ZmSMR10 Increases the Level of Endoreplication of Plants through Its Interactions with ZmPCNA2 and ZmCSN5B
Abstract
1. Introduction
2. Results
2.1. Cloning of ZmSMR10 and the Generation of Transgenic Plants
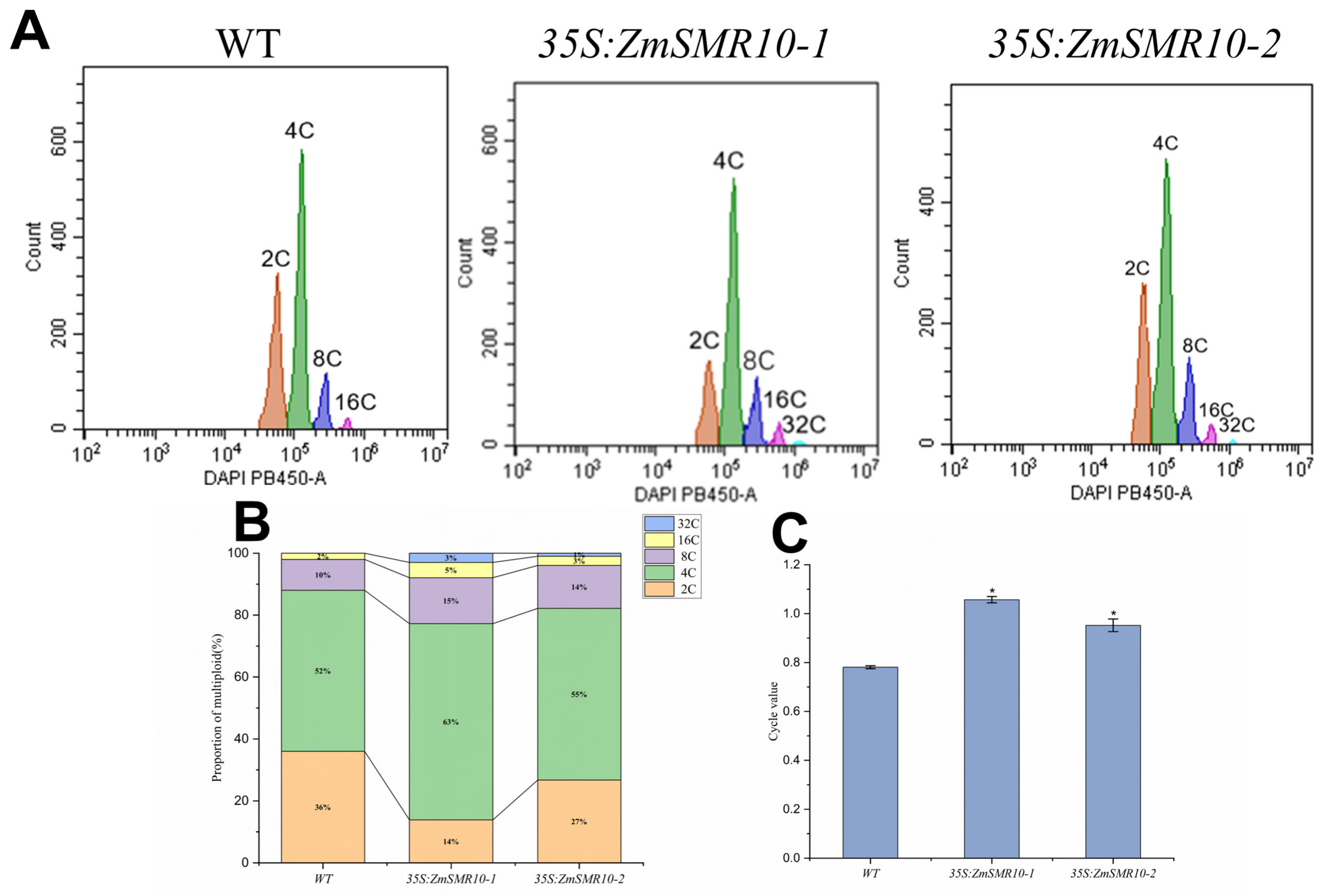
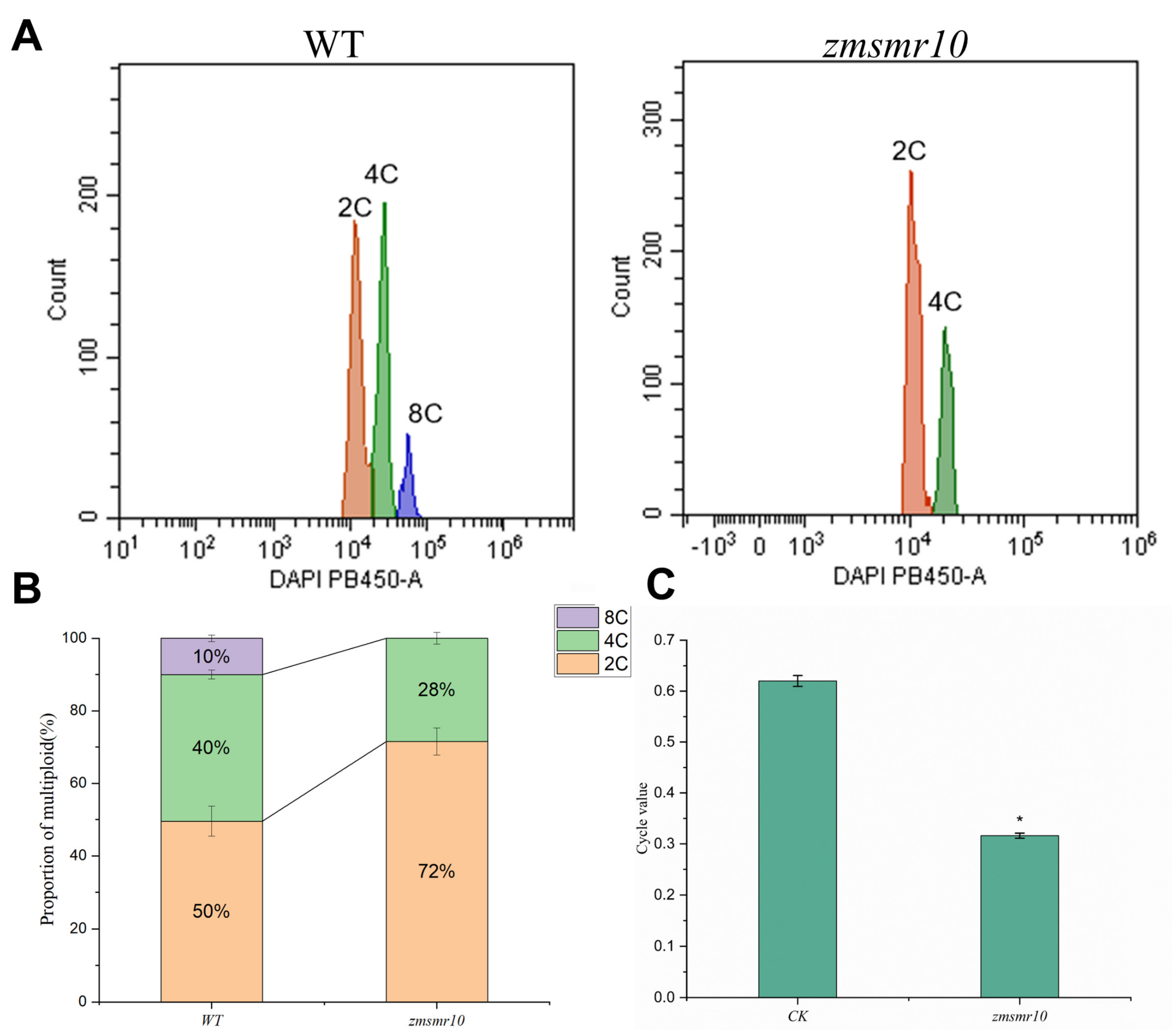
2.2. Changes in the Cell Ploidy of Transgenic Plants
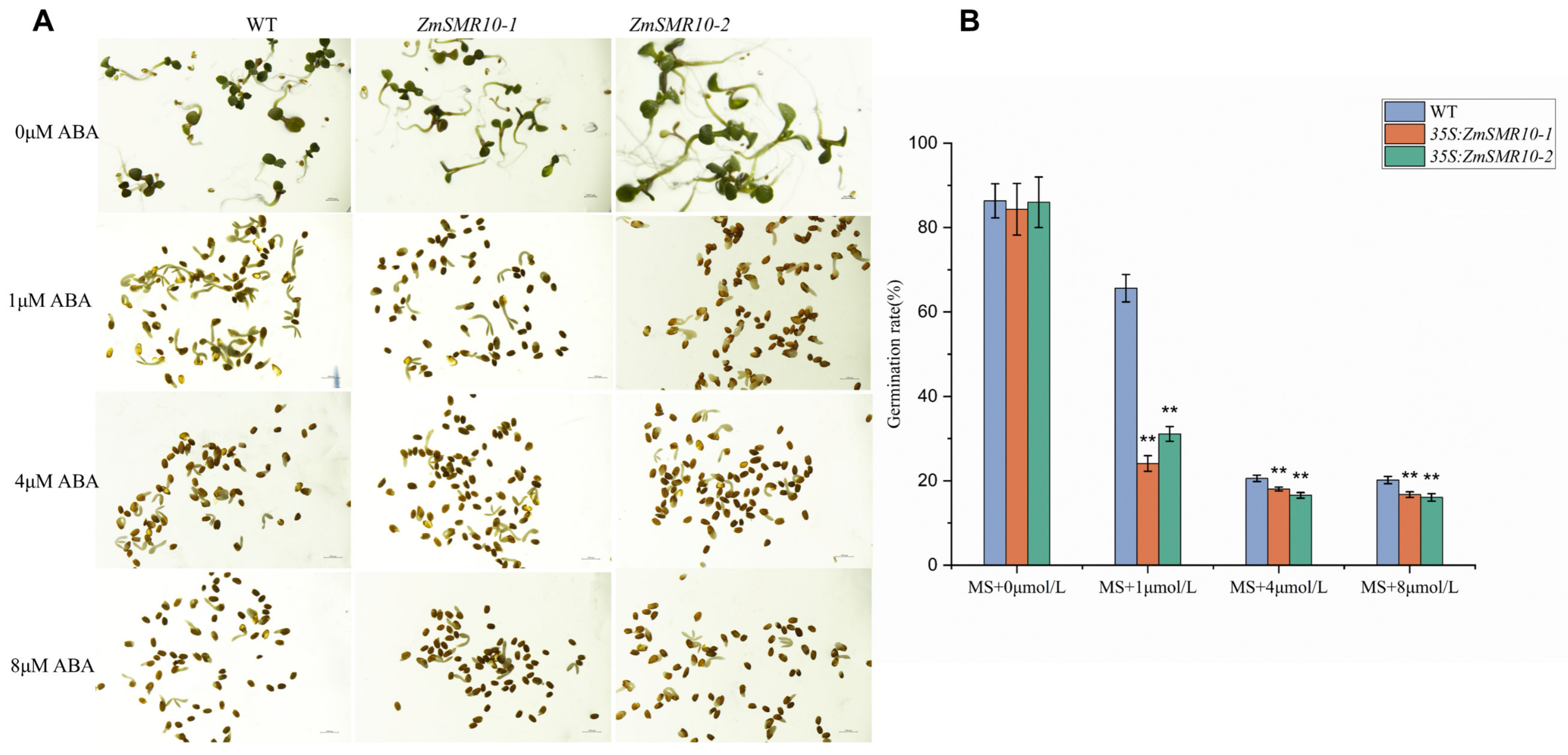
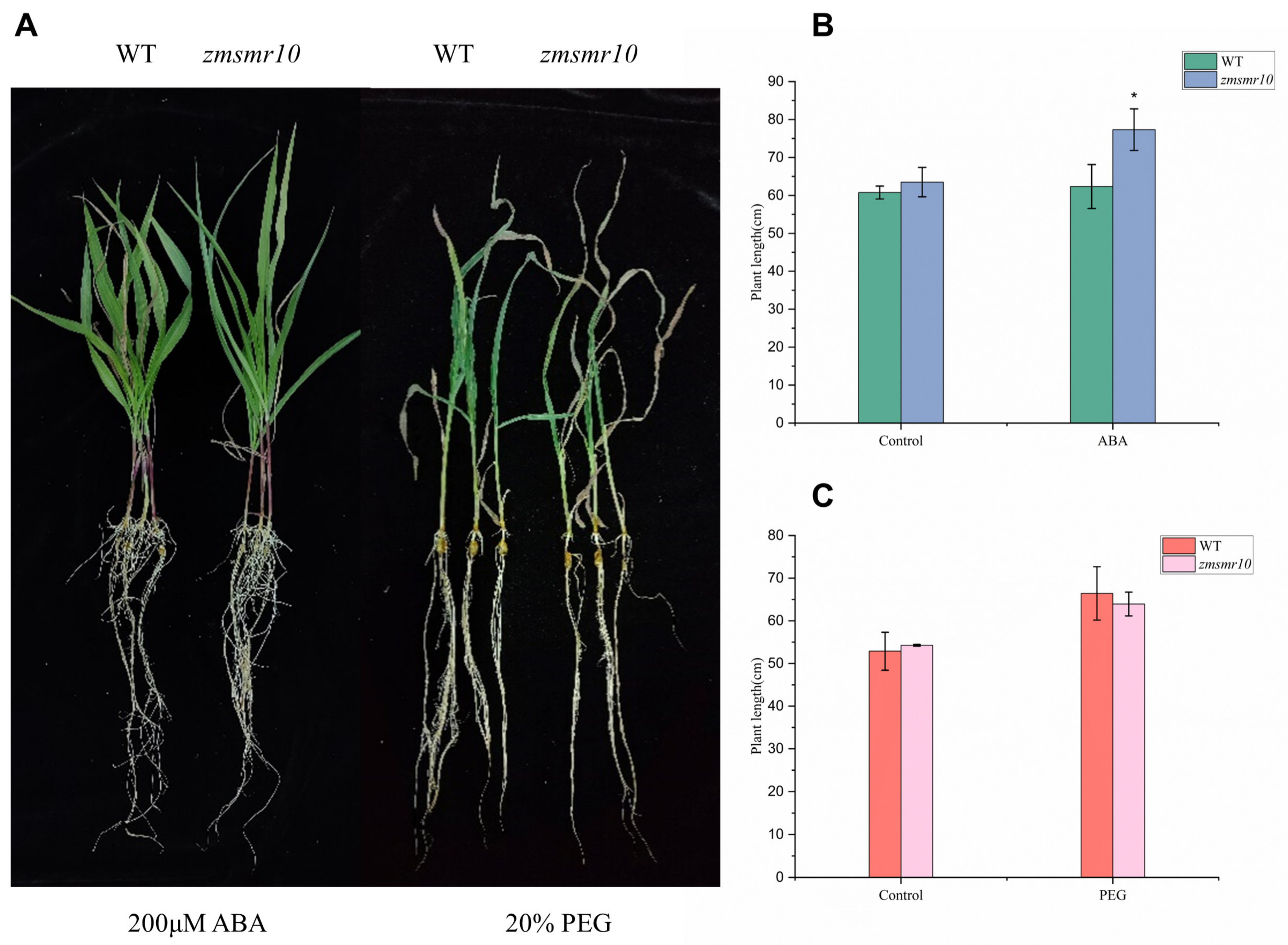
2.3. Effects of ZmSMR10 Gene on Seed Germination under Abscisic Acid Stress
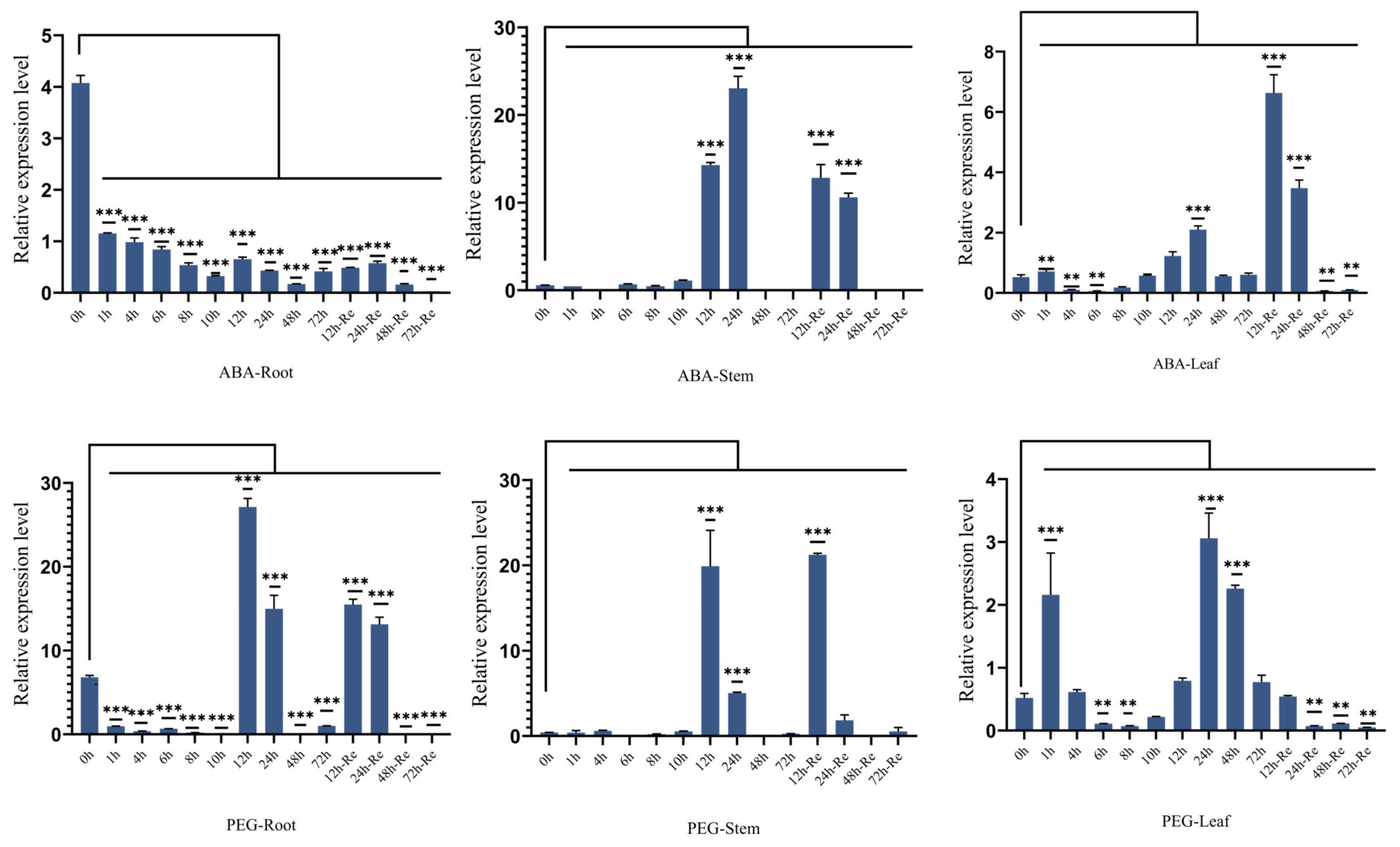
2.4. Effects of ZmSMR10 Gene on the Response to Drought and Salt Stress
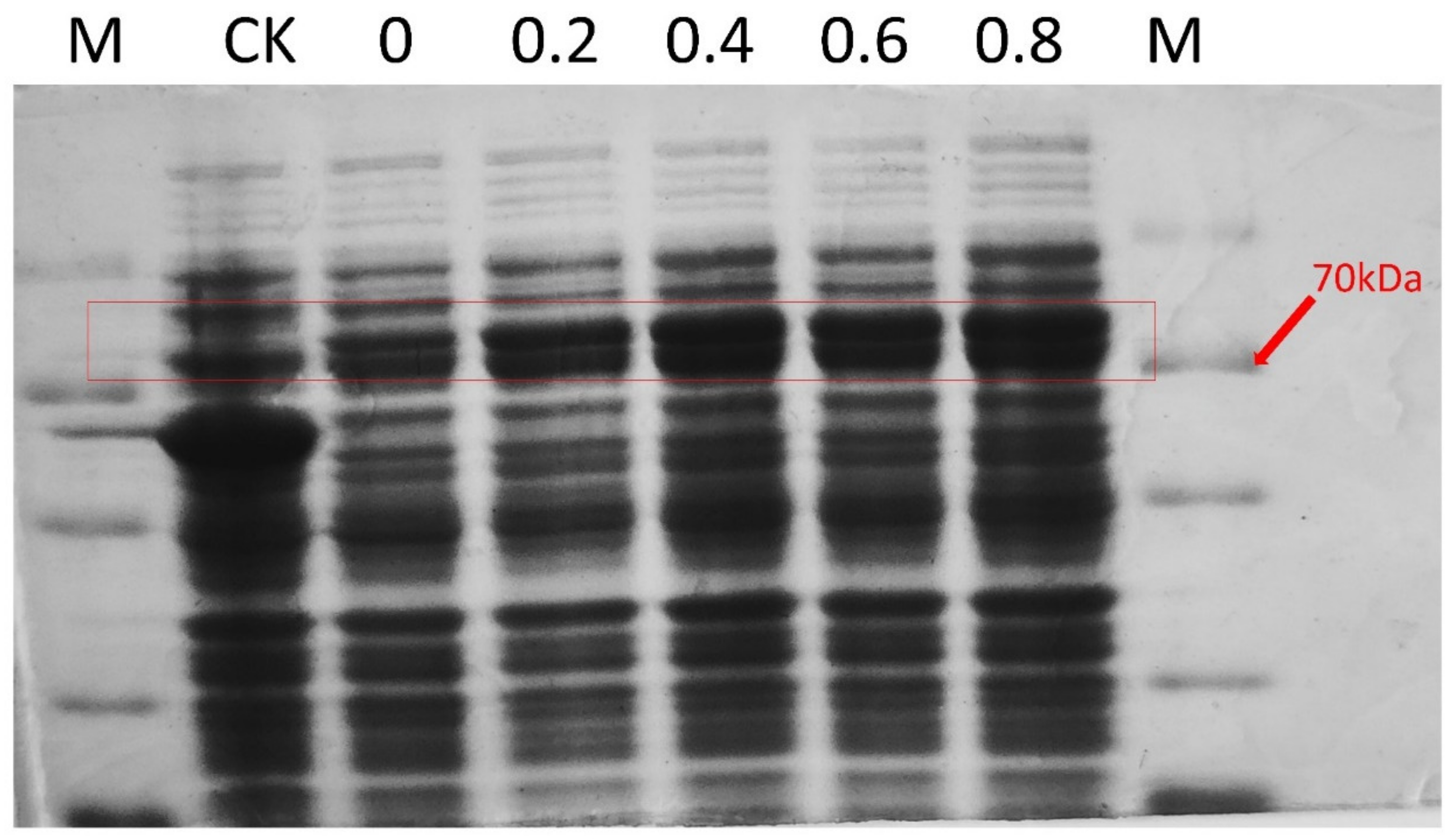
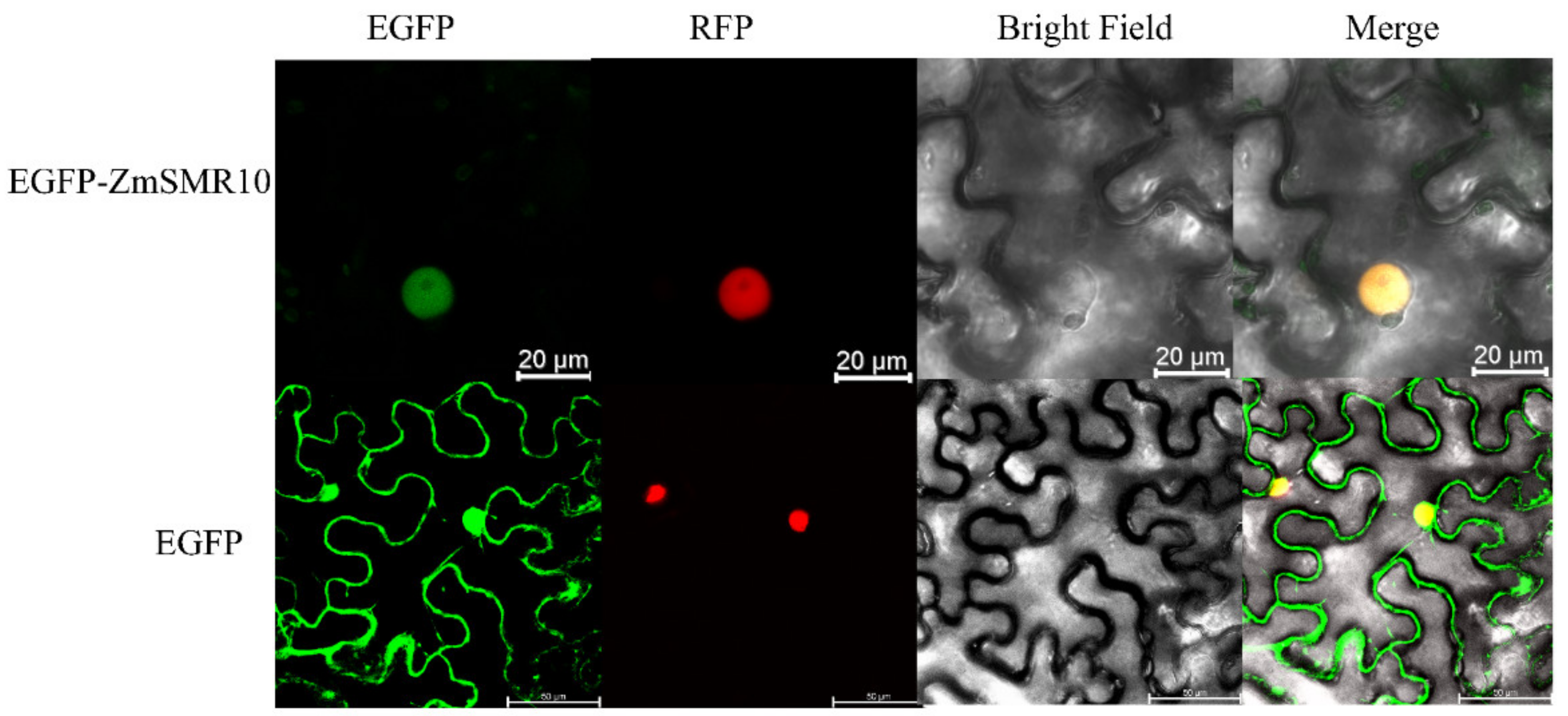
2.5. Prokaryotic Expression and Localization of the ZmSMR10 Protein
2.6. Interactions of the ZmSMR10 Protein
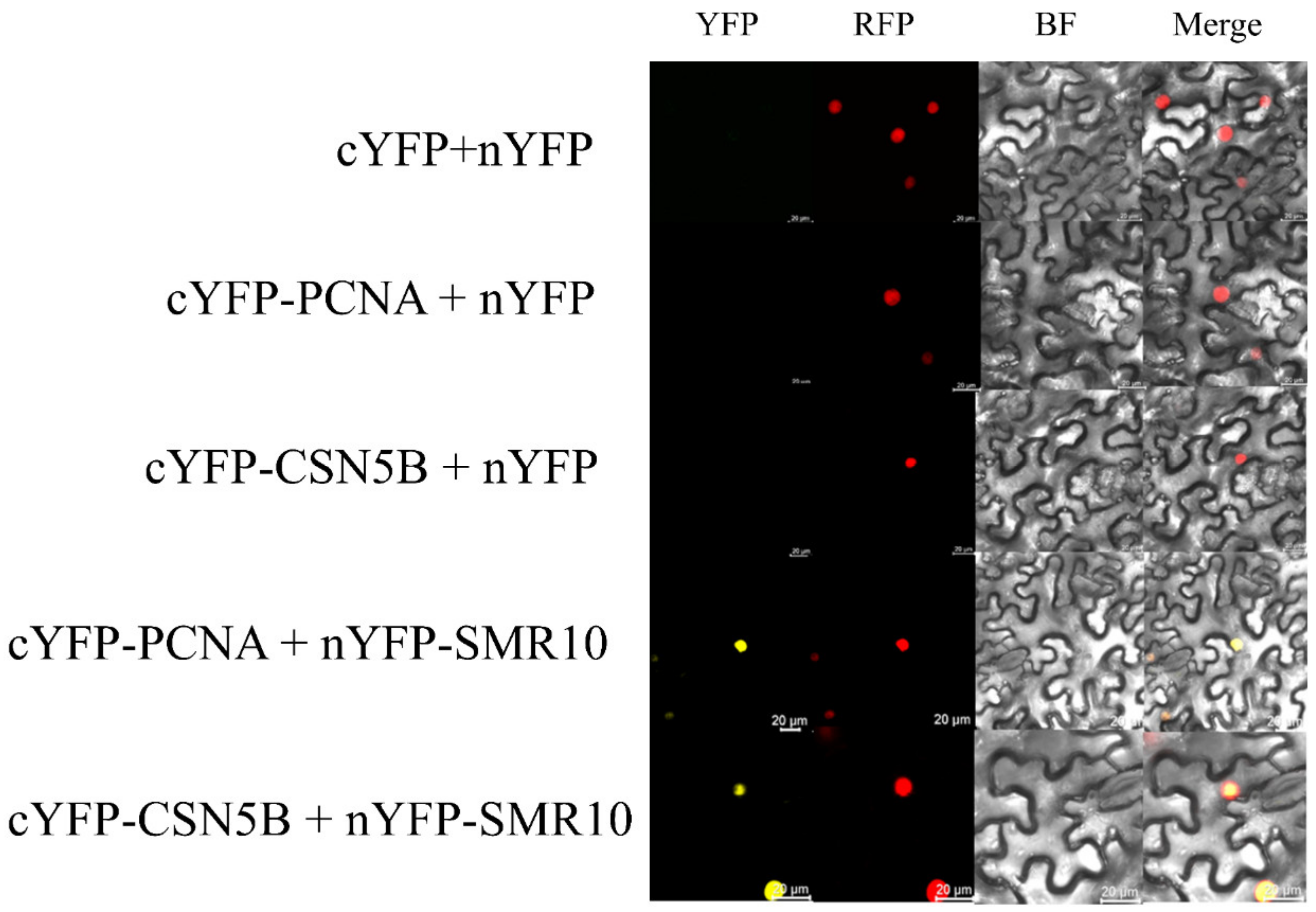
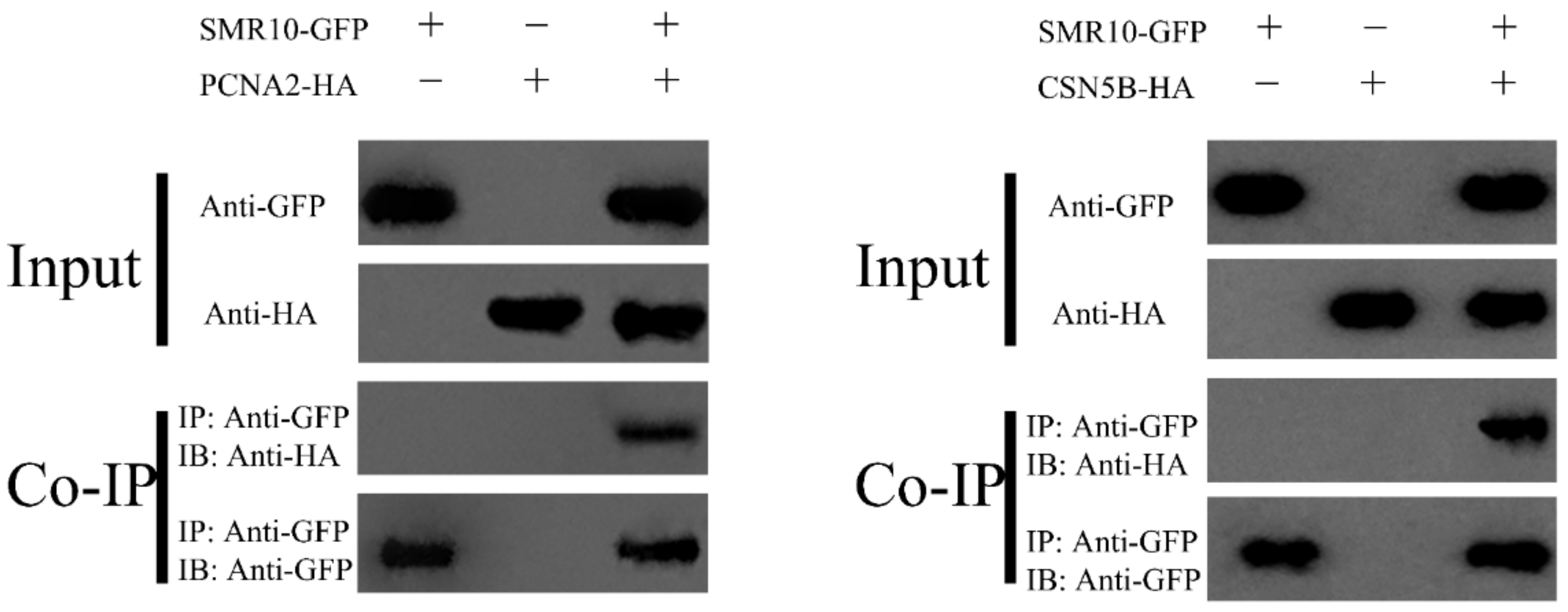
2.7. BiFC and CoIP Determination of the Interaction between ZmSMR10 and ZmPCNA2, ZmCSN5B
3. Discussion
3.1. The ZmSMR10 Protein in Maize Is Functionally Similar to the SMR Proteins in Other Plants
3.2. ZmSMR10 Is Sensitive to ABA and Resistant to PEG
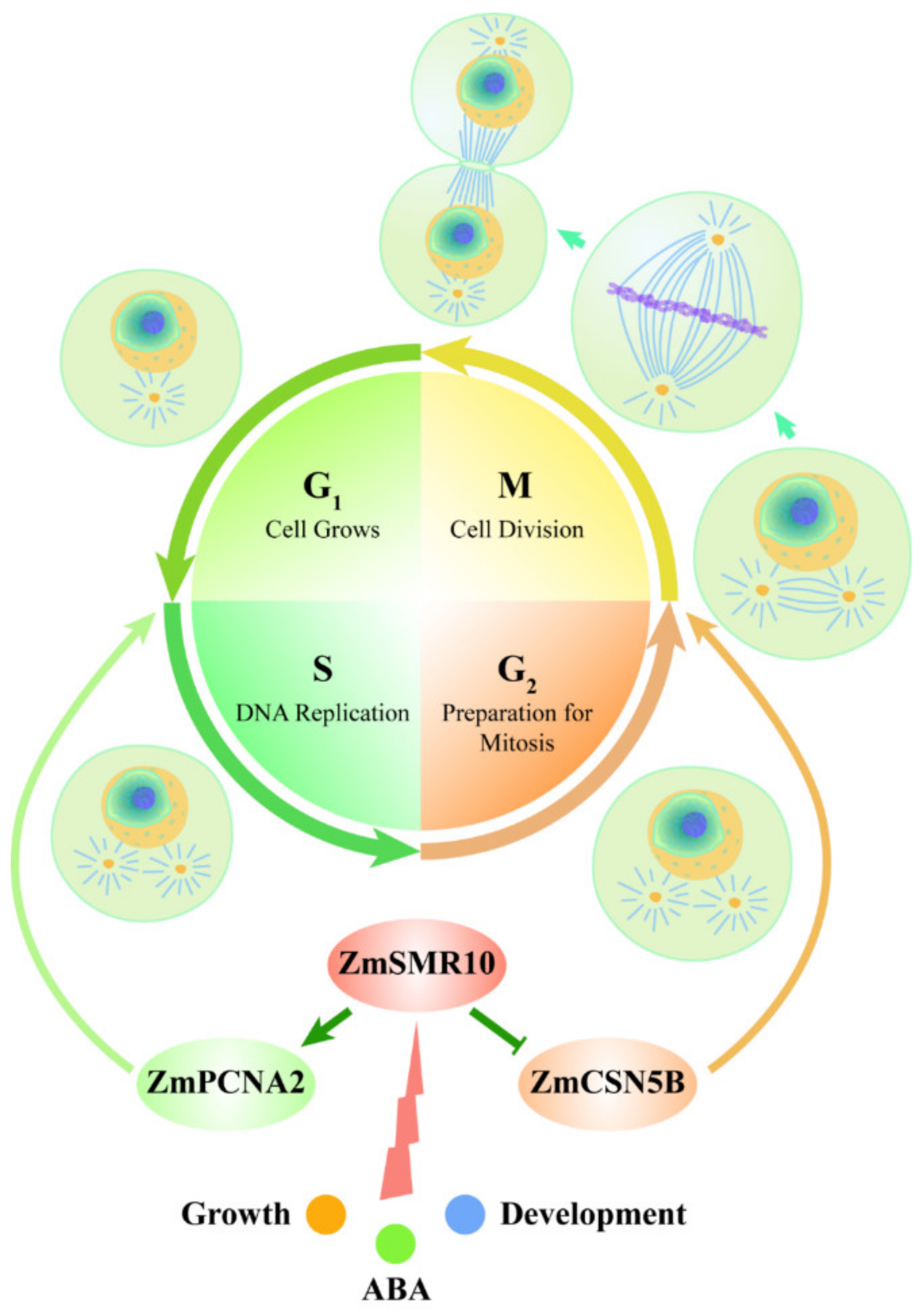
3.3. ZmSMR10 Interacts with ZmPCNA2/ZmCSN5B in the Nucleus
4. Materials and Methods
4.1. Experimental Materials
4.2. Cloning and Transformation of ZmSMR10
4.3. Quantitative Real-Time Reverse-Transcription PCR (qRT-PCR)
4.4. Plant Flow Cytometry Analysis
4.5. Subcellular Localization
4.6. Prokaryotic Expression of the ZmSMR10 Protein
4.7. Yeast Two-Hybrid Assay
4.8. Yeast Two-Hybrid Screen Library
4.9. Bimolecular Fluorescence Complementation (BiFC)
4.10. Co-Immunoprecipitation (CoIP)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffman, J.A. Cell cycle development. Dev. Cell 2004, 6, 321–327. [Google Scholar] [CrossRef]
- Zielke, N.; Edgar, B.A.; DePamphilis, M.L. Endoreplication. Cold Spring Harb. Perspect. Biol. 2013, 5, a012948. [Google Scholar] [CrossRef]
- Van den Heuvel, S.; Dyson, N.J. Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell Biol. 2008, 9, 713–724. [Google Scholar] [CrossRef]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Larkins, B.A. The contribution of cell cycle regulation to endosperm development. Sex. Plant Reprod. 2009, 22, 207–219. [Google Scholar] [CrossRef]
- Cheniclet, C.; Rong, W.Y.; Causse, M.; Frangne, N.; Bolling, L.; Carde, J.-P.; Renaudin, J.-P. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiol. 2005, 139, 1984–1994. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, B.; Dong, A.W. The Arabidopsis transcription factor AtTCP15 regulates endoreduplication by modulating expression of key cell-cycle genes. Mol. Plant 2012, 5, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, K.; Wang, Z.; Wei, D.; Tang, Q. Research progress in regulation model in different types of plant trichome. Sheng Wu Gong Cheng Xue Bao 2020, 36, 2051–2065. [Google Scholar] [PubMed]
- Hatano, H.; Mizuno, N.; Matsuda, R.; Shitsukawa, N.; Park, P.; Takumi, S. Dysfunction of mitotic cell division at shoot apices triggered severe growth abortion in interspecific hybrids between tetraploid wheat and Aegilops tauschii. New Phytol. 2012, 194, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, Z.; Wang, S.; Li, Y.; Wei, H.; Tian, X.; Kan, Q. Recent development of CDK inhibitors: An overview of CDK/inhibitor co-crystal structures. Eur. J. Med. Chem. 2019, 164, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Schnittger, A. Endoreplication—A means to an end in cell growth and stress response. Curr. Opin. Plant Biol. 2020, 54, 85–92. [Google Scholar] [CrossRef]
- Sun, X.; Cahill, J.; Van Hautegem, T.; Feys, K.; Whipple, C.; Novák, O.; Delbare, S.; Versteele, C.; Demuynck, K.; De Block, J.; et al. Altered expression of maize PLASTOCHRON1 enhances biomass and seed yield by extending cell division duration. Nat. Commun. 2017, 8, 14752. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gu, Y.; Zebell, S.G.; Anderson, L.K.; Wang, W.; Mohan, R.; Dong, X. A noncanonical role for the CKI-RB-E2F cell-cycle signaling pathway in plant effector-triggered immunity. Cell Host Microbe 2014, 16, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.M.; Dante, R.A.; Sabelli, P.A.; Sun, Y.; Dilkes, B.P.; Gordon-Kamm, W.J.; Larkins, B.A. Cyclin-dependent kinase inhibitors in maize endosperm and their potential role in endoreduplication. Plant Physiol. 2005, 138, 2323–2336. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, L.; Zhang, Z.; Li, T.; Feng, J.; Xu, S.; Zhang, R.; Guo, D.; Xue, J. ZmSMR4, a novel cyclin-dependent kinase inhibitor (CKI) gene in maize (Zea mays L.), functions as a key player in plant growth, development and tolerance to abiotic stress. Plant Sci. 2019, 280, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Research on the Occurrence Law of Intracellular Polyploidisation in Maize. Master’s Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2019. (In Chinese). [Google Scholar]
- Zhang, Z.; Qu, J.; Li, F.; Li, S.; Xu, S.; Zhang, R.; Xue, J.; Guo, D. Genome-wide evolutionary characterization and expression analysis of SIAMESE-RELATED family genes in maize. BMC Evol. Biol. 2020, 20, 91. [Google Scholar] [CrossRef]
- Malinowski, R.; Kasprzewska, A.; Fleming, A.J. Targeted manipulation of leaf form via local growth repression. Plant J. 2011, 66, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.P.; Leyser, O. Shoot branching. Curr. Opin. Plant Biol. 2004, 7, 73–78. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Li, W.; Cui, X.; Meng, Z.; Huang, X.; Xie, Q.; Wu, H.; Jin, H.; Zhang, D.; Liang, W. Transcriptional regulation of Arabidopsis MIR168a and argonaute1 homeostasis in abscisic acid and abiotic stress responses. Plant Physiol. 2012, 158, 1279–1292. [Google Scholar] [CrossRef]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef]
- Dohmann, E.M.; Levesque, M.P.; De Veylder, L.; Reichardt, I.; Jürgens, G.; Schmid, M.; Schwechheimer, C. The Arabidopsis COP9 signalosome is essential for G2 phase progression and genomic stability. Development 2008, 135, 2013–2022. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef]
- Churchman, M.L.; Brown, M.L.; Kato, N.; Kirik, V.; Hulskamp, M.; Inze, D.; De Veylder, L.; Walker, J.D.; Zheng, Z.; Oppenheimer, D.G.; et al. SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell 2006, 18, 3145–3157. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.; Churchman, M.L.; Hariharan, S.; Himanen, K.; Verkest, A.; Vandepoele, K.; Magyar, Z.; Hatzfeld, Y.; Van Der Schueren, E.; Beemster, G.T.S.; et al. Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J. Biol. Chem. 2007, 282, 25588–25596. [Google Scholar] [CrossRef]
- Van Leene, J.; Hollunder, J.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Stals, H.; Van Isterdael, G.; Verkest, A.; Neirynck, S.; Buffel, Y.; et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol. Syst. Biol. 2010, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fowke, L.; Wang, H. Plant CDK inhibitors: Studies of interactions with cell cycle regulators in the yeast two-hybrid system and functional comparisons in transgenic Arabidopsis plants. Plant Cell Rep. 2002, 20, 967–975. [Google Scholar] [CrossRef]
- Hamdoun, S.; Zhang, C.; Gill, M.; Kumar, N.; Churchman, M.; Larkin, J.C.; Kwon, A.; Lu, H. Differential roles of two homologous cyclin-dependent kinase inhibitor genes in regulating cell cycle and innate immunity in Arabidopsis. Plant Physiol. 2016, 170, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, Q.; Schorr, P.; Cutler, A.J.; Crosby, W.L.; Fowke, L.C. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 1998, 15, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P.; Mowforth, M.A. Variation in genome size—An ecological interpretation. Nature 1982, 299, 151–153. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Santantonio, E.; Marmottini, F.; Amzallag, G.N.; Cionini, P.G. Chromosome endoreduplication as a factor of salt adaptation in Sorghum bicolor. Protoplasma 2006, 227, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, K.; Boudolf, V.; Beemster, G.T.S.; Maes, S.; Magyar, Z.; Atanassova, A.; de Almeida Engler, J.; De Groodt, R.; Inzé, D.; De Veylder, L. The DP-E2F-like gene DEL1 controls the endocycle in Arabidopsis thaliana. Curr. Biol. 2005, 15, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dale, R.; Kemboi, D.; Zeringue, E.A.; Kato, N.; Larkin, J.C. Functional analysis of short linear motifs in the plant cyclin-dependent kinase inhibitor SIAMESE. Plant Physiol. 2018, 177, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Pines, J. Four-dimensional control of the cell cycle. Nat. Cell Biol. 1999, 1, E73–E79. [Google Scholar] [CrossRef]
- Kumar, N.; Harashima, H.; Kalve, S.; Bramsiepe, J.; Wang, K.; Sizani, B.L.; Bertrand, L.L.; Johnson, M.C.; Faulk, C.; Dale, R.; et al. Functional conservation in the SIAMESE-RELATED family of cyclin-dependent kinase inhibitors in land plants. Plant Cell. 2015, 27, 3065–3080. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, G.L.; Pfander, B.; Jentsch, S. PCNA, the maestro of the replication fork. Cell 2007, 129, 665–679. [Google Scholar] [CrossRef]
- Garg, P.; Stith, C.M.; Sabouri, N.; Johansson, E.; Burgers, P.M. Idling by DNA polymerase delta maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 2004, 18, 2764–2773. [Google Scholar] [CrossRef]
- Umar, A.; Buermeyer, A.B.; Simon, J.A.; Thomas, D.C.; Clark, A.B.; Liskay, R.; Kunkel, T.A. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell 1996, 87, 65–73. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, H.; Katzmann, D.; Hochstrasser, M.; Atanasova, E.; Zhang, Z. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc. Natl. Acad. Sci. USA 2005, 102, 13410–13415. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Beach, D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell. 1993, 4, 897–906. [Google Scholar] [CrossRef]
- De la Paz, S.M.; Torres, A.; Boniotti, M.B.; Gutierrez, C.; Vázquez-Ramos, J.M. PCNA protein associates to Cdk-A type protein kinases in germinating maize. Plant Mol. Biol. 2002, 50, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Quiroz-Figueroa, F.; Vázquez-Ramos, J.M. Maize cyclin D2 expression, associated kinase activity and effect of phytohormones during germination. Plant Cell Physiol. 2005, 46, 166–173. [Google Scholar] [CrossRef]
- Wei, N.; Chamovitz, D.A.; Deng, X.W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell 1994, 78, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mundt, K.E.; Porte, J.; Murray, J.M.; Brikos, C.; Christensen, P.U.; Caspari, T.; Hagan, I.M.; Millar, J.B.A.; Simanis, V.; Hofmann, K.; et al. The COP9/signalosome complex is conserved in fission yeast and has a role in S phase. Curr. Biol. 1999, 9, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Eckert, S.E.; Krappmann, S.; Braus, G.H. The COP9 signalosome is an essential regulator of development in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2003, 49, 717–730. [Google Scholar] [CrossRef]
- Wei, N.; Deng, X.W. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 2003, 19, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.S.; Zundel, W. The emerging role of the COP9 signalosome in cancer. Mol. Cancer Res. 2005, 3, 645–653. [Google Scholar] [CrossRef]
- Gusmaroli, G.; Feng, S.; Deng, X.W. The Arabidopsis CSN5A and CSN5B subunits are present in distinct COP9 signalosome complexes, and mutations in their JAMM domains exhibit differential dominant negative effects on development. Plant Cell 2004, 16, 2984–3001. [Google Scholar] [CrossRef]
- Grafi, G.; Larkins, B.A. Endoreduplication in maize endosperm: Involvement of M phase--promoting factor inhibition and induction of s phase--related kinases. Science 1995, 269, 1262–1264. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Ren, W.; Yang, Q.; Chai, Z.; Chen, R.; Wang, L.; Zhao, J.; Lang, Z.; Wang, H.; et al. Gene-indexed mutations in maize. Mol. Plant. 2018, 11, 496–504. [Google Scholar] [CrossRef]
- Topping, J.F.; Wei, W.; Clarke, M.C.; Muskett, P.; Lindsey, K. Agrobacterium-mediated transformation of Arabidopsis thaliana application in T-DNA tagging. Methods Mol. Biol. 1995, 49, 63–76. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, T.S.; Hnasko, R.M. The western blot. Methods Mol. Biol. 2015, 1318, 87–96. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, L.; Si, J.; Zhai, M.; Liu, N.; Qu, H.; Capulong, C.; Li, J.; Liu, Q.; Liu, Y.; Huang, C.; et al. ZmSMR10 Increases the Level of Endoreplication of Plants through Its Interactions with ZmPCNA2 and ZmCSN5B. Int. J. Mol. Sci. 2024, 25, 3356. https://doi.org/10.3390/ijms25063356
Bao L, Si J, Zhai M, Liu N, Qu H, Capulong C, Li J, Liu Q, Liu Y, Huang C, et al. ZmSMR10 Increases the Level of Endoreplication of Plants through Its Interactions with ZmPCNA2 and ZmCSN5B. International Journal of Molecular Sciences. 2024; 25(6):3356. https://doi.org/10.3390/ijms25063356
Chicago/Turabian StyleBao, Lulu, Jihao Si, Mingming Zhai, Na Liu, Haoran Qu, Christian Capulong, Jinyuan Li, Qianqian Liu, Yilin Liu, Chenggang Huang, and et al. 2024. "ZmSMR10 Increases the Level of Endoreplication of Plants through Its Interactions with ZmPCNA2 and ZmCSN5B" International Journal of Molecular Sciences 25, no. 6: 3356. https://doi.org/10.3390/ijms25063356
APA StyleBao, L., Si, J., Zhai, M., Liu, N., Qu, H., Capulong, C., Li, J., Liu, Q., Liu, Y., Huang, C., Zhang, M., Ao, Z., Yang, A., Qin, C., & Guo, D. (2024). ZmSMR10 Increases the Level of Endoreplication of Plants through Its Interactions with ZmPCNA2 and ZmCSN5B. International Journal of Molecular Sciences, 25(6), 3356. https://doi.org/10.3390/ijms25063356




