Gender-Specific Renoprotective Pathways in αMUPA Transgenic Mice Subjected to Acute Kidney Injury
Abstract
1. Introduction
2. Results
2.1. Impact of I/R-Induced AKI following Gonadectomy or L-NAME Treatment on Body and Kidney Weight
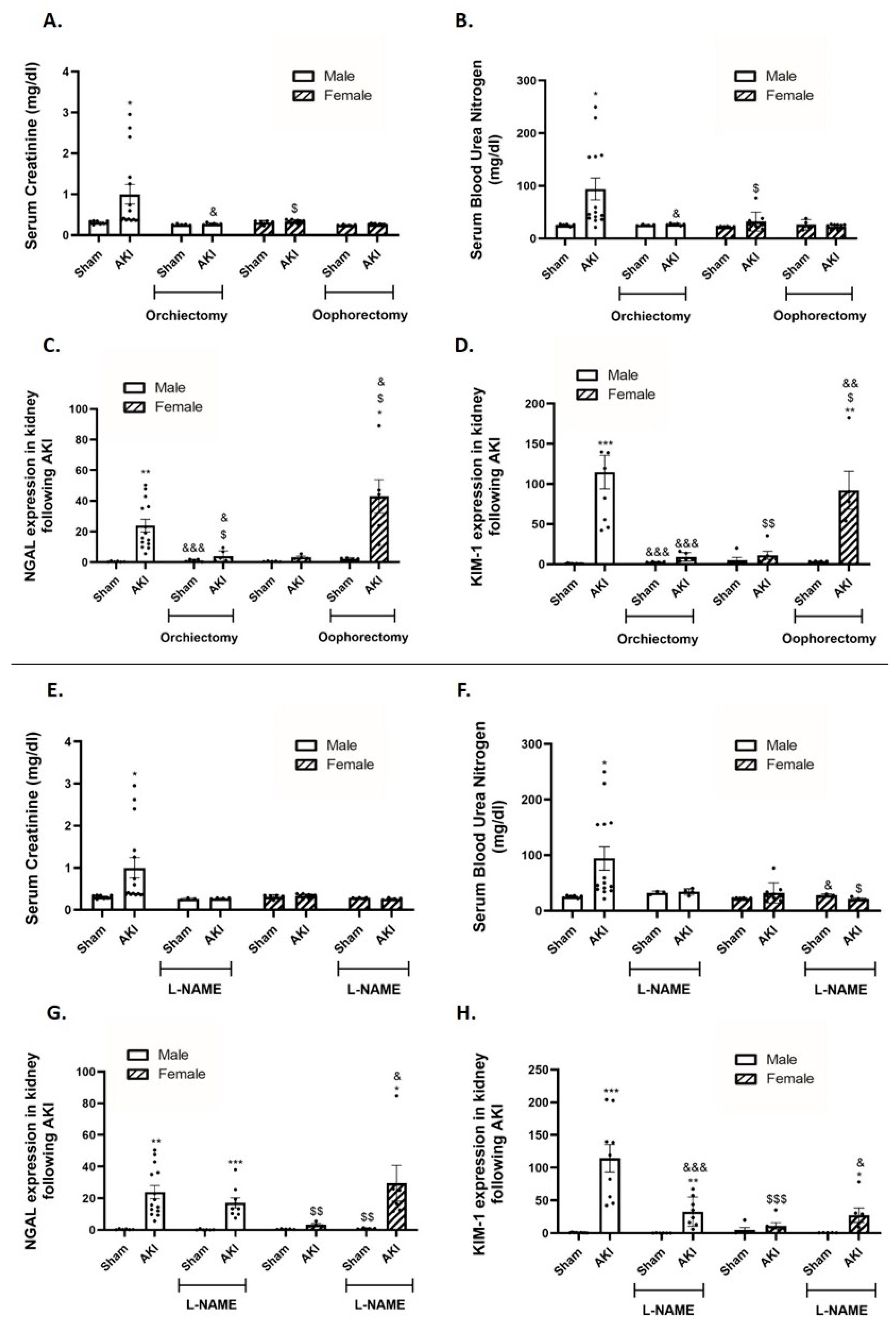
2.2. Kidney Function and Renal Injury Biomarkers
2.3. Renal Histology
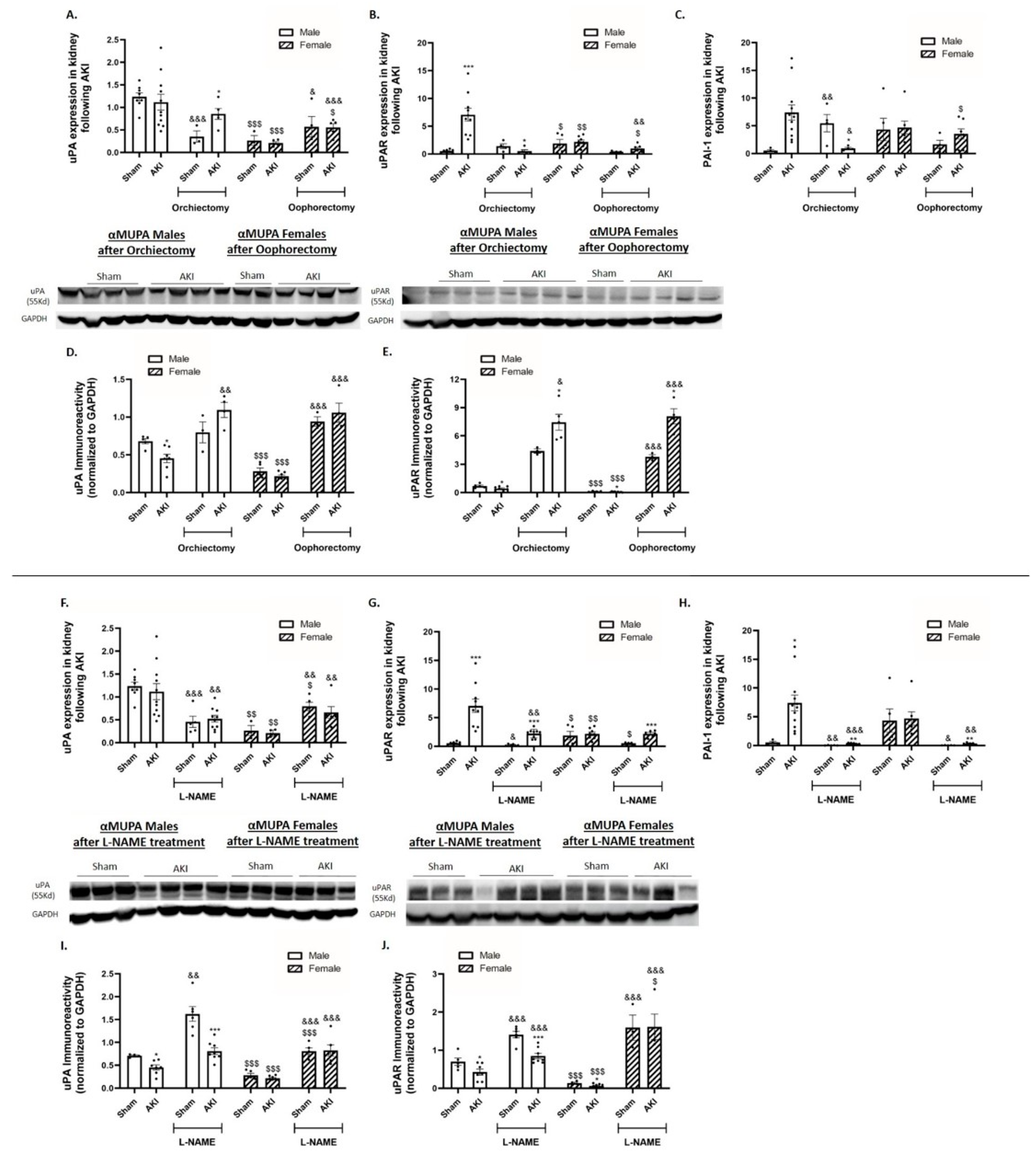
2.4. Renal uPA and uPA Receptor (uPAR)
2.5. Renal Leptin, Insulin Receptor and Peroxisome Proliferator-Activated Receptor-gamma Coactivator (PGC1α)
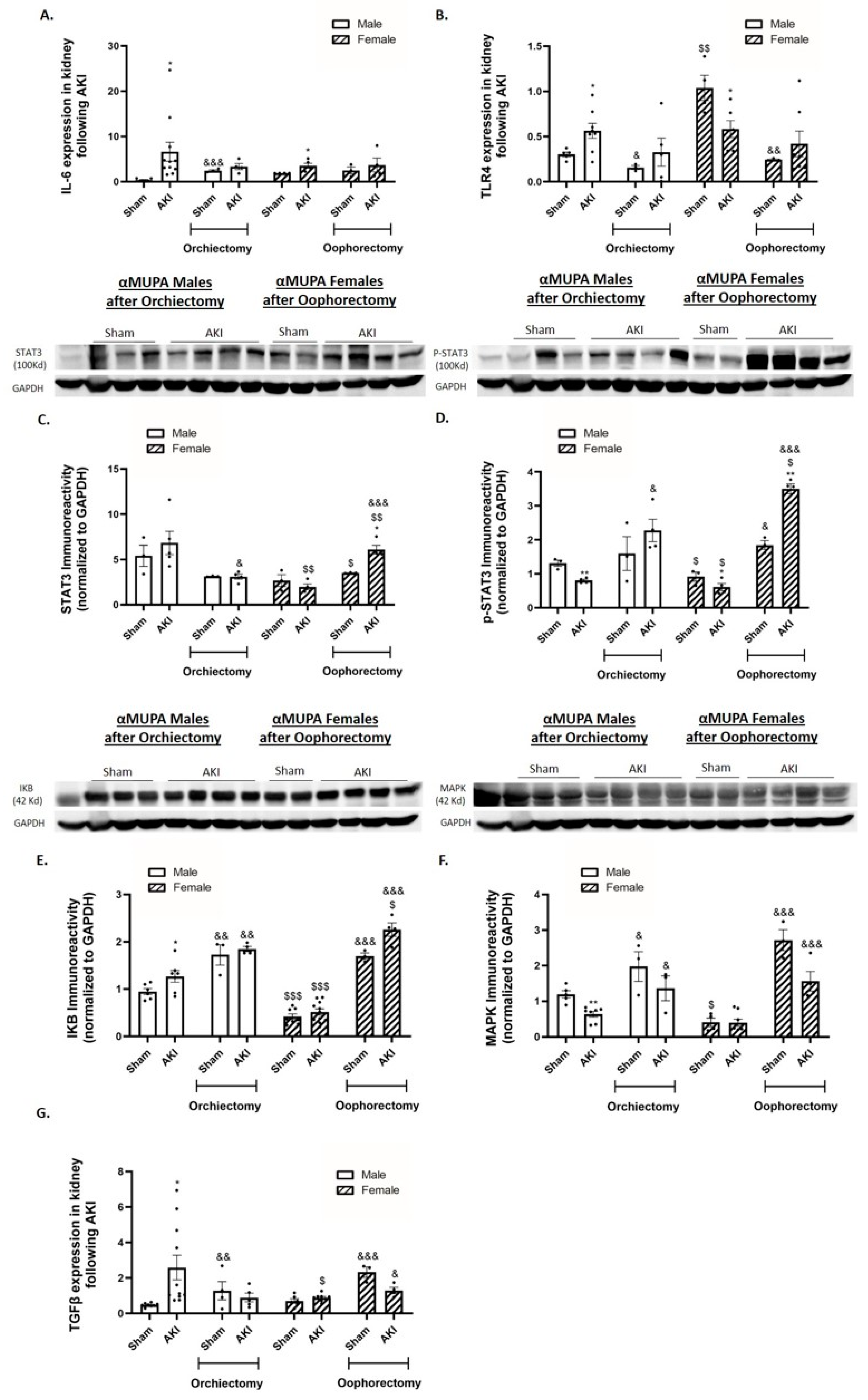
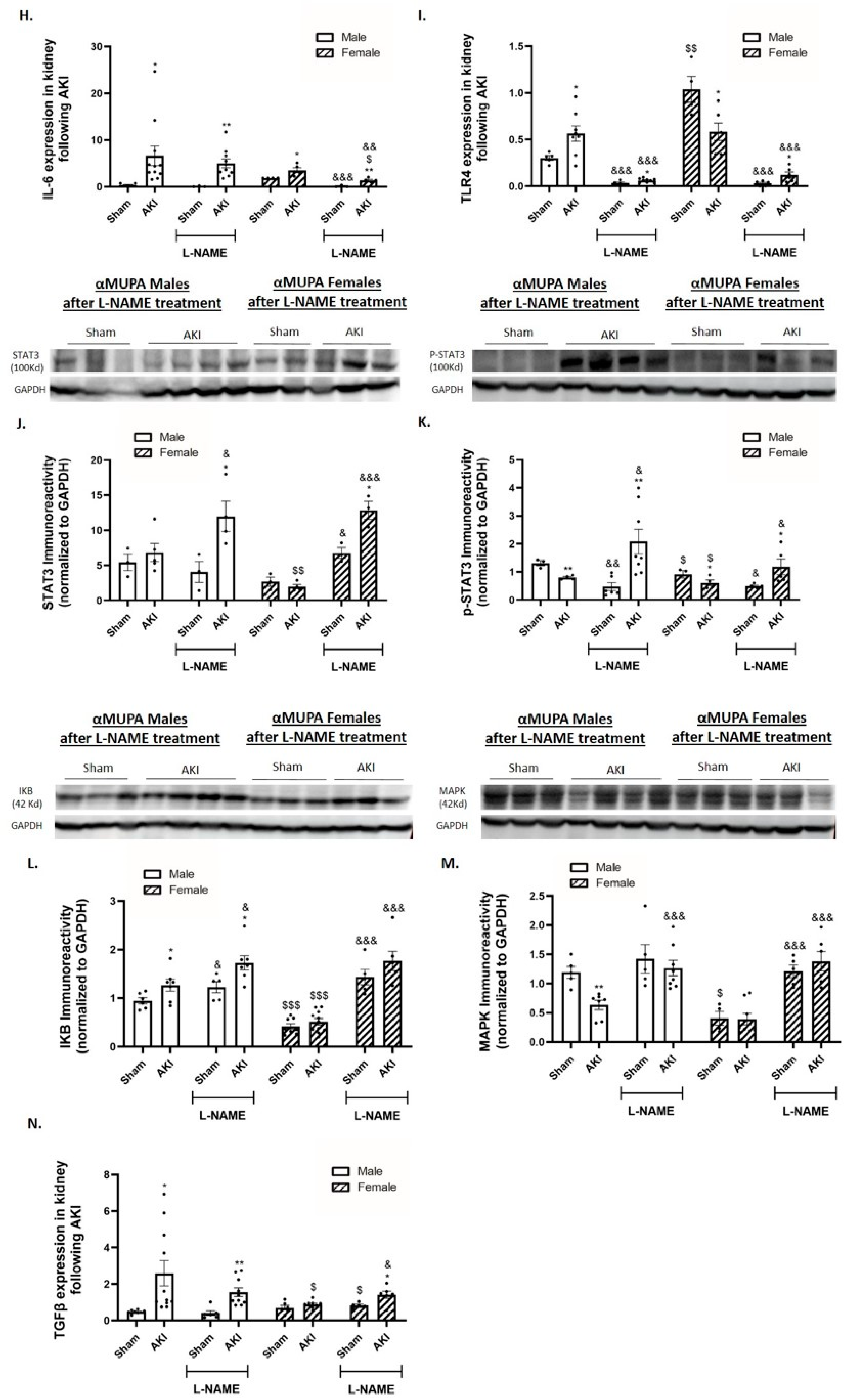
2.6. Renal Expression of Inflammatory and Fibrotic Markers
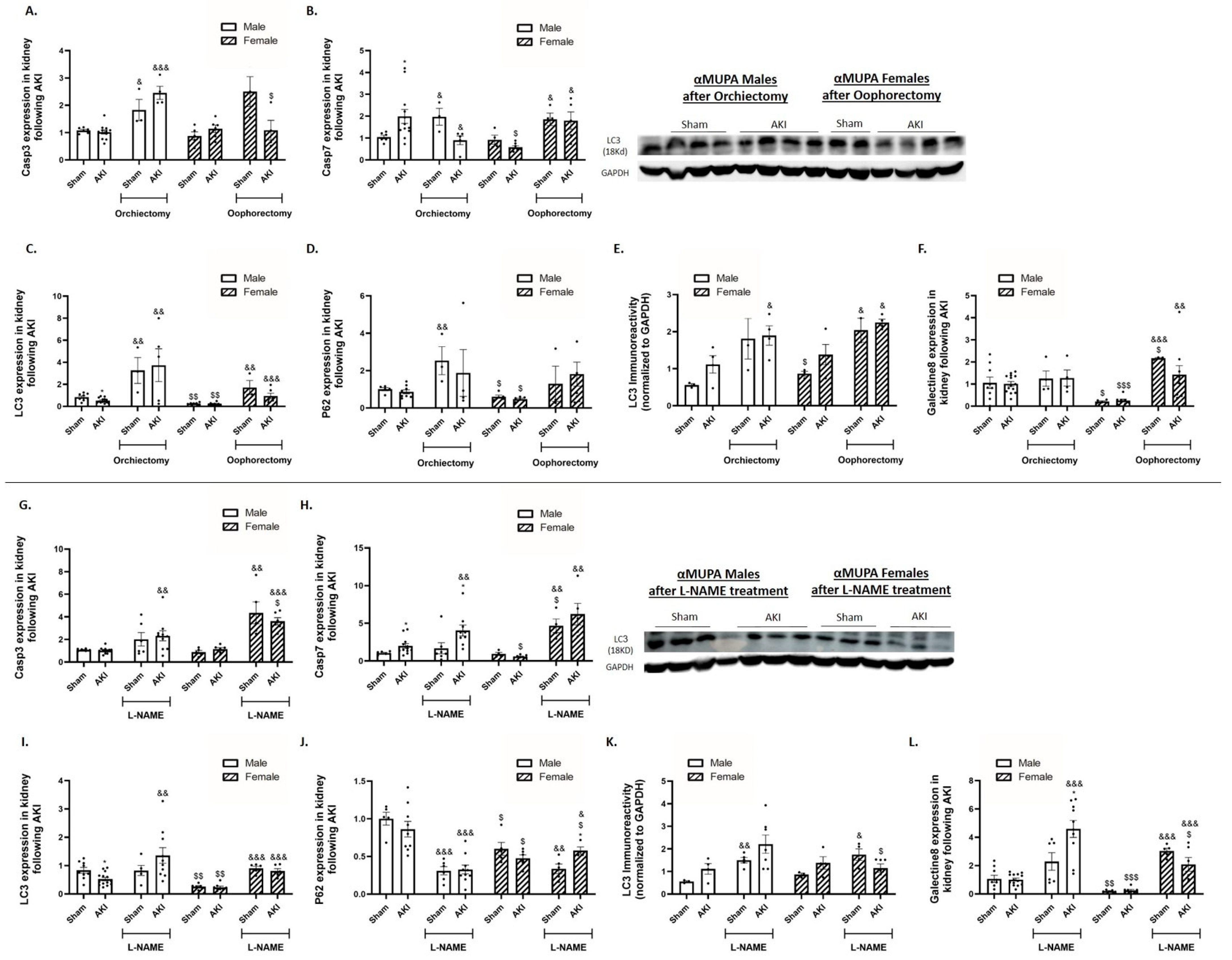
2.7. Renal Apoptotic and Autophagy Markers
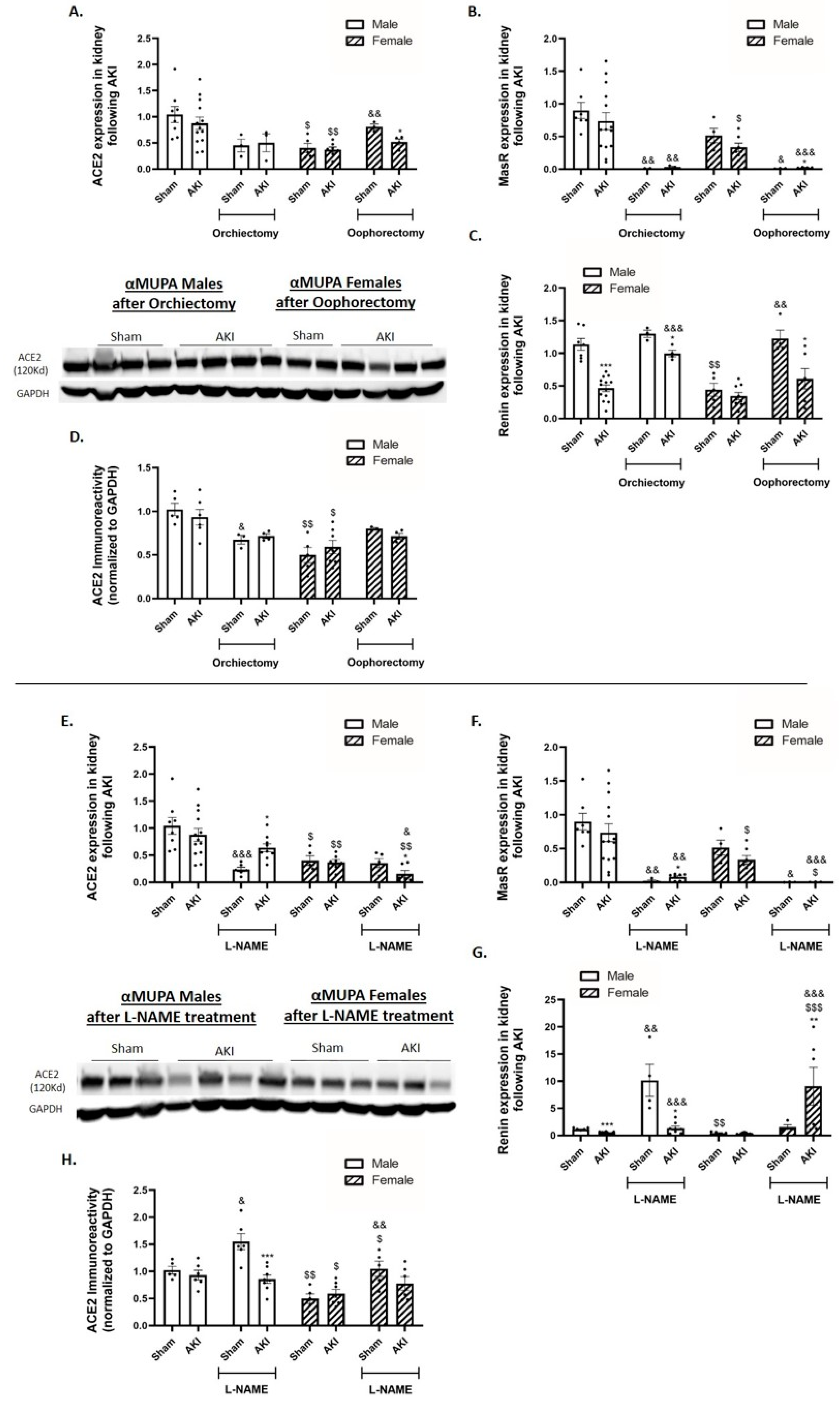
2.8. Renal Angiotensin-Converting Enzyme 2 (ACE2) and Mas Receptor (MasR)
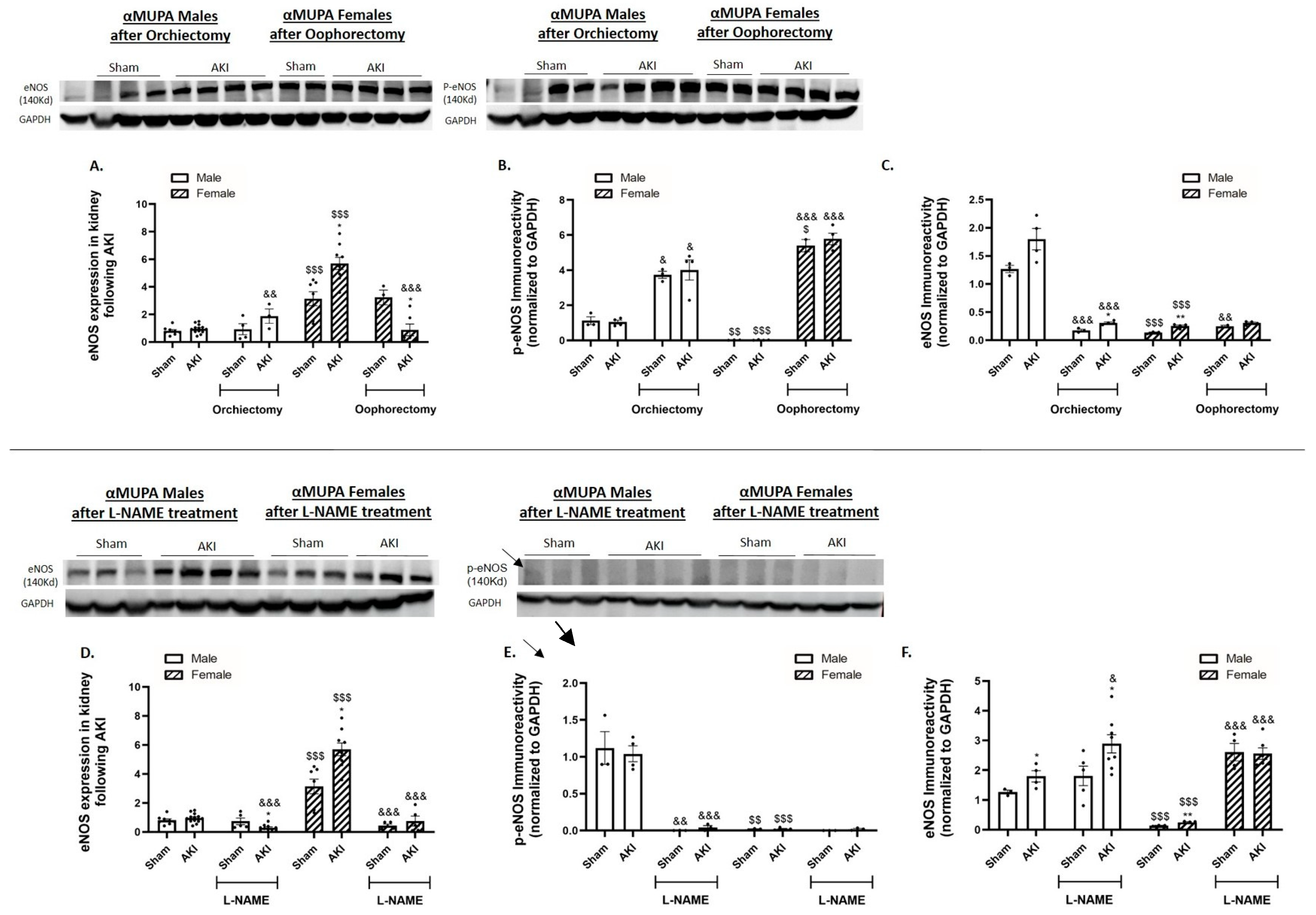
2.9. Renal Endothelial Nitric Oxide Synthase (eNOS)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Evaluation of Blood Variables and Renal Functional Parameters
4.3. Histopathology
4.4. Real-Time PCR
- uPA: -F-AGAGTCTGAAAGTGACTATCTC,-R-CCTTCGATGTTACAGATAAGC
- uPAR: -F-TCTGGATCTTCAGAGCTTTC,-R-GCCTCTTACGGTATAACTCC
- PAI-1: -F-AGCAACAAGTTCAACTACAC,-R-CTTCCATTGTCTGATGAGTTC
- InsR: -F-AAGACCTTGGTTACCTTCTC,-R-GGATTAGTGGCATCTGTTTG
- eNOS: -F-AAAGCTGCAGGTATTTGATG,-R-AGATTGCCTCTATTTGTTGC
- ACE2: -F-CATTTGCTTGGTGATATGTG,-R-GCCTCTTGAAATATCCTTTCTG
- MasR: -F-GTTTAAGGAACTCTGGAAGATG,-R-TTAGTCAGTTAGTCAGTGGC
- Renin: -F-AGCCAAGGAGAAGAGAATAG,-R-CTCCTGTTGGGATACTGTAG
- IL-6: -F-GTCTATACCACTTCACAAGTC,-R-TGCATCATCGTTGTTCATAC
- TLR4: -F-TCCCTGCATAGAGGTAGTTCC,-R-TCCAGCCACTGAAGTTCTGA
- Leptin: -F-ACATTTCACACACGCAGTCGG,-R-GGACCTGTTGATAGACTGCCA
- LC3: -F-GAACCGCAGACGCATCTCT,-R-TGATCACCGGGATCTTACTGG
- P62: -F-AATGTGATCTGTGATGGTTG,-R-GAGAGAAGCTATCAGAGAGG
- Galectin 8: -F-ATATACAAAAGCCAGGCAAG,-R-CAAATGCTTTCACATTGAGG
- TGF-β: -F-GGATACCAACTATTGCTTCAG,-R-TGTCCAGGCTCCAAATATAG
- Caspase 3: -F-CATAAGAGCACTGGAATGTC,-R-GCTCCTTTTGCTATGATCTTC
- Caspase 7: -F-CAAAACCCTGTTAGAGAAACC,-R-CCATGAGTAATAACCTGGAAC
- KIM-1: -F-CTGGAGTAATCACACTGAAG,-R-AAGTATGTACCTGGTGATAGC
- NGAL: -F-ATATGCACAGGTATCCTCAG,-R-GAAACGTTCCTTCAGTTCAG
- PGC1α: -F-TCCTCTTCAAGATCCTGTTAC,-R-CACATACAAGGGAGAATTGC
- Rpl13a: -F-AAGCAGGTACTTCTGGGCCG,-R-GGGGTTGGTATTCATCCGCT
4.5. Western Blot
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raghay, K.; Akki, R.; Bensaid, D.; Errami, M. Ghrelin as an anti-inflammatory and protective agent in ischemia/reperfusion injury. Peptides 2020, 124, 170226. [Google Scholar] [CrossRef]
- Chiang, C.K.; Loh, J.Z.; Yang, T.H.; Huang, K.T.; Wu, C.T.; Guan, S.S.; Liu, S.H.; Hung, K.Y. Prevention of acute kidney injury by low intensity pulsed ultrasound via anti-inflammation and anti-apoptosis. Sci. Rep. 2020, 10, 14317. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar]
- Kher, A.; Meldrum, K.K.; Wang, M.; Tsai, B.M.; Pitcher, J.M.; Meldrum, D.R. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc. Res. 2005, 67, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hutchens, M.P.; Dunlap, J.; Hurn, P.D.; Jarnberg, P.O. Renal ischemia: Does sex matter? Anesth. Analg. 2008, 107, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, S.R.; Neugarten, J. The impact of gender on the progression of chronic renal disease. Am. J. Kidney Dis. 1995, 25, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C.; Corman, B. The aging kidney: Insights from experimental studies. J. Am. Soc. Nephrol. 1998, 9, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.J.; Karl, M.; Berho, M.; Potier, M.; Zheng, F.; Leclercq, B.; Striker, G.E.; Striker, L.J. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. Am. J. Pathol. 2003, 162, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, N.; Magro, A.M.; Han, Y.; Rudofsky, U.H. Contrasting effects of early and late orchiectomy on hypertension and renal disease in fawn-hooded rats. Life Sci. 1987, 41, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, T.; Ohtsuka, N.; Shouno, Y.; Morito, F. Effect of ovariectomy on glomerular injury in hypercholesterolemic female Imai rats. Nephron 1996, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sakemi, T.; Ohtsuka, N.; Tomiyosi, Y.; Morito, F. Attenuating effect of castration on glomerular injury is age-dependent in unilaterally nephrectomized male Sprague-Dawley rats. Nephron 1997, 75, 342–349. [Google Scholar] [CrossRef]
- Zheng, F.; Plati, A.R.; Potier, M.; Schulman, Y.; Berho, M.; Banerjee, A.; Leclercq, B.; Zisman, A.; Striker, L.J.; Striker, G.E. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am. J. Pathol. 2003, 162, 1339–1348. [Google Scholar] [CrossRef]
- Gross, M.L.; Adamczak, M.; Rabe, T.; Harbi, N.A.; Krtil, J.; Koch, A.; Hamar, P.; Amann, K.; Ritz, E. Beneficial Effects of Estrogens on Indices of Renal Damage in Uninephrectomized SHRsp Rats. J. Am. Soc. Nephrol. 2004, 15, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.D.; Komers, R.; Osman, S.A.; Oyama, T.T.; Lindsley, J.N.; Anderson, S. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. 2005, 68, 1729–1739. [Google Scholar] [CrossRef]
- Tofovic, S.P.; Dubey, R.; Salah, E.M.; Jackson, E.K. 2-Hydroxyestradiol attenuates renal disease in chronic puromycin aminonucleoside nephropathy. J. Am. Soc. Nephrol. 2002, 13, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, S.R.; Neugarten, J. The role of gender in the progression of renal disease. Adv. Ren. Replace Ther. 2003, 10, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.; Vannay, A.; Ver, A.; Rusai, K.; Muller, V.; Reusz, G.; Tulassay, T.; Szabo, A.J. Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2006, 291, F806–F811. [Google Scholar] [CrossRef]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Renal. Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef]
- Metcalfe, P.D.; Meldrum, K.K. Sex differences and the role of sex steroids in renal injury. J. Urol. 2006, 176, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.; Losonczy, G.; Heemann, U.; Vannay, A.; Fekete, A.; Reusz, G.; Tulassay, T.; Szabo, A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002, 62, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Takaoka, M.; Nishikawa, M.; Yuba, M.; Shibata, Y.; Okumura, K.; Kitano, K.; Tsutsui, H.; Fujii, K.; Kobuchi, S.; et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008, 73, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.; Baylis, C. Sex differences in renal hemodynamics in rats. Am. J. Physiol. 1988, 254, F223–F231. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Anacta, L.A.; Cattran, D.C. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999, 55, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Golestaneh, L. Sex Differences in Acute Kidney Injury. Semin. Nephrol. 2022, 42, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Changes in renal hemodynamics and structure in the aging kidney; sexual dimorphism and the nitric oxide system. Exp. Gerontol. 2005, 40, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Nieto-Ceron, S.; Fenoy, F.J.; Lopez, B.; Hernandez, I.; Martinez, R.R.; Soriano, M.J.; Salom, M.G. Sex differences in nitrosative stress during renal ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1387–R1395. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin. Proc. 2019, 94, 1339–1356. [Google Scholar] [CrossRef]
- Miskin, R.; Masos, T.; Yahav, S.; Shinder, D.; Globerson, A. AlphaMUPA mice: A transgenic model for increased life span. Neurobiol. Aging 1999, 20, 555–564. [Google Scholar] [CrossRef]
- Miskin, R.; Axelrod, J.H.; Griep, A.E.; Lee, E.; Belin, D.; Vassalli, J.D.; Westphal, H. Human and murine urokinase cDNAs linked to the murine alpha A-crystallin promoter exhibit lens and non-lens expression in transgenic mice. Eur. J. Biochem. 1990, 190, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Booth, N. Plasminogen Activators: From Cloning to Therapy; Abbate, R., Barni, T., Tsafriri, A., Eds.; Raven Press: New York, NY, USA, 1991. [Google Scholar]
- Miskin, R.; Masos, T. Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, B118–B124. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Kornowski, R.; Gavrieli, R.; Fratty, I.; Greenberg, G.; Waldman, M.; Birk, E.; Shainberg, A.; Akirov, A.; Miskin, R.; et al. Long-Lived alphaMUPA Mice Show Attenuation of Cardiac Aging and Leptin-Dependent Cardioprotection. PLoS ONE 2015, 10, e0144593. [Google Scholar] [CrossRef] [PubMed]
- Abd Alkhaleq, H.; Kornowski, R.; Waldman, M.; Levy, E.; Zemel, R.; Nudelman, V.; Shainberg, A.; Miskin, R.; Hochhauser, E. Leptin modulates gene expression in the heart and cardiomyocytes towards mitigating ischemia-induced damage. Exp. Cell Res. 2020, 397, 112373. [Google Scholar] [CrossRef] [PubMed]
- Abd Alkhaleq, H.; Kornowski, R.; Waldman, M.; Zemel, R.; Lev, D.L.; Shainberg, A.; Miskin, R.; Hochhauser, E. Leptin modulates gene expression in the heart, cardiomyocytes and the adipose tissue thus mitigating LPS-induced damage. Exp. Cell Res. 2021, 404, 112647. [Google Scholar] [CrossRef] [PubMed]
- Alkhaleq, H.A.; Karram, T.; Fokra, A.; Hamoud, S.; Kabala, A.; Abassi, Z. The Protective Pathways Activated in Kidneys of alphaMUPA Transgenic Mice Following Ischemia\Reperfusion-Induced Acute Kidney Injury. Cells 2023, 12, 2497. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, P.; Leon-Contreras, J.C.; Aparicio-Trejo, O.E.; Reyes-Ocampo, J.G.; Medina-Campos, O.N.; Jimenez-Osorio, A.S.; Gonzalez-Reyes, S.; Marquina-Castillo, B.; Hernandez-Pando, R.; Barrera-Oviedo, D.; et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, J.I.; Ahn, Y.; Bonventre, A.J.; Bonventre, J.V. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J. Biol. Chem. 2004, 279, 52282–52292. [Google Scholar] [CrossRef]
- Takayama, J.; Takaoka, M.; Sugino, Y.; Yamamoto, Y.; Ohkita, M.; Matsumura, Y. Sex difference in ischemic acute renal failure in rats: Approach by proteomic analysis. Biol. Pharm. Bull. 2007, 30, 1905–1912. [Google Scholar] [CrossRef]
- Tanaka, R.; Tsutsui, H.; Kobuchi, S.; Sugiura, T.; Yamagata, M.; Ohkita, M.; Takaoka, M.; Yukimura, T.; Matsumura, Y. Protective effect of 17beta-estradiol on ischemic acute kidney injury through the renal sympathetic nervous system. Eur. J. Pharmacol. 2012, 683, 270–275. [Google Scholar] [CrossRef]
- Vinas, J.L.; Porter, C.J.; Douvris, A.; Spence, M.; Gutsol, A.; Zimpelmann, J.A.; Tailor, K.; Campbell, P.A.; Burns, K.D. Sex diversity in proximal tubule and endothelial gene expression in mice with ischemic acute kidney injury. Clin. Sci. 2020, 134, 1887–1909. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Bahceci, S.; Lucan, B.; Ryan, M. A practical guide for the use of very low calorie diets in adults with chronic kidney disease. Nephrology 2020, 25, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Spath, M.R.; Koehler, F.C.; Hoyer-Allo, K.J.R.; Grundmann, F.; Burst, V.; Muller, R.U. Preconditioning strategies to prevent acute kidney injury. F1000Research 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Sherman, H.; Bhargava, G.; Chapnik, N.; Cohen, R.; Gutman, R.; Kronfeld-Schor, N.; Miskin, R. Spontaneous caloric restriction associated with increased leptin levels in obesity-resistant alphaMUPA mice. Int. J. Obes. 2011, 35, 226–235. [Google Scholar] [CrossRef][Green Version]
- Tirosh, O.; Pardo, M.; Schwartz, B.; Miskin, R. Long-lived alphaMUPA transgenic mice show reduced SOD2 expression, enhanced apoptosis and reduced susceptibility to the carcinogen dimethylhydrazine. Mech. Ageing Dev. 2005, 126, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Miskin, R.; Tirosh, O.; Pardo, M.; Zusman, I.; Schwartz, B.; Yahav, S.; Dubnov, G.; Kohen, R. AlphaMUPA mice: A transgenic model for longevity induced by caloric restriction. Mech. Ageing Dev. 2005, 126, 255–261. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sutton, T.A. Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int. 2004, 66, 496–499. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Patel, N.S.A.; Kvale, E.O.; Cuzzocrea, S.; Brown, P.A.J.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002, 61, 862–871. [Google Scholar] [CrossRef]
- Neugarten, J.; Medve, I.; Lei, J.; Silbiger, S.R. Estradiol suppresses mesangial cell type I collagen synthesis via activation of the MAP kinase cascade. Am. J. Physiol. 1999, 277, F875–F881. [Google Scholar] [CrossRef]
- Zdunek, M.; Silbiger, S.; Lei, J.; Neugarten, J. Protein kinase CK2 mediates TGF-beta1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 2001, 60, 2097–2108. [Google Scholar] [CrossRef][Green Version]


| Group | Body Weight before Surgery (g.) | Body Weight after Surgery (g.) | % Change in BW | Kidney Weight (KW) (g.) | Kidney/Body Weight Ratio (%) |
|---|---|---|---|---|---|
| αMUPA male—Sham | 23.76 ± 0.57 | 23.94 ± 0.8 | +0.75% | 0.16 ± 0.005 | 0.68 ± 0.01 |
| αMUPA male—AKI | 23.97 ± 0.41 | 21.97 ± 0.3 | −8.34% ### | 0.174 ± 0.005 | 0.79 ± 0.02 ** |
| αMUPA male—Sham after orchiectomy | 25.48 ± 0.45 | 24.4 ± 0.77 | −4.2% | 0.13 ± 0.003 | 0.54 ± 0.02 &&& |
| αMUPA male—AKI after orchiectomy | 25.17 ± 0.42 | 23.68 ± 0.39 | −5.9% | 0.142 ± 0.003 | 0.6 ± 0.003 *,&&& |
| αMUPA male—Sham after L-NAME treatment | 27.11 ± 0.4 | 26.92 ± 0.34 | −0.7% | 0.174 ± 0.005 | 0.64 ± 0.01 & |
| αMUPA male—AKI after L-NAME treatment | 25.97 ± 0.53 | 24.16 ± 0.41 | −6.97% # | 0.185 ± 0.005 | 0.76 ± 0.01 ***,& |
| αMUPA female—Sham | 19.76 ± 0.83 | 19.85 ± 1.1 | +0.45% | 0.11 ± 0.005 | 0.53 ± 0.02 $$$ |
| αMUPA female—AKI | 20.71 ± 0.48 | 19.66 ± 0.49 | −5% | 0.117 ± 0.005 | 0.62 ± 0.02 *,$$$ |
| αMUPA female—Sham after oophorectomy | 21.81 ± 1.12 | 21.2 ± 0.94 | −2.8% | 0.124 ± 0.012 | 0.58 ± 0.02 |
| αMUPA female—AKI after oophorectomy | 21.7 ± 0.49 | 19.92 ± 0.5 | −8.2% # | 0.132 ± 0.005 | 0.68± 0.02 *,&,$$ |
| αMUPA female—Sham after L-NAME treatment | 22.2 ± 0.8 | 21.36 ± 0.7 | −3.7% | 0.123 ± 0.002 | 0.57 ± 0.007 $ |
| αMUPA female—AKI after L-NAME treatment | 22 ± 0.52 | 20.25 ± 0.49 | −7.9% # | 0.139 ± 0.006 | 0.68 ± 0.01 **,$,& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhaleq, H.A.; Hamoud, S.; Hacker, I.; Karram, T.; Fokra, A.; Kabala, A.; Abassi, Z. Gender-Specific Renoprotective Pathways in αMUPA Transgenic Mice Subjected to Acute Kidney Injury. Int. J. Mol. Sci. 2024, 25, 3544. https://doi.org/10.3390/ijms25063544
Alkhaleq HA, Hamoud S, Hacker I, Karram T, Fokra A, Kabala A, Abassi Z. Gender-Specific Renoprotective Pathways in αMUPA Transgenic Mice Subjected to Acute Kidney Injury. International Journal of Molecular Sciences. 2024; 25(6):3544. https://doi.org/10.3390/ijms25063544
Chicago/Turabian StyleAlkhaleq, Heba Abd, Shadi Hamoud, Israel Hacker, Tony Karram, Ahmad Fokra, Aviva Kabala, and Zaid Abassi. 2024. "Gender-Specific Renoprotective Pathways in αMUPA Transgenic Mice Subjected to Acute Kidney Injury" International Journal of Molecular Sciences 25, no. 6: 3544. https://doi.org/10.3390/ijms25063544
APA StyleAlkhaleq, H. A., Hamoud, S., Hacker, I., Karram, T., Fokra, A., Kabala, A., & Abassi, Z. (2024). Gender-Specific Renoprotective Pathways in αMUPA Transgenic Mice Subjected to Acute Kidney Injury. International Journal of Molecular Sciences, 25(6), 3544. https://doi.org/10.3390/ijms25063544






