Dihydropashanone Isolated from Lindera erythrocarpa, a Potential Natural Product for the Treatment of Neurodegenerative Diseases
Abstract
1. Introduction
2. Results
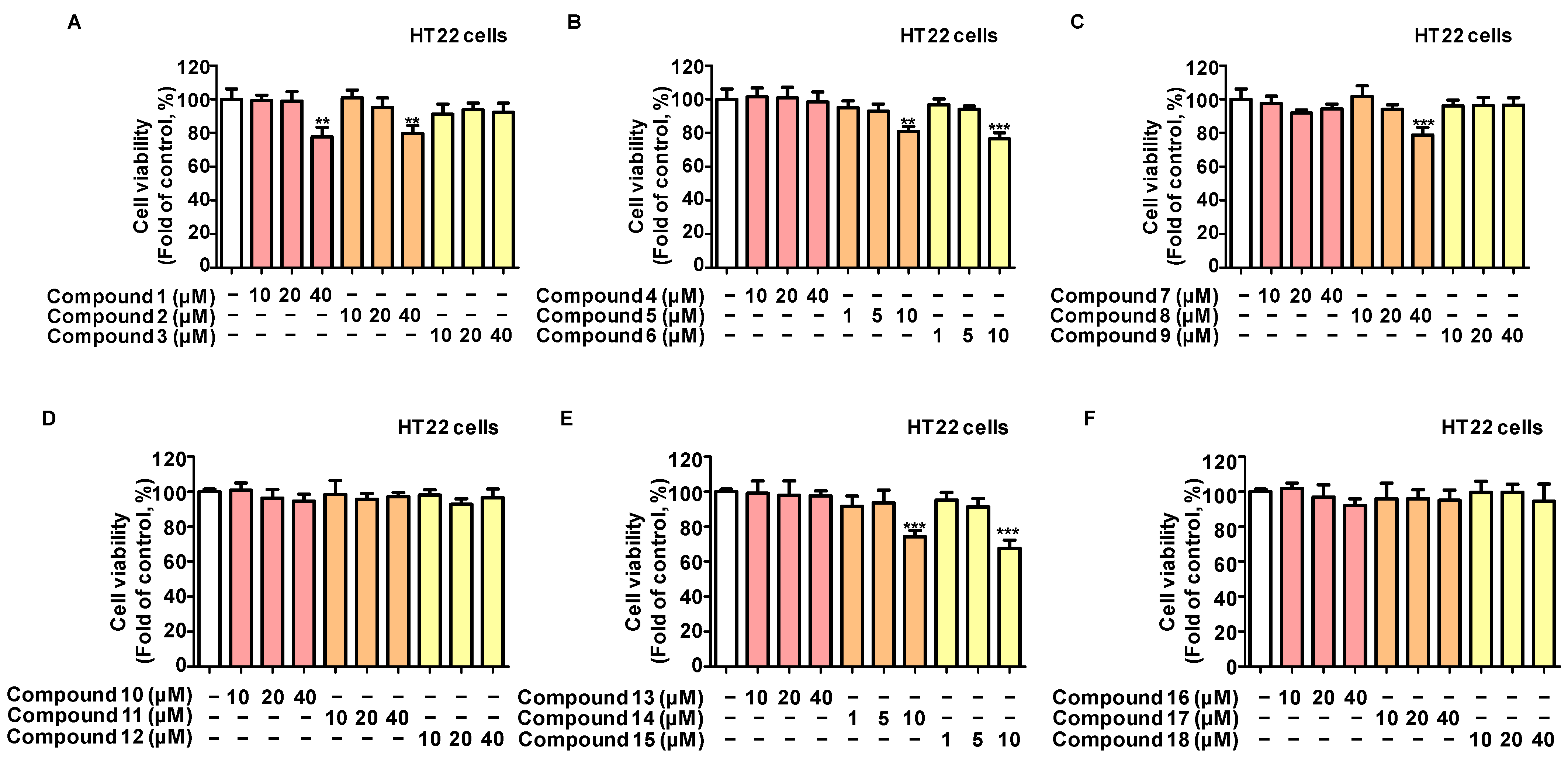
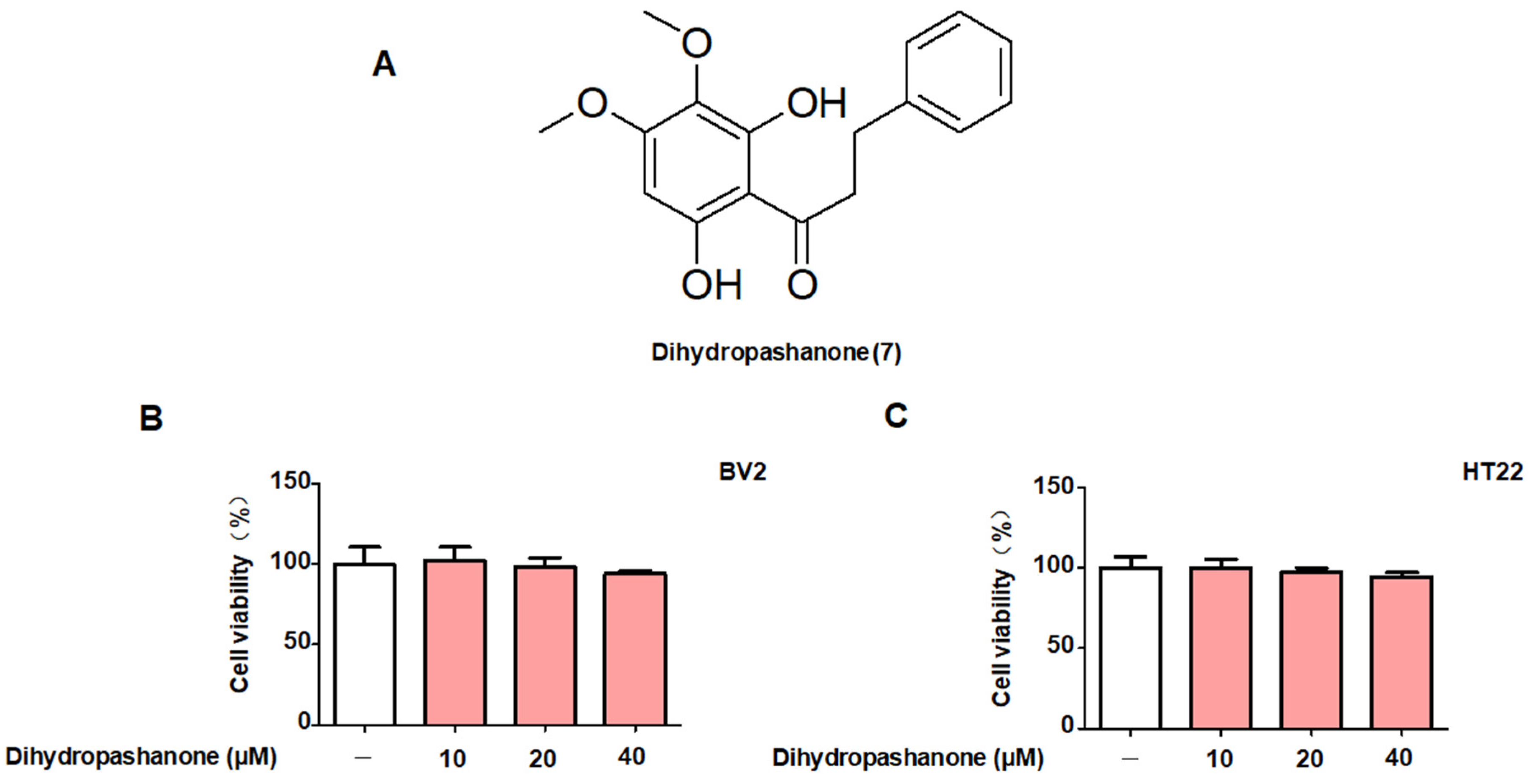
2.1. Protective Effects of the 18 Compounds Isolated from L. erythrocarpa on HT22 Cells
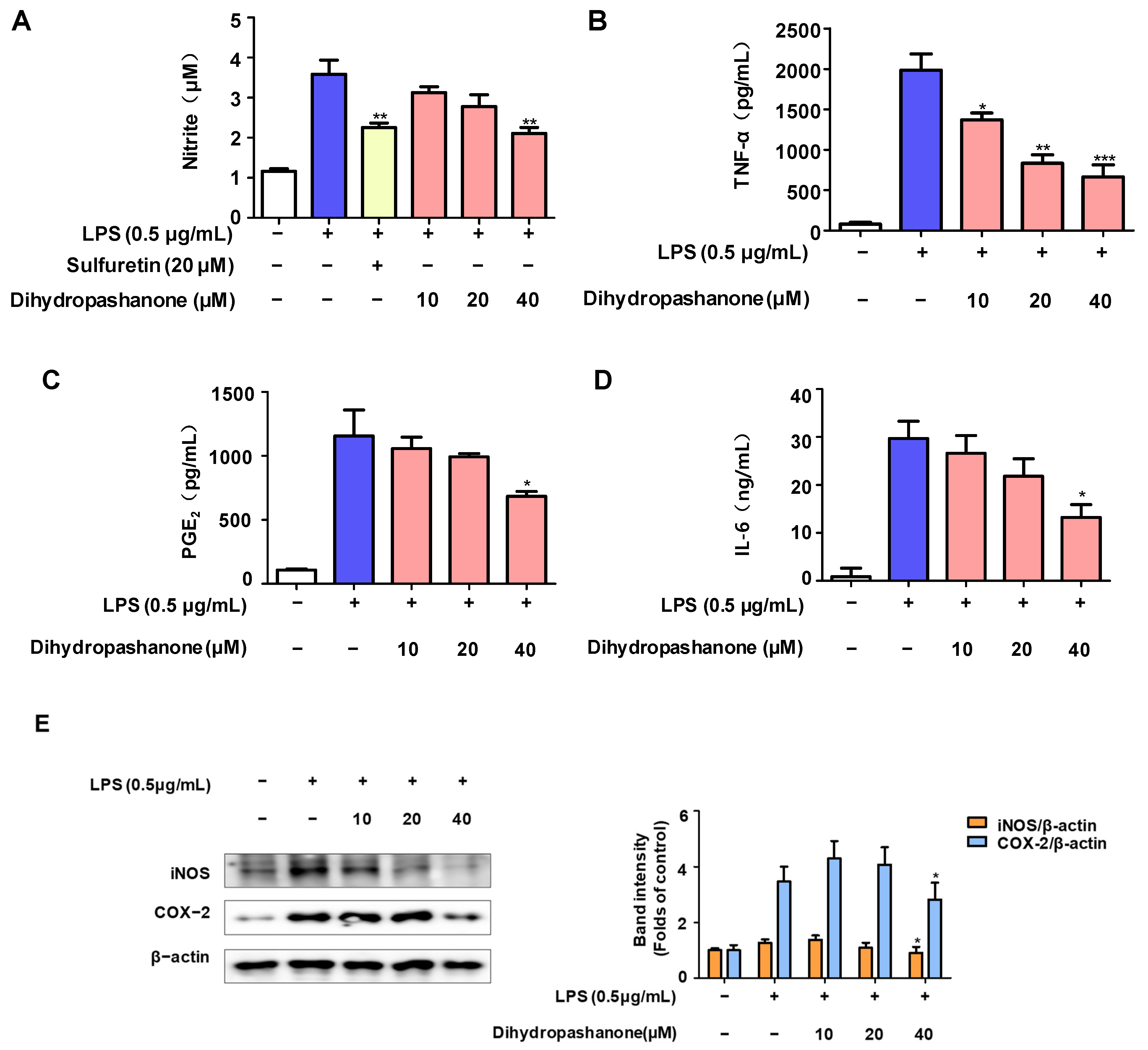
2.2. Effects of Dihydropashanone on Inflammatory Response in BV2 Cells
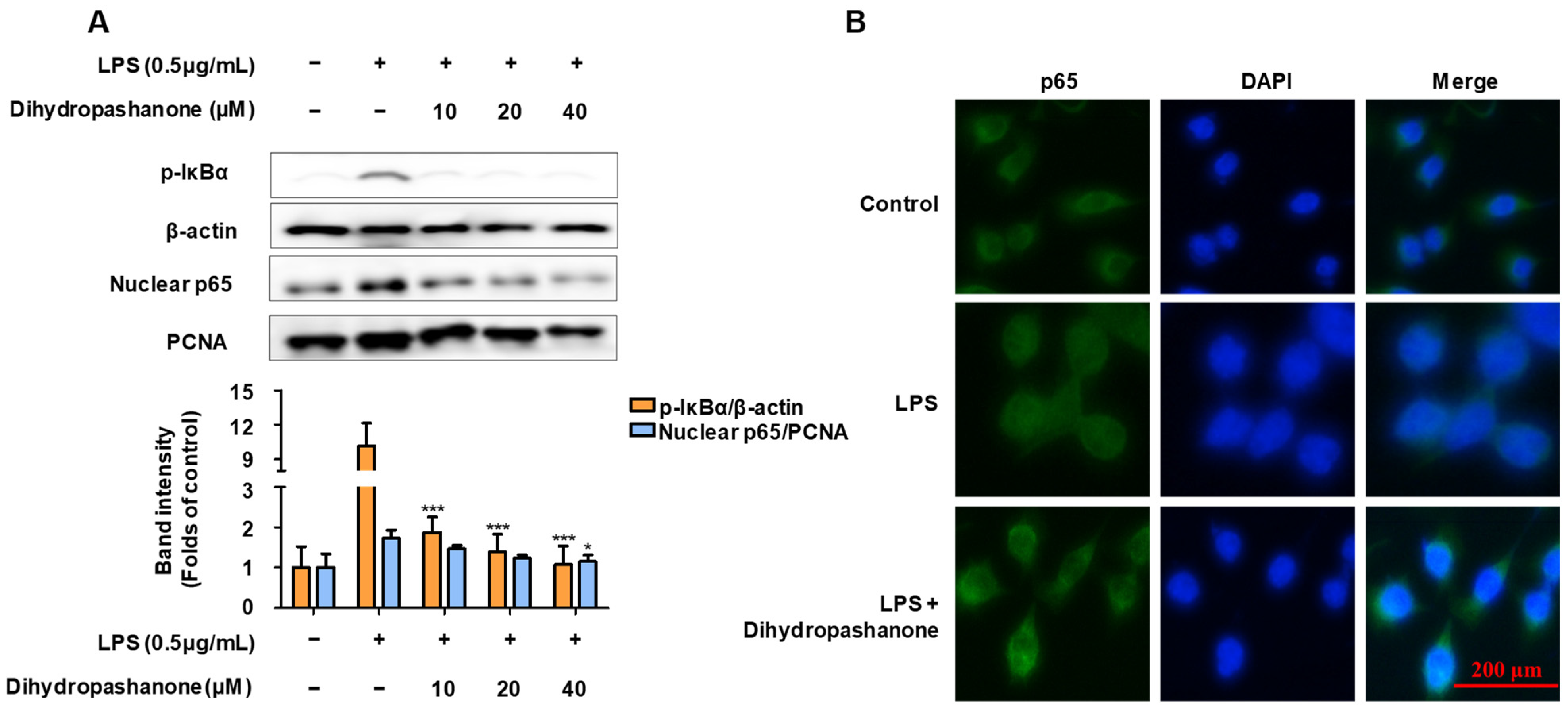
2.3. Effects of Dihydropashanone on the Regulation of NF-κB Pathway in BV2 Cells
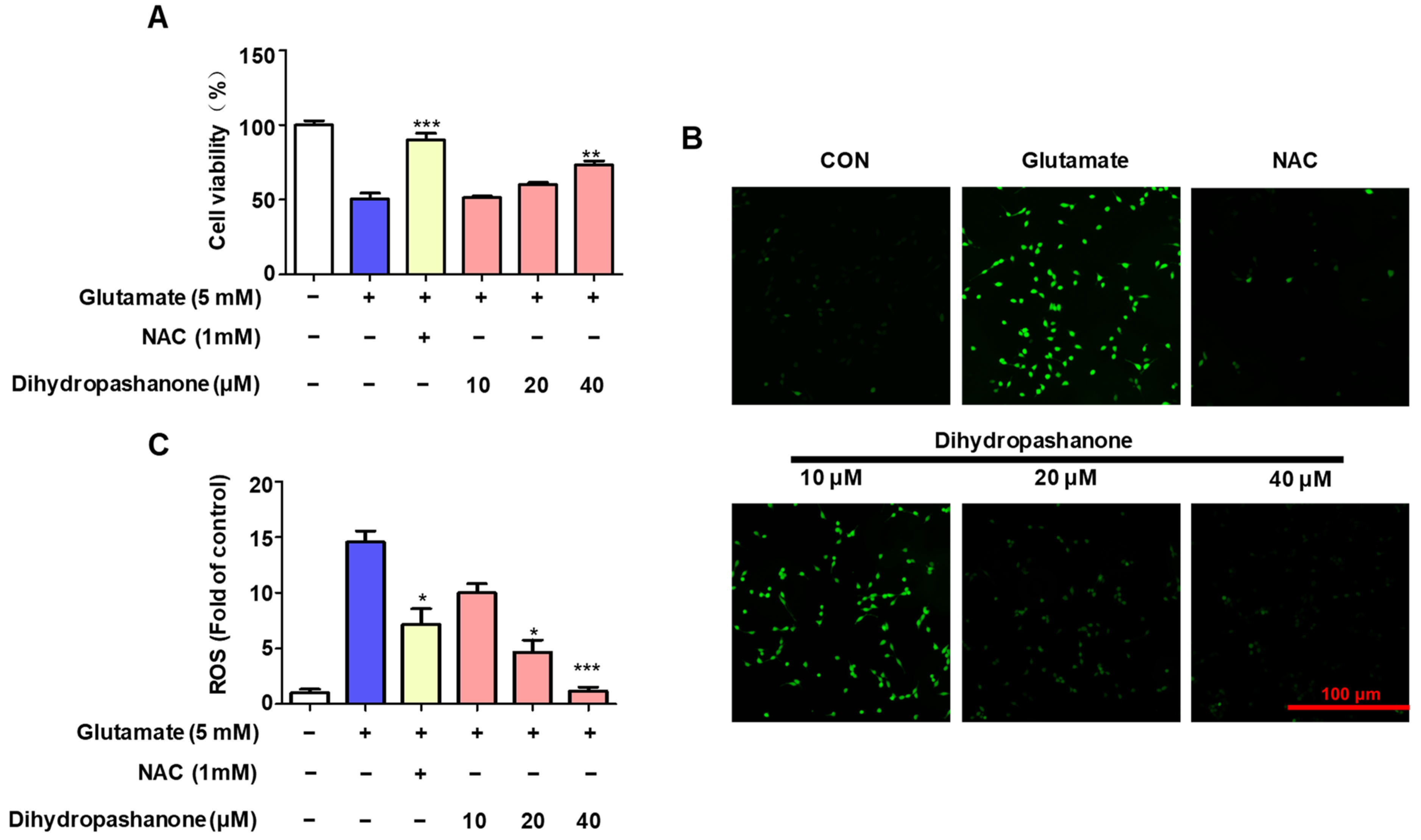
2.4. Effects of Dihydropashanone on Glutamate-Induced HT22 Cell Damage
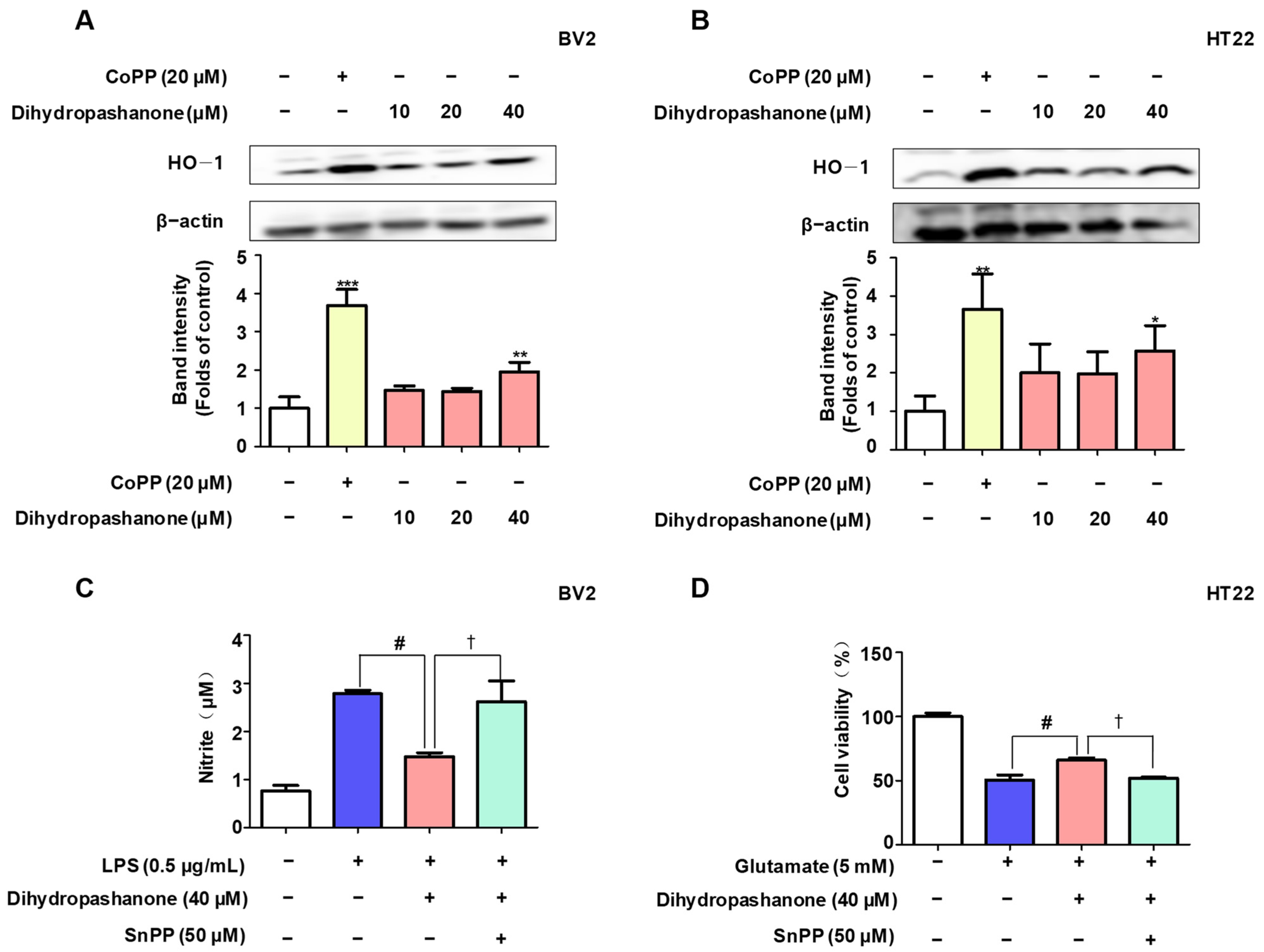
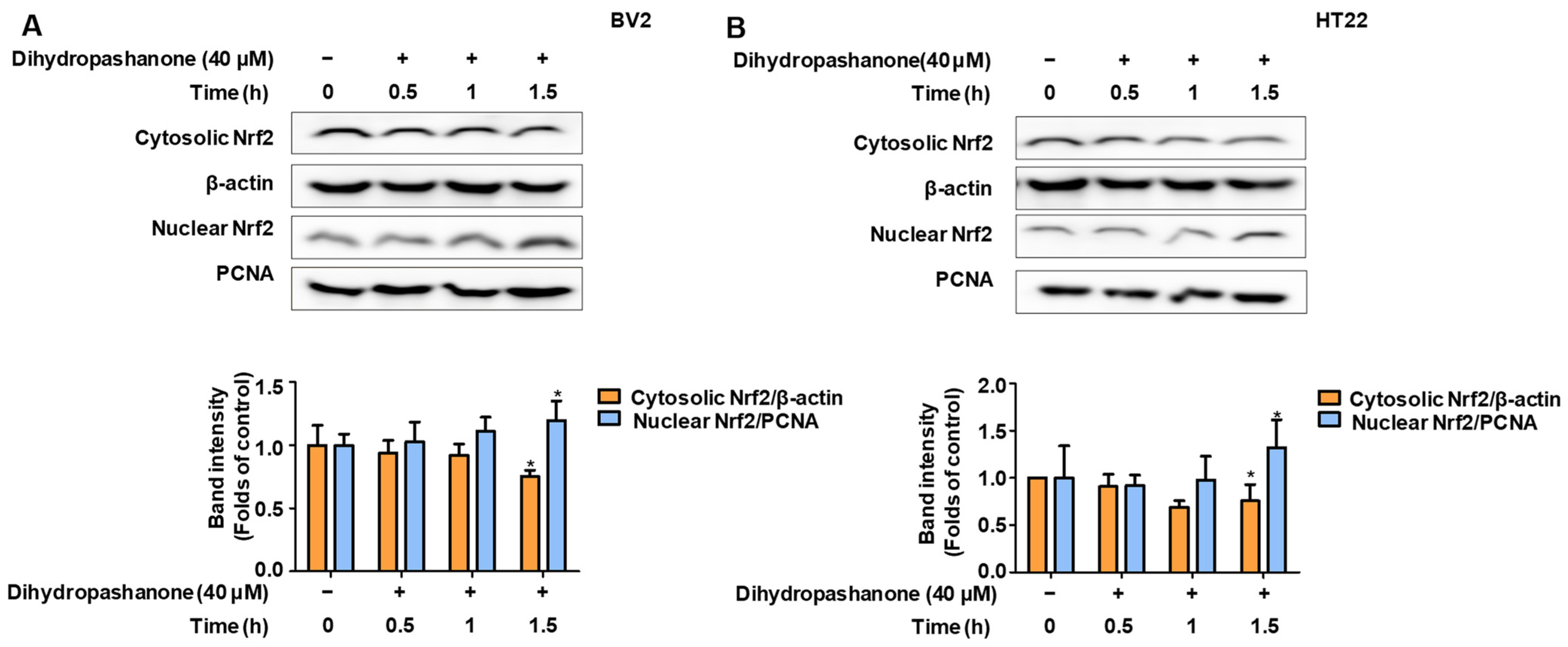
2.5. Effects of Dihydropashanone on Regulation of Nrf2/HO-1 Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Cell Viability Assays
4.3. Measurement of NO Generation
4.4. ELISA
4.5. Western Blot Analysis
4.6. NF-κB (p65) Localization
4.7. Determination of ROS Levels in HT22 Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoshida, K.; Kato, D.; Sugio, S.; Takeda, I.; Wake, H. Activity-dependent oligodendrocyte calcium dynamics and their changes in Alzheimer’s disease. Front. Cell. Neurosci. 2023, 17, 1154196. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Z.; Quan, Z.; Lu, Y.; Ren, Y.; He, Y. PEDF Prevents Mitochondrial Function Decay and ER Stress Induced by Rotenone in Aging RPE Cells. Front. Biosci. Landmark 2023, 28, 319. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Gangwar, P.; Yadav, A.; Yadav, B.; Rao, R.; Kaur, S.; Kumar, P.; Dhiman, M.; Taglialatela, G.; Mantha, A.K. Understanding the neuronal synapse and challenges associated with the mitochondrial dysfunction in mild cognitive impairment and Alzheimer’s disease. Mitochondrion 2023, 73, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tuo, P.; Zhang, W.; Wang, S. Inhibition of the TLR4/NF-κB pathway promotes the polarization of LPS-induced BV2 microglia toward the M2 phenotype. NeuroReport 2023, 34, 834–844. [Google Scholar] [CrossRef]
- Jing, S.; Wang, X.; Zhang, Z.; Cao, D.; Huang, K.; Wang, Y.; Liu, Z.; Su, S.; Wang, Q. Hesperetin attenuates cognitive dysfunction via SIRT6/NLRP3 pathway in scopolamine-induced mice. Metab. Brain Dis. 2023, 38, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.; Rosario-Claudio, J.; Sim, J.; Inyang, K.E.; Dagenais, A.; Monahan, K.; Lee, B.; Ramakrishnan, H.; Parmar, V.; Geron, M.; et al. Interleukin-10 signaling in somatosensory neurons controls CCL2 release and inflammatory response. Brain Behav. Immun. 2024, 116, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ma, Y.; Ma, Y.; Wang, Y.; Zhao, X.; Jin, D.; Xu, L.; Ge, S. Rutin prevents pyroptosis and M1 microglia via Nrf2/Mac-1/caspase-1-mediated inflammasome axis to improve POCD. Int. Immunopharmacol. 2023, 127, 111290. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Wang, X.; Pan, H.; Yan, C. Baicalein promotes the microglia M2 polarization and suppresses apoptosis by targeting HMOX1/PDE4D to alleviate Alzheimer’s disease. Immunobiology 2023, 228, 152761. [Google Scholar] [CrossRef]
- Ni, H.; Liao, Y.; Zhang, Y.; Lu, H.; Huang, Z.; Huang, F.; Zhang, Z.; Dong, Y.; Wang, Z.; Huang, Y. Levistilide A ameliorates neuroinflammation via inhibiting JAK2/STAT3 signaling for neuroprotection and cognitive improvement in scopolamine-induced Alzheimer’s disease mouse model. Int. Immunopharmacol. 2023, 124 Pt A, 110783. [Google Scholar] [CrossRef]
- Espinosa, J.M.; Quintero-Flórez, A.; Carrasquilla, N.; Montero, E.; Rodríguez-Rodríguez, A.; Castellano, J.M.; Perona, J.S. Bioactive compounds in pomace olive oil modulate the inflammatory response elicited by postprandial triglyceride-rich lipoproteins in BV-2 cells. Food Funct. 2023, 14, 8987–8999. [Google Scholar] [CrossRef]
- Rahmatkar, S.N.; Rana, A.K.; Kumar, R.; Singh, D. Fagopyrum tataricum (L.) Gaertn interacts with Gsk-3β/Nrf-2 signalling to protect neurotoxicity in a zebrafish model. J. Ethnopharmacol. 2024, 319 Pt 2, 117187. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, Y.; Meng, Q.; Xu, L.; Chen, G.; Zhou, D.; Lin, B.; Hou, Y.; Li, N. Potential inhibitors of microglial activation from the roots of Wikstroemia lichiangensis WW Sm. Phytochemistry 2023, 213, 113767. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Q.; Huang, W.P.; Jiang, Y.C.; Xu, H.; Duan, C.S.; Chen, N.H.; Liu, Y.J.; Fu, X.M. The function of astrocytes and their role in neurological diseases. Eur. J. Neurosci. 2023, 58, 3932–3961. [Google Scholar] [CrossRef] [PubMed]
- Jekabsone, A.; Jankeviciute, S.; Pampuscenko, K.; Borutaite, V.; Morkuniene, R. The Role of Intracellular Ca2+ and Mitochondrial ROS in Small Aβ1-42 Oligomer-Induced Microglial Death. Int. J. Mol. Sci. 2023, 24, 12315. [Google Scholar] [CrossRef]
- Du, J.; Song, D.; Li, Y.; Liu, J.; Huang, X.; Li, B.; Li, L. Saikosaponin-D Mitigates Oxidation in SH-SY5Y Cells Stimulated by Glutamate through Activation of Nrf2 Pathway: Involvement of PI3K. Neurotox. Res. 2022, 40, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Marmolejo-Garza, A.; Krabbendam, I.E.; Luu, M.D.A.; Brouwer, F.; Trombetta-Lima, M.; Unal, O.; O’Connor, S.J.; Majerníková, N.; Elzinga, C.R.S.; Mammucari, C.; et al. Negative modulation of mitochondrial calcium uniporter complex protects neurons against ferroptosis. Cell Death Dis. 2023, 14, 772. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Lee, H.; Liu, Z.; Lee, H.K.; Lee, D.S. Effects of Compounds Isolated from Lindera erythrocarpa on Anti-Inflammatory and Anti-Neuroinflammatory Action in BV2 Microglia and RAW264.7 Macrophage. Int. J. Mol. Sci. 2022, 23, 7122. [Google Scholar] [CrossRef]
- Liu, Z.; Yoon, C.S.; Lee, H.; Lee, H.K.; Lee, D.S. Linderone Isolated from Lindera erythrocarpa Exerts Antioxidant and Anti-Neuroinflammatory Effects via NF-κB and Nrf2 Pathways in BV2 and HT22 Cells. Int. J. Mol. Sci. 2023, 24, 7569. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Li, M. The Role of Traditional Chinese Medicine Natural Products in β-Amyloid Deposition and Tau Protein Hyperphosphorylation in Alzheimer’s Disease. Drug Des. Dev. Ther. 2023, 17, 3295–3323. [Google Scholar] [CrossRef]
- Wu, X.; Cao, S.; Zou, Y.; Wu, F. Traditional Chinese Medicine studies for Alzheimer’s disease via network pharmacology based on entropy and random walk. PLoS ONE 2023, 18, e0294772. [Google Scholar] [CrossRef]
- Ma, C.T.; Lee, S.B.; Cho, I.H.; Yu, J.S.; Huang, T.; Lee, T.M.; Ly, T.L.; Kwon, S.W.; Park, J.H.; Yang, H.O. Agarperoxinols A and B: Two Unprecedented Tricyclic 6/6/7 Rearranged Humulene-Type Sesquiterpenoids That Attenuated the Neuroinflammation in LPS-Stimulated Microglial Models. ACS Omega 2023, 8, 43873–43882. [Google Scholar] [CrossRef]
- da Silva, A.G.; Alves, M.D.M.; da Cunha, A.A.; Caires, G.A.; Kerkis, I.; Vigerelli, H.; Sciani, J.M. Echinometra lucunter molecules reduce Aβ42-induced neurotoxicity in SH-SY5Y neuron-like cells: Effects on disaggregation and oxidative stress. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230031. [Google Scholar] [CrossRef]
- Oh, J.; Kwon, T.W.; Choi, J.H.; Kim, Y.; Moon, S.K.; Nah, S.Y.; Cho, I.H. Ginsenoside-Re inhibits experimental autoimmune encephalomyelitis as a mouse model of multiple sclerosis by downregulating TLR4/MyD88/NF-κB signaling pathways. Phytomedicine 2024, 122, 155065. [Google Scholar] [CrossRef]
- Dong, W.; Gong, T.; Zhao, S.; Wen, S.; Chen, Q.; Jiang, M.; Ye, W.; Huang, Q.; Wang, C.; Yang, C.; et al. A Novel Extract from Ginkgo biloba Inhibits Neuroinflammation and Maintains White Matter Integrity in Experimental Stroke. Neuroscience 2023, 523, 7–19. [Google Scholar] [CrossRef]
- Liu, S.; Che, N.; Ou, W.; Yan, M.; Liao, Y.; Cheng, Y. Bullatine A exerts anti-inflammatory effects by inhibiting the ROS/JNK/NF-κB pathway and attenuating systemic inflammatory responses in mice. Pharm. Biol. 2022, 60, 1840–1849. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Zhu, Z.; Aisa, H.A.; Xin, X. Anti-inflammatory effect and mechanism of active parts of Artemisia mongolica in LPS-induced Raw264.7 cells based on network pharmacology analysis. J. Ethnopharmacol. 2023, 321, 117509. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, M.; Zhang, H.; Wang, Y.; Chen, B.; Shao, J. Diosmin ameliorates LPS-induced depression-like behaviors in mice: Inhibition of inflammation and oxidative stress in the prefrontal cortex. Brain Res. Bull. 2023, 206, 110843. [Google Scholar] [CrossRef]
- Li, T.; Zhai, Y.X.; Zheng, T.; Xu, B. Neferine exerts anti-inflammatory activity in BV-2 microglial cells and protects mice with MPTP-induced Parkinson’s disease by inhibiting NF-κB activation. Mol. Med. Rep. 2023, 28, 235. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhuo, Z.; Wang, X.; Zhang, Y.; Qian, Y.; Lin, F. Secretome of EMSCs neutralizes LPS-induced acute lung injury via aerosol administration. Int. J. Mol. Med. 2023, 52, 104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wan, Q.; Wang, X.; Di, L.; Li, X.; Kang, R.; Li, S.; Huang, L. LXA4 attenuates perioperative neurocognitive disorders by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2023, 123, 110788. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, T.; Lotfealian, A.; Anvari, M.; Namavar, M.; Karbalaei, N.; Shahedi, A.; Bokkon, I.; Salari, V.; Oblak, D. Effect of methamphetamine on ultraweak photon emission and level of reactive oxygen species in male rat brain. Neurosci. Lett. 2023, 801, 137136. [Google Scholar] [CrossRef]
- Selvaraj, B.; Nguyen, U.T.T.; Huh, G.; Nguyen, D.H.; Mok, I.K.; Lee, H.; Kang, K.; Bae, A.N.; Kim, D.W.; Lee, J.W. Synthesis and biological evaluation of chalcone derivatives as neuroprotective agents against glutamate-induced HT22 mouse hippocampal neuronal cell death. Bioorg. Med. Chem. Lett. 2020, 30, 127597. [Google Scholar] [CrossRef]
- Gomez, C.D.; Aguilera, P.; Ortiz-Plata, A.; López, F.N.; Chánez-Cárdenas, M.E.; Flores-Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Exp. Med. 2019, 28, 1609–1614. [Google Scholar] [CrossRef]
- Lee, D.S.; Cha, B.Y.; Woo, J.T.; Kim, Y.C.; Jang, J.H. Acerogenin A from Acer nikoense Maxim Prevents Oxidative Stress-Induced Neuronal Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression in Mouse Hippocampal HT22 Cell Line. Molecules 2015, 20, 12545–12557. [Google Scholar] [CrossRef]
- Song, J.; Zhao, X.; Bo, J.; Lv, Z.; Li, G.; Chen, Y.; Liang, J.; Zhang, C.; Jin, X.; Liu, C.; et al. A polysaccharide from Alhagi honey protects the intestinal barrier and regulates the Nrf2/HO-1-TLR4/MAPK signaling pathway to treat alcoholic liver disease in mice. J. Ethnopharmacol. 2023, 321, 117552. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Li, Y.; Mao, X.; Xie, K.; Zheng, Y.; Yu, Y.; Yu, Y. Effects of the PI3K/Akt/HO-1 pathway on autophagy in a sepsis-induced acute lung injury mouse model. Int. Immunopharmacol. 2023, 124 Pt B, 111063. [Google Scholar] [CrossRef]
- Xiang, S.; Zhao, L.; Tang, C.; Ling, L.; Xie, C.; Shi, Y.; Liu, W.; Li, X.; Cao, Y. Icariin inhibits osteoblast ferroptosis via Nrf2/HO-1 signaling and enhances healing of osteoporotic fractures. Eur. J. Pharmacol. 2023, 965, 176244. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yoon, C.-S.; Lee, H.; Lee, H.-K.; Lee, D.-S. Dihydropashanone Isolated from Lindera erythrocarpa, a Potential Natural Product for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2545. https://doi.org/10.3390/ijms25052545
Liu Z, Yoon C-S, Lee H, Lee H-K, Lee D-S. Dihydropashanone Isolated from Lindera erythrocarpa, a Potential Natural Product for the Treatment of Neurodegenerative Diseases. International Journal of Molecular Sciences. 2024; 25(5):2545. https://doi.org/10.3390/ijms25052545
Chicago/Turabian StyleLiu, Zhiming, Chi-Su Yoon, Hwan Lee, Hyeong-Kyu Lee, and Dong-Sung Lee. 2024. "Dihydropashanone Isolated from Lindera erythrocarpa, a Potential Natural Product for the Treatment of Neurodegenerative Diseases" International Journal of Molecular Sciences 25, no. 5: 2545. https://doi.org/10.3390/ijms25052545
APA StyleLiu, Z., Yoon, C.-S., Lee, H., Lee, H.-K., & Lee, D.-S. (2024). Dihydropashanone Isolated from Lindera erythrocarpa, a Potential Natural Product for the Treatment of Neurodegenerative Diseases. International Journal of Molecular Sciences, 25(5), 2545. https://doi.org/10.3390/ijms25052545





