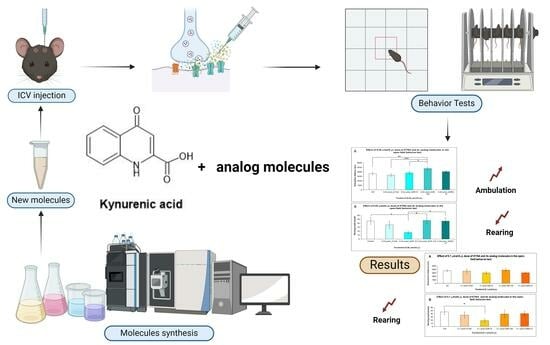
The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain
Abstract
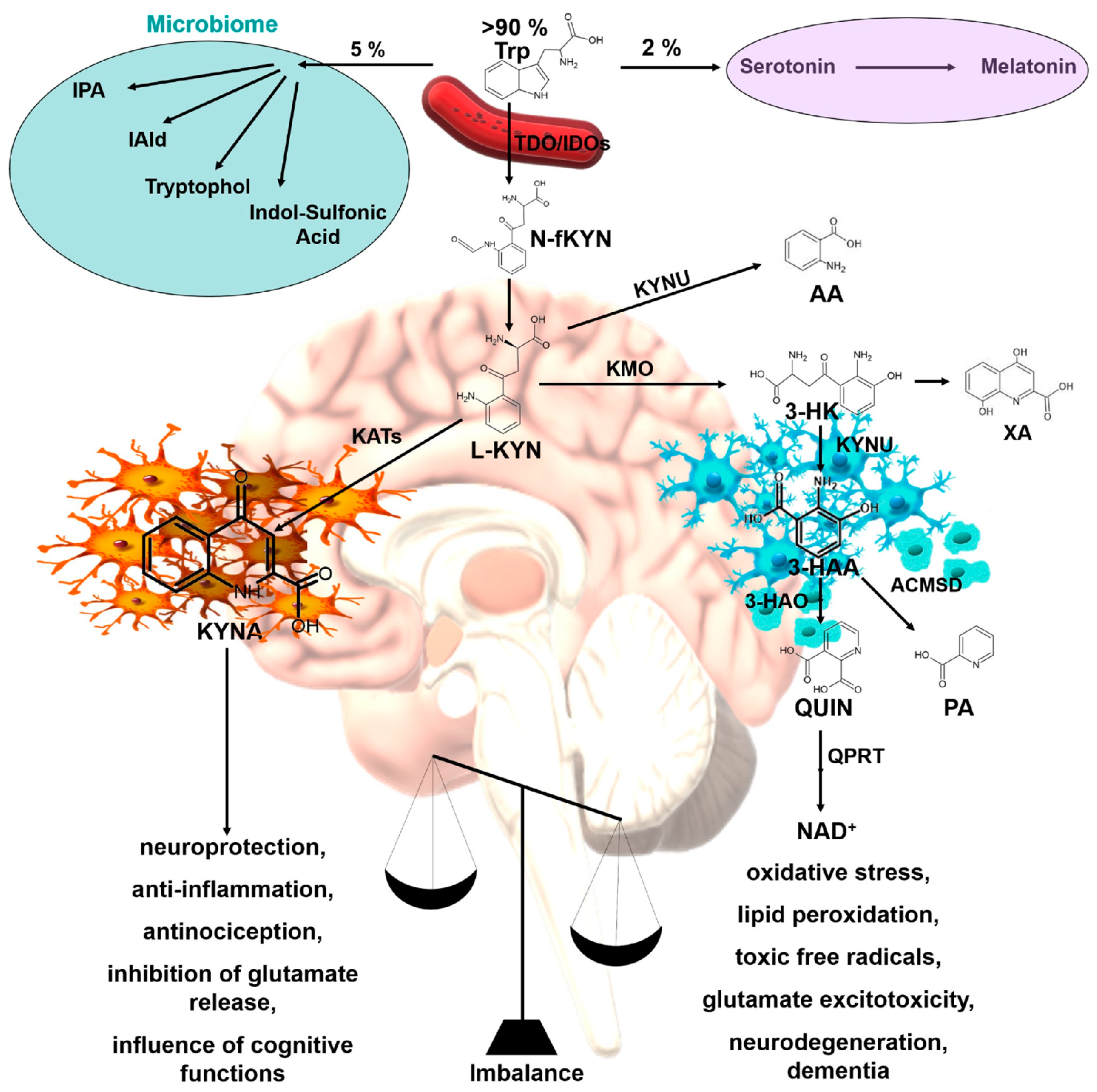
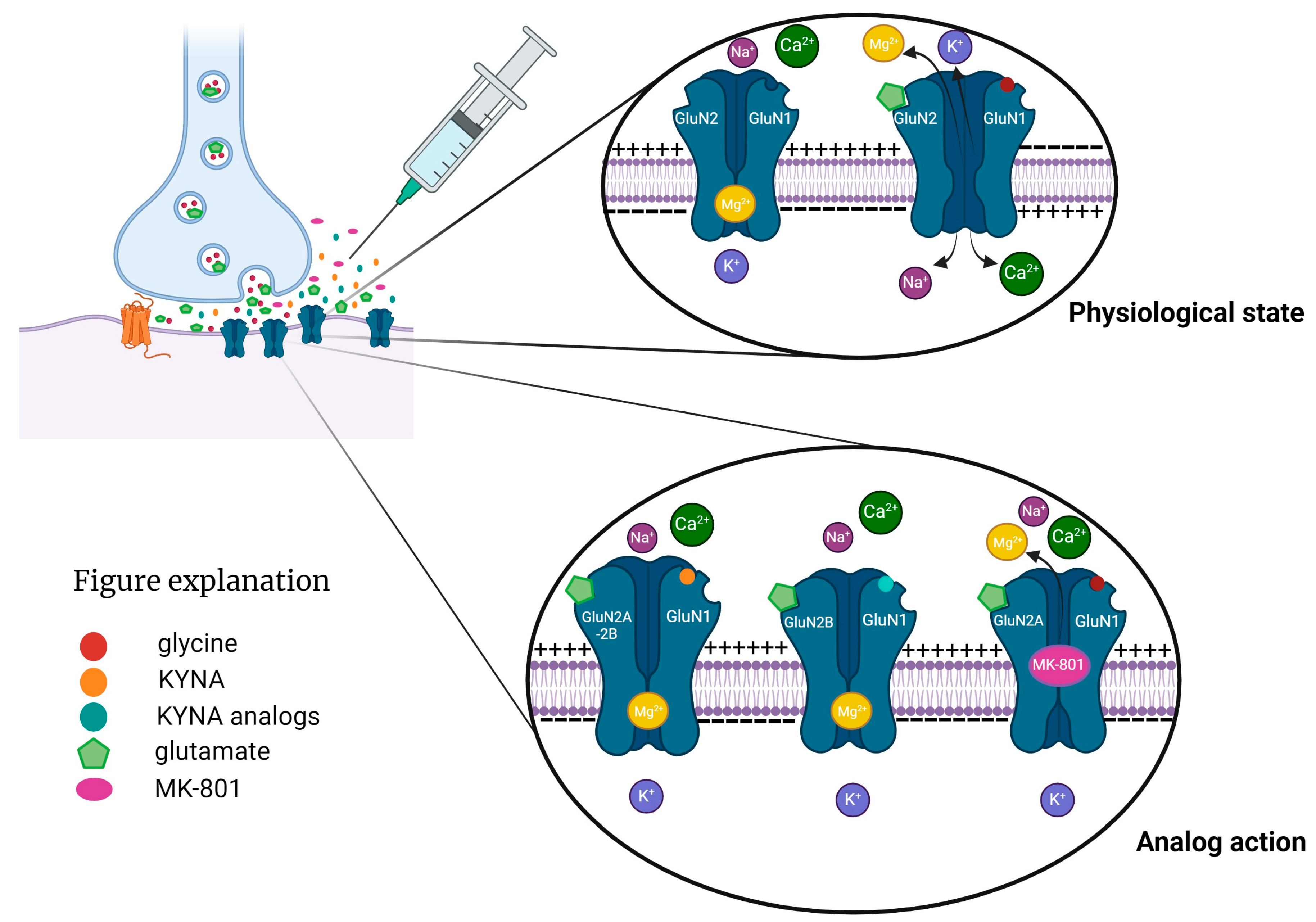
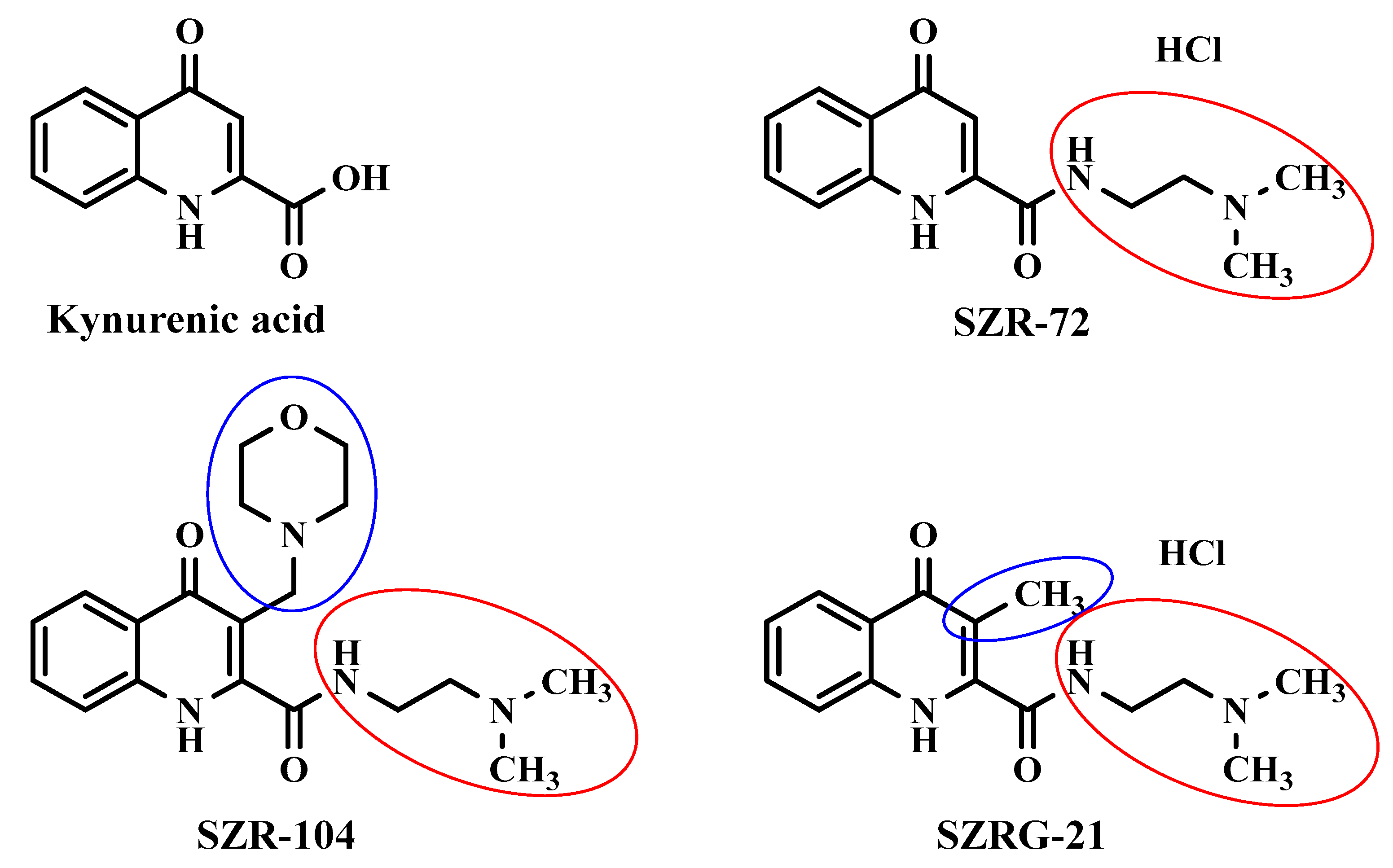
1. Introduction
2. Results
2.1. Behavioral Tests
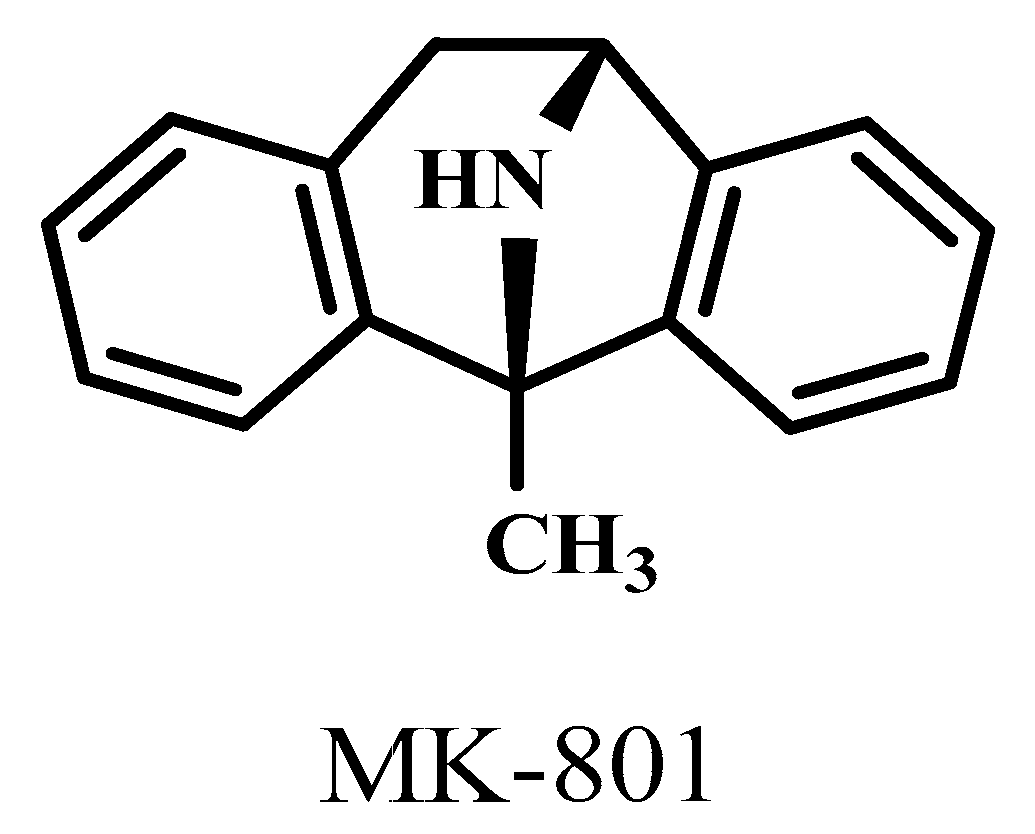
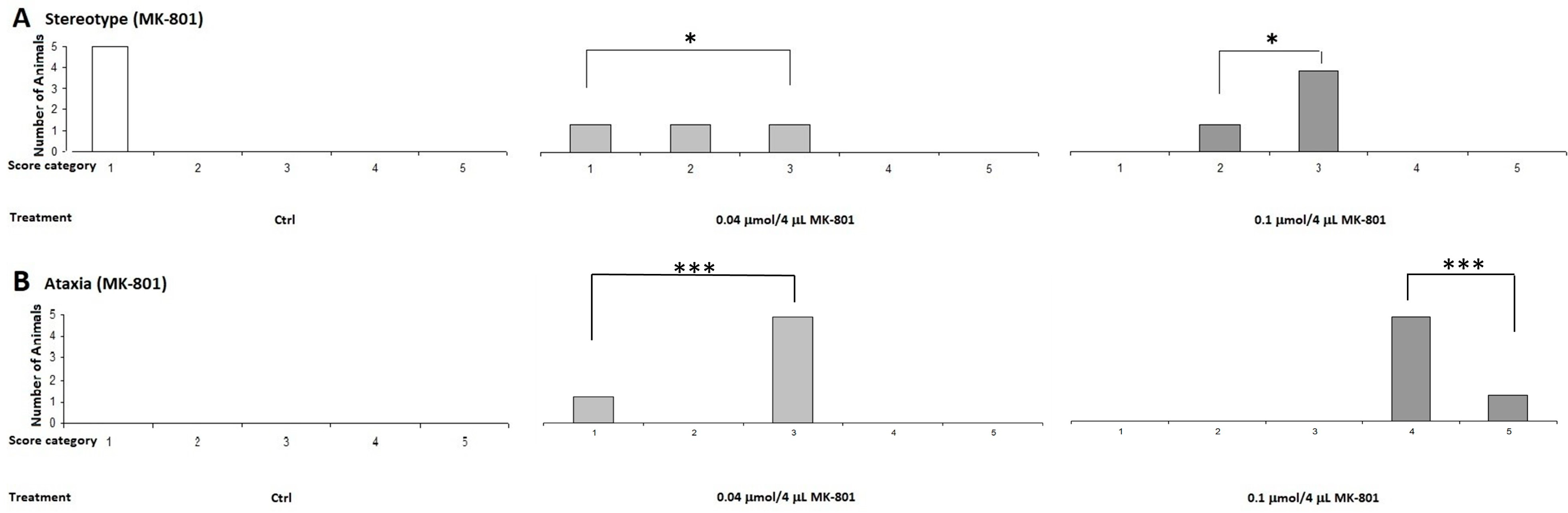
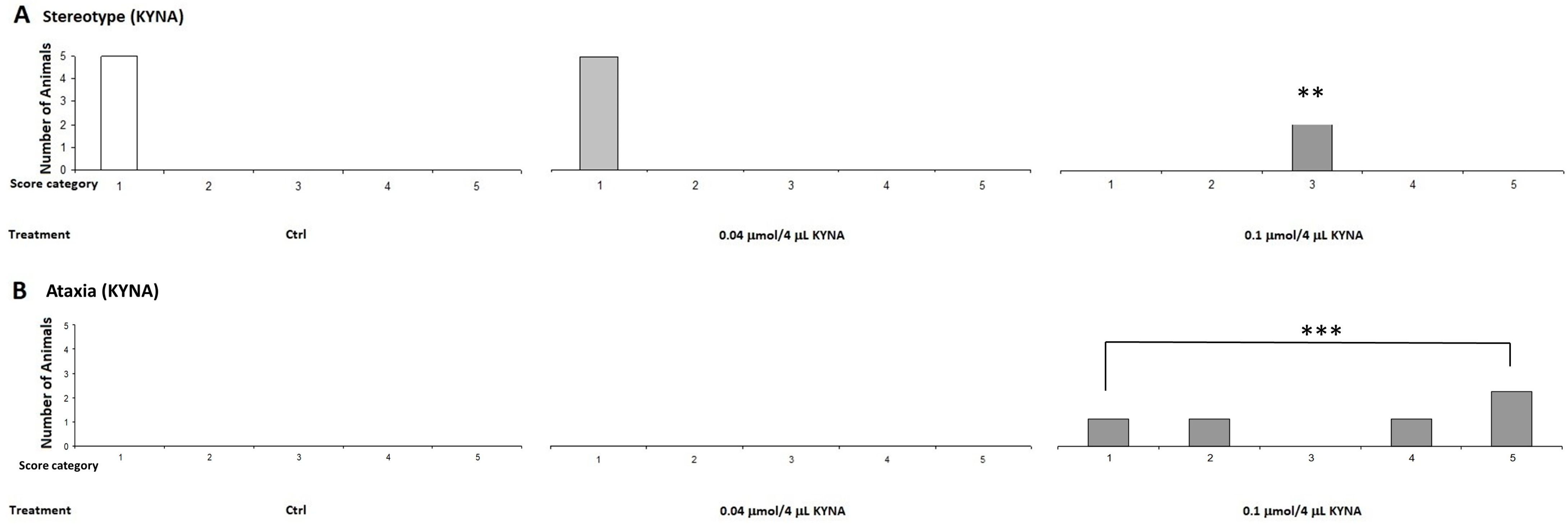
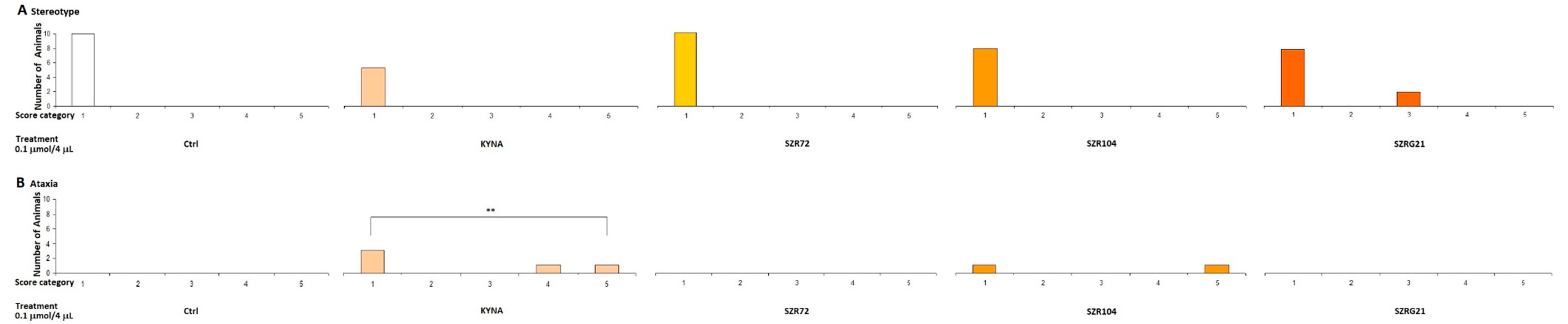
2.1.1. Pilot study
2.1.2. Stereotype/Ataxia Test
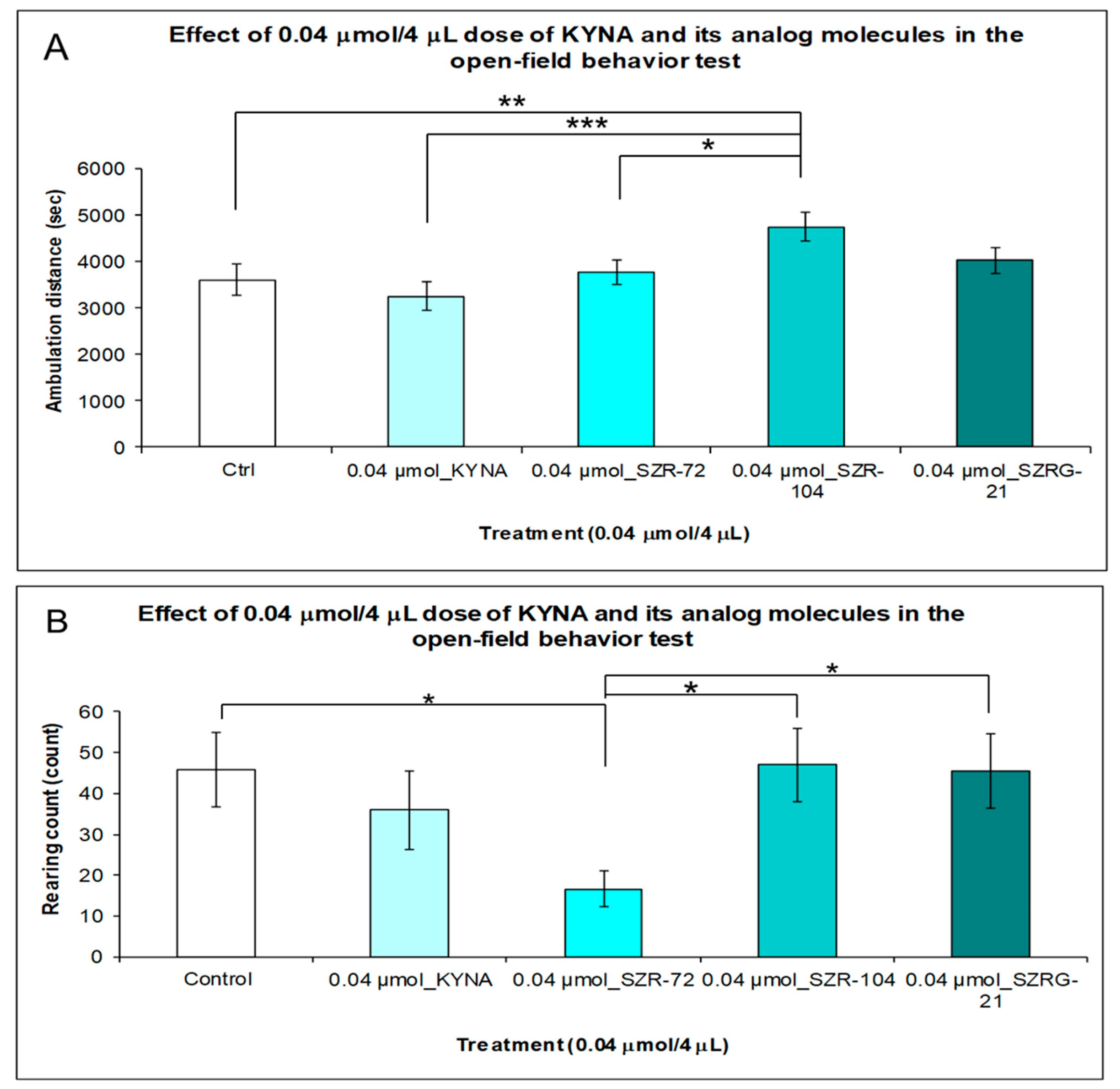
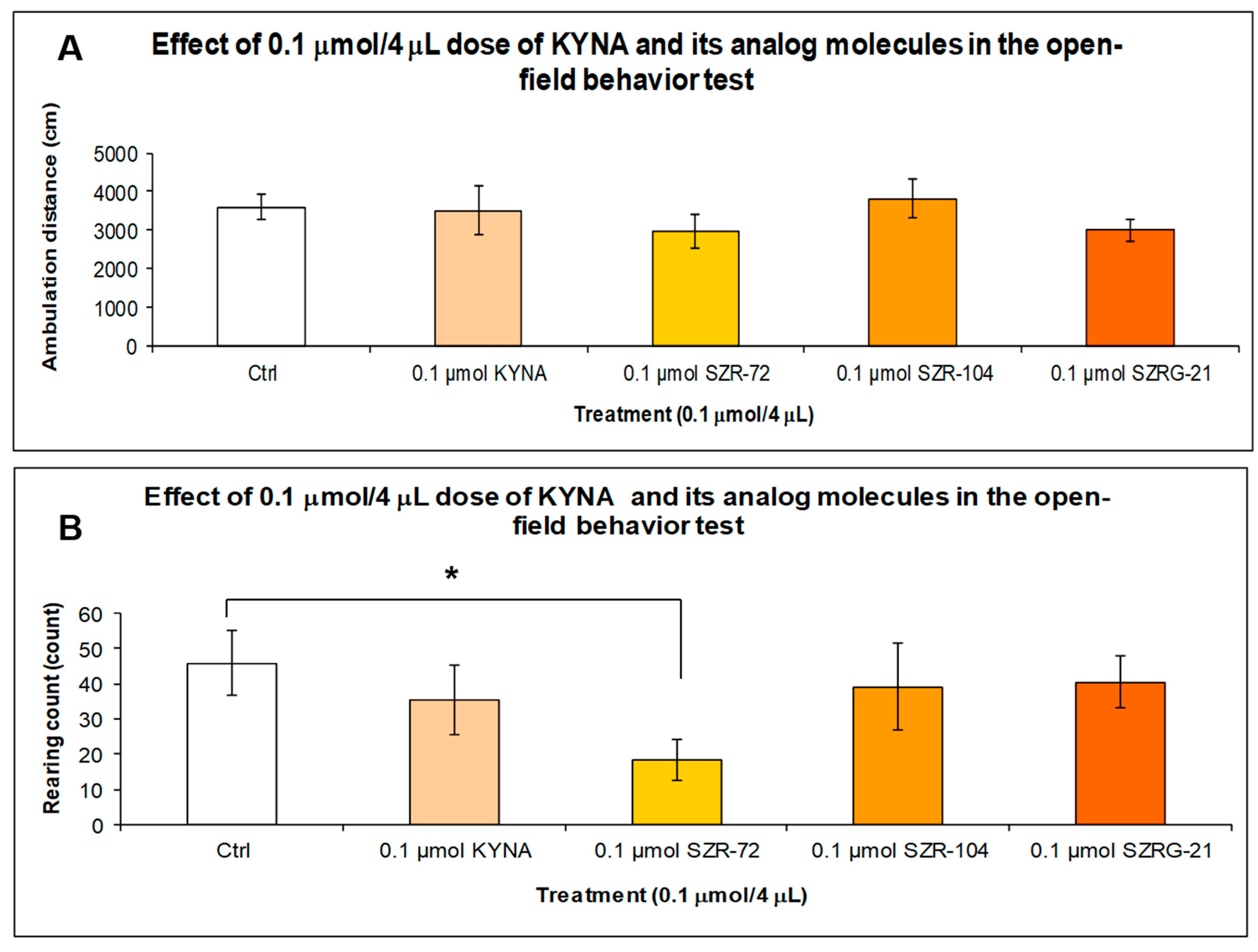
2.1.3. Open-Field (OF) Test
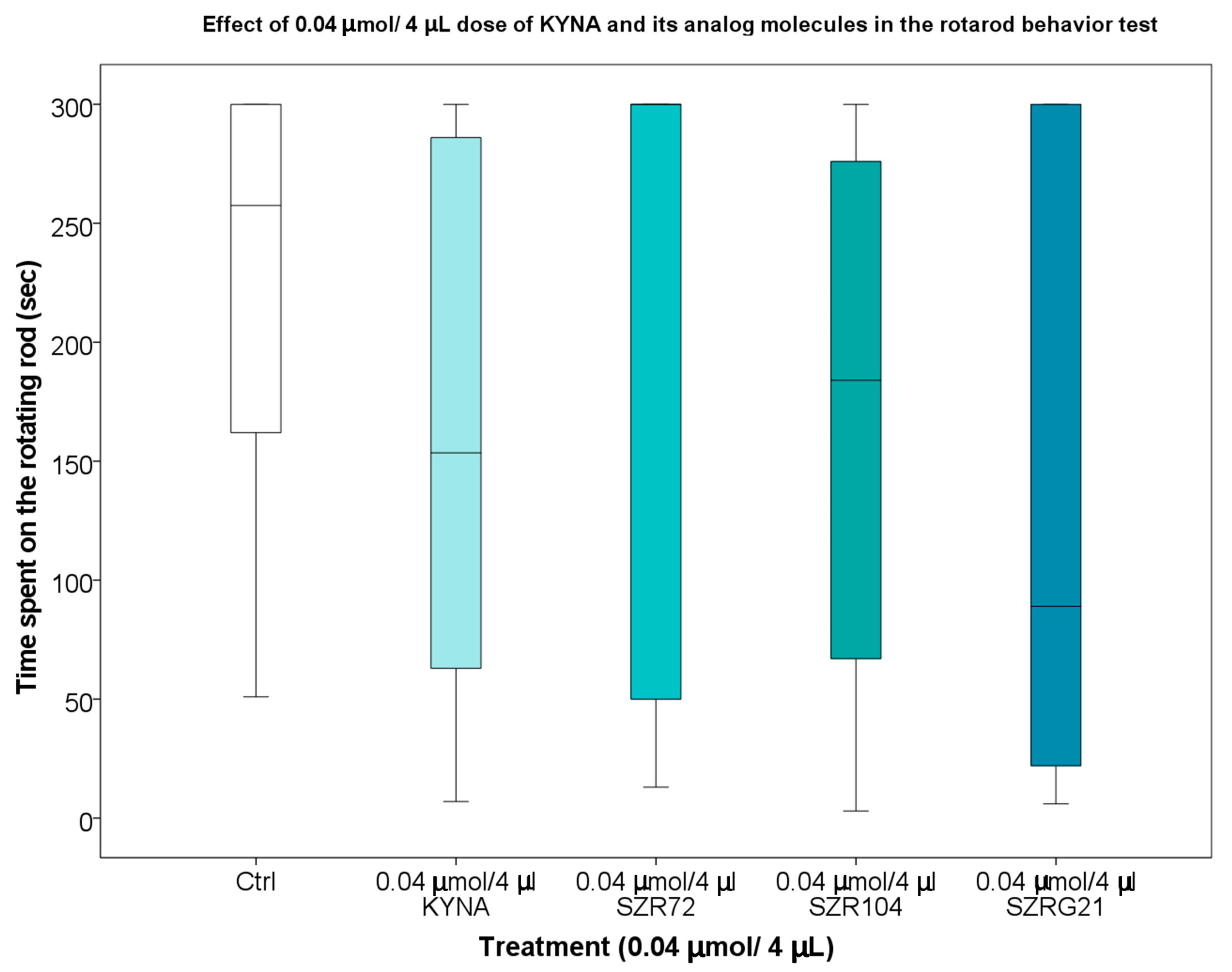
2.1.4. Rotarod (RR) Test
3. Discussion
4. Materials and Methods
4.1. Materials
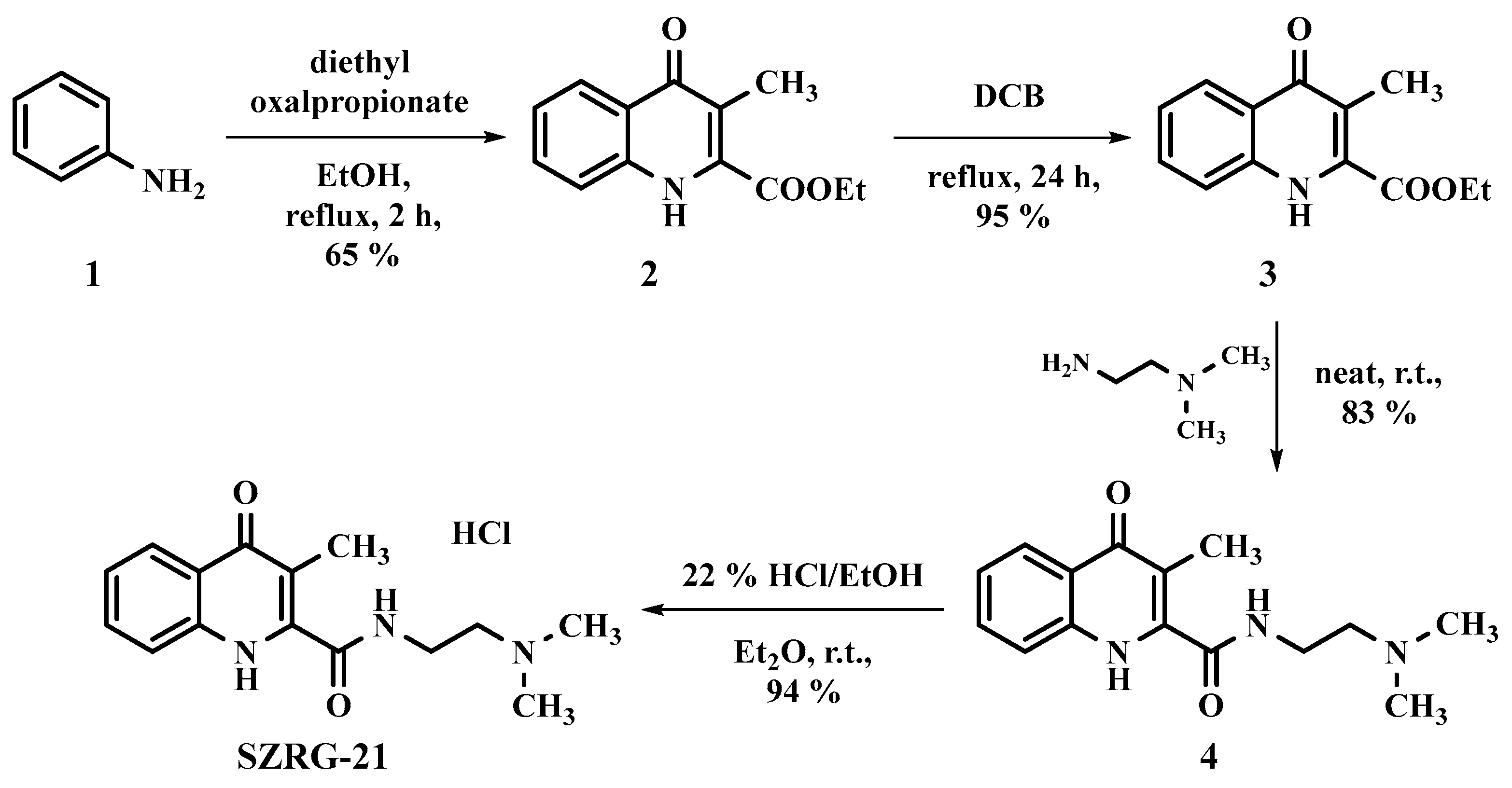
4.2. Kynurenic Acid Analog Synthesis
4.3. Animals
4.4. Surgery
4.5. Behavioral Tests
4.5.1. Pilot Study
4.5.2. Stereotype Behavior and Ataxia
4.5.3. Open-Field (OF) Test
4.5.4. Rotarod (RR) Test
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | anthranilic acid |
| ACMSD | 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase |
| AD | Alzheimer’s disease |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| IDOs | indoleamine 2,3-dioxygenases |
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxy-L-kynurenine |
| 3-HAO | 3-hydroxyanthranilate oxidase |
| KAT | kynurenine aminotransferase |
| KMO | kynurenine 3-monooxygenase |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
| MK-801 | 1-methyl-8-azabicyclo[3.2.1]octane |
| NADH | nicotinamide adenine dinucleotide + hydrogen |
| N-fKYN | N-formyl-kynurenine |
| NMDA | N-methyl-D-aspartic acid |
| PA | picolinic acid |
| PD | Parkinson’s disease |
| QPRT | quinolinate phosphoribosyltransferase |
| QUIN | quinolinic acid |
| SZR-72 | N-(2-(dimethylamino)ethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide |
| SZR-104 | N-(2-(dimethylamino)ethyl)-3-(morpholinomethyl)-4-oxo-1,4-dihydroquinoline-2-carboxamide |
| SZRG-21 | N-(2-(dimethylamino)ethyl)-3-methyl-4-oxo-1,4-dihydroquinoline-2-carboxamide |
| TDO | tryptophan 2,3-dioxygenase |
| Trp | tryptophan |
| XA | xanthurenic acid |
References
- Derryberry, D.; Tucker, D.M. Neural mechanisms of emotion. J. Consult. Clin. Psychol. 1992, 60, 329–338. [Google Scholar] [CrossRef]
- Pessoa, L. A Network Model of the Emotional Brain. Trends Cogn. Sci. 2017, 21, 357–371. [Google Scholar] [CrossRef]
- Jászberényi, M.; Thurzó, B.; Bagosi, Z.; Vécsei, L.; Tanaka, M. The Orexin/Hypocretin System, the Peptidergic Regulator of Vigilance, Orchestrates Adaptation to Stress. Biomedicines 2024, 12, 448. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring novel therapeutic targets in the common pathogenic factors in migraine and neuropathic pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef]
- Zigmond, M.J.; Wiley, C.A.; Chesselet, M.-F. Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Bors, L.A.; Erdő, F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Barchet, T.M.; Amiji, M.M. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin. Drug Deliv. 2009, 6, 211–225. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-brain barrier overview: Structural and functional correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: Understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zinserling, V. Defense Mechanisms and Local Immunity of the Brain. In Infectious Lesions of the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2022; pp. 5–24. [Google Scholar]
- Fong, C.W. Permeability of the blood–brain barrier: Molecular mechanism of transport of drugs and physiologically important compounds. J. Membr. Biol. 2015, 248, 651–669. [Google Scholar] [CrossRef]
- Seelig, A. The role of size and charge for blood–brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 2007, 33, 32–41. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Segal, M.; Zlokovic, B.V. The Blood-Brain Barrier, Amino Acids and Peptides; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Oldendorf, W.H. The blood-brain barrier. Exp. Eye Res. 1977, 25, 177–190. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. A comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood-brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar] [CrossRef]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr. Med. Chem. 2007, 14, 1199–1206. [Google Scholar] [CrossRef]
- Witt, K.A.; Gillespie, T.J.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Peptide drug modifications to enhance bioavailability and blood-brain barrier permeability. Peptides 2001, 22, 2329–2343. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: A recent perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Chung, S.; Yi, Y.; Ullah, I.; Chung, K.; Park, S.; Lim, J.; Kim, C.; Pyun, S.-H.; Kim, M.; Kim, D. Systemic Treatment with Fas-Blocking Peptide Attenuates Apoptosis in Brain Ischemia. Int. J. Mol. Sci. 2024, 25, 661. [Google Scholar] [CrossRef]
- Peraro, L.; Kritzer, J.A. Emerging methods and design principles for cell-penetrant peptides. Angew. Chem. Int. Ed. 2018, 57, 11868–11881. [Google Scholar] [CrossRef]
- Malakoutikhah, M.; Prades, R.; Teixido, M.; Giralt, E. N-methyl phenylalanine-rich peptides as highly versatile blood–brain barrier shuttles. J. Med. Chem. 2010, 53, 2354–2363. [Google Scholar] [CrossRef]
- Sanchez-Navarro, M.; Teixido, M.; Giralt, E. Jumping hurdles: Peptides able to overcome biological barriers. Acc. Chem. Res. 2017, 50, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009, 6, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Rosch, J.; Putnam, D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. J. Control. Release 2016, 240, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S. Drug development for central nervous system diseases using in vitro blood-brain barrier models and drug repositioning. Curr. Pharm. Des. 2020, 26, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Török, N.; Vécsei, L. Are 5-HT1 receptor agonists effective anti-migraine drugs? Expert Opin. Pharmacother. 2021, 22, 1221–1225. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Melatonin: Synthesis from tryptophan and its role in higher plant. In Amino Acids in Higher Plants; CAB International: Wallingford, UK, 2015; pp. 390–435. [Google Scholar]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Barik, S. The uniqueness of tryptophan in biology: Properties, metabolism, interactions and localization in proteins. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef]
- Peyrot, F.; Ducrocq, C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J. Pineal Res. 2008, 45, 235–246. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed.) 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.-B. Kynurenine pathway and human systems. Exp. Gerontol. 2020, 129, 110770. [Google Scholar] [CrossRef]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- Dezsi, L.; Tuka, B.; Martos, D.; Vecsei, L. Alzheimer’s disease, astrocytes and kynurenines. Curr. Alzheimer Res. 2015, 12, 462–480. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial impairment: A common motif in neuropsychiatric presentation? The link to the tryptophan–kynurenine metabolic system. Cells 2022, 11, 2607. [Google Scholar] [CrossRef]
- Muneer, A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: Pathophysiologic and therapeutic considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef]
- Iwaoka, K.; Otsuka, C.; Maeda, T.; Yamahara, K.; Kato, K.; Takahashi, K.; Takahashi, K.; Terayama, Y. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci. Lett. 2020, 714, 134576. [Google Scholar] [CrossRef]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer’s disease. J. Neural Transm. 1999, 106, 165–181. [Google Scholar] [CrossRef]
- Tan, L.; Yu, J.-T.; Tan, L. The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J. Neurol. Sci. 2012, 323, 1–8. [Google Scholar] [CrossRef]
- Urbańska, E.M.; Chmiel-Perzyńska, I.; Perzyński, A.; Derkacz, M.; Owe-Larsson, B. Endogenous kynurenic acid and neurotoxicity. In Handbook of Neurotoxicity; Springer: Cham, Switzerland, 2021; pp. 1–31. [Google Scholar]
- Sharma, R.; Razdan, K.; Bansal, Y.; Kuhad, A. Rollercoaster ride of kynurenines: Steering the wheel towards neuroprotection in Alzheimer’s disease. Expert Opin. Ther. Targets 2018, 22, 849–867. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Polyák, H.; Galla, Z.; Nánási, N.; Cseh, E.K.; Rajda, C.; Veres, G.; Spekker, E.; Szabó, Á.; Klivényi, P.; Tanaka, M. The tryptophan-kynurenine metabolic system is suppressed in cuprizone-induced model of demyelination simulating progressive multiple sclerosis. Biomedicines 2023, 11, 945. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Barone, P. The ‘Yin’and the ‘Yang’of the kynurenine pathway: Excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behav. Pharmacol. 2019, 30, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.; Drevets, W.C.; Smith, C.M.; Victor, T.A.; Wurfel, B.E.; Bellgowan, P.S.; Bodurka, J.; Teague, T.K.; Dantzer, R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015, 40, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2015; pp. 134–141. [Google Scholar]
- Pérez-De La Cruz, V.; Königsberg, M.; Santamaría, A. Kynurenine pathway and disease: An overview. CNS Neurol. Disord. Drug Targets 2007, 6, 398–410. [Google Scholar]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yan, M.; Wang, L.; Zhou, Y.; Wang, Z.; Xia, T.; Liu, X.; Pan, R.; Chang, Q. Homeostasis imbalance of microglia and astrocytes leads to alteration in the metabolites of the kynurenine pathway in LPS-induced depressive-like mice. Int. J. Mol. Sci. 2020, 21, 1460. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Tutakhail, A.; Boulet, L.; Khabil, S.; Nazari, Q.A.; Hamid, H.; Coudoré, F. Neuropathology of kynurenine pathway of tryptophan metabolism. Curr. Pharmacol. Rep. 2020, 6, 8–23. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan–kynurenine metabolic pathway in depression. Rev. Neurosci. 2023, 34, 313–324. [Google Scholar] [CrossRef]
- Deora, G.S.; Kantham, S.; Chan, S.; Dighe, S.N.; Veliyath, S.K.; McColl, G.; Parat, M.O.; McGeary, R.P.; Ross, B.P. Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacology, and Molecular Modeling Studies. ACS Chem. Neurosci. 2017, 8, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The mechanism of the neuroprotective effect of kynurenic acid in the experimental model of neonatal hypoxia–ischemia: The link to oxidative stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the etiological links behind neurodegenerative diseases: Inflammatory cytokines and bioactive kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: Metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2, 3-Dioxygenase 1 Influenced the Age Onset of Parkinson’s Disease. Front. Biosci. (Landmark Ed.) 2022, 27, 265. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vecsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan–kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N. Y. Acad. Sci. 2007, 1122, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.R.; Di Fazio, C.; Battaglia, S. Activated Tryptophan-Kynurenine metabolic system in the human brain is associated with learned fear. Front. Mol. Neurosci. 2023, 16, 1217090. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Vitale, F.; Battaglia, S.; de Vega, M.; Avenanti, A. Task-related modulation of motor response to emotional bodies: A TMS motor-evoked potential study. Cortex 2024, 171, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The role of Serotonin in fear learning and memory: A systematic review of human studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Heart’s tale of trauma: Fear-conditioned heart rate changes in post-traumatic stress disorder. Acta Psychiatr. Scand. 2023, 148, 463–466. [Google Scholar] [CrossRef]
- Talarowska, M.; Galecki, P. Cognition and emotions in recurrent depressive disorders-the role of inflammation and the kynurenine pathway. Curr. Pharm. Des. 2016, 22, 955–962. [Google Scholar] [CrossRef]
- Ren, W.H.; Guo, J.D.; Cao, H.; Wang, H.; Wang, P.F.; Sha, H.; Ji, R.R.; Zhao, Z.Q.; Zhang, Y.Q. Is endogenous d-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J. Neurochem. 2006, 96, 1636–1647. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural Correlates and Molecular Mechanisms of Memory and Learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2022, 27, 784–786. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the role of vmPFC in the acquisition of Pavlovian threat conditioning in humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-dependent TMS over prefrontal cortex disrupts fear-memory reconsolidation and prevents the return of fear. Curr. Biol. 2020, 30, 3672–3679.e4. [Google Scholar] [CrossRef]
- Samavati, R.; Zádor, F.; Szűcs, E.; Tuka, B.; Martos, D.; Veres, G.; Gáspár, R.; Mándity, I.M.; Fülöp, F.; Vécsei, L. Kynurenic acid and its analogue can alter the opioid receptor G-protein signaling after acute treatment via NMDA receptor in rat cortex and striatum. J. Neurol. Sci. 2017, 376, 63–70. [Google Scholar] [CrossRef]
- Morales-Puerto, N.; Gimenez-Gomez, P.; Perez-Hernandez, M.; Abuin-Martinez, C.; de Biedma-Elduayen, L.G.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef]
- Klausing, A.D. Effects of Acute Stress on Discriminative Fear Conditioning: A Key Role of Kynurenic Acid in the Medial Prefrontal Cortex. Ph.D. Thesis, University of Maryland, Baltimore, MD, USA, 2020. [Google Scholar]
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, T.K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 2016, 53, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, V.; Costafreda, S.G.; Rimmer, R.M.; Rasenick, M.M.; Marangell, L.B.; Fu, C.H. Brain-derived neurotrophic factor association with amygdala response in major depressive disorder. J. Affect. Disord. 2020, 267, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, H.A.; Gandhi, C.P.; Hill, M.N. Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plast. 2016, 2016, 7215684. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Lőrinczi, B.; Szatmári, I.; Fülöp, F.; Vécsei, L. Antidepressant-like Effects of Kynurenic Acid Analogues. Presented at the 1st International Electronic Conference on Biomedicine, Online, 1–26 June 2021; p. 26. Available online: https://sciforum.net/paper/view/10301 (accessed on 12 March 2024).
- Myint, A.M.; Halaris, A. Imbalances in kynurenines as potential biomarkers in the diagnosis and treatment of psychiatric disorders. Front. Psychiatry 2022, 13, 913303. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological modulation of N-methyl-D-aspartate, noradrenaline and endocannabinoid receptors in fear extinction learning: Synaptic transmission and plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Meier, T.B.; Savitz, J. The kynurenine pathway in traumatic brain injury: Implications for psychiatric outcomes. Biol. Psychiatry 2022, 91, 449–458. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef]
- Bai, M.Y.; Lovejoy, D.B.; Guillemin, G.J.; Kozak, R.; Stone, T.W.; Koola, M.M. Galantamine-memantine combination and kynurenine pathway enzyme inhibitors in the treatment of neuropsychiatric disorders. Complex Psychiatry 2021, 7, 19–33. [Google Scholar] [CrossRef]
- Chen, P.; Geng, X. Research progress on the kynurenine pathway in the prevention and treatment of Parkinson’s disease. J. Enzym. Inhib. Med. Chem. 2023, 38, 2225800. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging Dis. 2023, 14, 858. [Google Scholar] [CrossRef]
- Sheibani, M.; Shayan, M.; Khalilzadeh, M.; Soltani, Z.E.; Jafari-Sabet, M.; Ghasemi, M.; Dehpour, A.R. Kynurenine pathway and its role in neurologic, psychiatric, and inflammatory bowel diseases. Mol. Biol. Rep. 2023, 50, 10409–10425. [Google Scholar] [CrossRef]
- S Jayawickrama, G.; R Sadig, R.; Sun, G.; Nematollahi, A.; A Nadvi, N.; R Hanrahan, J.; D Gorrell, M.; Bret Church, W. Kynurenine aminotransferases and the prospects of inhibitors for the treatment of schizophrenia. Curr. Med. Chem. 2015, 22, 2902–2918. [Google Scholar] [CrossRef]
- Kloog, Y.; Lamdani-Itkin, H.; Sokolovsky, M. The glycine site of the N-methyl-D-aspartate receptor channel: Differences between the binding of HA-966 and of 7-chlorokynurenic acid. J. Neurochem. 1990, 54, 1576–1583. [Google Scholar] [CrossRef]
- Mok, M.S.; Fricker, A.-C.; Weil, A.; Kew, J.N. Electrophysiological characterisation of the actions of kynurenic acid at ligand-gated ion channels. Neuropharmacology 2009, 57, 242–249. [Google Scholar] [CrossRef]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef]
- Rózsa, E.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. 2008, 115, 1087–1091. [Google Scholar] [CrossRef]
- Gladding, C.M.; Raymond, L.A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell. Neurosci. 2011, 48, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Petralia, R.S. Distribution of extrasynaptic NMDA receptors on neurons. Sci. World J. 2012, 2012, 267120. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate Jr, C.A. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry 2017, 81, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Pribiag, H.; Jefferson, S.J.; Shorey, M.; Fuchs, T.; Stellwagen, D.; Luscher, B. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol. Psychiatry 2016, 80, 457–468. [Google Scholar] [CrossRef]
- Kasper, J.M.; McCue, D.L.; Milton, A.J.; Szwed, A.; Sampson, C.M.; Huang, M.; Carlton, S.; Meltzer, H.Y.; Cunningham, K.A.; Hommel, J.D. Gamma-Aminobutyric Acidergic Projections From the Dorsal Raphe to the Nucleus Accumbens Are Regulated by Neuromedin U. Biol. Psychiatry 2016, 80, 878–887. [Google Scholar] [CrossRef]
- Adeel, M.; Chen, C.-C.; Lin, B.-S.; Chen, H.-C.; Liou, J.-C.; Li, Y.-T.; Peng, C.-W. Safety of Special Waveform of Transcranial Electrical Stimulation (TES): In Vivo Assessment. Int. J. Mol. Sci. 2022, 23, 6850. [Google Scholar] [CrossRef]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A.; et al. Kynurenic Acid and Its Synthetic Derivatives Protect against Sepsis-Associated Neutrophil Activation and Brain Mitochondrial Dysfunction in Rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Szabo, M.; Lajkó, N.; Dulka, K.; Szatmári, I.; Fülöp, F.; Mihály, A.; Vécsei, L.; Gulya, K. Kynurenic Acid and Its Analog SZR104 Exhibit Strong Antiinflammatory Effects and Alter the Intracellular Distribution and Methylation Patterns of H3 Histones in Immunochallenged Microglia-Enriched Cultures of Newborn Rat Brains. Int. J. Mol. Sci. 2022, 23, 1079. [Google Scholar] [CrossRef]
- Gregorio, F.; Battaglia, S. Advances in EEG-based functional connectivity approaches to the study of the central nervous system in health and disease. Adv. Clin. Exp. Med. 2023, 32, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e) motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; di Pellegrino, G. Don’t hurt me no more: State-dependent transcranial magnetic stimulation for the treatment of specific phobia. J. Affect. Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Almeida, P.G.; Nani, J.V.; Oses, J.P.; Brietzke, E.; Hayashi, M.A. Neuroinflammation and glial cell activation in mental disorders. Brain Behav. Immun.-Health 2020, 2, 100034. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front. Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef]
- Di Gregorio, F.; La Porta, F.; Petrone, V.; Battaglia, S.; Orlandi, S.; Ippolito, G.; Romei, V.; Piperno, R.; Lullini, G. Accuracy of EEG biomarkers in the detection of clinical outcome in disorders of consciousness after severe acquired brain injury: Preliminary results of a pilot study using a machine learning approach. Biomedicines 2022, 10, 1897. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. The influence of vicarious fear-learning in “infecting” reactive action inhibition. Front. Behav. Neurosci. 2022, 16, 946263. [Google Scholar] [CrossRef]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Veres, G.; Fejes-Szabo, A.; Zadori, D.; Nagy-Grocz, G.; Laszlo, A.M.; Bajtai, A.; Mandity, I.; Szentirmai, M.; Bohar, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: A behavioral, immunohistochemical and pharmacokinetic study. J. Neural Transm. 2017, 124, 99–112. [Google Scholar] [CrossRef]
- Tanaka, M.; Chen, C. Towards a mechanistic understanding of depression, anxiety, and their comorbidity: Perspectives from cognitive neuroscience. Front. Behav. Neurosci. 2023, 17, 1268156. [Google Scholar] [CrossRef]
- Quadt, L.; Critchley, H.; Nagai, Y. Cognition, emotion, and the central autonomic network. Auton. Neurosci. 2022, 238, 102948. [Google Scholar] [CrossRef]
- Lewis, M.D.; Todd, R.M. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cogn. Dev. 2007, 22, 406–430. [Google Scholar] [CrossRef]
- Maiese, M. Embodiment, Emotion, and Cognition; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharm. 2006, 147 (Suppl. S1), S232–S240. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Eccles, J.; Garfinkel, S.N. Interaction between cognition, emotion, and the autonomic nervous system. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 117, pp. 59–77. [Google Scholar]
- Šumec, R.; Rektorová, I.; Jech, R.; Menšíková, K.; Roth, J.; Růžička, E.; Sochorová, D.; Dušek, L.; Kaňovský, P.; Rektor, I. Motion and emotion: Anxiety–axial connections in Parkinson’s disease. J. Neural Transm. 2017, 124, 369–377. [Google Scholar] [CrossRef]
- Jawer, M.A. Sensitive Soul: The Unseen Role of Emotion in Extraordinary States; Simon and Schuster: New York, NY, USA, 2020. [Google Scholar]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: How to “open” the blood brain barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Davis, T.P.; Ronaldson, P.T. Transporters at CNS barrier sites: Obstacles or opportunities for drug delivery? Curr. Pharm. Des. 2014, 20, 1422–1449. [Google Scholar] [CrossRef]
- De Boer, A.; Van Der Sandt, I.; Gaillard, P. The role of drug transporters at the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 629–656. [Google Scholar] [CrossRef]
- Shityakov, S.; Neuhaus, W.; Dandekar, T.; Förster, C. Analysing molecular polar surface descriptors to predict blood-brain barrier permeation. Int. J. Comput. Biol. Drug Des. 2013, 6, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L.; Firth, J.A.; Goldstein, G.W. Polarity of the blood-brain barrier: Distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 1980, 192, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the blood-brain barrier permeability of new drug-like compounds via HPLC with various stationary phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the CNS: Strategies to overcome the BBB. Int. J. Pharm. 2023, 636, 122759. [Google Scholar] [CrossRef]
- Liu, H.-M.; Liu, X.-F.; Yao, J.-L.; Wang, C.-L.; Yu, Y.; Wang, R. Utilization of combined chemical modifications to enhance the blood-brain barrier permeability and pharmacological activity of endomorphin-1. J. Pharmacol. Exp. Ther. 2006, 319, 308–316. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016, 8, 749. [Google Scholar] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012, 279, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Myint, A.-M.; Schwarz, M.J. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders-relation to drug treatment. Dialogues Clin. Neurosci. 2009, 11, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Amaral, M.; Outeiro, T.F.; Scrutton, N.S.; Giorgini, F. The causative role and therapeutic potential of the kynurenine pathway in neurodegenerative disease. J. Mol. Med. 2013, 91, 705–713. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine Metabolism and Alzheimer’s Disease: The Potential Targets and Approaches. Neurochem. Res. 2022, 47, 1459–1476. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Ruas, J.L. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol.-Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, R.; Kumar, A.; Mehta, P.K.; Yadav, A.K. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol. Genet. Eng. Rev. 2021, 37, 105–153. [Google Scholar] [CrossRef]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine pathway metabolites in humans: Disease and healthy states. Int. J. Tryptophan Res. 2009, 2, IJTR.S2097. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, tryptophan metabolism, and kynurenine pathway: A complex interconnected loop influencing human health status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef]
- Lin, J.; Sun-Waterhouse, D.; Cui, C. The therapeutic potential of diet on immune-related diseases: Based on the regulation on tryptophan metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 8793–8811. [Google Scholar] [CrossRef] [PubMed]
- Günther, J.; Fallarino, F.; Fuchs, D.; Wirthgen, E. Immunomodulatory roles of tryptophan metabolites in inflammation and cancer. Front. Immunol. 2020, 11, 1497. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic acid in the digestive system—New facts, new challenges. Int. J. Tryptophan Res. 2013, 6, IJTR.S12536. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: The tryptophan-kynurenine metabolic pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between existential phenomenological psychotherapy and neurological sciences in mood and anxiety disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef]
- Schwarcz, R. The kynurenine pathway of tryptophan degradation as a drug target. Curr. Opin. Pharm. 2004, 4, 12–17. [Google Scholar] [CrossRef]
- Balla, Z.; Kormányos, E.S.; Kui, B.; Bálint, E.R.; Fűr, G.; Orján, E.M.; Iványi, B.; Vécsei, L.; Fülöp, F.; Varga, G.; et al. Kynurenic Acid and Its Analogue SZR-72 Ameliorate the Severity of Experimental Acute Necrotizing Pancreatitis. Front. Immunol. 2021, 12, 702764. [Google Scholar] [CrossRef]
- Gellért, L.; Varga, D.; Ruszka, M.; Toldi, J.; Farkas, T.; Szatmári, I.; Fülöp, F.; Vécsei, L.; Kis, Z. Behavioural studies with a newly developed neuroprotective KYNA-amide. J. Neural Transm. 2012, 119, 165–172. [Google Scholar] [CrossRef]
- Vámos, E.; Párdutz, A.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The Opposite Effects of Kynurenic Acid and Different Kynurenic Acid Analogs on Tumor Necrosis Factor-α (TNF-α) Production and Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Demeter, I.; Nagy, K.; Gellért, L.; Vécsei, L.; Fülöp, F.; Toldi, J. A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: Special issue related to kynurenine. J. Neural Transm. 2012, 119, 151–154. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory enhancement with kynurenic acid and its mechanisms in neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.; Roberts, M.S. Challenges and Innovations of Controlled Drug Delivery. In Fundamentals of Drug Delivery; Wiley: Hoboken, NJ, USA, 2021; pp. 1–14. [Google Scholar]
- McCarson, K.E. Strategies for behaviorally phenotyping the transgenic mouse. In Transgenic Mouse: Methods and Protocols; Humana: New York, NY, USA, 2020; pp. 171–194. [Google Scholar]
- Sukoff Rizzo, S.J.; Crawley, J.N. Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annu. Rev. Anim. Biosci. 2017, 5, 371–389. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-players in chronic pain: Neuroinflammation and the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Molnár, K.; Lőrinczi, B.; Fazakas, C.; Szatmári, I.; Fülöp, F.; Kmetykó, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I. Szr-104, a novel kynurenic acid analogue with high permeability through the blood–brain barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef]
- Castillo-Mariqueo, L.; Giménez-Llort, L. Impact of Behavioral Assessment and Re-Test as Functional Trainings That Modify Survival, Anxiety and Functional Profile (Physical Endurance and Motor Learning) of Old Male and Female 3xTg-AD Mice and NTg Mice with Normal Aging. Biomedicines 2022, 10, 973. [Google Scholar] [CrossRef]
- Lee, E.C.; Hong, D.-Y.; Lee, D.-H.; Park, S.-W.; Lee, J.Y.; Jeong, J.H.; Kim, E.-Y.; Chung, H.-M.; Hong, K.-S.; Park, S.-P. Inflammation and rho-associated protein kinase-induced brain changes in vascular dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment affects anxious behaviors and theExpression of serotonergic genes in Midbrain Raphe nuclei of defeated male mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef]
- Vila-Merkle, H.; González-Martínez, A.; Campos-Jiménez, R.; Martínez-Ricós, J.; Teruel-Martí, V.; Blasco-Serra, A.; Lloret, A.; Celada, P.; Cervera-Ferri, A. The Oscillatory Profile Induced by the Anxiogenic Drug FG-7142 in the Amygdala–Hippocampal Network Is Reversed by Infralimbic Deep Brain Stimulation: Relevance for Mood Disorders. Biomedicines 2021, 9, 783. [Google Scholar] [CrossRef]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Fine-Tuning the PI3K/Akt Signaling Pathway Intensity by Sex and Genotype-Load: Sex-Dependent Homozygotic Threshold for Somatic Growth but Feminization of Anxious Phenotype in Middle-Aged PDK1 K465E Knock-In and Heterozygous Mice. Biomedicines 2021, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Marin-Pardo, D.; Marazuela, P.; Hernández-Guillamón, M. Survival Bias and Crosstalk between Chronological and Behavioral Age: Age-and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging. Biomedicines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Samra, A.I.; Kamel, A.S.; Abdallah, D.M.; El Fattah, M.A.A.; Ahmed, K.A.; El-Abhar, H.S. Preclinical Evidence for the Role of the Yin/Yang Angiotensin System Components in Autism Spectrum Disorder: A Therapeutic Target of Astaxanthin. Biomedicines 2023, 11, 3156. [Google Scholar] [CrossRef] [PubMed]
- Canfora, F.; Ottaviani, G.; Calabria, E.; Pecoraro, G.; Leuci, S.; Coppola, N.; Sansone, M.; Rupel, K.; Biasotto, M.; Di Lenarda, R. Advancements in Understanding and Classifying Chronic Orofacial Pain: Key Insights from Biopsychosocial Models and International Classifications (ICHD-3, ICD-11, ICOP). Biomedicines 2023, 11, 3266. [Google Scholar] [CrossRef]
- Cruz-Martínez, Y.; Aguilar-Ponce, L.; Romo-Araiza, A.; Chávez-Guerra, A.; Martiñón, S.; Ibarra-García, A.P.; Arias-Santiago, S.; Gálvez-Susano, V.; Ibarra, A. Supplementation with a Symbiotic Induced Neuroprotection and Improved Memory in Rats with Ischemic Stroke. Biomedicines 2024, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Baliellas, D.E.; Barros, M.P.; Vardaris, C.V.; Guariroba, M.; Poppe, S.C.; Martins, M.F.; Pereira, Á.A.; Bondan, E.F. Propentofylline Improves Thiol-Based Antioxidant Defenses and Limits Lipid Peroxidation following Gliotoxic Injury in the Rat Brainstem. Biomedicines 2023, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaokun, O.O. The Beneficial Role of Apigenin against Cognitive and Neurobehavioural Dysfunction: A Systematic Review of Preclinical Investigations. Biomedicines 2024, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, G.; Yi, J.; Huang, Y. Intraoperative Hypothermia Induces Vascular Dysfunction in the CA1 Region of Rat Hippocampus. Brain Sci. 2022, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, S.; Low, S.W.; Poore, C.P.; Lee, A.T.; Nilius, B.; Liao, P. TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines 2023, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Salafutdinov, I.I.; Gatina, D.Z.; Markelova, M.I.; Garanina, E.E.; Malanin, S.Y.; Gazizov, I.M.; Izmailov, A.A.; Rizvanov, A.A.; Islamov, R.R.; Palotás, A.; et al. A Biosafety Study of Human Umbilical Cord Blood Mononuclear Cells Transduced with Adenoviral Vector Carrying Human Vascular Endothelial Growth Factor cDNA In Vitro. Biomedicines 2023, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, A.J.; Finner-Prével, M.; Sudar, F.P.; Langer, F.H.; Keskin, F.; Gebel, A.; Zweerings, J.; Mathiak, K. The interplay between vitamin D, exposure of anticholinergic antipsychotics and cognition in schizophrenia. Biomedicines 2022, 10, 1096. [Google Scholar] [CrossRef]
- Simonato, M.; Dall’Acqua, S.; Zilli, C.; Sut, S.; Tenconi, R.; Gallo, N.; Sfriso, P.; Sartori, L.; Cavallin, F.; Fiocco, U. Tryptophan metabolites, cytokines, and fatty acid binding protein 2 in myalgic encephalomyelitis/chronic fatigue syndrome. Biomedicines 2021, 9, 1724. [Google Scholar] [CrossRef]
- Panov, G.; Dyulgerova, S.; Panova, P. Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines 2023, 11, 3114. [Google Scholar] [CrossRef]
- de Oliveira, M.; Santinelli, F.B.; Lisboa-Filho, P.N.; Barbieri, F.A. The blood concentration of metallic nanoparticles is related to cognitive performance in people with multiple sclerosis: An exploratory analysis. Biomedicines 2023, 11, 1819. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Biomarkers of Neurodegeneration in Post-Traumatic Stress Disorder: An Integrative Review. Biomedicines 2023, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, A.; Lakićević, N.; Pavlović, S. Mercury and Autism Spectrum Disorder: Exploring the Link through Comprehensive Review and Meta-Analysis. Biomedicines 2023, 11, 3344. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Kasselimis, D.; Laskaris, N.; Potagas, C. Unraveling the Thread of Aphasia Rehabilitation: A Translational Cognitive Perspective. Biomedicines 2023, 11, 2856. [Google Scholar] [CrossRef] [PubMed]
- Zakia, H.; Iskandar, S. Case report: Depressive disorder with peripartum onset camouflages suspected intracranial tuberculoma. Front. Psychiatry 2022, 13, 932635. [Google Scholar] [CrossRef]
- Liu, M.; Xie, X.; Xie, J.; Tian, S.; Du, X.; Feng, H.; Zhang, H. Early-onset Alzheimer’s disease with depression as the first symptom: A case report with literature review. Front. Psychiatry 2023, 14, 1192562. [Google Scholar] [CrossRef]
- Komatsu, H.; Watanabe, E.; Fukuchi, M. Psychiatric neural networks and precision therapeutics by machine learning. Biomedicines 2021, 9, 403. [Google Scholar] [CrossRef]
- Koul, A.M.; Ahmad, F.; Bhat, A.; Aein, Q.-U.; Ahmad, A.; Reshi, A.A.; Kaul, R.-U.-R. Unraveling Down Syndrome: From Genetic Anomaly to Artificial Intelligence-Enhanced Diagnosis. Biomedicines 2023, 11, 3284. [Google Scholar] [CrossRef]
- Ikonnikova, A.; Anisimova, A.; Galkin, S.; Gunchenko, A.; Abdukhalikova, Z.; Filippova, M.; Surzhikov, S.; Selyaeva, L.; Shershov, V.; Zasedatelev, A. Genetic Association Study and Machine Learning to Investigate Differences in Platelet Reactivity in Patients with Acute Ischemic Stroke Treated with Aspirin. Biomedicines 2022, 10, 2564. [Google Scholar] [CrossRef]
- Rassler, B.; Blinowska, K.; Kaminski, M.; Pfurtscheller, G. Analysis of Respiratory Sinus Arrhythmia and Directed Information Flow between Brain and Body Indicate Different Management Strategies of fMRI-Related Anxiety. Biomedicines 2023, 11, 1028. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.d.A.; Rubira, C.J.; Barbalho, S.M. The role of resveratrol in mild cognitive impairment and Alzheimer’s disease: A systematic review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Klon, A.E. Computational models for central nervous system penetration. Curr. Comput.-Aided Drug Des. 2009, 5, 71–89. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.d.A.; Tofano, R.J.; Carvalho, A.C.; Flato, U.A.P.; Capelluppi Tofano, V.A. Ginkgo biloba in the aging process: A narrative review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Koszła, O.; Stępnicki, P.; Zięba, A.; Grudzińska, A.; Matosiuk, D.; Kaczor, A.A. Current Approaches and Tools Used in Drug Development against Parkinson’s Disease. Biomolecules 2021, 11, 897. [Google Scholar] [CrossRef]
- Matias, J.N.; Achete, G.; Campanari, G.S.d.S.; Guiguer, E.L.; Araújo, A.C.; Buglio, D.S.; Barbalho, S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry 2021, 55, 451–462. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Senevirathne, D.K.L.; Mahboob, A.; Zhai, K.; Paul, P.; Kammen, A.; Lee, D.J.; Yousef, M.S.; Chaari, A. Deep Brain Stimulation beyond the Clinic: Navigating the Future of Parkinson’s and Alzheimer’s Disease Therapy. Cells 2023, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Wang, W.-L.; Shieh, Y.-H.; Peng, H.-Y.; Ho, C.-S.; Tsai, H.-C. Case Report: Low-Frequency Repetitive Transcranial Magnetic Stimulation to Dorsolateral Prefrontal Cortex and Auditory Cortex in a Patient with Tinnitus and Depression. Front. Psychiatry 2022, 13, 847618. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.; Wieczorek, T.; Fila-Witecka, K.; Smarzewska, K.; Weiser, A.; Piotrowski, P.; Tabakow, P. Various neuromodulation methods including Deep Brain Stimulation of the medial forebrain bundle combined with psychopharmacotherapy of treatment-resistant depression—Case report. Front. Psychiatry 2023, 13, 3014. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.D. Psychedelics in the Treatment of Headache and Chronic Pain Disorders. Curr. Top. Behav. Neurosci. 2022, 56, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tao, Q.; Niu, X.; Zhang, M.; Gao, X.; Yang, Z.; Yu, M.; Wang, W.; Han, S.; Cheng, J. Meta-analysis of structural and functional brain abnormalities in cocaine addiction. Front. Psychiatry 2022, 13, 927075. [Google Scholar] [CrossRef]
- Borbély, K.; Emri, M.; Kenessey, I.; Tóth, M.; Singer, J.; Barsi, P.; Vajda, Z.; Pál, E.; Tóth, Z.; Beyer, T. Pet/Mri in the presurgical evaluation of patients with epilepsy: A concordance analysis. Biomedicines 2022, 10, 949. [Google Scholar] [CrossRef]
- Okanda Nyatega, C.; Qiang, L.; Jajere Adamu, M.; Bello Kawuwa, H. Altered striatal functional connectivity and structural dysconnectivity in individuals with bipolar disorder: A resting state magnetic resonance imaging study. Front. Psychiatry 2022, 13, 1054380. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Hong, Y.; Huo, J.; Chang, T.; Wang, H.; Huang, Y.; Li, W.; Zhang, Y. Altered brain activities in mesocorticolimbic pathway in primary dysmenorrhea patients of long-term menstrual pain. Front. Neurosci. 2023, 17, 1098573. [Google Scholar] [CrossRef]
- Du, H.; Yang, B.; Wang, H.; Zeng, Y.; Xin, J.; Li, X. The non-linear correlation between the volume of cerebral white matter lesions and incidence of bipolar disorder: A secondary analysis of data from a cross-sectional study. Front. Psychiatry 2023, 14, 1149663. [Google Scholar] [CrossRef] [PubMed]
- Adamu, M.J.; Qiang, L.; Nyatega, C.O.; Younis, A.; Kawuwa, H.B.; Jabire, A.H.; Saminu, S. Unraveling the pathophysiology of schizophrenia: Insights from structural magnetic resonance imaging studies. Front. Psychiatry 2023, 14, 1188603. [Google Scholar] [CrossRef] [PubMed]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson’s disease: A voxel-based morphometry study. Front. Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Kim, S.-H.; Han, C.; Jeong, H.-G.; Lee, M.-S.; Kim, J. Antidepressant-induced mania in panic disorder: A single-case study of clinical and functional connectivity characteristics. Front. Psychiatry 2023, 14, 1205126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, R.; DeSouza, J.F.; Shen, Y.; Zhang, H.; Zhu, C.; Huang, P.; Wang, C. Differential responses from the left postcentral gyrus, right middle frontal gyrus, and precuneus to meal ingestion in patients with functional dyspepsia. Front. Psychiatry 2023, 14, 1184797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cao, Y.; Deng, G.; Fang, J.; Qiu, C. Transient splenial lesion syndrome in bipolar-II disorder: A case report highlighting reversible brain changes during hypomanic episodes. Front. Psychiatry 2023, 14, 1219592. [Google Scholar] [CrossRef] [PubMed]
- Manuello, J.; Costa, T.; Cauda, F.; Liloia, D. Six actions to improve detection of critical features for neuroimaging coordinate-based meta-analysis preparation. Neurosci. Biobehav. Rev. 2022, 137, 104659. [Google Scholar] [CrossRef]
- Salvaggio, F.; Hodgkinson, J.T.; Carro, L.; Geddis, S.M.; Galloway, W.R.; Welch, M.; Spring, D.R. The synthesis of quinolone natural products from Pseudonocardia sp. Eur. J. Org. Chem. 2015, 2016, 434–437. [Google Scholar] [CrossRef]
- Piochon, M.; Coulon, P.M.; Caulet, A.; Groleau, M.-C.; Déziel, E.; Gauthier, C. Synthesis and antimicrobial activity of Burkholderia-related 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs) and their N-oxide counterparts. J. Nat. Prod. 2020, 83, 2145–2154. [Google Scholar] [CrossRef]
- Nam, D.H.; Lee, K.S.; Kim, S.H.; Kim, S.M.; Jung, S.Y.; Chung, S.H.; Kim, H.J.; Kim, N.D.; Jin, C.; Lee, Y.S. Design and synthesis of 4-quinolinone 2-carboxamides as calpain inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 205–209. [Google Scholar] [CrossRef]
- Vyas, V.K.; Variya, B.; Ghate, M.D. Design, synthesis and pharmacological evaluation of novel substituted quinoline-2-carboxamide derivatives as human dihydroorotate dehydrogenase (hDHODH) inhibitors and anticancer agents. Eur. J. Med. Chem. 2014, 82, 385–393. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, J.S.; Moon, Y.S.; Song, D.K. Central or peripheral norepinephrine depletion enhances MK-801-induced plasma corticosterone level in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 45–48. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: From in vitro to in vivo models. Int. J. Mol. Sci. 2023, 24, 15739. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, R.D.; Fessler, R.G.; Meltzer, H.Y. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur. J. Pharm. 1979, 59, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Contreras, P.C. D-serine antagonized phencyclidine- and MK-801-induced stereotyped behavior and ataxia. Neuropharmacology 1990, 29, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.M.; Platt, B.; Janhunen, S.K.; Riedel, G. Comparison of automated video tracking systems in the open field test: ANY-Maze versus EthoVision XT. J. Neurosci. Methods 2023, 397, 109940. [Google Scholar] [CrossRef]
- Stanford, S.C. The Open Field Test: Reinventing the wheel. J. Psychopharmacol. 2007, 21, 134–135. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pritchett, K.; Mulder, G.B. The rotarod. Contemp. Top. Lab. Anim. Sci. 2003, 42, 49. [Google Scholar] [PubMed]
- Osmon, K.J.; Vyas, M.; Woodley, E.; Thompson, P.; Walia, J.S. Battery of Behavioral Tests Assessing General Locomotion, Muscular Strength, and Coordination in Mice. J. Vis. Exp. 2018, 131, e55491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. https://doi.org/10.3390/ijms25063394
Martos D, Lőrinczi B, Szatmári I, Vécsei L, Tanaka M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. International Journal of Molecular Sciences. 2024; 25(6):3394. https://doi.org/10.3390/ijms25063394
Chicago/Turabian StyleMartos, Diána, Bálint Lőrinczi, István Szatmári, László Vécsei, and Masaru Tanaka. 2024. "The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain" International Journal of Molecular Sciences 25, no. 6: 3394. https://doi.org/10.3390/ijms25063394
APA StyleMartos, D., Lőrinczi, B., Szatmári, I., Vécsei, L., & Tanaka, M. (2024). The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. International Journal of Molecular Sciences, 25(6), 3394. https://doi.org/10.3390/ijms25063394