence of prolapse after procedure for prolapse and haemorrhoids. Color. Dis. 2012, 14, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Zmora, O.; Colquhoun, P.; Abramson, S.; Weiss, E.G.; Efron, J.; Vernava, A.M., 3rd; Nogueras, J.J.; Wexner, S.D. Can the procedure for prolapsing hemorrhoids (PPH) be done twice? Results of a porcine model. Surg. Endosc. 2004, 18, 757–761. [Google Scholar] [CrossRef]
- Wei, D.; Jiang, P.; Gao, R.; Zhao, Y. Prevention and Treatment of Anastomotic Strictures After Procedure for Prolapse and Hemorrhoids. Risk Manag. Healthc. Policy 2023, 16, 1351–1357. [Google Scholar] [CrossRef]
- Singh, A.V.; Varma, M.; Rai, M.; Singh, S.P.; Bansod, G.; Laux, P.; Luch, A. Advancing Predictive Risk Assessment of Chemicals via Integrating Machine Learning, Computational Modeling, and Chemical/Nano-Quantitative Structure-Activity Relationship Approaches. Adv. Intell. Syst. 2024, 2300366. [Google Scholar] [CrossRef]
- Cremers, N.A.; Suttorp, M.; Gerritsen, M.M.; Wong, R.J.; van Run-van Breda, C.; van Dam, G.M.; Brouwer, K.M.; Kuijpers-Jagtman, A.M.; Carels, C.E.; Lundvig, D.M.; et al. Mechanical Stress Changes the Complex Interplay Between HO-1, Inflammation and Fibrosis, During Excisional Wound Repair. Front. Med. 2015, 2, 86. [Google Scholar] [CrossRef]
- Son, D.O.; Hinz, B. A Rodent Model of Hypertrophic Scarring: Splinting of Rat Wounds. Methods Mol. Biol. 2021, 2299, 405–417. [Google Scholar]
- Jimi, S.; Saparov, A.; Koizumi, S.; Miyazaki, M.; Takagi, S. A novel mouse wound model for scar tissue formation in abdominal muscle wall. J. Vet. Med. Sci. 2021, 83, 1933–1942. [Google Scholar] [CrossRef]
- Aarabi, S.; Bhatt, K.A.; Shi, Y.; Paterno, J.; Chang, E.I.; Loh, S.A.; Holmes, J.W.; Longaker, M.T.; Yee, H.; Gurtner, G.C. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007, 21, 3250–3261. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Zan, T.; Li, Q.; Li, H. A modified scar model with controlled tension on secondary wound healing in mice. Burn. Trauma. 2020, 8, tkaa013. [Google Scholar] [CrossRef]
- Chin, M.S.; Ogawa, R.; Lancerotto, L.; Pietramaggiori, G.; Schomacker, K.T.; Mathews, J.C.; Scherer, S.S.; Van Duyn, P.; Prsa, M.J.; Ottensmeyer, M.P.; et al. In vivo acceleration of skin growth using a servo-controlled stretching device. Tissue Eng. Part C Methods 2010, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.P.; Burgess, L.P.; Livermore, G.H.; Tzikas, T.L.; Vossoughi, J. Wound healing. Tensile strength vs healing time for wounds closed under tension. Arch. Otolaryngol. Head. Neck Surg. 1996, 122, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Leventoglu, S.; Mentes, B.; Balci, B.; Kebiz, H.C. New Techniques in Hemorrhoidal Disease but the Same Old Problem: Anal Stenosis. Medicina 2022, 58, 362. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, P.D.; Marle, J.V.; Kuehne, A.; Schouten, H.J.; Gaffney, E.A.; Maini, P.K.; Middelkoop, E.; Zuijlen, P.P. Collagen bundle morphometry in skin and scar tissue: A novel distance mapping method provides superior measurements compared to Fourier analysis. J. Microsc. 2012, 245, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.; LeRoy, E.C.; Trojanowska, M. Oncostatin M stimulates transcription of the human alpha2(I) collagen gene via the Sp1/Sp3-binding site. J. Biol. Chem. 1997, 272, 24666–24672. [Google Scholar] [CrossRef]
- Tzortzaki, E.G.; Koutsopoulos, A.V.; Dambaki, K.I.; Lambiri, I.; Plataki, M.; Gordon, M.K.; Gerecke, D.R.; Siafakas, N.M. Active remodeling in idiopathic interstitial pneumonias: Evaluation of collagen types XII and XIV. J. Histochem. Cytochem. 2006, 54, 693–700. [Google Scholar] [CrossRef]
- Tzortzaki, E.G.; Tischfield, J.A.; Sahota, A.; Siafakas, N.M.; Gordon, M.K.; Gerecke, D.R. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 275, 1073–1080. [Google Scholar] [CrossRef]
- Imagami, T.; Takayama, S.; Maeda, Y.; Hattori, T.; Matsui, R.; Sakamoto, M.; Kani, H. Surgical Resection of Anastomotic Stenosis after Rectal Cancer Surgery Using a Circular Stapler and Colostomy with Double Orifice. Case Rep. Surg. 2019, 2019, 2898691. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell 2001, 12, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Varney, S.D.; Betts, C.B.; Zheng, R.; Wu, L.; Hinz, B.; Zhou, J.; Van De Water, L. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J. Cell Sci. 2016, 129, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Munakata, H. Connective tissue growth factor binds to fibronectin through the type I repeat modules and enhances the affinity of fibronectin to fibrin. Biochim. Biophys. Acta 2007, 1770, 672–680. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; He, Y.; Zhang, D.; Zou, S.; Xie, J.; Zhou, C. Connective tissue growth factor promotes cell-to-cell communication in human periodontal ligament stem cells via MAPK and PI3K pathway. J. Periodontol. 2022, 93, e60–e72. [Google Scholar] [CrossRef]
- Kossakowska, A.E.; Edwards, D.R.; Lee, S.S.; Urbanski, L.S.; Stabbler, A.L.; Zhang, C.L.; Phillips, B.W.; Zhang, Y.; Urbanski, S.J. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am. J. Pathol. 1998, 153, 1895–1902. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Ding, F.; Chen, P.; Tang, M. Inhibition of liver fibrosis using vitamin A-coupled liposomes to deliver matrix metalloproteinase-2 siRNA in vitro. Mol. Med. Rep. 2015, 12, 3453–3461. [Google Scholar] [CrossRef]
- Morozov, D.; Morozova, O.; Severgina, L.; Mokrushina, O.; Marchuk, T.; Budnik, I.; Ozbey, H.; Morozov, D. Effects of extensive mobilization and tension anastomosis in anorectal reconstruction (experimental study). Pediatr. Surg. Int. 2022, 39, 10. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Ma, D.; Chen, L.; Shi, J.; Zhao, Y.; Vasani, S.; Chen, K.; Romana-Souza, B.; Henkin, J.; DiPietro, L.A. Pigment epithelium-derived factor attenuates angiogenesis and collagen deposition in hypertrophic scars. Wound Repair. Regen. 2020, 28, 684–695. [Google Scholar] [CrossRef]
- Lu, J.; Guan, F.; Cui, F.; Sun, X.; Zhao, L.; Wang, Y.; Wang, X. Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regen. Biomater. 2019, 6, 325–334. [Google Scholar] [CrossRef]
- Zarrinpashneh, E.; Poggioli, T.; Sarathchandra, P.; Lexow, J.; Monassier, L.; Terracciano, C.; Lang, F.; Damilano, F.; Zhou, J.Q.; Rosenzweig, A.; et al. Ablation of SGK1 impairs endothelial cell migration and tube formation leading to decreased neo-angiogenesis following myocardial infarction. PLoS ONE 2013, 8, e80268. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Xie, Z.; Wang, J.; Ho, C.K.; Zhang, Y.; Li, Q. Mechanical strain promotes skin fibrosis through LRG-1 induction mediated by ELK1 and ERK signalling. Commun. Biol. 2019, 2, 359. [Google Scholar] [CrossRef]
- Gong, L.; Gu, Y.; Han, X.; Luan, C.; Liu, C.; Wang, X.; Sun, Y.; Zheng, M.; Fang, M.; Yang, S.; et al. Spatiotemporal Dynamics of the Molecular Expression Pattern and Intercellular Interactions in the Glial Scar Response to Spinal Cord Injury. Neurosci. Bull. 2023, 39, 213–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, L.Z.; Xue, B. Recombinant human endostatin reduces hypertrophic scar formation in rabbit ear model through down-regulation of VEGF and TIMP-1. Afr. Health Sci. 2016, 16, 542–553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Gurevich, D.B. Macrophage regulation of angiogenesis in health and disease. Semin. Cell Dev. Biol. 2021, 119, 101–110. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Kuninaka, Y.; Ishida, Y.; Ishigami, A.; Nosaka, M.; Matsuki, J.; Yasuda, H.; Kofuna, A.; Kimura, A.; Furukawa, F.; Kondo, T. Macrophage polarity and wound age determination. Sci. Rep. 2022, 12, 20327. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Chen, Y.; Liu, Y.; Ma, M.; Ma, Z.; Wang, C.; Zeng, H.; Xue, L.; Yue, C.; et al. The Dynamic Feature of Macrophage M1/M2 Imbalance Facilitates the Progression of Non-Traumatic Osteonecrosis of the Femoral Head. Front. Bioeng. Biotechnol. 2022, 10, 912133. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006, 119, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]

| Hemorrhoidectomy | PPH | p-Value | |
|---|---|---|---|
| n | 99 | 75 | |
| Gender | 0.962 | ||
| Male (%) | 53 (53.5) | 39 (52.0) | |
| Female (%) | 46 (46.5) | 36 (48.0) | |
| Age (mean (SD)) | 51.87 (15.95) | 51.01 (12.28) | 0.697 |
| Grade 4 (%) | 66 (66.7) | 47 (62.7) | 0.699 |
| 3 | 33 (33.3) | 28 (37.3) | |
| 4 | 66 (66.7) | 47 (62.7) | |
| ASA (%) | 0.338 | ||
| 1 | 34 (34.3) | 34 (45.3) | |
| 2 | 52 (52.5) | 33 (44.0) | |
| 3 | 13 (13.1) | 8 (10.7) | |
| Operation Time (mean (SD)) | 51.72 (17.85) | 41.60 (13.59) | <0.001 * |
| Length of stay (LOS) (mean (SD)) | 1.44 (0.66) | 1.51 (0.67) | 0.54 |
| Constipation History | 0.727 | ||
| Yes | 50 (50.5) | 35 (46.7) | |
| No | 49 (49.5) | 40 (53.3) | |
| Complications (%) | 3 (3.0) | 8 (12.5) | 0.042 * |
| Anatomical anal stenosis (%) | 0 (0.0) | 5 (6.7) | 0.032 * |
| Bleeding (%) | 0 (0.0) | 1 (1.3) | 0.889 |
| Urine retention (%) | 3 (3.0) | 2 (2.7) | 1 |
| Painkiller in 1st OPD (%) | 0.002 * | ||
| Yes | 41 (41.4) | 14 (18.7) | |
| No | 58 (58.6) | 61 (81.3) | |
| Recurrence (%) | 0.808 | ||
| Yes | 1 (1.0) | 2 (2.7) | |
| No | 98 (99.0) | 73 (97.3) |
| Odds Ratio | 95% CI | p-Value | |||
|---|---|---|---|---|---|
| Surgery | |||||
| Hemorroidectomy | 1.0 (reference) | ||||
| PPH | 5.913 | 4.903 | ~ | 40.541 | 0.049 * |
| Sex | |||||
| Male | 1.0 (reference) | ||||
| Female | 0.667 | 0.506 | ~ | 3.429 | 0.577 |
| Age | 1.009 | 0.062 | ~ | 2.084 | 0.774 |
| Grade | |||||
| 3 | 1.0 (reference) | ||||
| 4 | 2.174 | 1.740 | ~ | 13.056 | 0.344 |
| ASA | 0.532 | 0.380 | ~ | 2.398 | 0.324 |
| operation time | 1.014 | 0.049 | ~ | 2.079 | 0.581 |
| Length of stay (LOS) | 2.827 | 1.615 | ~ | 9.420 | 0.016 * |
| Constipation History | |||||
| No | 1.0 (reference) | ||||
| Yes | 2.598 | 2.003 | ~ | 13.948 | 0.204 |
| Reference | Animal Species | Primary Mechanism | Advantages | Limitations |
|---|---|---|---|---|
| Present study | Rat | A spreader compressed and fixed at both ends of the incision line and generated horizontal tension focused on the incision line (85-g force). | Less harmful to the surrounding skin | A transverse incision in rats is to simulate a small portion of the PPH wound. |
| Cremers et al. [38] Son et al. [39] | Mice | Splinted the edges of full-thickness wounds. (The mechanical force is not stated) | A historical, robust, and reliable model to study myofibroblast biology | Harmful to the surrounding skin. |
| JIMI et al. [40] | Mice | The abdominal muscle wall may be potentially/physiologically under longitudinal static tension to some extent (1.8 mN, 1.84-g force). | Under physiological conditions without using any artificial factors | The tension that can be formed is weak, easily affected by animal activities, and has large variations. |
| Aarabi et al. [41] Wang et al. [42] | Mice | Biomechanical loading device engineered from expansion screws (1.5–2.7 N/mm2, 153–275-g force). | Capable of producing horizontal or vertical mechanical force stably | The device affects the animals’ daily activities and causes discomfort. |
| Chin et al. [43] | Mice | A servo-controlled skin-stretching device to produce predetermined tension in mice (35–90-g force). | Can achieve cyclically or static stretch. | Must be operated under anesthesia and cannot be used for long periods of time. |
| Pickett et al. [44] | Rat | Excise some skin between incisions to produce closing tensions (70–120-g force). | Without using any artificial factors | Skin loss up to 50–60 mm widths. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.-C.; Huang, S.-M.; Wang, Y.-W. Anatomical Anal Stenosis after PPH: Insights from a Retrospective Study and Rat Model. Int. J. Mol. Sci. 2024, 25, 3543. https://doi.org/10.3390/ijms25063543
Wen C-C, Huang S-M, Wang Y-W. Anatomical Anal Stenosis after PPH: Insights from a Retrospective Study and Rat Model. International Journal of Molecular Sciences. 2024; 25(6):3543. https://doi.org/10.3390/ijms25063543
Chicago/Turabian StyleWen, Chia-Cheng, Shih-Ming Huang, and Yi-Wen Wang. 2024. "Anatomical Anal Stenosis after PPH: Insights from a Retrospective Study and Rat Model" International Journal of Molecular Sciences 25, no. 6: 3543. https://doi.org/10.3390/ijms25063543
APA StyleWen, C.-C., Huang, S.-M., & Wang, Y.-W. (2024). Anatomical Anal Stenosis after PPH: Insights from a Retrospective Study and Rat Model. International Journal of Molecular Sciences, 25(6), 3543. https://doi.org/10.3390/ijms25063543







