Abstract
In recent years, newly emerging therapies, such as immune checkpoint inhibitors and antibody-drug conjugates, have further improved outcomes for breast cancer patients. However, recurrent and metastatic breast cancer often eventually develops resistance to these drugs, and cure is still rare. As such, the development of new therapies for refractory breast cancer that differ from conventional mechanisms of action is necessary. Sphingosine-1-phosphate (S1P) is a key molecule with a variety of bioactive activities, including involvement in cancer cell proliferation, invasion, and metastasis. S1P also contributes to the formation of the cancer microenvironment by inducing surrounding vascular- and lymph-angiogenesis and regulating the immune system. In this article, we outline the basic mechanism of action of S1P, summarize previous findings on the function of S1P in cancer cells and the cancer microenvironment, and discuss the clinical significance of S1P in breast cancer and the therapeutic potential of targeting S1P signaling.
1. Introduction
Breast cancer is the most common cancer among women worldwide, and despite advances in treatment, many women still die from it [1,2]. In recent years, newly emerging therapies, such as immune checkpoint inhibitors and antibody-drug conjugates, have further improved outcomes for breast cancer patients [3,4,5,6,7,8,9,10,11,12]. However, recurrent and metastatic breast cancer often eventually develop resistance to these drugs, and cure is still rare [13,14,15,16,17,18,19]. Further development of new therapies for refractory breast cancer that do not have conventional mechanisms of action is necessary.
Recently, lipid mediators have attracted attention as signaling molecules that play important roles in cancer [20,21,22,23,24,25,26,27]. Among the lipid mediators, sphingosine-1-phosphate (S1P) is a key molecule with a variety of bioactive activities, including those involved in cancer cell proliferation, invasion, and metastasis; S1P has also been shown to contribute to the formation of the cancer microenvironment by inducing surrounding vascular- and lymph-angiogenesis and regulating the immune system [28,29,30,31,32,33,34,35,36]. More than 30 years of research have led to today’s focus on S1P as a factor that controls cellular physiological activity and plays an important role in cancer.
In the first half of the 1990s, Spiegel et al. reported that S1P is a lipid mediator that acts on cells in a way similar to protein growth factors that regulate cell growth [37,38,39,40]. Subsequently, Spiegel et al. and other researchers around the world have clarified the details of S1P-producing enzymes and the specific receptors for S1P, and the wide range of biological activities of S1P have been revealed [41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. However, the clinical significance of S1P in cancer patients had not been well understood until recently.
Because S1P is a lipid, it is not easy to accurately quantify its levels, and the significance of S1P in cancer has not been fully elucidated. We have demonstrated that S1P production is upregulated in various cancer types, including breast cancer, and that it contributes to lymphatic metastasis, and is associated with prognosis based on the histological evaluation of phosphorylation of sphingosine kinase type 1 (SphK1), the S1P-producing enzyme [61,62,63,64,65,66,67,68,69]. In this article, we outline the basic mechanism of action of S1P, summarize previous findings on the function of S1P in cancer cells and the cancer microenvironment, and discuss the clinical significance of S1P in breast cancer patients and the therapeutic potential of targeting S1P signaling.
2. Molecular Mechanisms of S1P Regulation of Cellular Physiological Functions
Sphingosine kinases produce S1P by phosphorylating sphingosine produced from ceramide, a component of the cell membrane, via the catalytic action of ceramidase [70,71,72,73] (Figure 1). There are two types of sphingosine kinases, SphK1 and SphK2, each with different subcellular localizations [74,75,76,77]; SphK1 is mainly found in the cytoplasm near the plasma membrane, while SphK2 is mainly found in the nucleus and mitochondria [74,75,76,77]. S1P, produced mainly by SphK1 in the cytosol, is released extracellularly by transporters on the plasma membrane and acts as a signaling molecule that regulates cellular functions by acting on S1P-specific G protein-coupled receptors on the cell surface via autocrine and paracrine modes of action [78,79,80,81,82]. ATP-binding cassette (ABC) transporters, such as ABCC1 and ABCG2, and the S1P-specific transporter, Spns2, transport intracellular S1P to the cell exterior [82,83,84,85,86,87,88,89]. Extracellularly released S1P stimulates one of five S1P receptors (S1PR1-5) that exhibit tissue-specific expression patterns and each S1P receptor binds to a different G protein and regulates a wide range of downstream signaling pathways and numerous biological processes [90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108]. The intracellular production of S1P, followed by its release and extracellular action, is called “inside-out” signaling and is characteristic of S1P signaling [109,110].
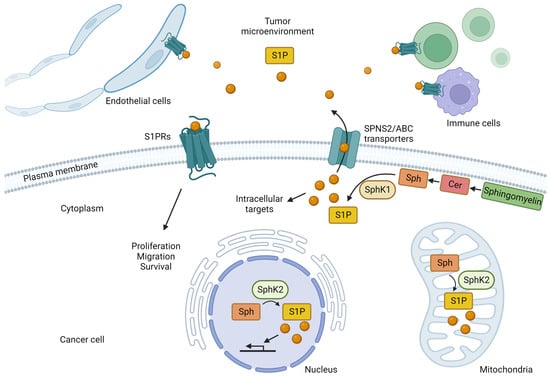
Figure 1.
The roles of sphingosine-1-phosphate (S1P) produced by cancer cells. In cancer cells, sphingosine (Sph) produced from ceramide (Cer), one of the components of the cell membrane, is phosphorylated by sphingosine kinases (SphK1 and SphK2) to produce S1P. S1P produced in the cytoplasm by SphK1 is released into the extracellular space by transporters, such as SPNS2 and the ATP-binding cassette (ABC) transporters. Extracellular S1P acts on cancer cells themselves in an autocrine manner and also activates S1P receptors (S1PR) on the cell surface of surrounding cancer cells, immune cells, and endothelial cells in the cancer microenvironment in a paracrine manner, thereby triggering intracellular signals that regulate their physiological functions. SphK1 regulates physiological functions by activating S1P receptors (S1PR) on the cell surface of cancer cells, immune cells, endothelial cells, and other cell types. Stimulation of S1P receptors on cancer cells promotes cancer growth, migration, and survival. S1P produced by SphK2 in the nucleus is thought to contribute to the transcriptional regulation of genes. S1P produced by SphK2 in the mitochondria is also involved in the electron transport system.
In addition to “inside-out” signaling, S1P also acts directly via intracellular targets. For example, tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) increase the levels of intracellular S1P that binds directly to TNF-α receptor-associated factor 2 by activating SphK1 [111]. TNF-α receptor-associated factor 2 is an important regulator of nuclear factor-κB signaling and cellular apoptosis suppression 2, and plays an important role in the regulation of apoptosis. In addition, it acts via lysine-63 binding polyubiquitination to enhance E3 ubiquitin ligase activity [111]. SphK2 exists in the nucleus and mitochondria and plays important roles in these organelles (Figure 1). S1P produced by SphK2 in the nucleus specifically binds to histone deacetylase (HDAC) 1 and HDAC2, inhibiting their enzymatic activity and preventing the removal of acetyl groups from lysine residues within histone tails [112]. As a result, S1P acts as an HDAC inhibitor that is involved in the transcriptional regulation of genes, and it plays a role in higher-order functional regulation in the brain [112,113]. We also reported that SphK2 and S1P regulate the transcription of genes encoding enzymes involved in metabolism in the liver [114,115,116,117,118]. In mitochondria, it has been suggested that SphK2 and S1P may be involved in energy metabolism via the electron transfer system [119]. In brief, S1P, mainly produced by SphK2 in mitochondria, binds to prohibitin 2, which plays an important role in regulating cytochrome-c oxidase assembly and mitochondrial respiration [119].
The concentration levels of S1P have been found to be very tightly regulated by the balance between its synthesis and degradation. S1P is converted to sphingosine by two specific S1P phosphatases (SPP1 and SPP2) belonging to the magnesium-dependent, N-ethylmaleimide-insensitive type 2 lipid phosphate phosphohydrolase family in the endoplasmic reticulum, where phosphate groups are removed [120,121]. S1P is also irreversibly degraded to hexadecenal and phosphoethanolamine by pyridoxal phosphate-dependent S1P lyase [122,123]. Phosphoethanolamine is subsequently recycled for phosphatidylethanolamine biosynthesis [124].
In the human body, the blood concentration of S1P is finely regulated to keep it at the relatively high level of 1–2 µM [125]. In mice, the half-life of S1P in plasma has been reported to be about 15 min, suggesting rapid clearance by enzymes, such as S1P phosphatase and S1P lyase, or the rapid uptake of S1P into cells [126,127,128]. This rapid turnover of S1P in the blood suggests the presence of a large number of S1P-supplying cells that are involved in maintaining high S1P levels in the blood [128]. The cells responsible for S1P synthesis and secretion into the blood include erythrocytes, endothelial cells, platelets, macrophages, and mast cells [129,130,131,132,133,134].
Compared to the S1P concentration in the blood, the concentration in lymphatic fluid has rarely been measured. The difficulty in collecting lymphatic fluid from clinical samples, and even in animal experiments, may be the reason for the paucity of data. We collected lymphatic fluid from the cisterna chyli of mice using filter paper, extracted S1P from it and quantified its concentration using mass spectrometry. S1P concentrations in lymphatic fluid were in the range of 0.1 to 0.3 μM, while those in plasma were greater than 0.5 μM [135]. The levels of S1P in lymphatic fluid were in agreement with previous reports from other groups [136,137]. We confirmed that the concentration of S1P in lymphatic fluid was significantly higher than that in normal tissue, such as mesenteric lymph nodes [135]. The main source of S1P in the lymphatic fluid is thought to be produced by SphK1 in lymphatic endothelial cells, which is thought to be released into the lymphatic fluid by the Spns2 transporter [138].
In peripheral tissues, S1P concentrations are maintained in the lower range of several nM to several tens of nM by the action of S1P-degrading enzymes, such as S1P phosphatases and S1P lyases [139]. This concentration gradient of S1P among the blood, lymph, and peripheral tissues plays an essential role in regulating immune cell trafficking [138]. In Spns2-deficient mice, S1P concentrations are increased in several specific tissues, including lymph nodes, interstitial fluid, and lymph fluid, suggesting that Spns2 deficiency causes dysregulation of the S1P concentration gradient [140]. As a result, the lymphatic vascular network is disrupted, and the number of lymphocytes in the lymph nodes is reduced in Spns2-deficient mice [140].
S1P is important in the egress of lymphocytes from the lymphoid organs, including the thymus and lymph nodes [141,142]. S1P is considered a circulation marker that allows immune cells to find blood and lymphatic vessels and stabilizes the vascular system by acting on endothelial cells [143]. S1PR1 and sphingosine kinases have an important role in the maturation of vascular and lymphatic vessels [143,144,145,146]. T-cells develop in the thymus, a primary lymphoid organ and S1PR1 signaling is essential for T-cells to exit the thymus after maturation; when S1PR1 is lost in T-cells, mature T-cells outside the thymus are almost completely lost, but mature T-cells within the thymus accumulate [147].
After maturation, lymphocytes circulate through secondary lymphoid organs, such as the lymph nodes, spleen, and Peyer’s patches, to encounter antigens [138,148]. In vivo imaging of S1PR1-deficient cells and control T-cells exiting the lymph nodes suggest that the primary role of S1PR1 is to allow cells to cross the endothelial barrier; both S1PR1-deficient cells and control cells reach the endothelial intercellular spaces in the cortical lymph sinuses and extend their projections, but control T-cells migrate 10 times more frequently than S1PR1-deficient cells [149,150]. In T-cells, S1PR1-mediated exit signals compete with retention signals from chemokine receptor 7 and C-X-C chemokine receptor type 4 [151,152].
S1P is a lipid chemoattractant that directs cells not only from lymphoid tissues but also non-lymphoid tissue with relatively low S1P concentrations to circulating fluids with relatively high S1P concentrations [153,154,155,156]. The role of S1P signaling in T-cell egress from non-lymphoid tissues has been well studied in memory T-cells [141]. Memory T-cells remain at the site of infection and provide strong protection against reinfection. Transcriptional analysis of memory T-cells has revealed that S1pr1 is strongly downregulated in these cells [157,158,159]. Recent experiments further support the hypothesis that S1P levels increase in inflammation, prolonging T-cell residence time in the location of inflammation [160].
3. S1P in Cancer Cells and the Interaction between Cancer and the Microenvironment
Cancer cells often have abundant SphK1 in the cytoplasm, and S1P production is enhanced [161,162]. In cancer development and progression, S1P “inside-out” signaling plays a critical role, and S1P acts on S1P receptors on the plasma membrane of the cancer cell itself and in the surrounding microenvironment via autocrine and paracrine modes of action [29,163]. The expression of S1PR1 and S1PR3 is often higher in breast cancer, suggesting that signaling through these receptors contributes to cancer growth and invasion [164,165,166,167,168,169]. S1P also acts on S1PR1 on vascular endothelial cells to induce angiogenesis [170,171] and on S1PR1 on immune cells to promote migration [172]. Thus, the SphKs/S1P/S1PRs signaling pathway is thought to contribute to the formation of the cancer immune microenvironment [34,173].
The cancer microenvironment is composed of host tissues, including blood vessels, lymphatic vessels, immune cells, stromal cells, extracellular matrix, and the stromal fluid that fills this space [174,175,176,177]. The interaction between cancer cells and their microenvironment is important during cancer development and progression, and it is essential to understand the molecular mechanisms involved in this interaction [174,175,176,177,178,179]. Cancer cells are thought to secrete bioactive molecules, such as cytokines, chemokines, and lipid mediators, into the microenvironment to influence cancer progression [179]. On the other hand, noncancerous cells in the microenvironment also release bioactive molecules that influence cancer cells. S1P regulates the development and progression of cancer by promoting cell proliferation, migration, angiogenesis, and lymphangiogenesis [170,179]. The effects of S1P on the interaction between cancer and the microenvironment include the following: (1) S1P produced by cancer cells acts on cancer cells themselves to promote proliferation, migration, and viability; (2) S1P produced by cancer cells acts on the microenvironment to induce vascular and lymphangiogenesis, immune responses, chronic inflammation, and stromal reactions; and (3) cytokines, such as IL-6 and TNF-α, produced by cancer cells act on stromal cells in the microenvironment, and the stromal cells produce S1P and affect the cancer cells [32,179,180].
In mice transplanted with the 4T1 breast cancer cell line, S1P levels in the tumor gradually increase with tumor growth, reaching twice the level of S1P in the normal mammary gland, and serum S1P levels increase significantly, from 800 pmol/mL to about 1200 pmol/mL [181]. SK1-I, an inhibitor of SphK1, reduces S1P levels in tumors to levels comparable to those in the mammary glands of mice without transplanted tumors, and serum S1P levels are also reduced to levels comparable to those in mice without transplanted tumors [181]. Furthermore, stage IIIA breast cancer patients with lymph node metastases have a two-fold increase in serum S1P concentrations compared to age– and ethnicity-matched healthy volunteers [181]. Taken together, it appears that S1P produced by the tumor not only affects the S1P concentration in the tumor but also affects the S1P concentration in the systemic circulation, which is related to cancer progression.
Tissue interstitial fluid surrounds cells in the microenvironment and is drained into lymphatic vessels to become lymph fluid. It eventually enters the veins and joins the systemic circulation. In normal tissues, S1P in tissue fluid is maintained at a low concentration by the aforementioned S1P-degrading enzyme. When S1P is measured after collection by low-speed centrifugation, the concentration in the interstitial fluid of human breast cancer tissue is about several hundred picomoles, approximately three times higher than in the interstitial fluid of normal human mammary glands [139]. This suggests that the S1P concentration in cancer tissue interstitial fluid is increased by the enhanced production and release of S1P in cancer cells and that higher concentrations of S1P flow into lymph vessels. The possibility that S1P released from cancer cells acts on lymphatic endothelial cells and promotes metastasis of cancer cells has been confirmed in animal experiments [181].
Angiogenesis brings oxygen and nutrients to cancer cells as new blood vessels extend into the cancer, provides a conduit for cancer cells to metastasize, and regulates the rate of cancer growth and progression [171,182]. Neutralization of extracellular S1P by anti-S1P antibodies has an inhibitory effect on angiogenesis, tumor growth, and metastasis in animal models, indicating that extracellular S1P regulates angiogenesis in cancer [183]. A study reported that the addition of 1 mg/mL of anti-S1P monoclonal antibody to the medium inhibits the S1P-induced migration of human umbilical vein endothelial cells by 70% [183]. The study also evaluated the ability of S1P to promote vascular endothelial cell infiltration into Matrigel plugs in vivo and found that animals implanted with Matrigel containing 5 mM S1P have a significant, 6-fold increase in vascular endothelial cell density compared to those implanted with Matrigel lacking S1P [183]. Treatment with an anti-S1P monoclonal antibody also suppresses vascular endothelial cell infiltration to a level comparable to antibody-treated animals and controls that did not receive S1P [183]. Data from mouse models and human patient samples suggest that SphK1-upregulated tumors themselves may be an important source of S1P [181,184,185,186]. More importantly, endothelial cells have also been found to synthesize and release S1P [127,187,188,189].
S1P signaling also plays an important role in lymphangiogenesis [170,190,191]. Specifically, S1P induces lymphatic endothelial cell tube formation in an S1PR1-dependent manner, and S1P is also involved in cancer-induced lymphangiogenesis [181,192,193]. Lymphangiogenesis induced by angiopoietin-2 is suppressed by SphK1-specific pharmacological inhibitors, suggesting that there is cross-talk between angiopoietin-2 and S1P signaling pathways and that both interactively contribute to lymphangiogenesis [170,181]. The lymphatic endothelial cell-specific deletion of SphK1 in SphK2 knockout mice results in a complete loss of S1P production in lymphatic vessel endothelial cells, thereby inhibiting lymphatic vessel maturation, suggesting that SphKs and S1P are required for proper development of lymphatic vessel endothelial cells [194].
S1P is also associated with inflammation in the interaction between cancer and the microenvironment [34,195,196]. We have shown that S1P is involved in chronic intestinal inflammation and colitis-associated cancer in a mouse model of inflammatory carcinogenesis [197]; that is, S1P contributes to the production of IL-6, which is regulated by nuclear factor kappa B and to the constant activation of the transcription factor, signal transducer and activator of transcription 3 (STAT3), resulting in the upregulation of S1PR1, and consequently the high expression of S1PR1 [197,198]. In addition, epidemiological data have shown that obesity is a risk factor for postmenopausal breast cancer and exacerbates cancer progression, and the upregulation of S1P/S1PR1/STAT3 signaling has also been implicated in a mouse model of breast cancer associated with obesity [196]. This suggests that S1P plays a key role in the maintenance of chronic inflammation and cancer progression in obesity-associated breast cancer.
Since S1P is strongly involved in immune cell migration, it is assumed to play a significant role in the formation of the tumor immune microenvironment [199]. Memory T-cells in tumor tissue have been reported to be characterized by the absence of S1PR1 [200], which may suggest that memory T-cells are able to remain in the location of tumor tissue for long periods of time by not being receptive to S1P signaling via S1PR1. On the other hand, for regulatory T-cells (Tregs), Stat3-mediated S1PR1 signaling has been shown to be important for Treg migration to tumors [201]. One study showed that increased S1PR1 in CD4+ T-cells promotes STAT3 activation and JAK/STAT3-dependent Treg tumor migration, while ablation of STAT3 in T-cells reduces tumor-associated Treg accumulation and tumor migration [202]. These results demonstrate the importance of S1PR1 signaling in peripheral and tumor Treg cells. In our study, which analyzed the association between immune-related genes and Sphk1 gene expression in breast cancer using the Cancer Genome Atlas database, breast cancers with high Sphk1 expression are associated with increased expression of immune-related genes, such as CD68, CD163, CD4, and forkhead box protein 3, which are associated with increased myeloid-derived suppressor cells (MDSCs), and Treg infiltration is suggested to be increased [203]. Based on previous findings, S1P signaling appears to act in the direction of promoting the migration of immune cells that suppress tumor immunity in the tumor immune microenvironment. The function of S1P in tumor immunity, including its association with immune checkpoint inhibitors, is intriguing and deserves further study.
4. Clinical Significance of S1P in Breast Cancer Patients
Our previous studies have revealed various roles for S1P in the interaction between cancer and the microenvironment [34], and we speculated that S1P may play a major role in lymphatic metastasis (Figure 2). In brief, S1P produced by cancer is released into the interstitial fluid and contributes to the interaction between cancer cells and the microenvironment, together with cytokines and chemokines, which also regulate the physiological functions of cancer cells and mesenchymal cells in the cancer microenvironment. In the cancer microenvironment, S1P promotes cancer cell proliferation and invasion and contributes to the formation of a favorable microenvironment for cancer progression via angiogenesis, lymphangiogenesis, and immune cell mobilization. S1P may contribute to lymphangiogenesis by acting on lymphatic endothelial cells, and S1P in lymphatic vessels may also contribute to the survival of free-floating cancer cells and lymph node metastasis based on our hypothesis.
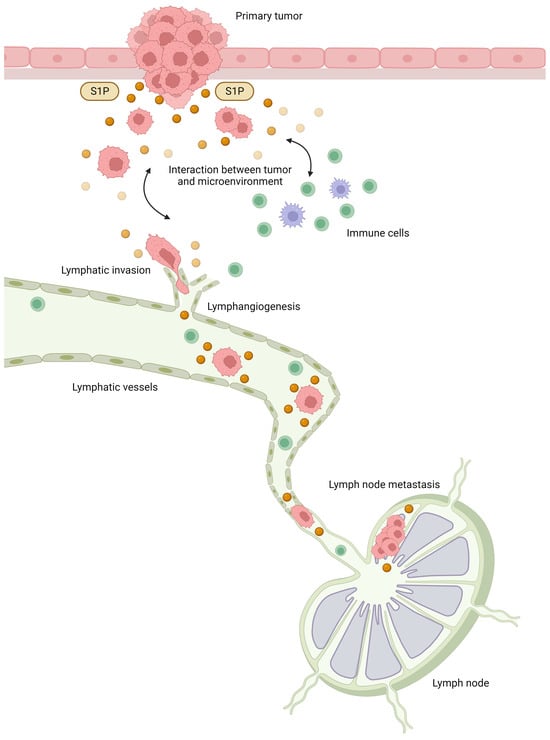
Figure 2.
The roles of sphingosine-1-phosphate (S1P) in lymphatic metastasis. S1P produced by cancer cells is released into the interstitial fluid and contributes to the interaction between cancer and the microenvironment, together with cytokines and chemokines. In the cancer microenvironment, S1P promotes cancer cell proliferation and invasion and contributes to the formation of a favorable microenvironment for cancer progression through angiogenesis, lymphangiogenesis, and immune cell mobilization. S1P may contribute to lymphangiogenesis by acting on lymphatic endothelial cells, and S1P in lymphatic vessels may also contribute to the survival of free-floating cancer cells and lymph node metastasis.
S1P is expected to be associated with clinical outcomes because it affects the tumor microenvironment and is involved in the developmental progression of cancer. Indeed, previous studies have reported that S1P and SphK1 are associated with outcomes in breast cancer patients [204,205,206]. High SphK1 expression by microarray analysis was associated with significantly worse disease-free survival in combined datasets of 968 breast cancer patients [204]. It had been expected that the high expression levels of SphK1 might result in high levels of S1P in the cancer tissue. We previously confirmed that patients with positive-expression of phosphorylated SphK1 (pSphK1) on breast cancer specimens do indeed show significantly higher levels of S1P in the breast cancer tissue as determined using mass spectrometry [65]. Mass spectrometry analysis of frozen cancer tissue samples in many cancer types also shows significantly higher concentrations of S1P in cancer tissues compared to normal tissues [61,62,63,64]. Regarding breast cancer, our analysis using mass spectrometry shows that S1P concentrations are approximately twice as high in breast cancer as in normal mammary glands [61]. Interestingly, S1P levels in the breast cancer tissue measured using mass spectrometry in patients with lymph node metastasis are significantly higher than those in patients with negative nodes [65].
Immunostaining for pSphK1 is much cheaper and simpler to perform than mass spectrometry and can be analyzed using paraffin blocks rather than fresh frozen samples, making it suitable for studying a larger number of clinical cases. We performed immunostaining using pSphK1 antibodies on surgical specimens from cancer patients and found that pSphK1 is overexpressed in many types of cancer, including breast, gastric, esophageal, pancreatic, biliary, and liver cancers [62,63,64,65,66,67,68,69]. We previously examined 275 breast cancer patients utilizing pSphK1 immunostaining, and the association between pSphK1 expression and clinicopathological factors was analyzed [206]. pSphK1 positivity was about 20% in patients without lymph node metastasis, whereas pSphK1 positivity was about 40% in patients with lymph node metastasis, showing a significant association of the presence of lymph node metastasis with the expression of pSphK1. Furthermore, the clinical stage was also correlated with pSphK1 expression; pSphK1 positivity was approximately 20% in stage I breast cancer but was significantly increased to 60% in stage III cases. Furthermore, survival analysis of those patients revealed that patients with breast cancers that express both pSphK1 and ABCC1 have significantly shorter disease-free survival compared to the others [206]. It was also revealed that increased S1P production, suggested by higher expression of pSphK1, is associated with lymphatic metastasis in various cancer types other than breast cancer, supporting the hypothesis based on previous basic experimental data [62,63,64,65,66,67,68,69].
Breast cancer is treated based on subtype defined by hormone receptors and human epidermal growth factor receptor 2 (HER2) receptor expression; pharmacological inhibition of 17β-estradiol (E2) production or binding of E2 to estrogen receptor (ER) is an effective treatment for patients with ER-positive breast cancer, and ER status is an important prognostic factor [207]. We previously reported that there is an S1P-mediated pathway in E2 signaling [82]. The binding of E2 to ER stimulates the release of S1P via the ABC transporters, ABCC1 and ABCG2, and the released S1P binds to and activates S1P receptors [82]. Activation of S1P receptors promotes breast cancer growth, progression, and invasion by stimulating downstream ERK1/2 [82]. Thus, S1P may contribute to the non-genomic signaling of E2 at the plasma membrane of cells, where they can propagate signal transduction through kinase pathways, such as AKT and MAPK pathways [82,208]. In ER-positive breast cancer, high expression of SphK1 has been reported to be associated with poor prognosis, and SphK1 has been shown to be associated with the development of tamoxifen-resistant early recurrence during tamoxifen treatment [164,209].
HER2 overexpression is a major determinant of breast cancer progression, and S1P signaling may contribute to this; S1PR4 stimulates the ERK1/2 pathway in ER-negative HER2-positive MDA-MB-453 breast cancer cells through a HER2-dependent mechanism [210]. S1PR4 and high expression of SphK1 are associated with shorter survival in breast cancer patients, indicating the importance of S1PR4 and SphK1 in the progression of breast cancer [205]. Interestingly, for S1P concentrations in breast cancer tissues, we found that stronger HER2 expression was associated with lower S1P concentrations [65]. Since both HER2 and SphK1 are strong activators of survival signaling pathways, such as the MAPK pathway, and the HER2 signaling is highly autonomous, it is possible that negative feedback from HER2 signaling suppresses SphK1 activation in HER2-positive breast cancer. However, further studies are needed to clarify the relationship between S1P signaling and HER2 expression.
Triple-negative breast cancer has a particularly poor prognosis because it is often biologically more malignant than other subtypes and has limited treatment options [211]. LM2-4 cells, which have acquired a lung metastasis phenotype from the triple-negative cell line, MDA-MB-231, require SphKs/S1P/S1PRs signaling for their growth, survival, and cell motility [212]. PF-543, a selective and potent inhibitor of SphK1, does not inhibit proliferative signaling in parental MDA-MB-231 cells but inhibits the proliferative signaling of AKT, ERK, and p38 MAP kinase pathways in LM2-4 cells [212]. These observations suggest a contribution of SphKs/S1P/S1PRs signaling in metastatic triple-negative breast cancer and that S1P signaling may be a therapeutic target in triple-negative breast cancer [213].
Although S1P levels in cancer tissue have been shown to correlate with lymphatic metastasis and patient prognosis, the clinical significance of S1P levels in the blood of cancer patients has been less well understood. We investigated ER-positive HER2-negative breast cancer and quantified plasma S1P in patients using mass spectrometry and compared S1P levels with clinicopathological factors [214]. We found that higher plasma S1P levels are associated with larger tumor size, positive lymph nodes, and advanced-stage cancer [214]. Considering that ER-positive HER2-negative breast cancer patients have particularly high S1P levels in cancer tissues compared with other subtypes and that previous basic studies have shown that “inside-out” signaling of S1P contributes to the signaling of the ER non-genomic pathway, S1P is predicted to play an important role in ER-positive breast cancer in combination with estrogen signaling [82]. Therefore, the fact that the S1P concentration in the peripheral blood of patients with ER-positive breast cancer is associated with the degree of cancer progression suggests that the function of S1P in cancer linked to estrogen signaling may be reflected in changes in the peripheral blood.
As mentioned above, animal studies have suggested that S1P links cancer and inflammation, but translational research using clinical specimens has also confirmed this cancer-inflammation relationship [67]. Serum S1P levels are found to be significantly increased in breast cancer patients with obesity [196]. This finding may be a corollary to the involvement of the S1P/S1PR1/STAT3 signaling in breast cancer patients with obesity, similar to the results of animal studies. S1P produced by cancer cells and released into the microenvironment may act on immune cells to promote cancer development and progression. According to the results of the Cancer Genome Atlas database analysis, increased expression of sphk1 in HER2-negative breast cancer tissue is associated with increased expression of immune-related molecules, such as TNF-α, IL-6, and transforming growth factor-β, and probably immune cells that suppress tumor immunity, such as Tregs and MDSCs [203]. Further clarification of the mechanism of interaction between S1P and the suppressive immune cells, Tregs, and MDSCs, and their therapeutic applications are expected in the future.
5. Targeting S1P as Therapy for Advanced Breast Cancer
In addition to treatment with anti-S1P antibodies targeting S1P itself [183,215], treatment with inhibitors of S1P receptors and S1P-producing enzymes is being considered to target the S1P signaling pathway [207], and new agents are being developed. FTY720 (fingolimod), the first molecularly targeted drug approved for multiple sclerosis, has been shown to inhibit S1P receptors other than S1PR2 and also inhibit SphK1 in basic studies [216,217,218,219]. Thus, by inhibiting S1P signaling, FTY720 has been shown to suppress the growth and metastasis of various cancers in cellular and animal studies [220,221,222,223]. Because FTY720 has the side effect of immunosuppression, which lowers the number of lymphocytes in the blood, its clinical application as an anticancer drug has not progressed, but efforts are being made to develop new drugs to overcome these side effects [224].
Technological advances have also led to the development of new S1P signaling inhibitors [225]. The function of S1P in nuclear gene regulation may also play an important role in cancer and is a subject for future research [77]. Antibody drugs against S1P have also been developed and are currently in clinical development [183,215]. A number of molecularly targeted drugs that act more specifically on S1P receptors have been developed and are being tested in clinical trials as potential treatments for multiple sclerosis and inflammatory bowel disease [226,227,228,229].
Technological advances have also led to the development of new S1P signaling inhibitors. The function of S1P in nuclear gene regulation may also play an important role in cancer and is a subject for future research [40]. Antibody drugs against S1P have also been developed and are currently in clinical development [103,129]. A number of molecularly targeted drugs that act more specifically on S1P receptors have been developed and are being tested in clinical trials as potential treatments for multiple sclerosis and inflammatory bowel disease [138,139,140,141].
Since the importance of S1P in cancer varies depending on the cancer type, subtype, degree of progression, presence or absence of metastases and the organs to which it spreads, and the cancer microenvironment, clinical application of therapies targeting S1P signaling will require identifying the patient population for whom the therapy will be most effective [230,231]. Therefore, the development of therapies targeting S1P signaling requires the selection of target patient groups, the development of S1P signaling inhibitors, and consideration of combination therapy with anti-cancer drugs and molecular-targeted drugs. A variety of agents have been developed to regulate S1P signaling, each with different targets, and clinical development strategies must be tailored to the pathophysiology of the cancer [181,226,227,228,229,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247]. S1P signaling inhibitors developed to date, which could be applied to cancer therapy, are listed in Table 1. Since S1P contributes to inflammation-based carcinogenesis, as in the case of colorectal cancer carcinogenesis from ulcerative colitis, suppression of S1P signaling may be expected to have a preventive effect on carcinogenesis. However, chemoprevention of cancer using drugs would be a later priority in its development from a safety perspective. More realistically, clinical development of the drugs listed in Table 1 as single agents or in combination with existing therapies for the treatment of advanced cancer with metastasis is desirable.

Table 1.
List of major drugs targeting S1P signaling that could be applied to cancer therapy.
Therapy targeting S1P is thought to affect the microenvironment while suppressing the cancer itself. In particular, it may be effective in inflammation-associated cancers because of its strong anti-inflammatory effect [248,249,250]. In the field of breast cancer, it may be effective, especially in breast cancer associated with obesity and inflammatory breast cancer. Moreover, FTY720, Ozanimod, Siponimod, and Ponesimod are in clinical use for the treatment of multiple sclerosis, meaning that these drugs are effective in crossing the blood–brain barrier. Its potential as a therapeutic agent for brain metastases appears to be promising. Considering the significant impact of S1P on tumor immunity, further studies are needed, but there is great promise for the future use of S1P signaling modulation in immunotherapy.
6. Conclusions
In this article, the basic mechanism of action of S1P, a lipid mediator, the function of S1P in cancer cells and the cancer microenvironment based on in vitro and in vivo experiments, and the clinical significance of S1P based on clinical studies are introduced, and its potential for therapeutic application is discussed. Advances in science and technology have made it possible to quantify S1P, which was previously difficult, and to elucidate its clinical significance. Further technological innovations may enable the clinical application of S1P as a biomarker and therapeutic target, and we look forward to the development of future translational research.
Author Contributions
Conceptualization, M.N.; writing—original draft preparation, M.N.; writing—review and editing, Y.M.; supervision, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number 22H03140 and 21K19522 for M.N., 22K08764 for Y.M.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this study.
Acknowledgments
All the figures were generated using biorender.com.
Conflicts of Interest
M.N. received honoraria from Chugai, AstraZeneca, Eli Lilly, Pfizer, Novartis, Taiho, Daiichi Sankyo, Esai, Kyowa-Kirin, MSD, and Denka. Y.M. received research funding and honoraria from Esai, Chugai, AstraZeneca, Eli Lilly, Pfizer, MSD, Kyowa-Kirin, Daiichi-Sankyo, and Taiho. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Masuda, N.; Takano, T.; Tsugawa, K.; Inoue, K.; Matsumoto, K.; Ishikawa, T.; Itoh, M.; Yasojima, H.; Tanabe, Y.; et al. Pembrolizumab plus chemotherapy in Japanese patients with triple-negative breast cancer: Results from KEYNOTE-355. Cancer Med. 2023, 12, 10280–10293. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Jhaveri, K.; Kalinsky, K.; Pernas, S.; Tsurutani, J.; Xu, B.; Hamilton, E.; Im, S.A.; Nowecki, Z.; Sohn, J.; et al. TROPION-Breast01: Datopotamab Deruxtecan vs Chemotherapy in Pre-Treated Inoperable or Metastatic HR+/HER2- Breast Cancer. Future Oncol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Bhardwaj, P.V.; Abdou, Y.G. The Evolving Landscape of Immune Checkpoint Inhibitors and Antibody Drug Conjugates in the Treatment of Early-Stage Breast Cancer. Oncologist 2023, 28, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; De Caluwé, A.; Wang, X.; Piccart-Gebhart, M.; Tuohy, V.K.; Romano, E.; Buisseret, L. Immunotherapy in breast cancer: An overview of current strategies and perspectives. NPJ Breast Cancer 2023, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Alataki, A.; Dowsett, M. Human epidermal growth factor receptor-2 and endocrine resistance in hormone-dependent breast cancer. Endocr. Relat. Cancer 2022, 29, R105–R122. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yu, X.; Qin, J.J. Drug-resistant HER2-positive breast cancer: Molecular mechanisms and overcoming strategies. Front. Pharmacol. 2022, 13, 1012552. [Google Scholar] [CrossRef]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers 2023, 15, 1278. [Google Scholar] [CrossRef]
- Marra, A.; Trapani, D.; Ferraro, E.; Curigliano, G. Mechanisms of Endocrine Resistance in Hormone Receptor-Positive Breast Cancer. Cancer Treat. Res. 2023, 188, 219–235. [Google Scholar]
- Antonarelli, G.; Taurelli Salimbeni, B.; Marra, A.; Esposito, A.; Locatelli, M.A.; Trapani, D.; Pescia, C.; Fusco, N.; Curigliano, G.; Criscitiello, C. The CDK4/6 inhibitors biomarker landscape: The most relevant biomarkers of response or resistance for further research and potential clinical utility. Crit. Rev. Oncol. Hematol. 2023, 192, 104148. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.; Bieberich, E. Ceramide and Exosomes: A Novel Target in Cancer Biology and Therapy. Adv. Cancer Res. 2018, 140, 121–154. [Google Scholar]
- Luo, X.; Zhao, X.; Cheng, C.; Li, N.; Liu, Y.; Cao, Y. The implications of signaling lipids in cancer metastasis. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- García-González, V.; Díaz-Villanueva, J.F.; Galindo-Hernández, O.; Martínez-Navarro, I.; Hurtado-Ureta, G.; Pérez-Arias, A.A. Ceramide Metabolism Balance, a Multifaceted Factor in Critical Steps of Breast Cancer Development. Int. J. Mol. Sci. 2018, 19, 2527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Czarnota, G.J. Involvement of Ceramide Signalling in Radiation-Induced Tumour Vascular Effects and Vascular-Targeted Therapy. Int. J. Mol. Sci. 2022, 23, 6671. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.C.E.; Chaves-Filho, A.B.; Schulze, A. Lipids as mediators of cancer progression and metastasis. Nat. Cancer 2024, 5, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Tang, X.; Brindley, D.N.; Takabe, K. Autotaxin and Lysophosphatidate Signaling: Prime Targets for Mitigating Therapy Resistance in Breast Cancer. World J. Oncol. 2024, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 2002, 277, 25851–25854. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Nagahashi, M.; Ramachandran, S.; Rashid, O.M.; Takabe, K. Lymphangiogenesis: A new player in cancer progression. World J. Gastroenterol. 2010, 16, 4003–4012. [Google Scholar] [CrossRef]
- Gandy, K.A.; Obeid, L.M. Regulation of the Sphingosine Kinase/Sphingosine 1-Phosphate Pathway. In Sphingolipids in Disease; Springer: Vienna, Austria, 2013; pp. 275–303. [Google Scholar]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef]
- Zhang, H.; Desai, N.N.; Olivera, A.; Seki, T.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991, 114, 155–167. [Google Scholar] [CrossRef]
- Olivera, A.; Buckley, N.E.; Spiegel, S. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J. Biol. Chem. 1992, 267, 26121–26127. [Google Scholar] [CrossRef]
- Olivera, A.; Spiegel, S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef]
- Spiegel, S.; Olivera, A.; Carlson, R.O. The role of sphingosine in cell growth regulation and transmembrane signaling. Adv. Lipid Res. 1993, 25, 105–129. [Google Scholar]
- Mazurek, N.; Megidish, T.; Hakomori, S.; Igarashi, Y. Regulatory effect of phorbol esters on sphingosine kinase in BALB/C 3T3 fibroblasts (variant A31): Demonstration of cell type-specific response--a preliminary note. Biochem. Biophys. Res. Commun. 1994, 198, 1–9. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Dickson, R.C.; Nagiec, E.E.; Skrzypek, M.; Tillman, P.; Wells, G.B.; Lester, R.L. Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem. 1997, 272, 30196–30200. [Google Scholar] [CrossRef]
- Blakesley, V.A.; Beitner-Johnson, D.; Van Brocklyn, J.R.; Rani, S.; Shen-Orr, Z.; Stannard, B.S.; Spiegel, S.; LeRoith, D. Sphingosine 1-phosphate stimulates tyrosine phosphorylation of Crk. J. Biol. Chem. 1997, 272, 16211–16215. [Google Scholar] [CrossRef]
- Rani, C.S.; Wang, F.; Fuior, E.; Berger, A.; Wu, J.; Sturgill, T.W.; Beitner-Johnson, D.; LeRoith, D.; Varticovski, L.; Spiegel, S. Divergence in signal transduction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors. Involvement of sphingosine 1-phosphate in PDGF but not EGF signaling. J. Biol. Chem. 1997, 272, 10777–10783. [Google Scholar] [CrossRef]
- Wang, F.; Nobes, C.D.; Hall, A.; Spiegel, S. Sphingosine 1-phosphate stimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem. J. 1997, 324 Pt 2, 481–488. [Google Scholar] [CrossRef]
- Kohama, T.; Olivera, A.; Edsall, L.; Nagiec, M.M.; Dickson, R.; Spiegel, S. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 1998, 273, 23722–23728. [Google Scholar] [CrossRef]
- Wang, F.; Van Brocklyn, J.R.; Hobson, J.P.; Movafagh, S.; Zukowska-Grojec, Z.; Milstien, S.; Spiegel, S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 1999, 274, 35343–35350. [Google Scholar] [CrossRef]
- Rosenfeldt, H.M.; Hobson, J.P.; Maceyka, M.; Olivera, A.; Nava, V.E.; Milstien, S.; Spiegel, S. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 2001, 15, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Benaud, C.; Oberst, M.; Hobson, J.P.; Spiegel, S.; Dickson, R.B.; Lin, C.Y. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J. Biol. Chem. 2002, 277, 10539–10546. [Google Scholar] [CrossRef] [PubMed]
- English, D.; Brindley, D.N.; Spiegel, S.; Garcia, J.G. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim. Biophys. Acta 2002, 1582, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Lacana, E.; Maceyka, M.; Milstien, S.; Spiegel, S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. J. Biol. Chem. 2002, 277, 32947–32953. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Cinamon, G.; Matloubian, M.; Lesneski, M.J.; Xu, Y.; Low, C.; Lu, T.; Proia, R.L.; Cyster, J.G. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 2004, 5, 713–720. [Google Scholar] [CrossRef]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef]
- Allende, M.L.; Dreier, J.L.; Mandala, S.; Proia, R.L. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 2004, 279, 15396–15401. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 2007, 282, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Spiegel, S. The role of sphingosine-1-phosphate and its receptors in asthma. Drug News Perspect. 2008, 21, 89–96. [Google Scholar] [PubMed]
- Blaho, V.A.; Hla, T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef]
- Chawla, S.; Saxena, S. Differential modulation of S1PR(1-5) and specific activities of SphK and nSMase in pulmonary and cerebral tissues of rats exposed to hypobaric hypoxia. Lipids 2015, 50, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Tsuchida, J.; Moro, K.; Hasegawa, M.; Tatsuda, K.; Woelfel, I.A.; Takabe, K.; Wakai, T. High levels of sphingolipids in human breast cancer. J. Surg. Res. 2016, 204, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, T.; Nagahashi, M.; Ichikawa, H.; Ishikawa, T.; Kobayashi, T.; Wakai, T. Expression of phosphorylated sphingosine kinase 1 is associated with diffuse type and lymphatic invasion in human gastric cancer. Surgery 2018, 163, 1301–1306. [Google Scholar] [CrossRef]
- Hirose, Y.; Nagahashi, M.; Katsuta, E.; Yuza, K.; Miura, K.; Sakata, J.; Kobayashi, T.; Ichikawa, H.; Shimada, Y.; Kameyama, H.; et al. Generation of sphingosine-1-phosphate is enhanced in biliary tract cancer patients and is associated with lymphatic metastasis. Sci. Rep. 2018, 8, 10814. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Nagahashi, M.; Prasoon, P.; Hirose, Y.; Kobayashi, T.; Sakata, J.; Abe, M.; Sakimura, K.; Matsuda, Y.; Butash, A.L.; et al. Dysregulation of sphingolipid metabolic enzymes leads to high levels of sphingosine-1-phospate and ceramide in human hepatocellular carcinoma. Hepatol. Res. 2021, 51, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, J.; Nagahashi, M.; Nakajima, M.; Moro, K.; Tatsuda, K.; Ramanathan, R.; Takabe, K.; Wakai, T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J. Surg. Res. 2016, 205, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yuza, K.; Nakajima, M.; Nagahashi, M.; Tsuchida, J.; Hirose, Y.; Miura, K.; Tajima, Y.; Abe, M.; Sakimura, K.; Takabe, K.; et al. Different Roles of Sphingosine Kinase 1 and 2 in Pancreatic Cancer Progression. J. Surg. Res. 2018, 232, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Yuza, K.; Nagahashi, M.; Shimada, Y.; Nakano, M.; Tajima, Y.; Kameyama, H.; Nakajima, M.; Takabe, K.; Wakai, T. Upregulation of phosphorylated sphingosine kinase 1 expression in colitis-associated cancer. J. Surg. Res. 2018, 231, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Ichikawa, H.; Nagahashi, M.; Hanyu, T.; Ishikawa, T.; Kano, Y.; Muneoka, Y.; Wakai, T. Phospho-Sphingosine Kinase 1 Expression in Lymphatic Spread of Esophageal Squamous Cell Carcinoma. J. Surg. Res. 2019, 234, 123–131. [Google Scholar] [CrossRef]
- Nagaro, H.; Ichikawa, H.; Takizawa, K.; Nagahashi, M.; Abe, S.; Hirose, Y.; Moro, K.; Miura, K.; Nakano, M.; Shimada, Y.; et al. Clinical Significance of Phosphorylated Sphingosine Kinase 1 Expression in Pancreatic Ductal Adenocarcinoma. Anticancer. Res. 2023, 43, 3969–3977. [Google Scholar] [CrossRef]
- Baran, Y.; Salas, A.; Senkal, C.E.; Gunduz, U.; Bielawski, J.; Obeid, L.M.; Ogretmen, B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J. Biol. Chem. 2007, 282, 10922–10934. [Google Scholar] [CrossRef]
- Geffken, K.; Spiegel, S. Sphingosine kinase 1 in breast cancer. Adv. Biol. Regul. 2018, 67, 59–65. [Google Scholar] [CrossRef]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef]
- Maceyka, M.; Rohrbach, T.; Milstien, S.; Spiegel, S. Role of Sphingosine Kinase 1 and Sphingosine-1-Phosphate Axis in Hepatocellular Carcinoma. In Lipid Signaling in Human Diseases; Springer: Cham, Switzerland, 2020; Volume 259, pp. 3–17. [Google Scholar]
- Liu, H.; Toman, R.E.; Goparaju, S.K.; Maceyka, M.; Nava, V.E.; Sankala, H.; Payne, S.G.; Bektas, M.; Ishii, I.; Chun, J.; et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 2003, 278, 40330–40336. [Google Scholar] [CrossRef]
- Okada, T.; Ding, G.; Sonoda, H.; Kajimoto, T.; Haga, Y.; Khosrowbeygi, A.; Gao, S.; Miwa, N.; Jahangeer, S.; Nakamura, S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 2005, 280, 36318–36325. [Google Scholar] [CrossRef]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef]
- Nagahashi, M.; Matsuda, Y.; Moro, K.; Tsuchida, J.; Soma, D.; Hirose, Y.; Kobayashi, T.; Kosugi, S.; Takabe, K.; Komatsu, M.; et al. DNA damage response and sphingolipid signaling in liver diseases. Surg. Today 2016, 46, 995–1005. [Google Scholar] [CrossRef]
- Pitson, S.M.; Xia, P.; Leclercq, T.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Wattenberg, B.W.; Vadas, M.A. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J. Exp. Med. 2005, 201, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.E.; Milstien, S.; Spiegel, S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 2007, 18, 300–307. [Google Scholar] [CrossRef]
- Shida, D.; Fang, X.; Kordula, T.; Takabe, K.; Lepine, S.; Alvarez, S.E.; Milstien, S.; Spiegel, S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008, 68, 6569–6577. [Google Scholar] [CrossRef]
- Snider, A.J.; Orr Gandy, K.A.; Obeid, L.M. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010, 92, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Kim, R.H.; Allegood, J.C.; Mitra, P.; Ramachandran, S.; Nagahashi, M.; Harikumar, K.B.; Hait, N.C.; Milstien, S.; Spiegel, S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010, 285, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Oskeritzian, C.A.; Payne, S.G.; Beaven, M.A.; Milstien, S.; Spiegel, S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16394–16399. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Takabe, K.; Milstien, S.; Spiegel, S. Export and functions of sphingosine-1-phosphate. Biochim. Biophys. Acta 2009, 1791, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Hisano, Y.; Kobayashi, N.; Kawahara, A.; Yamaguchi, A.; Nishi, T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 2011, 286, 1758–1766. [Google Scholar] [CrossRef]
- Hisano, Y.; Kobayashi, N.; Yamaguchi, A.; Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 2012, 7, e38941. [Google Scholar] [CrossRef]
- Donoviel, M.S.; Hait, N.C.; Ramachandran, S.; Maceyka, M.; Takabe, K.; Milstien, S.; Oravecz, T.; Spiegel, S. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 2015, 29, 5018–5028. [Google Scholar] [CrossRef]
- Spiegel, S.; Maczis, M.A.; Maceyka, M.; Milstien, S. New insights into functions of the sphingosine-1-phosphate transporter SPNS2. J. Lipid Res. 2019, 60, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Candelore, M.R.; Wright, M.J.; Tota, L.M.; Milligan, J.; Shei, G.J.; Bergstrom, J.D.; Mandala, S.M. Phytosphingosine 1-phosphate: A high affinity ligand for the S1P(4)/Edg-6 receptor. Biochem. Biophys. Res. Commun. 2002, 297, 600–606. [Google Scholar] [CrossRef]
- Ishii, I.; Ye, X.; Friedman, B.; Kawamura, S.; Contos, J.J.; Kingsbury, M.A.; Yang, A.H.; Zhang, G.; Brown, J.H.; Chun, J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J. Biol. Chem. 2002, 277, 25152–25159. [Google Scholar] [CrossRef]
- Dolezalova, H.; Shankar, G.; Huang, M.C.; Bikle, D.D.; Goetzl, E.J. Biochemical regulation of breast cancer cell expression of S1P2 (Edg-5) and S1P3 (Edg-3) G protein-coupled receptors for sphingosine 1-phosphate. J. Cell Biochem. 2003, 88, 732–743. [Google Scholar] [CrossRef]
- Dorsam, G.; Graeler, M.H.; Seroogy, C.; Kong, Y.; Voice, J.K.; Goetzl, E.J. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J. Immunol. 2003, 171, 3500–3507. [Google Scholar] [CrossRef]
- Rosen, H.; Alfonso, C.; Surh, C.D.; McHeyzer-Williams, M.G. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 10907–10912. [Google Scholar] [CrossRef]
- Rosen, H.; Liao, J. Sphingosine 1-phosphate pathway therapeutics: A lipid ligand-receptor paradigm. Curr. Opin. Chem. Biol. 2003, 7, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.G.; Liao, J.; Jo, E.; Alfonso, C.; Ahn, M.Y.; Peterson, M.S.; Webb, B.; Lefebvre, S.; Chun, J.; Gray, N.; et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004, 279, 13839–13848. [Google Scholar] [CrossRef]
- Lepley, D.; Paik, J.H.; Hla, T.; Ferrer, F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005, 65, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Graeler, M.H.; Goetzl, E.J. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005, 19, 1731–1733. [Google Scholar] [CrossRef]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arter. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef]
- Serriere-Lanneau, V.; Teixeira-Clerc, F.; Li, L.; Schippers, M.; de Wries, W.; Julien, B.; Tran-Van-Nhieu, J.; Manin, S.; Poelstra, K.; Chun, J.; et al. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007, 21, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Niessen, F.; Schaffner, F.; Furlan-Freguia, C.; Pawlinski, R.; Bhattacharjee, G.; Chun, J.; Derian, C.K.; Andrade-Gordon, P.; Rosen, H.; Ruf, W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 2008, 452, 654–658. [Google Scholar] [CrossRef]
- Jongsma, M.; van Unen, J.; van Loenen, P.B.; Michel, M.C.; Peters, S.L.; Alewijnse, A.E. Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P(3)). Br. J. Pharmacol. 2009, 156, 1305–1311. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Luth, A.; Chun, J.; Huwiler, A.; Pfeilschifter, J.; Schafer-Korting, M.; Kleuser, B. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J. Mol. Med. 2009, 87, 645–657. [Google Scholar] [CrossRef]
- Skoura, A.; Hla, T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc. Res. 2009, 82, 221–228. [Google Scholar] [CrossRef]
- Murakami, A.; Takasugi, H.; Ohnuma, S.; Koide, Y.; Sakurai, A.; Takeda, S.; Hasegawa, T.; Sasamori, J.; Konno, T.; Hayashi, K.; et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol. Pharmacol. 2010, 77, 704–713. [Google Scholar] [CrossRef]
- Van Doorn, R.; Van Horssen, J.; Verzijl, D.; Witte, M.; Ronken, E.; Van Het Hof, B.; Lakeman, K.; Dijkstra, C.D.; Van Der Valk, P.; Reijerkerk, A.; et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 2010, 58, 1465–1476. [Google Scholar] [CrossRef]
- Jenne, C.N.; Enders, A.; Rivera, R.; Watson, S.R.; Bankovich, A.J.; Pereira, J.P.; Xu, Y.; Roots, C.M.; Beilke, J.N.; Banerjee, A.; et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009, 206, 2469–2481. [Google Scholar] [CrossRef]
- Olesch, C.; Sirait-Fischer, E.; Berkefeld, M.; Fink, A.F.; Susen, R.M.; Ritter, B.; Michels, B.E.; Steinhilber, D.; Greten, F.R.; Savai, R.; et al. S1PR4 ablation reduces tumor growth and improves chemotherapy via CD8+ T cell expansion. J. Clin. Investig. 2020, 130, 5461–5476. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef]
- Nagahashi, M.; Takabe, K.; Terracina, K.P.; Soma, D.; Hirose, Y.; Kobayashi, T.; Matsuda, Y.; Wakai, T. Sphingosine-1-phosphate transporters as targets for cancer therapy. BioMed Res. Int. 2014, 2014, 651727. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Wise, L.E.; Allegood, J.C.; O’Brien, M.; Avni, D.; Reeves, T.M.; Knapp, P.E.; Lu, J.; Luo, C.; Miles, M.F.; et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat. Neurosci. 2014, 17, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Studer, E.; Zhou, X.; Zhao, R.; Wang, Y.; Takabe, K.; Nagahashi, M.; Pandak, W.M.; Dent, P.; Spiegel, S.; Shi, R.; et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology 2012, 55, 267–276. [Google Scholar] [CrossRef]
- Nagahashi, M.; Takabe, K.; Liu, R.; Peng, K.; Wang, X.; Wang, Y.; Hait, N.C.; Wang, X.; Allegood, J.C.; Yamada, A.; et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 2015, 61, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Yuza, K.; Hirose, Y.; Nakajima, M.; Ramanathan, R.; Hait, N.C.; Hylemon, P.B.; Zhou, H.; Takabe, K.; Wakai, T. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J. Lipid Res. 2016, 57, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Hylemon, P.B.; Takabe, K.; Dozmorov, M.; Nagahashi, M.; Zhou, H. Bile acids as global regulators of hepatic nutrient metabolism. Liver Res. 2017, 1, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]
- Le Stunff, H.; Peterson, C.; Liu, H.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta 2002, 1582, 8–17. [Google Scholar] [CrossRef]
- Ogawa, C.; Kihara, A.; Gokoh, M.; Igarashi, Y. Identification and characterization of a novel human sphingosine-1-phosphate phosphohydrolase, hSPP2. J. Biol. Chem. 2003, 278, 1268–1272. [Google Scholar] [CrossRef]
- Zhou, J.; Saba, J.D. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem. Biophys. Res. Commun. 1998, 242, 502–507. [Google Scholar] [CrossRef]
- Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase in immunity and cancer: Silencing the siren. Trends Mol. Med. 2007, 13, 210–217. [Google Scholar] [CrossRef]
- Kiss, Z.; Crilly, K.S.; Anderson, W.H. Extracellular sphingosine 1-phosphate stimulates formation of ethanolamine from phosphatidylethanolamine: Modulation of sphingosine 1-phosphate-induced mitogenesis by ethanolamine. Biochem. J. 1997, 328 Pt 2, 383–391. [Google Scholar] [CrossRef]
- Hla, T.; Venkataraman, K.; Michaud, J. The vascular S1P gradient-cellular sources and biological significance. Biochim. Biophys. Acta 2008, 1781, 477–482. [Google Scholar] [CrossRef]
- Allende, M.L.; Sasaki, T.; Kawai, H.; Olivera, A.; Mi, Y.; van Echten-Deckert, G.; Hajdu, R.; Rosenbach, M.; Keohane, C.A.; Mandala, S.; et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004, 279, 52487–52492. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef]
- Salous, A.K.; Panchatcharam, M.; Sunkara, M.; Mueller, P.; Dong, A.; Wang, Y.; Graf, G.A.; Smyth, S.S.; Morris, A.J. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 2013, 54, 2775–2784. [Google Scholar] [CrossRef]
- Yatomi, Y.; Igarashi, Y.; Yang, L.; Hisano, N.; Qi, R.; Asazuma, N.; Satoh, K.; Ozaki, Y.; Kume, S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 1997, 121, 969–973. [Google Scholar] [CrossRef]
- Pyne, S.; Pyne, N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. Ther. 2000, 88, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Hanel, P.; Andreani, P.; Graler, M.H. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007, 21, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, R.; Nakamura, K.; Okubo, S.; Hosogaya, S.; Ozaki, Y.; Tozuka, M.; Osima, N.; Yokota, H.; Ikeda, H.; Yatomi, Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: Close correlation with red blood cell parameters. Ann. Clin. Biochem. 2008, 45, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yatomi, Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim. Biophys. Acta 2008, 1780, 606–611. [Google Scholar] [CrossRef]
- Thuy, A.V.; Reimann, C.M.; Hemdan, N.Y.; Graler, M.H. Sphingosine 1-phosphate in blood: Function, metabolism, and fate. Cell Physiol. Biochem. 2014, 34, 158–171. [Google Scholar] [CrossRef]
- Nagahashi, M.; Yamada, A.; Aoyagi, T.; Allegood, J.; Wakai, T.; Spiegel, S.; Takabe, K. Sphingosine-1-phosphate in the lymphatic fluid determined by novel methods. Heliyon 2016, 2, e00219. [Google Scholar] [CrossRef]
- Pappu, R.; Schwab, S.R.; Cornelissen, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.W.; Huang, Y.; Cyster, J.G.; et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef]
- Sensken, S.C.; Bode, C.; Nagarajan, M.; Peest, U.; Pabst, O.; Graler, M.H. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J. Immunol. 2010, 184, 4133–4142. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012, 30, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Yamada, A.; Miyazaki, H.; Allegood, J.C.; Tsuchida, J.; Aoyagi, T.; Huang, W.C.; Terracina, K.P.; Adams, B.J.; Rashid, O.M.; et al. Interstitial Fluid Sphingosine-1-Phosphate in Murine Mammary Gland and Cancer and Human Breast Tissue and Cancer Determined by Novel Methods. J. Mammary Gland. Biol. Neoplasia 2016, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Kim, E.Y.; Yamada, A.; Ramachandran, S.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Milstien, S.; Takabe, K.; Spiegel, S. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 2013, 27, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, A.A.L.; Schwab, S.R. Finding a Way Out: S1P Signaling and Immune Cell Migration. Annu. Rev. Immunol. 2020, 38, 759–784. [Google Scholar] [CrossRef] [PubMed]
- Hallisey, V.M.; Schwab, S.R. Get me out of here: Sphingosine 1-phosphate signaling and T cell exit from tissues during an immune response. Immunol. Rev. 2023, 317, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef]
- Allende, M.L.; Yamashita, T.; Proia, R.L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003, 102, 3665–3667. [Google Scholar] [CrossRef]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [PubMed]
- Weigel, C.; Bellaci, J.; Spiegel, S. Sphingosine-1-phosphate and its receptors in vascular endothelial and lymphatic barrier function. J. Biol. Chem. 2023, 299, 104775. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Shiow, L.R.; Rosen, D.B.; Brdickova, N.; Xu, Y.; An, J.; Lanier, L.L.; Cyster, J.G.; Matloubian, M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 2006, 440, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, I.L.; Schwab, S.R.; Phan, T.G.; Pham, T.H.; Okada, T.; Cyster, J.G. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat. Immunol. 2009, 10, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Benechet, A.P.; Menon, M.; Xu, D.; Samji, T.; Maher, L.; Murooka, T.T.; Mempel, T.R.; Sheridan, B.S.; Lemoine, F.M.; Khanna, K.M. T cell-intrinsic S1PR1 regulates endogenous effector T-cell egress dynamics from lymph nodes during infection. Proc. Natl. Acad. Sci. USA 2016, 113, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Okada, T.; Matloubian, M.; Lo, C.G.; Cyster, J.G. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity 2008, 28, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Hayano, Y.; Furuta, F.; Noda, M.; Suzuki, K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med. 2014, 211, 2583–2598. [Google Scholar] [CrossRef]
- Lo, C.G.; Xu, Y.; Proia, R.L.; Cyster, J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005, 201, 291–301. [Google Scholar] [CrossRef]
- Kabashima, K.; Haynes, N.M.; Xu, Y.; Nutt, S.L.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 2006, 203, 2683–2690. [Google Scholar] [CrossRef]
- Bode, C.; Graler, M.H. Immune regulation by sphingosine 1-phosphate and its receptors. Arch. Immunol. Ther. Exp. 2012, 60, 3–12. [Google Scholar] [CrossRef]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of S1P gradients. Biochim. Biophys. Acta 2013, 1831, 193–202. [Google Scholar] [CrossRef]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Wakim, L.M.; Woodward-Davis, A.; Liu, R.; Hu, Y.; Villadangos, J.; Smyth, G.; Bevan, M.J. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 2012, 189, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Iwasaki, A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014, 346, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, A.; Bracero, S.; Chaluvadi, V.S.; Khodadadi-Jamayran, A.; Cammer, M.; Schwab, S.R. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 2021, 592, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Ramanathan, R.; Takabe, K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag. 2015, 4, 241–244. [Google Scholar] [CrossRef]
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: A promising therapeutic target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef]
- Takabe, K.; Spiegel, S. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 2014, 55, 1839–1846. [Google Scholar] [CrossRef]
- Watson, C.; Long, J.S.; Orange, C.; Tannahill, C.L.; Mallon, E.; McGlynn, L.M.; Pyne, S.; Pyne, N.J.; Edwards, J. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 2010, 177, 2205–2215. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, J.S.; Kim, S.G.; Hwang, S.; Lee, C.H.; Moon, A. Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. J. Cell Sci. 2011, 124, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.U.; Cosan, D.T.; Mutlu, F. Effects of S1P1 and S1P3 in ER(+) and ER(-) Breast Cancer Cells. Anticancer Res. 2017, 37, 5469–5475. [Google Scholar] [PubMed]
- Rostami, N.; Nikkhoo, A.; Ajjoolabady, A.; Azizi, G.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Yousefi, B.; Yousefi, M.; Jadidi-Niaragh, F. S1PR1 as a Novel Promising Therapeutic Target in Cancer Therapy. Mol. Diagn. Ther. 2019, 23, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Lei, C.; Tan, Y.; Yi, G. Sphingosine-1-phosphate receptor 3 signaling. Clin. Chim. Acta 2021, 519, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Anu, B.; Namitha, N.N.; Harikumar, K.B. S1PR1 signaling in cancer: A current perspective. Adv. Protein Chem. Struct. Biol. 2021, 125, 259–274. [Google Scholar]
- Aoyagi, T.; Nagahashi, M.; Yamada, A.; Takabe, K. The role of sphingosine-1-phosphate in breast cancer tumor-induced lymphangiogenesis. Lymphat. Res. Biol. 2012, 10, 97–106. [Google Scholar] [CrossRef]
- Takabe, K.; Yamada, A.; Rashid, O.M.; Adams, B.J.; Huang, W.C.; Aoyagi, T.; Nagahashi, M. Twofer anti-vascular therapy targeting sphingosine-1-phosphate for breast cancer. Gland. Surg. 2012, 1, 80–83. [Google Scholar]
- Nagahashi, M.; Hait, N.C.; Maceyka, M.; Avni, D.; Takabe, K.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate in chronic intestinal inflammation and cancer. Adv. Biol. Regul. 2014, 54, 112–120. [Google Scholar] [CrossRef]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef]
- Wong, S.Y.; Hynes, R.O. Tumor-lymphatic interactions in an activated stromal microenvironment. J. Cell Biochem. 2007, 101, 840–850. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 2007, 28, 297–321. [Google Scholar] [CrossRef]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Nagahashi, M.; Rashid, O.M.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in the tumor microenvironment and its clinical implications. Tumour Biol. 2017, 39, 1010428317699133. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef]
- Huang, W.C.; Nagahashi, M.; Terracina, K.P.; Takabe, K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer, and Lymphangiogenesis. Biomolecules 2013, 3, 408–434. [Google Scholar] [CrossRef] [PubMed]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Selvam, S.P.; Mehrotra, S.; Kawamori, T.; Snider, A.J.; Obeid, L.M.; Shao, Y.; Sabbadini, R.; Ogretmen, B. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol. Med. 2012, 4, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Kaneshiro, T.; Okumura, M.; Maalouf, S.; Uflacker, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Venkataraman, K.; Hwang, S.I.; Han, D.K.; Hla, T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC). Prostaglandins Other Lipid Mediat. 2007, 84, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, P.; Proia, R.L.; Hla, T. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Investig. 2014, 124, 4823–4828. [Google Scholar] [CrossRef] [PubMed]
- Książek, M.; Chacińska, M.; Chabowski, A.; Baranowski, M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid Res. 2015, 56, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ören, B.; Mora, J.; Mertens, C.; Dziumbla, S.; Popp, R.; Weigert, A.; Grossmann, N.; Fleming, I.; Brüne, B. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci. Signal 2016, 9, ra64. [Google Scholar] [CrossRef]
- Ji, R.C. Lymph Nodes and Cancer Metastasis: New Perspectives on the Role of Intranodal Lymphatic Sinuses. Int. J. Mol. Sci. 2016, 18, 51. [Google Scholar] [CrossRef]
- Anelli, V.; Gault, C.R.; Snider, A.J.; Obeid, L.M. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010, 24, 2727–2738. [Google Scholar] [CrossRef]
- Yoon, C.M.; Hong, B.S.; Moon, H.G.; Lim, S.; Suh, P.G.; Kim, Y.K.; Chae, C.B.; Gho, Y.S. Sphingosine-1-phosphate promotes lymphangiogenesis by stimulating S1P1/Gi/PLC/Ca2+ signaling pathways. Blood 2008, 112, 1129–1138. [Google Scholar] [CrossRef]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.J.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.R.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Liang, J.; Nagahashi, M.; Avni, D.; Yamada, A.; Maceyka, M.; Wolen, A.R.; Kordula, T.; Milstien, S.; Takabe, K.; et al. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB J. 2016, 30, 2945–2958. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Yamada, A.; Katsuta, E.; Aoyagi, T.; Huang, W.C.; Terracina, K.P.; Hait, N.C.; Allegood, J.C.; Tsuchida, J.; Yuza, K.; et al. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation and breast cancer metastasis. Cancer Res. 2018, 78, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Deng, J.; Kujawski, M.; Yang, C.; Liu, Y.; Herrmann, A.; Kortylewski, M.; Horne, D.; Somlo, G.; Forman, S.; et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat. Med. 2010, 16, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Olesch, C.; Brüne, B.; Weigert, A. Keep a Little Fire Burning-The Delicate Balance of Targeting Sphingosine-1-Phosphate in Cancer Immunity. Int. J. Mol. Sci. 2022, 23, 1289. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M. Role of Tissue-Resident Memory in Intra-Tumor Heterogeneity and Response to Immune Checkpoint Blockade. Front. Immunol. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Rathinasamy, A.; Domschke, C.; Ge, Y.; Böhm, H.H.; Dettling, S.; Jansen, D.; Lasitschka, F.; Umansky, L.; Gräler, M.H.; Hartmann, J.; et al. Tumor specific regulatory T cells in the bone marrow of breast cancer patients selectively upregulate the emigration receptor S1P1. Cancer Immunol. Immunother. 2017, 66, 593–603. [Google Scholar] [CrossRef]
- Priceman, S.J.; Shen, S.; Wang, L.; Deng, J.; Yue, C.; Kujawski, M.; Yu, H. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep. 2014, 6, 992–999. [Google Scholar] [CrossRef]
- Tsuchida, J.; Nagahashi, M.; Nakajima, M.; Katsuta, E.; Rashid, O.M.; Qi, Q.; Yan, L.; Okuda, S.; Takabe, K.; Wakai, T. Sphingosine Kinase 1 is Associated with Immune Cell-Related Gene Expressions in Human Breast Cancer. J. Surg. Res. 2020, 256, 645–656. [Google Scholar] [CrossRef]
- Ruckhaberle, E.; Rody, A.; Engels, K.; Gaetje, R.; von Minckwitz, G.; Schiffmann, S.; Grosch, S.; Geisslinger, G.; Holtrich, U.; Karn, T.; et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res. Treat. 2008, 112, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Ohotski, J.; Long, J.S.; Orange, C.; Elsberger, B.; Mallon, E.; Doughty, J.; Pyne, S.; Pyne, N.J.; Edwards, J. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br. J. Cancer 2012, 106, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Nagahashi, M.; Aoyagi, T.; Huang, W.C.; Lima, S.; Hait, N.C.; Maiti, A.; Kida, K.; Terracina, K.P.; Miyazaki, H.; et al. ABCC1-Exported Sphingosine-1-phosphate, Produced by Sphingosine Kinase 1, Shortens Survival of Mice and Patients with Breast Cancer. Mol. Cancer Res. 2018, 16, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, J.; Nagahashi, M.; Takabe, K.; Wakai, T. Clinical Impact of Sphingosine-1-Phosphate in Breast Cancer. Mediat. Inflamm. 2017, 2017, 2076239. [Google Scholar] [CrossRef] [PubMed]
- Maczis, M.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate and estrogen signaling in breast cancer. Adv. Biol. Regul. 2016, 60, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Edwards, J.; Watson, C.; Tovey, S.; Mair, K.M.; Schiff, R.; Natarajan, V.; Pyne, N.J.; Pyne, S. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol. Cell Biol. 2010, 30, 3827–3841. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Fujiwara, Y.; Edwards, J.; Tannahill, C.L.; Tigyi, G.; Pyne, S.; Pyne, N.J. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J. Biol. Chem. 2010, 285, 35957–35966. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Ling, Y.; Toshikawa, C.; Hayashida, T.; Kitagawa, Y.; Futamura, M.; Kuwayama, T.; Nakamura, S.; Yamauchi, H.; Yamauchi, T.; et al. Copy number alteration is an independent prognostic biomarker in triple-negative breast cancer patients. Breast Cancer 2023, 30, 584–595. [Google Scholar] [CrossRef]
- Maiti, A.; Takabe, K.; Hait, N.C. Metastatic triple-negative breast cancer is dependent on SphKs/S1P signaling for growth and survival. Cell Signal 2017, 32, 85–92. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A. The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer. Mediat. Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, M.; Tsuchida, J.; Nagahashi, M.; Takeuchi, S.; Moro, K.; Toshikawa, C.; Abe, S.; Ichikawa, H.; Shimada, Y.; Sakata, J.; et al. Plasma Sphingosine-1-Phosphate Levels Are Associated with Progression of Estrogen Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 13367. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, R.A. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br. J. Pharmacol. 2011, 162, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef]
- White, C.; Alshaker, H.; Cooper, C.; Winkler, M.; Pchejetski, D. The emerging role of FTY720 (Fingolimod) in cancer treatment. Oncotarget 2016, 7, 23106–23127. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.W.; Man, K.; Sun, C.K.; Lee, T.K.; Poon, R.T.; Fan, S.T. Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Mol. Cancer Ther. 2005, 4, 1430–1438. [Google Scholar] [CrossRef]
- Hait, N.C.; Avni, D.; Yamada, A.; Nagahashi, M.; Aoyagi, T.; Aoki, H.; Dumur, C.I.; Zelenko, Z.; Gallagher, E.J.; Leroith, D.; et al. The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis 2015, 4, e156. [Google Scholar] [CrossRef]
- Singh, S.K.; Spiegel, S. Sphingosine-1-phosphate signaling: A novel target for simultaneous adjuvant treatment of triple negative breast cancer and chemotherapy-induced neuropathic pain. Adv. Biol. Regul. 2020, 75, 100670. [Google Scholar] [CrossRef]
- Kolodziej, M.A.; Al Barim, B.; Nagl, J.; Weigand, M.A.; Uhl, E.; Uhle, F.; Di Fazio, P.; Schwarm, F.P.; Stein, M. Sphingosine-1-phosphate analogue FTY720 exhibits a potent anti-proliferative effect on glioblastoma cells. Int. J. Oncol. 2020, 57, 1039–1046. [Google Scholar] [CrossRef]
- Alshaker, H.; Wang, Q.; Srivats, S.; Chao, Y.; Cooper, C.; Pchejetski, D. New FTY720-docetaxel nanoparticle therapy overcomes FTY720-induced lymphopenia and inhibits metastatic breast tumour growth. Breast Cancer Res. Treat. 2017, 165, 531–543. [Google Scholar] [CrossRef]
- Gupta, P.; Taiyab, A.; Hussain, A.; Alajmi, M.F.; Islam, A.; Hassan, M.I. Targeting the Sphingosine Kinase/Sphingosine-1-Phosphate Signaling Axis in Drug Discovery for Cancer Therapy. Cancers 2021, 13, 1898. [Google Scholar] [CrossRef]
- Choi, D.; Stewart, A.P.; Bhat, S. Ozanimod: A First-in-Class Sphingosine 1-Phosphate Receptor Modulator for the Treatment of Ulcerative Colitis. Ann. Pharmacother. 2022, 56, 592–599. [Google Scholar] [CrossRef]
- Swallow, E.; Pham, T.; Patterson-Lomba, O.; Yin, L.; Gomez-Lievano, A.; Liu, J.; Tencer, T.; Gupte-Singh, K. Comparative efficacy and safety of ozanimod and ponesimod for relapsing multiple sclerosis: A matching-adjusted indirect comparison. Mult. Scler. Relat. Disord. 2023, 71, 104551. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T. Siponimod: First Global Approval. Drugs 2019, 79, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Alnaif, A.; Oiler, I.; D’Souza, M.S. Ponesimod: An Oral Second-Generation Selective Sphingosine 1-Phosphate Receptor Modulator for the Treatment of Multiple Sclerosis. Ann. Pharmacother. 2023, 57, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, A.; Vignini, A.; Alia, S.; Membrino, V.; Delli Carpini, G.; Giannella, L.; Ciavattini, A. Pathogenic Role of the Sphingosine 1-Phosphate (S1P) Pathway in Common Gynecologic Disorders (GDs): A Possible Novel Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 13538. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Zhang, C.; Ding, P.; Tian, S.; Chen, J.; Ji, G.; Wu, T. Sphingosine 1-phosphate receptor, a new therapeutic direction in different diseases. Biomed. Pharmacother. 2022, 153, 113341. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 2011, 69, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.A.; Weinstock-Guttman, B. Fingolimod: An oral disease-modifying therapy for relapsing multiple sclerosis. Adv. Ther. 2011, 28, 270–278. [Google Scholar] [CrossRef]
- Paugh, S.W.; Paugh, B.S.; Rahmani, M.; Kapitonov, D.; Almenara, J.A.; Kordula, T.; Milstien, S.; Adams, J.K.; Zipkin, R.E.; Grant, S.; et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 2008, 112, 1382–1391. [Google Scholar] [CrossRef]
- Hazar-Rethinam, M.; de Long, L.M.; Gannon, O.M.; Topkas, E.; Boros, S.; Vargas, A.C.; Dzienis, M.; Mukhopadhyay, P.; Simpson, F.; Endo-Munoz, L.; et al. A novel E2F/sphingosine kinase 1 axis regulates anthracycline response in squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Gao, D.; Fang, Z.Y. Targeting colorectal cancer cells by a novel sphingosine kinase 1 inhibitor PF-543. Biochem. Biophys. Res. Commun. 2016, 470, 728–734. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, N.; Volpert, G.; Al Washih, M.; Watson, D.G.; Futerman, A.H.; Kennedy, S.; Pyne, S.; Pyne, N.J. Effect of the sphingosine kinase 1 selective inhibitor, PF-543 on arterial and cardiac remodelling in a hypoxic model of pulmonary arterial hypertension. Cell Signal 2016, 28, 946–955. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, C.; Ren, Y.; Yao, F.; Duan, J.; Zhao, H.; Du, Y.; Xiao, X.; Duan, H.; Shi, Y. Sphingosine kinase 1 protects renal tubular epithelial cells from renal fibrosis via induction of autophagy. Int. J. Biochem. Cell Biol. 2017, 90, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, B. Sphk1 promotes ulcerative colitis via activating JAK2/STAT3 signaling pathway. Hum. Cell 2020, 33, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.M.; Mak, B.; Yeung, N.; Huynh, K.; Meikle, T.G.; Mellett, N.A.; Kwan, E.M.; Fettke, H.; Tran, B.; Davis, I.D.; et al. Overcoming enzalutamide resistance in metastatic prostate cancer by targeting sphingosine kinase. EBioMedicine 2021, 72, 103625. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Tamashiro, P.M.; Shimizu, Y.; Iino, K.; Peres, R.; Chen, R.; Sun, Y.; Hannun, Y.A.; Obeid, L.M.; Kawamori, T. Sphingosine Kinase 1 expression in peritoneal macrophages is required for colon carcinogenesis. Carcinogenesis 2017, 38, 1218–1227. [Google Scholar] [CrossRef]
- Alshaker, H.; Srivats, S.; Monteil, D.; Wang, Q.; Low, C.M.R.; Pchejetski, D. Field template-based design and biological evaluation of new sphingosine kinase 1 inhibitors. Breast Cancer Res. Treat. 2018, 172, 33–43. [Google Scholar] [CrossRef]
- Kang, Y.; Sundaramoorthy, P.; Gasparetto, C.; Feinberg, D.; Fan, S.; Long, G.; Sellars, E.; Garrett, A.; Tuchman, S.A.; Reeves, B.N.; et al. Phase I study of opaganib, an oral sphingosine kinase 2-specific inhibitor, in relapsed and/or refractory multiple myeloma. Ann. Hematol. 2023, 102, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A Phase I Study of ABC294640, a First-in-Class Sphingosine Kinase-2 Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 4642–4650. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.R.; Powell, J.A.; Coolen, C.; Moretti, P.A.; Zebol, J.R.; Pham, D.H.; Finnie, J.W.; Don, A.S.; Ebert, L.M.; Bonder, C.S.; et al. A selective ATP-competitive sphingosine kinase inhibitor demonstrates anti-cancer properties. Oncotarget 2015, 6, 7065–7083. [Google Scholar] [CrossRef] [PubMed]
- Santos, W.L.; Lynch, K.R. Drugging sphingosine kinases. ACS Chem. Biol. 2015, 10, 225–323. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Drabkin, H.A.; Reeves, J.A.; Hainsworth, J.D.; Hazel, S.E.; Paggiarino, D.A.; Wojciak, J.; Woodnutt, G.; Bhatt, R.S. A phase 2 study of the sphingosine-1-phosphate antibody sonepcizumab in patients with metastatic renal cell carcinoma. Cancer 2017, 123, 576–582. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Furuya, H.; Ng, M.L.; Friedemann, M.; Menschikowski, M.; Tarasov, V.V.; Chubarev, V.N.; Klochkov, S.G.; Neganova, M.E.; Mangoni, A.A.; et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020, 207, 107464. [Google Scholar] [CrossRef]
- McGowan, E.M.; Lin, Y.; Chen, S. Targeting Chronic Inflammation of the Digestive System in Cancer Prevention: Modulators of the Bioactive Sphingolipid Sphingosine-1-Phosphate Pathway. Cancers 2022, 14, 535. [Google Scholar] [CrossRef]
- Espinoza, K.S.; Snider, A.J. Therapeutic Potential for Sphingolipids in Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).