Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities
Abstract
1. Introduction
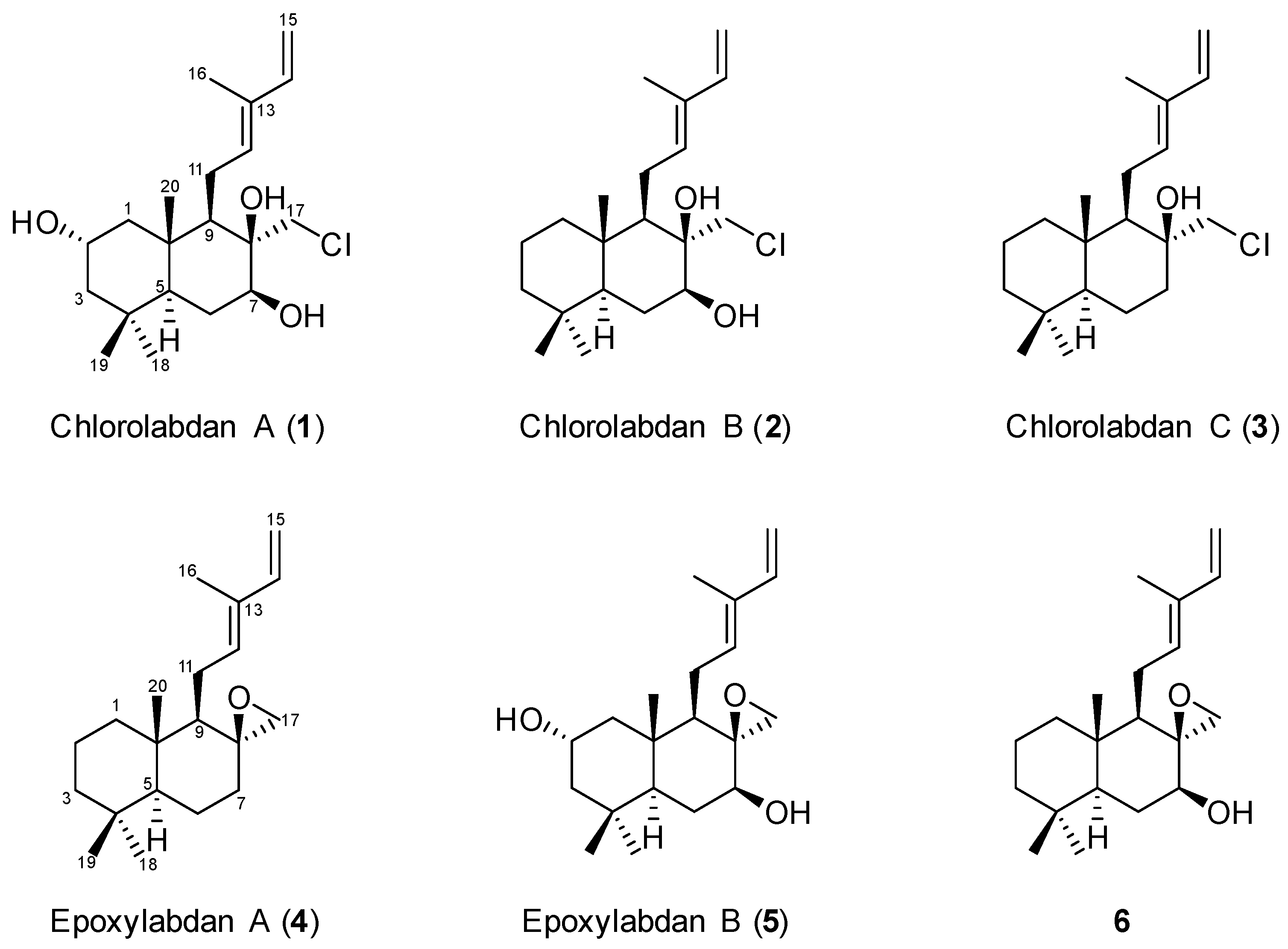
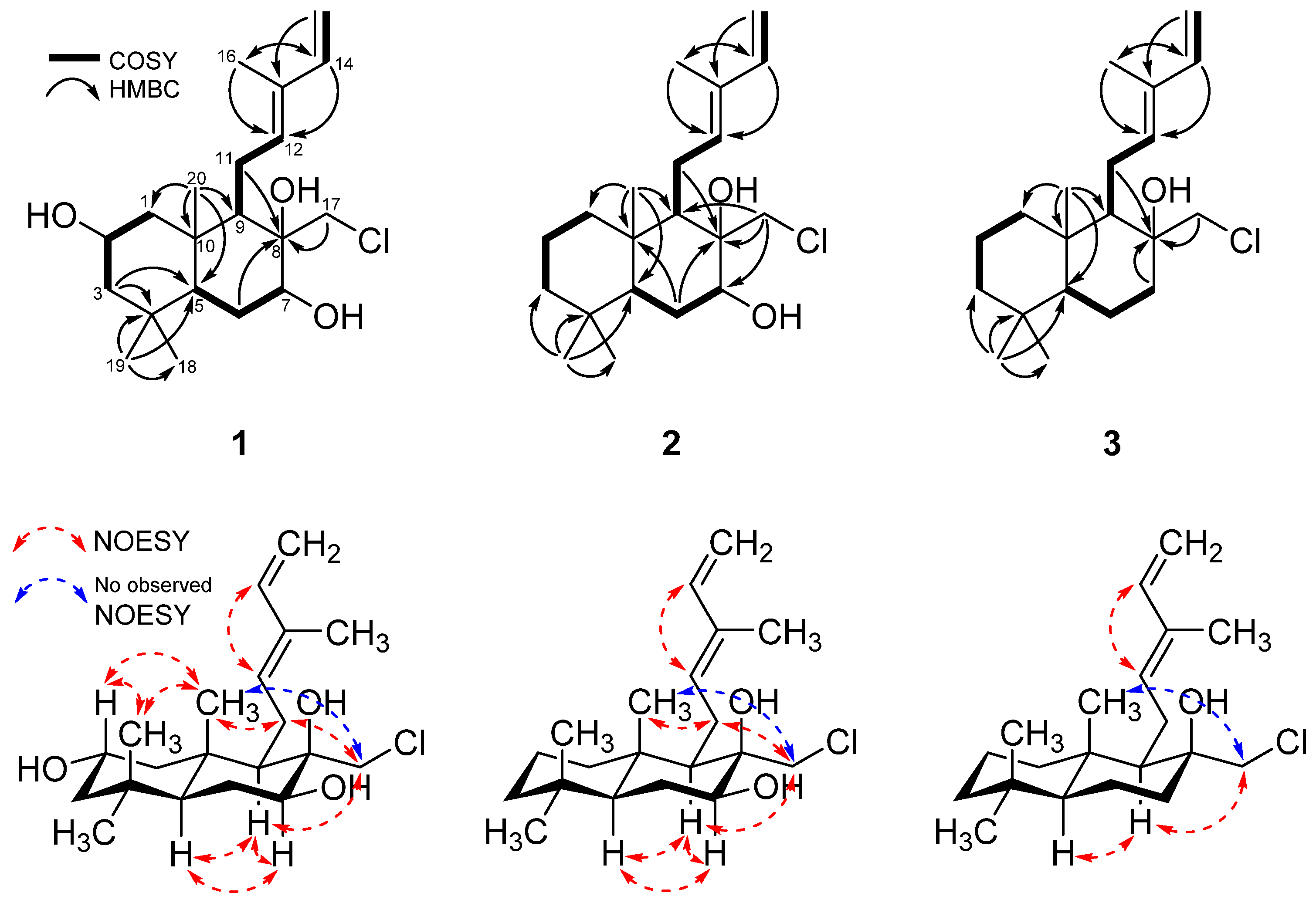
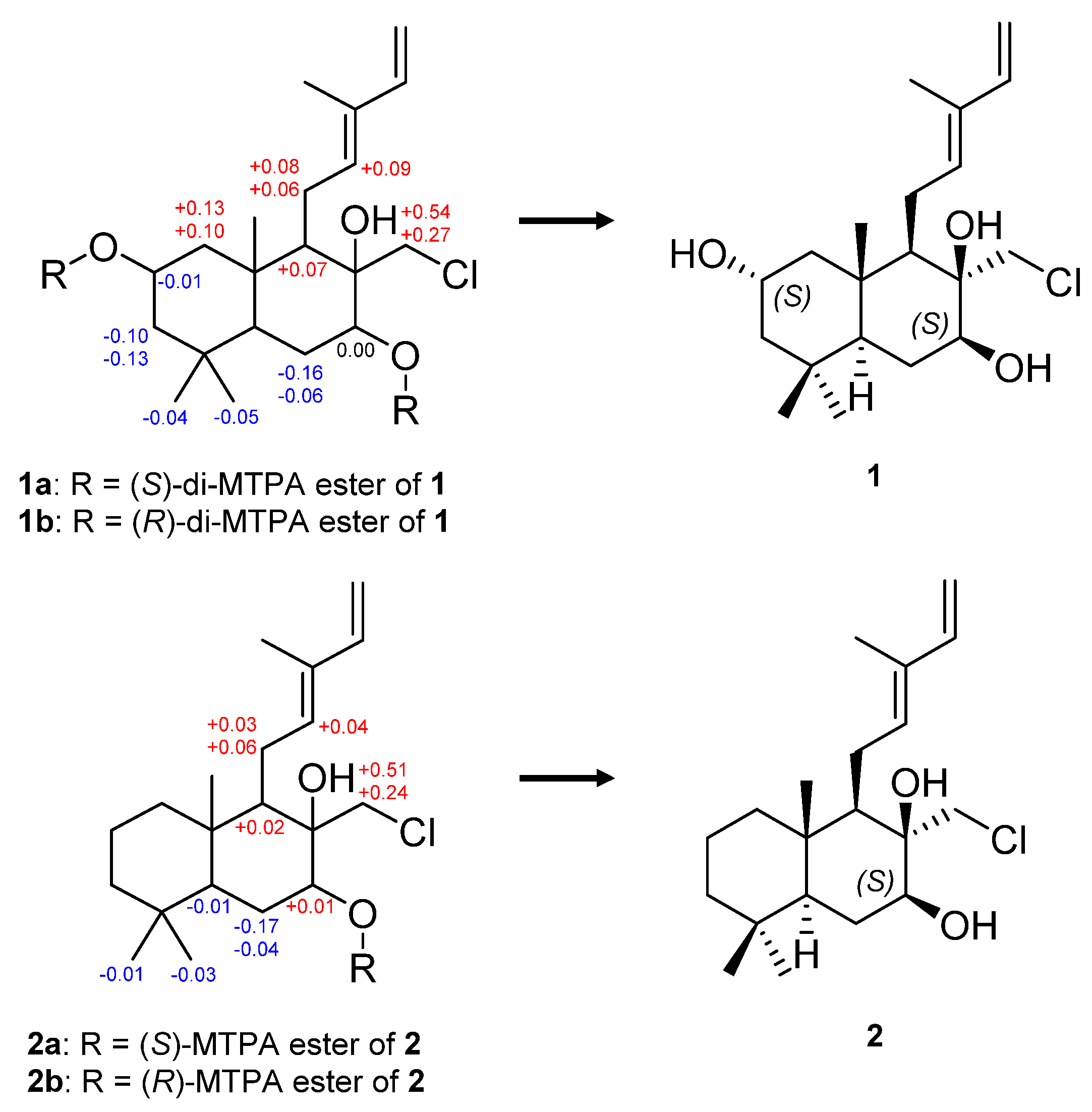
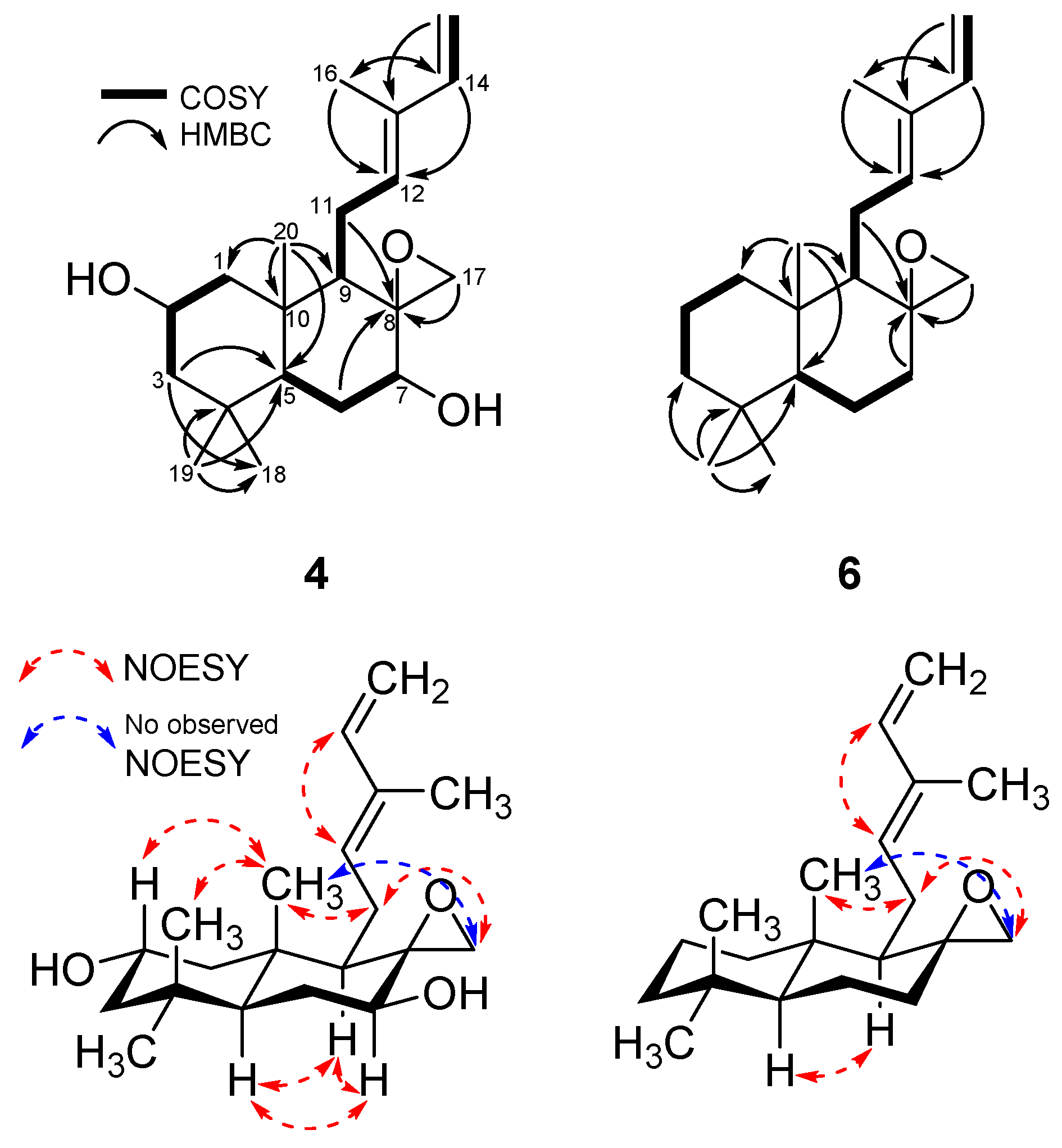
2. Results and Discussion
Bioactivities
3. Materials and Methods
3.1. General Experimental Procedures
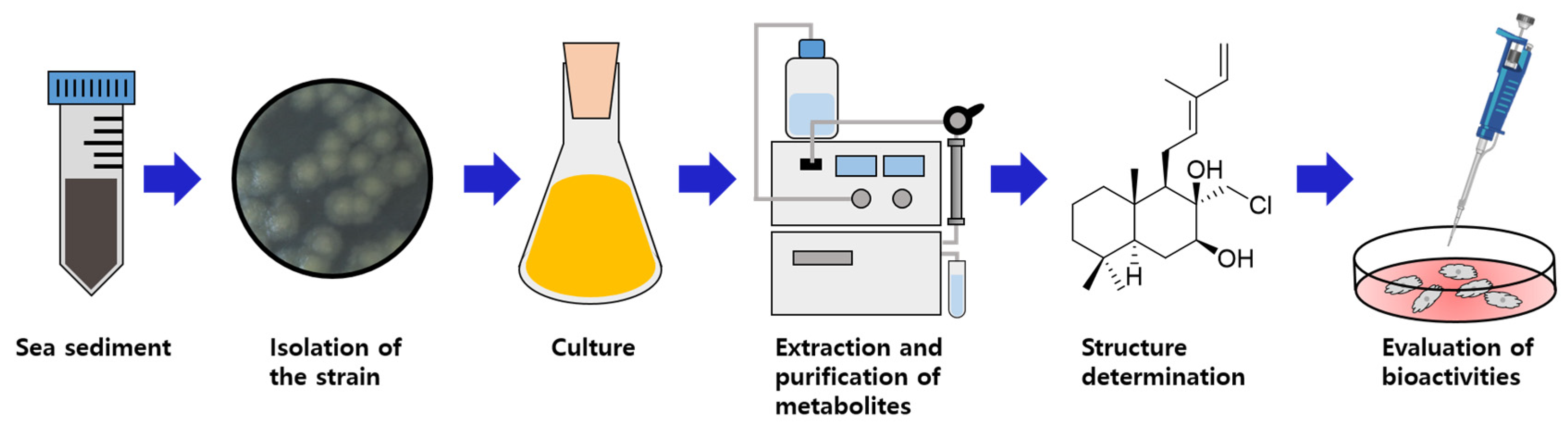
3.2. Isolation and Identification of Strain 2210JJ-087
3.3. Fermentation of Strain 2210JJ-087, Extraction, and Isolation of Metabolites
3.4. Preparations of the MTPA Ester of 1 and 2 Using the Modified Mosher’s Method
3.5. Antibacterial Assay
3.6. CellTiter-Glo (CTG) and Sulforhodamine B (SRB) Assay for Cytotoxicity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, C.-C.; Wei, W.-C.; Lin, L.-C. Diterpenoids and Bisnorditerpenoids from Blumea aromatica. J. Nat. Prod. 2019, 82, 3181–3185. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Shurpali, K.; Puranik, V.G.; Sarkar, D.; Joshi, S.P. Antimycobacterial Labdane Diterpenes from Leucas stelligera. J. Nat. Prod. 2013, 76, 1836–1841. [Google Scholar] [CrossRef]
- Hou, B.; Liu, S.; Huo, R.; Li, Y.; Ren, J.; Wang, W.; Wei, T.; Jiang, X.; Yin, W.; Liu, H.; et al. New Diterpenoids and Isocoumarin Derivatives from the Mangrove-Derived Fungus Hypoxylon sp. Mar. Drugs 2021, 19, 362. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, W.; Fan, R.; Han, S.; Li, Y.; Cui, X.; Zhang, J.; Wu, Y.; Lv, X.; Zhang, Y.; et al. Terpenoids from the Deep-Sea-Derived Fungus Penicillium thomii YPGA3 and Their Bioactivities. Mar. Drugs 2020, 18, 164. [Google Scholar] [CrossRef]
- Ohyoshi, T.; Zhao, Y.; Kigoshi, H. Isolation and Synthesis of Azuriaplysins A and B, Bromoditerpenes with an α-Methylene Carbonyl from the Sea Hare Aplysia kurodai. J. Nat. Prod. 2022, 85, 2082–2089. [Google Scholar] [CrossRef]
- Van Wyk, A.W.W.; Gray, C.A.; Whibley, C.E.; Osoniyi, O.; Hendricks, D.T.; Caira, M.R.; Davies-Coleman, M.T. Bioactive Metabolites from the South African Marine Mollusk Trimusculus costatus. J. Nat. Prod. 2008, 71, 420–425. [Google Scholar] [CrossRef]
- Manse, Y.; Ninomiya, K.; Nishi, R.; Hashimoto, Y.; Chaipech, S.; Muraoka, O.; Morikawa, T. Labdane-Type Diterpenes, Galangalditerpenes A-C, with Melanogenesis Inhibitory Activity from the Fruit of Alpinia galanga. Molecules 2017, 22, 2279. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C.; Dimas, K.S. Labdane-type diterpenes: Chemistry and biological activity. Stud. Nat. Prod. Chem 2001, 25, 235–292. [Google Scholar]
- Yamada, Y.; Komatsu, M.; Ikeda, H. Chemical diversity of labdane-type bicyclic diterpene biosynthesis in Actinomycetales microorganisms. J. Antibiot. 2016, 69, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Günnewich, N.; Higashi, Y.; Feng, X.; Choi, K.-B.; Schmidt, J.; Kutchan, T.M. A diterpene synthase from the clary sage Salvia sclarea catalyzes the cyclization of geranylgeranyl diphosphate to (8R)-hydroxy-copalyl diphosphate. Phytochemistry 2013, 91, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, C.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Extending the Structural Diversity of Labdane Diterpenoids from Marine-Derived Fungus Talaromyces sp. HDN151403 Using Heterologous Expression. Mar. Drugs 2023, 21, 628. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane diterpenoids as potential anti-inflammatory agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Ahmed, A.F.; Chao, C.-H.; Yen, C.-H.; Hwang, T.-L.; Chang, F.-R.; Huang, Y.M.; Sheu, J.-H. The Chemically Highly Diversified Metabolites from the Red Sea Marine Sponge Spongia sp. Mar. Drugs 2022, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Chini, M.G.; Parisi, V.; Mensitieri, F.; Malafronte, N.; Bifulco, G.; Bisio, A.; De Tommasi, N.; Bader, A. Diterpenoid Constituents of Psiadia punctulata and Evaluation of Their Antimicrobial Activity. J. Nat. Prod. 2022, 85, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Alilou, M.; Marzocco, S.; Hofer, D.; Rapa, S.F.; Asadpour, R.; Schwaiger, S.; Troppmair, J.; Stuppner, H. Labdane-Type Diterpenes from the Aerial Parts of Rydingia persica: Their Absolute Configurations and Protective Effects on LPS-Induced Inflammation in Keratinocytes. J. Nat. Prod. 2020, 83, 2456–2468. [Google Scholar] [CrossRef]
- Pal, M.; Mishra, T.; Kumar, A.; Tewari, S. Biological Evaluation of Terrestrial and Marine Plant Originated Labdane Diterpenes (A Review). Pharm. Chem. J. 2016, 50, 558–568. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, H.; Wang, R.; Zhou, K.; Jing, Y.; Qiu, F. ent-Labdane Diterpenoid Lactone Stereoisomers from Andrographis paniculata. J. Nat. Prod. 2008, 71, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Carullo, G.; Schiano Moriello, A.; Amodeo, P.; Di Marzo, V.; Vega-Holm, M.; Vitale, R.M.; Aiello, F.; Brizzi, A.; De Petrocellis, L. Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes. Mar. Drugs 2020, 18, 519. [Google Scholar] [CrossRef]
- Fouillaud, M.; Dufossé, L. Microbial Secondary Metabolism and Biotechnology. Microorganisms 2022, 10, 123. [Google Scholar] [CrossRef]
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the Biggest Producer of New Natural Secondary Metabolites, a Current Perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Ohtawa, M.; Hishinuma, Y.; Takagi, E.; Yamada, T.; Ito, F.; Arima, S.; Uchida, R.; Kim, Y.P.; Ōmura, S.; Tomoda, H.; et al. Synthesis and Structural Revision of Cyslabdan. Chem. Pharm. Bull. 2016, 64, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Z.; Tang, C.; Quinn, R.J.; Feng, Y.; Ke, C.-Q.; Yao, S.; Ye, Y. ent-Labdane Diterpenes from the Stems of Mallotus japonicus. J. Nat. Prod. 2013, 76, 1580–1585. [Google Scholar] [CrossRef]
- Shen, Y.-H.; Li, R.-T.; Xiao, W.-L.; Xu, G.; Lin, Z.-W.; Zhao, Q.-S.; Sun, H.-D. ent-Labdane Diterpenoids from Andrographis paniculata. J. Nat. Prod. 2006, 69, 319–322. [Google Scholar] [CrossRef]
- Carman, R. Optical rotation and structure in the labdane series of diterpenoids. Aust. J. Chem. 1966, 19, 629–642. [Google Scholar] [CrossRef]
- Ikeda, H.; Shin-ya, K.; Nagamitsu, T.; Tomoda, H. Biosynthesis of mercapturic acid derivative of the labdane-type diterpene, cyslabdan that potentiates imipenem activity against methicillin-resistant Staphylococcus aureus: Cyslabdan is generated by mycothiol-mediated xenobiotic detoxification. J. Ind. Microbiol. Biotechnol. 2016, 43, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Kim, Y.P.; Hanaki, H.; Shiomi, K.; Tomoda, H.; Omura, S. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04-0144. II. Biological activities. J. Antibiot. 2008, 61, 7–10. [Google Scholar] [CrossRef]
- Gaspar-Marques, C.; Simões, M.F.; Duarte, A.; Rodríguez, B. Labdane and kaurane diterpenoids from Plectranthus fruticosus. J. Nat. Prod. 2003, 66, 491–496. [Google Scholar] [CrossRef]
- Voon, K.J.; Sivasothy, Y.; Sundralingam, U.; Lalmahomed, A.; Goh, A.P.-T. Cytotoxic Labdane Diterpenes, Norlabdane Diterpenes and Bis-Labdanic Diterpenes from the Zingiberaceae: A Systematic Review. Pharmaceuticals 2022, 15, 1517. [Google Scholar] [CrossRef]
- Kumrit, I.; Suksamrarn, A.; Meepawpan, P.; Songsri, S.; Nuntawong, N. Labdane-type diterpenes from Hedychium gardnerianum with potent cytotoxicity against human small cell lung cancer cells. Phytother. Res. 2010, 24, 1009–1013. [Google Scholar] [CrossRef]
- Songsri, S.; Nuntawong, N. Cytotoxic Labdane Diterpenes from Hedychium ellipticum Buch.-Ham. ex Sm. Molecules 2016, 21, 749. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Choi, B.-K.; Trinh, P.T.H.; Lee, H.-S.; Choi, B.-W.; Kang, J.S.; Ngoc, N.T.D.; Van, T.T.T.; Shin, H.J. New Ophiobolin Derivatives from the Marine Fungus Aspergillus flocculosus and Their Cytotoxicities against Cancer Cells. Mar. Drugs 2019, 17, 346. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Cho, H.; Lee, D.K.; Ha, J.; Choi, B.J.; Jeong, J.H.; Ryu, J.-H.; Kang, J.S.; Jeon, R. Discovery of 5-Phenoxy-2-aminopyridine Derivatives as Potent and Selective Irreversible Inhibitors of Bruton’s Tyrosine Kinase. Int. J. Mol. Sci. 2020, 21, 8006. [Google Scholar] [CrossRef] [PubMed]
| No | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | δC, Type | |
| 1a | 0.81, dd (12.0, 2.0) | 49.9, CH2 | 0.90, ol | 41.1, CH2 | 0.91, ol | 41.0, CH2 |
| 1b | 2.03, m | 1.72, ol | 1.73, ol | |||
| 2a | 3.82, m | 65.1, CH | 1.42, ol | 19.4, CH2 | 1.41, ol | 19.4, CH2 |
| 2b | 1.64, m | 1.63, m | ||||
| 3a | 1.12, m | 51.6, CH2 | 1.21, m | 43.0, CH2 | 1.19, m | 43.1, CH2 |
| 3b | 1.75, ol | 1.42, ol | 1.40, ol | |||
| 4 | 35.5, C | 34.1, C | 34.2, C | |||
| 5 | 0.88, dd (12.4, 1.8) | 53.1, CH | 0.90, ol | 53.6, CH | 0.89, ol | 57.0, CH |
| 6a | 1.61, dd (12.4, 11.9) | 27.4, CH2 | 1.61, m | 27.6, CH2 | 1.56, m | 18.9, CH2 |
| 6b | 1.75, ol | 1.73, ol | 1.61, m | |||
| 7a | 3.81, ol | 71.0, CH | 3.79, dd (11.6, 5.1) | 71.1, CH | 1.71, ol | 37.9, CH2 |
| 7b | 1.80, m | |||||
| 8 | 77.9, C | 78.0, C | 75.7, C | |||
| 9 | 1.50, dd (6.3, 2.5) | 53.0, CH | 1.46, dd (6.4, 2.5) | 53.1, CH | 1.33, dd (6.1, 2.6) | 56.1, CH |
| 10 | 41.2, C | 39.7, C | 40.1, C | |||
| 11a | 2.19, dd (16.7, 4.8) | 24.2, CH2 | 2.14, dd (17.6, 4.3) | 24.2, CH2 | 2.13, dd (18.0, 4.4) | 24.4, CH2 |
| 11b | 2.52, m | 2.47, m | 2.38, m | |||
| 12 | 5.58, t (6.9) | 136.5, CH | 5.56, t (6.8) | 136.9, CH | 5.48, t (6.8) | 137.1, CH |
| 13 | 133.8, C | 133.5, C | 133.6, C | |||
| 14 | 6.33, dd (17.4, 10.8) | 142.9, CH | 6.33, dd (17.4, 10.8) | 142.9, CH | 6.33, dd (17.4, 10.7) | 142.9, CH |
| 15a | 4.87, ol | 110.4, CH2 | 4.87, d (10.7) | 110.3, CH2 | 4.87, ol | 110.4, CH2 |
| 15b | 5.06, d (17.4) | 5.05, d (17.4) | 5.05, d (17.4) | |||
| 16 | 1.79, s | 12.0, CH3 | 1.76, s | 12.0, CH3 | 1.76, s | 12.0, CH3 |
| 17a | 3.28, d (10.8) | 45.6, CH2 | 3.27, d (10.7) | 45.7, CH2 | 3.23, d (10.9) | 53.4, CH2 |
| 17b | 3.60, d (10.8) | 3.59, d (10.7) | 3.38, d (10.9) | |||
| 18 | 0.93, s | 23.1, CH3 | 0.89, s | 22.3, CH3 | 0.87, s | 22.2, CH3 |
| 19 | 0.99, s | 34.1, CH3 | 0.93, s | 33.9, CH3 | 0.90, s | 34.0, CH3 |
| 20 | 1.06, s | 16.8, CH3 | 1.01, s | 15.8, CH3 | 1.03, s | 15.8, CH3 |
| No | 4 | 5 | ||
|---|---|---|---|---|
| δH, Mult (J in Hz) | δC, Type | δH, Mult (J in Hz) | δC, Type | |
| 1a | 1.02, td (13.0, 3.4) | 40.3, CH2 | 0.90, ol | 49.6, CH2 |
| 1b | 1.84, ol | 2.09, ol | ||
| 2a | 1.46, m | 19.7, CH2 | 3.85, m | 65.2, CH |
| 2b | 1.64, m | |||
| 3a | 1.23, td (13.3, 3.8) | 43.1, CH2 | 1.16, d (13.2) | 51.6, CH2 |
| 3b | 1.43, m | 1.77, m | ||
| 4 | 34.4, C | 35.7, C | ||
| 5 | 1.10, dd (12.4, 2.6) | 56.2, CH | 1.13, dd (12.2, 2.0) | 53.3, CH |
| 6a | 1.61, m | 21.2, CH2 | 1.53, m, CH2 | 30.5, CH2 |
| 6b | 1.71, m | 1.97, m | ||
| 7a | 1.31, m | 37.1, CH2 | 3.72, dd (11.7, 5.0) | 70.3, CH |
| 7b | 1.97, td (13.7, 5.0) | |||
| 8 | 59.3, C | 60.9, C | ||
| 9 | 1.58, m | 54.4, CH | 1.58, m | 53.2, CH |
| 10 | 40.9, C | 42.2, C | ||
| 11a | 1.73, m | 21.6, CH2 | 1.86, m | 21.7, CH2 |
| 11b | 2.04, dd (16.7, 5.4) | 2.12, m | ||
| 12 | 5.34, t (6.8) | 136.2, CH | 5.36, t (6.8) | 135.5, CH |
| 13 | 134.7, C | 135.1, C | ||
| 14 | 6.32, dd (17.4, 10.8) | 142.5, CH | 6.34, dd (17.4, 10.7) | 142.4, CH |
| 15a | 4.88, ol | 110.7, CH2 | 4.89, ol | 110.9, CH2 |
| 15b | 5.05, d (17.4) | 5.08, d (17.4) | ||
| 16 | 1.70, s | 12.1, CH3 | 1.73, s | 12.2, CH3 |
| 17a | 2.26, d (4.3) | 50.0, CH2 | 2.41, d (4.8) | 45.0, CH2 |
| 17b | 2.56, d (4.3) | 2.81, d (4.8) | ||
| 18 | 0.89, s | 22.2, CH3 | 0.94, s | 23.0, CH3 |
| 19 | 0.92, s | 34.0, CH3 | 1.00, s | 34.0, CH3 |
| 20 | 0.93, s | 15.2, CH3 | 0.96, s | 16.3, CH3 |
| Strains | MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Kanamycin | |
| Bacillus subtilis | >128 | 4 | 32 | >128 | >128 | 32 | 0.5 |
| Micrococcus luteus | >128 | 8 | >128 | >128 | >128 | >128 | 0.5 |
| Staphylococcus aureus | >128 | 8 | >128 | >128 | >128 | >128 | 1 |
| Cell Lines | IC50 (µM) | |||
|---|---|---|---|---|
| 1 | 2 | 4 | Doxorubicin | |
| HL-60 | >30 | >30 | >30 | 0.018 |
| Raji | 15.520 | 3.406 | 17.110 | 0.011 |
| WSU-DLCL2 | >30 | 1.182 | 14.170 | 0.006 |
| NALM6 C. G5 | >30 | 22.460 | >30 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, C.-S.; Kang, J.S.; Yang, J.-W.; Lee, M.A.; Lee, H.-S.; Shin, H.J. Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities. Int. J. Mol. Sci. 2024, 25, 3311. https://doi.org/10.3390/ijms25063311
Heo C-S, Kang JS, Yang J-W, Lee MA, Lee H-S, Shin HJ. Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities. International Journal of Molecular Sciences. 2024; 25(6):3311. https://doi.org/10.3390/ijms25063311
Chicago/Turabian StyleHeo, Chang-Su, Jong Soon Kang, Jeong-Wook Yang, Min Ah Lee, Hwa-Sun Lee, and Hee Jae Shin. 2024. "Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities" International Journal of Molecular Sciences 25, no. 6: 3311. https://doi.org/10.3390/ijms25063311
APA StyleHeo, C.-S., Kang, J. S., Yang, J.-W., Lee, M. A., Lee, H.-S., & Shin, H. J. (2024). Labdane-Type Diterpenoids from Streptomyces griseorubens and Their Antimicrobial and Cytotoxic Activities. International Journal of Molecular Sciences, 25(6), 3311. https://doi.org/10.3390/ijms25063311





