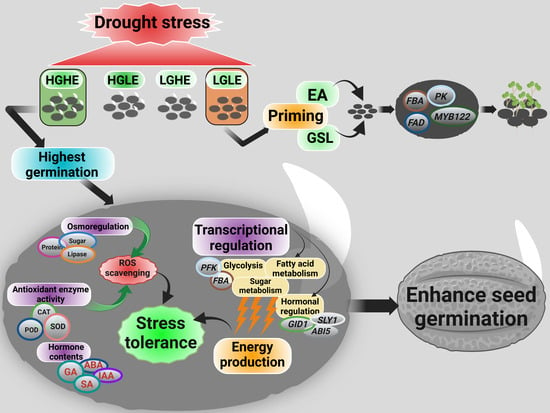
Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed
Abstract
1. Introduction
2. Results
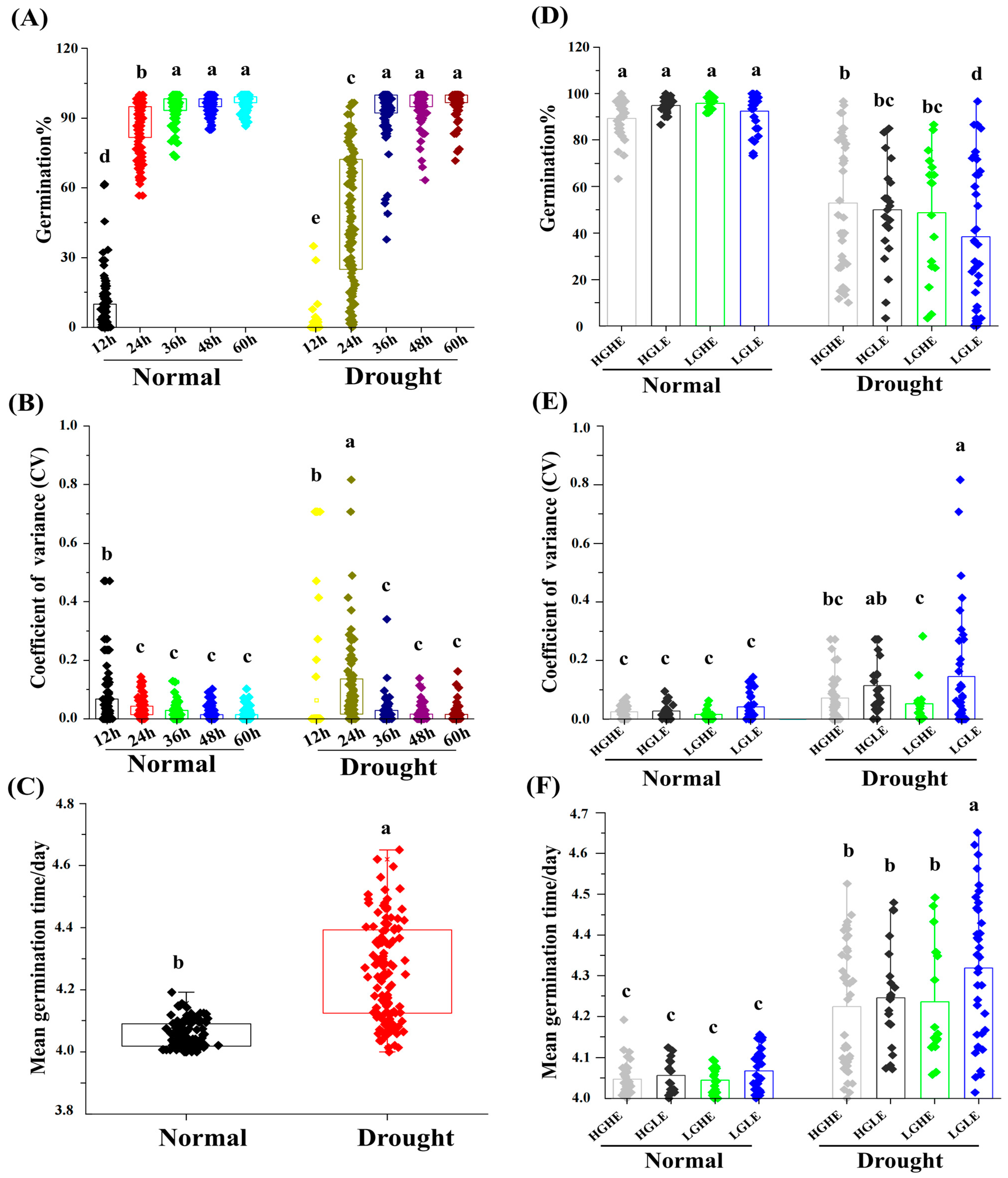
2.1. Variations in the Germination Rates of Various Accessions under Drought Stress
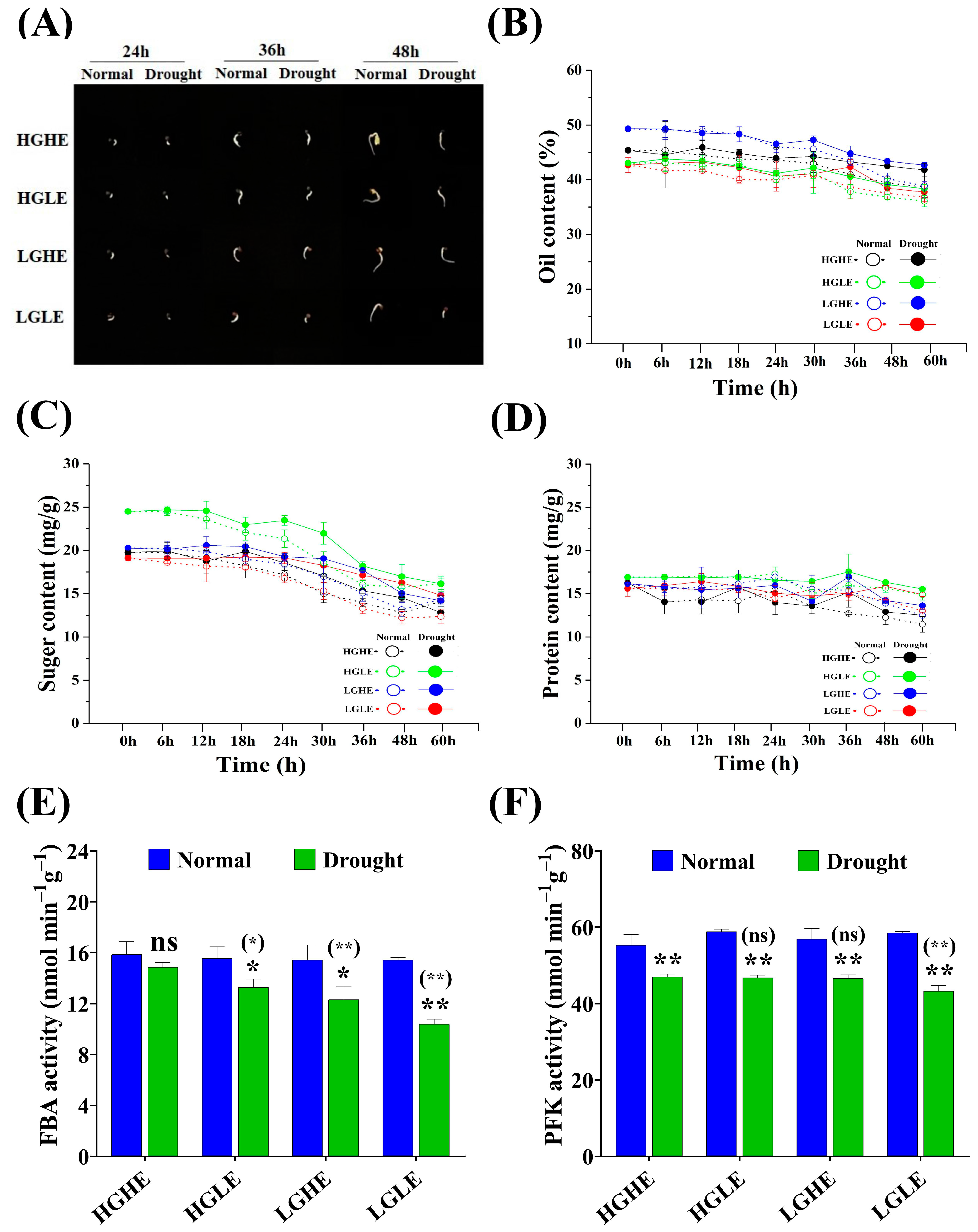
2.2. Alteration of Phenotype, Osmolytes, and Glucose Metabolism-Related Enzymes in Different Types of Rapeseed Seeds under Drought Stress Conditions
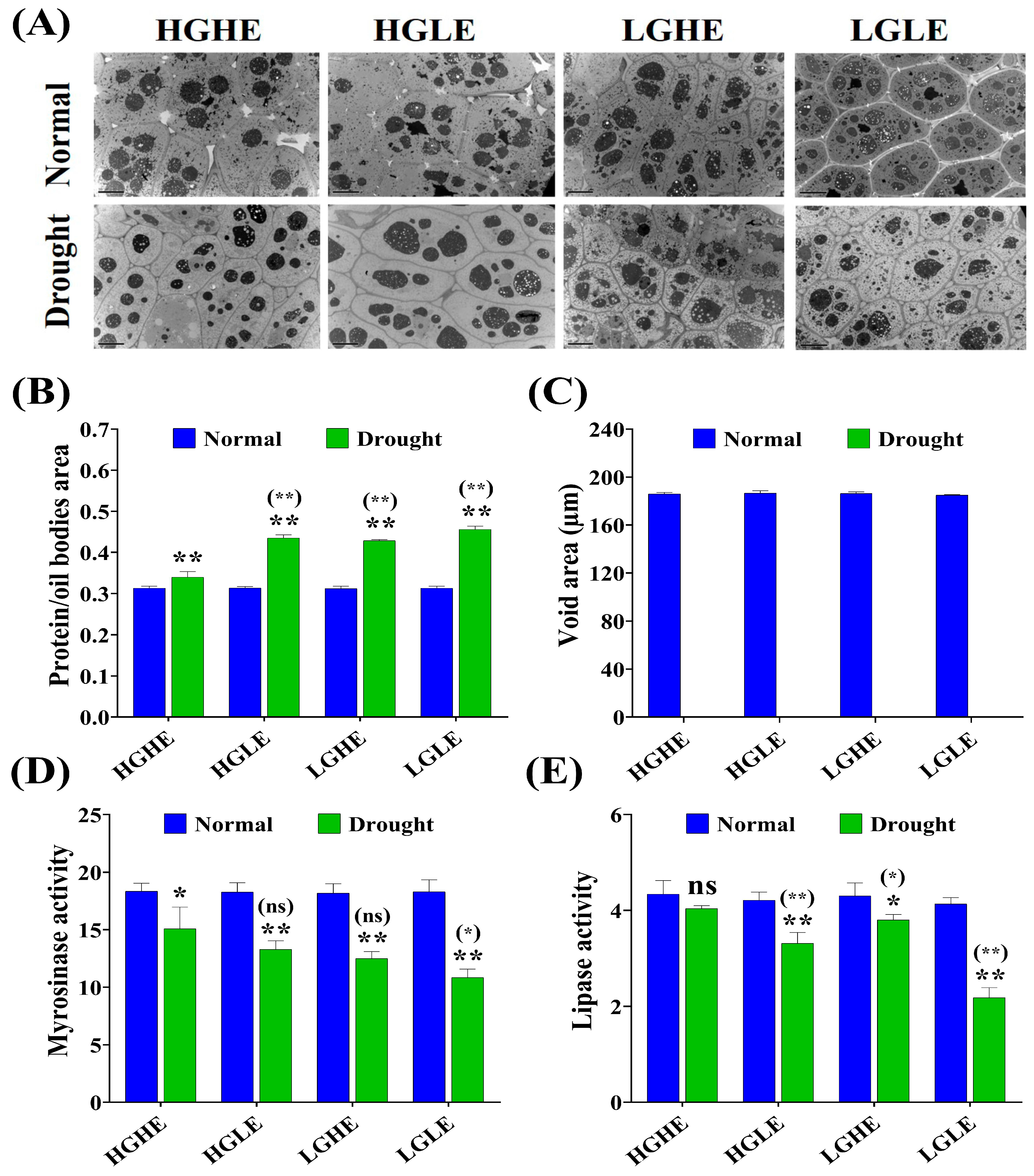
2.3. Microstructural-Related Traits and Myrosinase and Lipase Activities Varied in Different Rapeseed Seed Types under Drought Stress Conditions
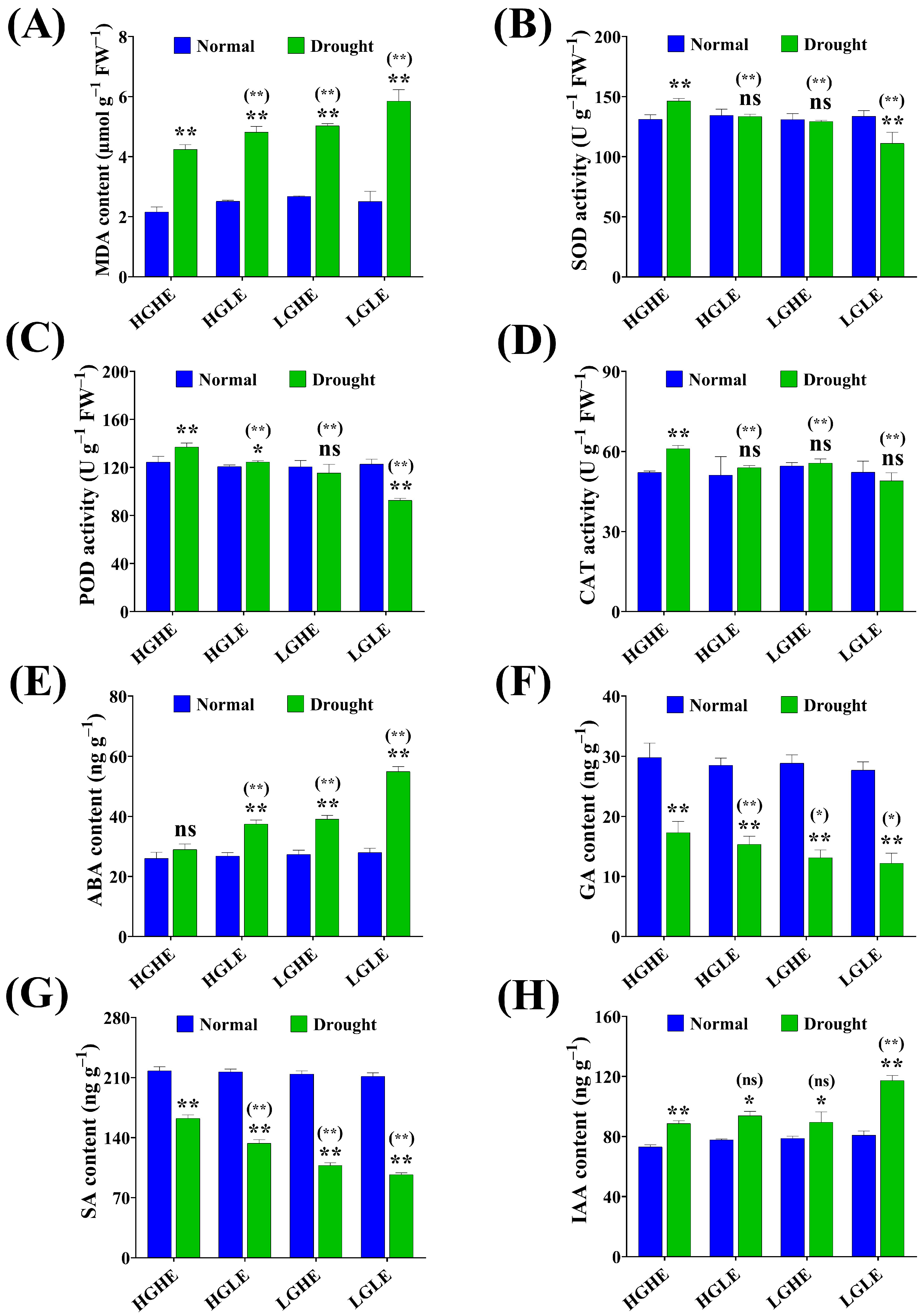
2.4. Effect of Drought Stress on the Seed’s Defense and Hormonal Systems in Different Types of Rapeseed
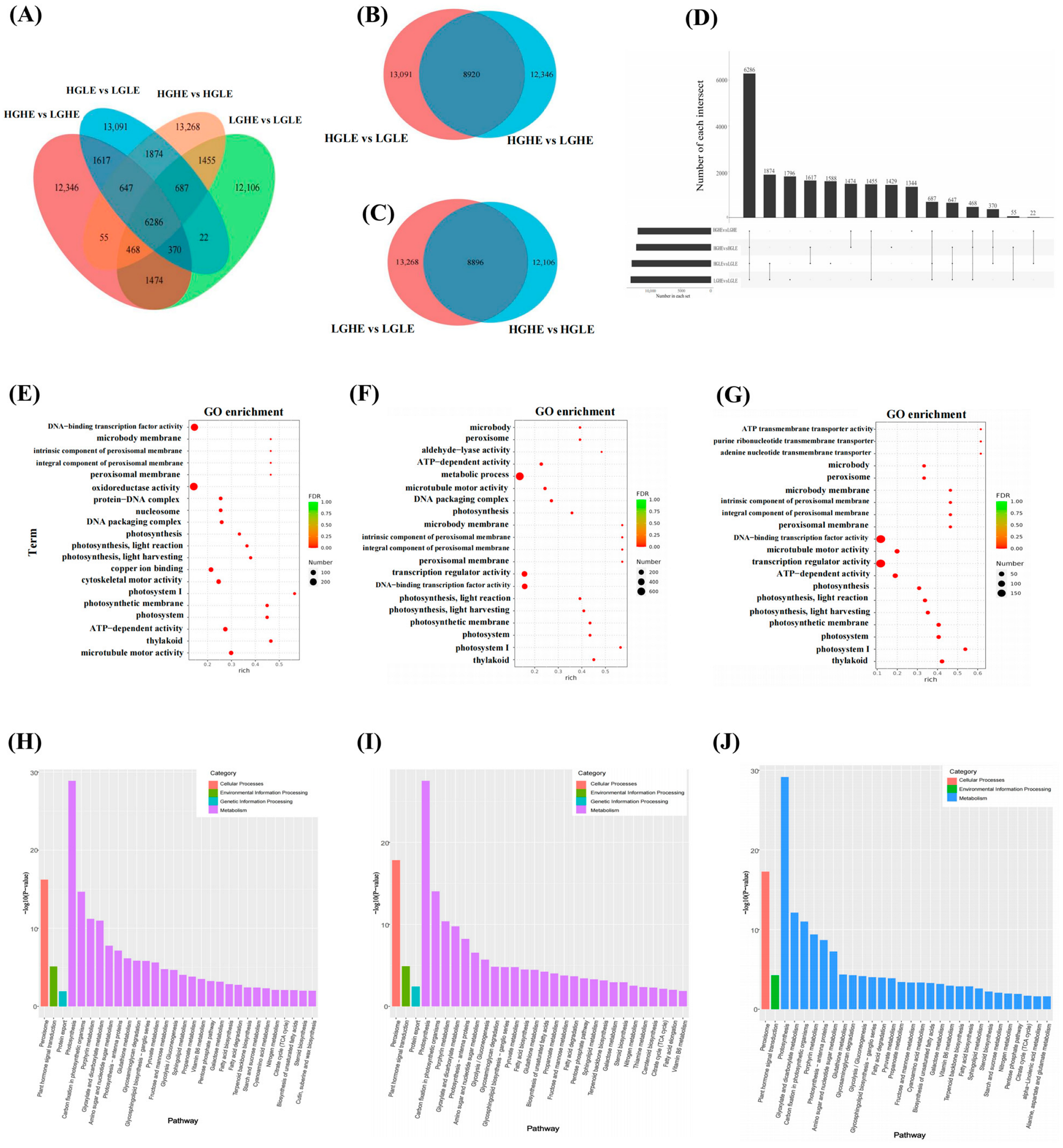
2.5. Transcriptome Analysis and Metabolic-Related Pathways in Response to Drought Stress
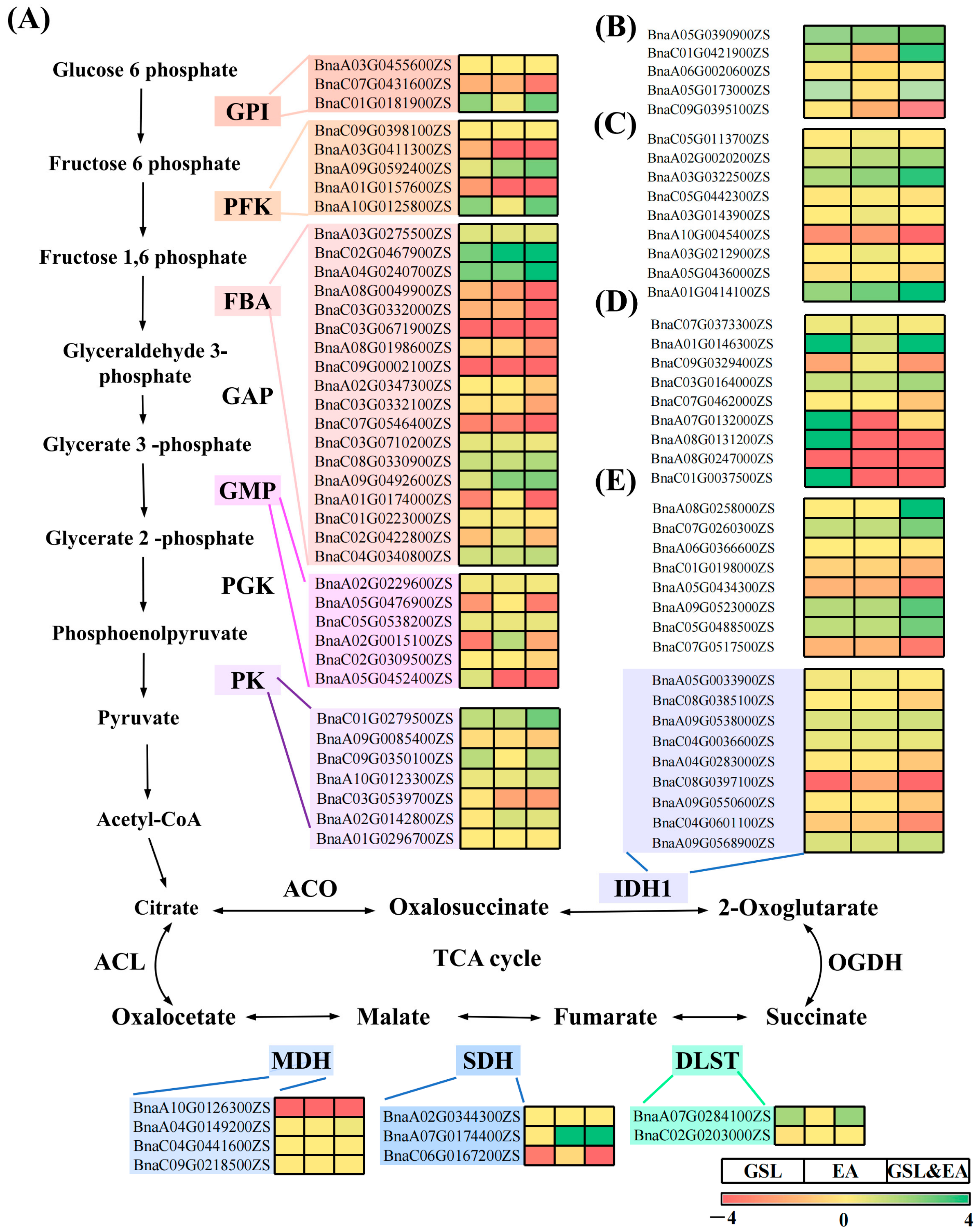
2.6. Gene Expression of Key Metabolic Pathways Associated with Drought Stress
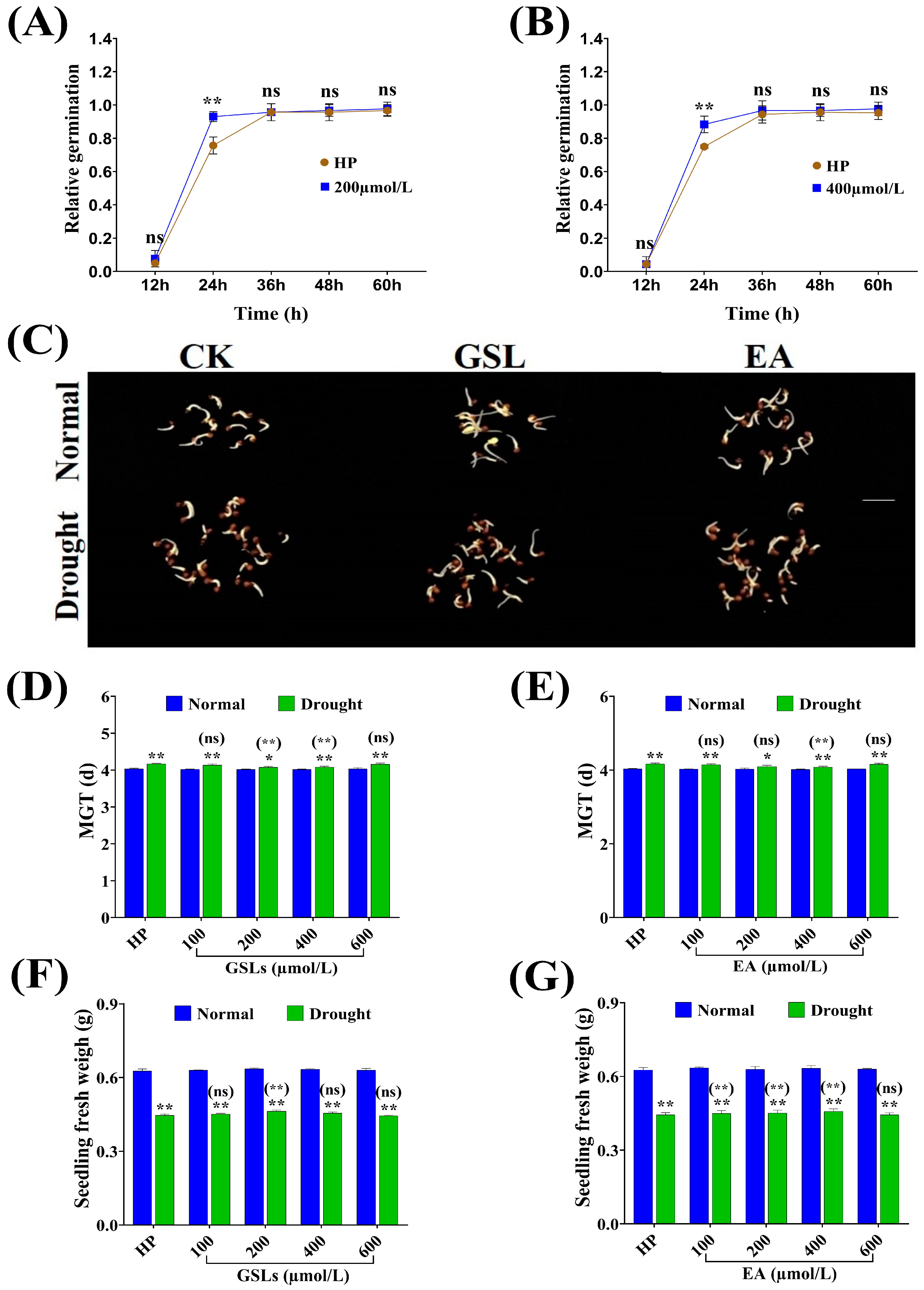
2.7. Influence of GSL and EA Priming on Seed Germination-Related Traits under Drought Stress Conditions
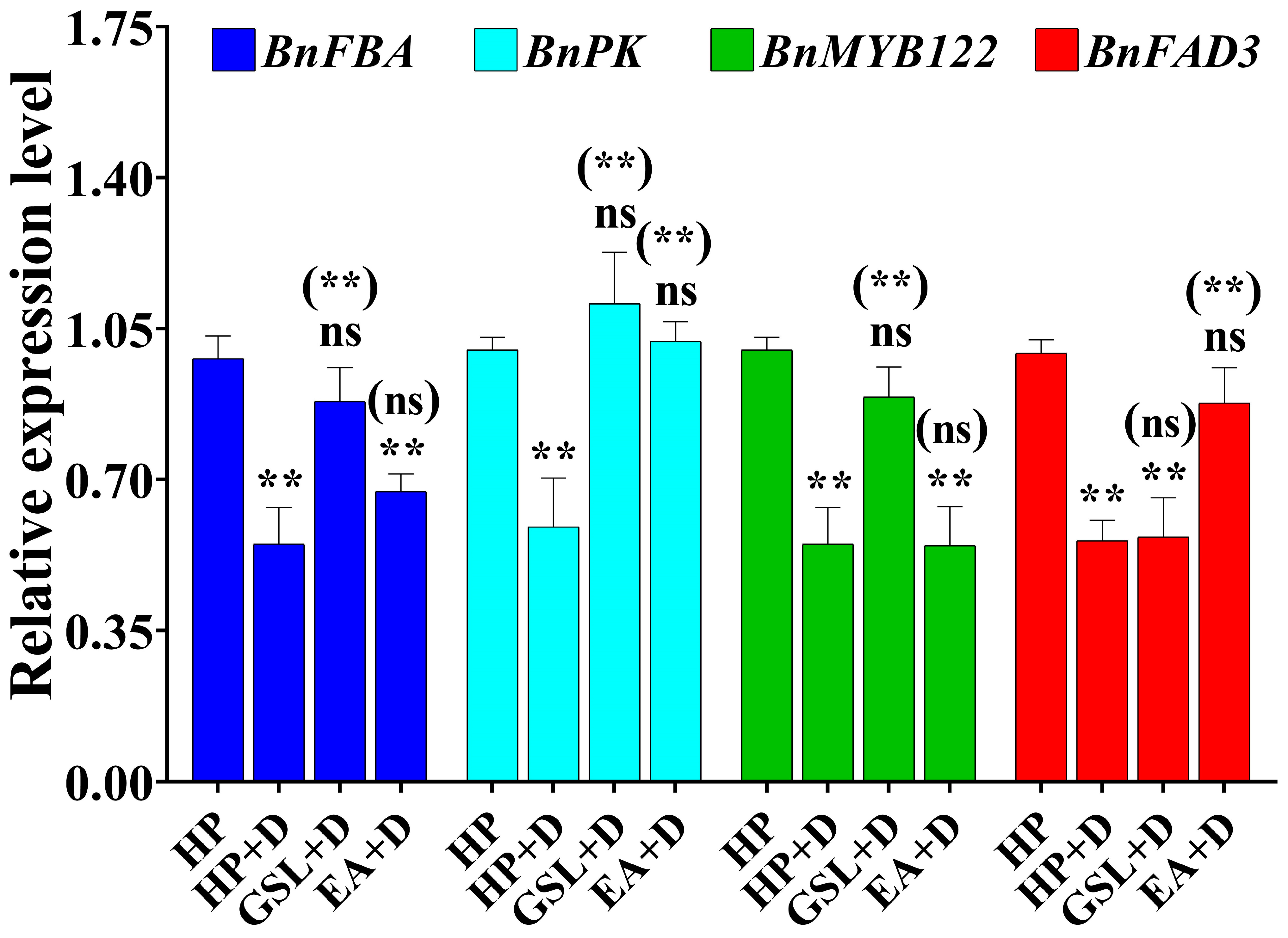
2.8. Differentail Gene Expression with GSL and EA Priming under Drought Stress Conditions
3. Discussion
3.1. Different GSL and EA Contents Influenced the Seed Germination-Related Traits under Drought Stress
3.2. Different GSL and EA Contents Modulated the Physiochemical Indices in Response to Drought Stress
3.3. Transcriptional Regulation of GSL- and EA-Related Drought Stress-Responsive Pathways during theSeed Germination
3.4. GSL and EA Priming Enhanced Drought Tolerance during the Seed Germination of LGLE-Type Seeds
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination
- Gt: number of germinated seeds on day t
- Dt: day after sowing
4.3. Transmission Electron Microscopy (TEM) Analysis
4.4. Oil Content Determination
4.5. Total Soluble Sugar and Protein Contents
4.6. Myrosinase, Lipase, FBA and PFK Activity Determination
4.7. Determination of MDA Content and Antioxidant Enzyme Activity
4.8. Endogenous Hormones Determination
4.9. Transcriptome Sequencing and Differential Gene Analysis
4.9.1. Sample Preparation and RNA Extraction
4.9.2. RNA-Seq Analysis
4.9.3. Analysis of Differentially Expressed Genes (DEGs)
4.9.4. Gene Functional Enrichment Analysis
4.10. Exogenous Application of GSL and EA
4.11. RNA Extraction, cDNA Synthesis, and Quantitative RT-PCR
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Q.; Liu, K. Worldwide rapeseed (Brassica napus L.) research: A bibliometric analysis during 2011–2021. Oil Crop Sci. 2022, 7, 157–165. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, C.; Guo, M.; El-Badri, A.M.; Batool, M.; Kuai, J.; Wang, J.; Wang, B.; Zhou, G. Rapeseed-maize double-cropping with high biomass and high economic benefits is a soil environment-friendly forage production mode in the yangtze river basin. Eur. J. Agron. 2023, 142, 126675–126685. [Google Scholar] [CrossRef]
- Wang, C.; Yan, Z.; Wang, Z.; Batool, M.; El-Badri, A.M.; Bai, F.; Li, Z.; Wang, B.; Zhou, G.; Kuai, J. Subsoil tillage promotes root and shoot growth of rapeseed in paddy fields and dryland in yangtze river basin soils. Eur. J. Agron. 2021, 130, 126351–126363. [Google Scholar] [CrossRef]
- Secchi, M.A.; Fernandez, J.A.; Stamm, M.J.; Durrett, T.; Prasad, P.V.V.; Messina, C.D.; Ciampitti, I.A. Effects of heat and drought on canola (Brassica napus L.) yield, oil, and protein: A meta-analysis. Field Crops Res. 2023, 293, 108848–108861. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, S.; Deihimfard, R.; Nazari, M.R.; Mohammadi-Ahmadmahmoudi, E.; Chenu, K. Understanding wheat growth and the seasonal climatic characteristics of major drought patterns occurring in cold dryland environments from iran. Eur. J. Agron. 2023, 145, 126772–126785. [Google Scholar] [CrossRef]
- Kim, J.-B.; Kim, S.-H.; Bae, D.-H. The impacts of global warming on arid climate and drought features. Theor. Appl. Climatol. 2023, 152, 693–708. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Bahrani, M.J. Yield and yield components of rapeseed as influenced by water stress at different growth stages and nitrogen levels. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 755–761. [Google Scholar]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Saha, D.; Choyal, P.; Mishra, U.N.; Dey, P.; Bose, B.; Md, P.; Gupta, N.K.; Mehta, B.K.; Kumar, P.; Pandey, S.; et al. Drought stress responses and inducing tolerance by seed priming approach in plants. Plant Stress 2022, 4, 100066–100079. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Qin, F. Genetic dissection of drought resistance for trait improvement in crops. Crop J. 2023, 11, 975–985. [Google Scholar] [CrossRef]
- Heng-Heng, X.; Ni, L.; Shujun, L.; Weiqing, W.; Weiping, W.; Hong, Z.; Hongyan, C.; Songquan, S. Research progress in seed germination and its control. Acta Agron. Sin. 2014, 40, 1141–1156. [Google Scholar]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Wang, J.; Wang, T.; Wei, L.; Li, J.; Liu, L. Identification of rapeseed micrornas involved in early stage seed germination under salt and drought stresses. Front. Plant Sci. 2016, 7, 658. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; de Carvalho Gonçalves, J.F.; Fernandes, A.V.; da Costa, K.C.P.; de Lima e Borges, E.E.; Araújo, W.L.; Nunes-Nesi, A.; Ramos, M.V.; Rathinasabapathi, B. Reserve mobilization and the role of primary metabolites during the germination and initial seedling growth of rubber tree genotypes. Acta Physiol. Plant. 2022, 44, 80–95. [Google Scholar] [CrossRef]
- Bai, B.; Peviani, A.; van der Horst, S.; Gamm, M.; Snel, B.; Bentsink, L.; Hanson, J. Extensive translational regulation during seed germination revealed by polysomal profiling. New Phytol. 2017, 214, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.A.; Quettier, A.-L.; Shaw, E.; Eastmond, P.J. Seed storage oil mobilization is important but not essential for germination or seedling establishment in arabidopsis. Plant Physiol. 2011, 157, 866–875. [Google Scholar] [CrossRef]
- Gu, J.; Hou, D.; Li, Y.; Chao, H.; Zhang, K.; Wang, H.; Xiang, J.; Raboanatahiry, N.; Wang, B.; Li, M. Integration of proteomic and genomic approaches to dissect seed germination vigor in Brassica napus seeds differing in oil content. BMC Plant Biol. 2019, 19, 21. [Google Scholar] [CrossRef]
- Batool, M.; El-Badri, A.M.; Wang, C.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Bashir, F.; Xu, Z.; Wang, J.; Kuai, J.; et al. The role of storage reserves and their mobilization during seed germination under drought stress conditions of rapeseed cultivars with high and low oli contents. Crop Environ. 2022, 1, 231–240. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant metabolomics in biotic and abiotic stress: A critical overview. Phytochem. Rev. 2022, 21, 503–524. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695–112707. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-L.; Li, J.; Wang, H.-C.; Zhang, C.-L.; Naeem, M.S. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. Under water stress. Plant Physiol. Biochem. 2018, 129, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-B.; Kim, Y.-H.; Lee, H.-S.; Kim, K.-Y.; Deng, X.-P.; Kwak, S.-S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Agami, R.; Elrewainy, I.M.; Wang, B.; Zhou, G. Role of phytomelatonin in promoting ion homeostasis during salt stress. In Melatonin: Role in Plant Signaling, Growth and Stress Tolerance: Phytomelatonin in Normal and Challenging Environments; Mukherjee, S., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 313–342. [Google Scholar]
- Lee, S.C.; Luan, S. Aba signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Hatzig, S.; Breuer, F.; Nesi, N.; Ducournau, S.; Wagner, M.-H.; Leckband, G.; Abbadi, A.; Snowdon, R.J. Hidden effects of seed quality breeding on germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2018, 9, 419. [Google Scholar] [CrossRef]
- Schreiner, M.; Beyene, B.; Krumbein, A.; Stützel, H. Ontogenetic changes of 2-propenyl and 3-indolylmethyl glucosinolates in Brassica carinata leaves as affected by water supply. J. Agric. Food Chem. 2009, 57, 7259–7263. [Google Scholar] [CrossRef]
- Li, M.; Xie, F.; Li, J.; Sun, B.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Zhang, F.; Zhang, Y.; et al. Tumorous stem development of Brassica juncea: A complex regulatory network of stem formation and identification of key genes in glucosinolate biosynthesis. Plants 2020, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pujante, P.J.; Borja-Martínez, M.; Pedreño, M.Á.; Almagro, L. Biosynthesis and bioactivity of glucosinolates and their production in plant in vitro cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.X.; Phua, S.Y.; Van Breusegem, F. Secondary sulfur metabolism in cellular signalling and oxidative stress responses. J. Exp. Bot. 2019, 70, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- Yifei, W.; Jianhua, H.; Ruiju, L.; Runmei, Z. Effect of nacl stress on seed germination and stem apexes growth of rapeseed cultivars with high and low-erucic acid. J. Nucl. Agric. Sci. 2008, 22, 762–765. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Long, J.; Dong, M.; Wang, C.; Miao, Y. Effects of drought and salt stress on seed germination and seedling growth of Elymus nutans. PeerJ 2023, 11, e15968–e15990. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Clay, H.A.; Lynn, J.R.; Morris, K. Towards a genetic understanding of seed vigour in small-seeded crops using natural variation in Brassica oleracea. Plant Sci. 2010, 179, 582–589. [Google Scholar] [CrossRef]
- Boter, M.; Calleja-Cabrera, J.; Carrera-Castaño, G.; Wagner, G.; Hatzig, S.V.; Snowdon, R.J.; Legoahec, L.; Bianchetti, G.; Bouchereau, A.; Nesi, N.; et al. An integrative approach to analyze seed germination in Brassica napus. Front. Plant Sci. 2019, 10, 1342. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, R.; Zheng, J.; Wang, Z.; Gao, T.e.; Qin, M.; Hu, X.; Wang, Y.; Yang, S.; Li, T. Insights into glucosinolate accumulation and metabolic pathways in isatis indigotica fort. BMC Plant Biol. 2022, 22, 78. [Google Scholar] [CrossRef]
- Safoora, B.; Mohsenzadeh, S.; Kahrizi, D. Water-deficit stress and genotype variation induced alteration in seed characteristics of Camelina sativa. Rhizosphere 2021, 20, 100427–100493. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, X.; Zhang, X.; Wu, G.; Liu, F. A review of erucic acid production in brassicaceae oilseeds: Progress and prospects for the genetic engineering of high and low-erucic acid rapeseeds (Brassica napus). Front. Plant Sci. 2022, 13, 899076. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of peg-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Pitann, B.; Schubert, S.; Mühling, K.H. Decline in leaf growth under salt stress is due to an inhibition of h+-pumping activity and increase in apoplastic ph of maize leaves. J. Plant Nutr. Soil Sci. 2009, 172, 535–543. [Google Scholar] [CrossRef]
- Li, P.; Ma, B.; Palta, J.A.; Ding, T.; Cheng, Z.; Lv, G.; Xiong, Y. Wheat breeding highlights drought tolerance while ignores the advantages of drought avoidance: A meta-analysis. Eur. J. Agron. 2021, 122, 126196–126210. [Google Scholar] [CrossRef]
- Farhad, W.; Cheema, M.A.; Saleem, M.F.; Saqib, M. Evaluation of drought tolerance in maize hybrids. Int. J. Agric. Biol. 2011, 13, 523–528. [Google Scholar]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern china. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Zhang, L.; Kawaguchi, R.; Morikawa-Ichinose, T.; Allahham, A.; Kim, S.-J.; Maruyama-Nakashita, A. Sulfur deficiency-induced glucosinolate catabolism attributed to two β-glucosidases, bglu28 and bglu30, is required for plant growth maintenance under sulfur deficiency. Plant Cell Physiol. 2020, 61, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Kask, K.; Kännaste, A.; Talts, E.; Copolovici, L.; Niinemets, Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant Cell Environ. 2016, 39, 2027–2042. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. Seedlings. 3 Biotech 2018, 8, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sturtevant, D.; Aziz, M.; Jin, C.; Li, Q.; Chapman, K.D.; Guo, L. Spatial analysis of lipid metabolites and expressed genes reveals tissue-specific heterogeneity of lipid metabolism in high- and low-oil Brassica napus L. Seeds. Plant J. 2018, 94, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Li, Q.; Xu, Y.; Yang, L.; Bi, H.; Ai, X. Genome-wide analysis of the fructose 1,6-bisphosphate aldolase (fba) gene family and functional characterization of fba7 in tomato. Plant Physiol. Biochem. 2016, 108, 251–265. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Sivasubramaniam, K.; Dadlani, M. Seed dormancy and regulation of germination. In Seed Science and Technology: Biology, Production, Quality; Dadlani, M., Yadava, D.K., Eds.; Springer Nature: Singapore, 2023; pp. 39–66. [Google Scholar]
- Lu, W.; Tang, X.; Huo, Y.; Xu, R.; Qi, S.; Huang, J.; Zheng, C.; Wu, C.-A. Identification and characterization of fructose 1,6-bisphosphate aldolase genes in arabidopsis reveal a gene family with diverse responses to abiotic stresses. Gene 2012, 503, 65–74. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Hossain, A.; Barutçular, C.; Gormus, O.; Ahmad, Z.; Hussain, S.; Islam, M.S.; Alharby, H.F.; Bamagoos, A.A.; Kumar, N.; et al. Effects of drought stress on the quality of major oilseed crops: Implication and possible mitigation strategies—A review. Appl. Ecol. Environ. Res. 2019, 17, 4019–4043. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Hu, S.; Zhang, J.; Wang, L.; Ping, X.; Wang, J.; Li, J.; Lu, K.; Tang, Z.; et al. Transcriptome and proteome analyses of the molecular mechanisms underlying changes in oil storage under drought stress in Brassica napus L. GCB Bioenergy 2021, 13, 1071–1086. [Google Scholar] [CrossRef]
- Parul, C. Glucosinolates and its role in mitigating abiotic and biotic stress in Brassicaceae. In Plant Stress Physiology; Mirza, H., Kamran, N., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Mongrand, S.; Chua, N.-H. A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the abi5 transcription factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2001, 98, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bousquet, A.; Harris, J.M. Abscisic acid and lateral root organ defective/numerous infections and polyphenolics modulate root elongation via reactive oxygen species in Medicago truncatula. Plant Physiol. 2014, 166, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Barker, G.C.; Walley, P.G.; Lynn, J.R.; Finch-Savage, W.E. Trait to gene analysis reveals that allelic variation in three genes determines seed vigour. New Phytol. 2016, 212, 964–976. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Ali, L.G.; Nulit, R.; Ibrahim, M.H.; Yien, C.Y.S. Efficacy of KNO3, SIO2 and sa priming for improving emergence, seedling growth and antioxidant enzymes of rice (Oryza sativa), under drought. Sci. Rep. 2021, 11, 3864. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive aux/iaa proteins mediate drought tolerance in arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yan, H.; Zhang, F.; Wang, Q. Effects of plant hormones on main health-promoting compounds and antioxidant capacity of chinese kale. Food Res. Int. 2012, 48, 359–366. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Kim, S.-H.; Chung, I.-M. Exogenous phytohormones increase the accumulation of health-promoting metabolites, and influence the expression patterns of biosynthesis related genes and biological activity in chinese cabbage (Brassica rapa spp. Pekinensis). Sci. Hortic. 2015, 193, 136–146. [Google Scholar] [CrossRef]
- He, A.; Dean, J.M.; Lodhi, I.J. Peroxisomes as cellular adaptors to metabolic and environmental stress. Trends Cell Biol. 2021, 31, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of abi5 (aba-insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Li, J.-Z.; Li, M.-Q.; Han, Y.-C.; Sun, H.-Z.; Du, Y.-X.; Zhao, Q.-Z. The crucial role of gibberellic acid on germination of drought-resistant upland rice. Biol. Plant. 2019, 63, 529–535. [Google Scholar]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040–101060. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Qu, X.; Wang, H.; Chen, M.; Liao, J.; Yuan, J.; Niu, G. Drought stress–induced physiological and metabolic changes in leaves of two oil tea cultivars. J. Am. Soc. Hortic. Sci. 2019, 144, 439–447. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Shen, X.; Xia, Z.; Zhou, X.; Chen, X.; Lu, C.; Wang, W. Genome-wide survey of the phosphofructokinase family in cassava and functional characterization in response to oxygen-deficient stress. BMC Plant Biol. 2021, 21, 376. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Bellieny-Rabelo, D.; de Oliveira, E.A.G.; Ribeiro, E.d.S.; Costa, E.P.; Oliveira, A.E.A.; Venancio, T.M. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Sci. Rep. 2016, 6, 36009. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, N. Chemo-priming with mannose, mannitol and H2O2 mitigate drought stress in wheat. Cereal Res. Commun. 2014, 42, 450–462. [Google Scholar] [CrossRef]
- Hu, M.; Shi, Z.; Zhang, Z.; Zhang, Y.; Li, H. Effects of exogenous glucose on seed germination and antioxidant capacity in wheat seedlings under salt stress. Plant Growth Regul. 2012, 68, 177–188. [Google Scholar] [CrossRef]
- Luo, T.; Sheng, Z.; Zhang, C.; Li, Q.; Liu, X.; Qu, Z.; Xu, Z. Seed characteristics affect low-temperature stress tolerance performance of rapeseed (Brassica napus L.) during seed germination and seedling emergence stages. Agronomy 2022, 12, 1969. [Google Scholar] [CrossRef]
- Khanna, S.M.; Taxak, P.C.; Jain, P.K.; Saini, R.; Srinivasan, R. Glycolytic enzyme activities and gene expression in cicer arietinum exposed to water-deficit stress. Appl. Biochem. Biotechnol. 2014, 173, 2241–2253. [Google Scholar] [CrossRef]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. Myb transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Aarabi, F.; Kusajima, M.; Tohge, T.; Konishi, T.; Gigolashvili, T.; Takamune, M.; Sasazaki, Y.; Watanabe, M.; Nakashita, H.; Fernie, A.R.; et al. Sulfur deficiency–induced repressor proteins optimize glucosinolate biosynthesis in plants. Sci. Adv. 2016, 2, e1601087–e1601103. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Hristov, A.N.; Domitrovich, C.; Wachter, A.; Cassidy, T.; Lee, C.; Shingfield, K.J.; Kairenius, P.; Davis, J.; Brown, J. Effect of replacing solvent-extracted canola meal with high-oil traditional canola, high-oleic acid canola, or high-erucic acid rapeseed meals on rumen fermentation, digestibility, milk production, and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2011, 94, 4057–4074. [Google Scholar] [CrossRef]
- Yan, G.; Li, D.; Cai, M.; Gao, G.; Chen, B.; Xu, K.; Li, J.; Li, F.; Wang, N.; Qiao, J.; et al. Characterization of fae1 in the zero erucic acid germplasm of Brassica rapa L. Breed. Sci. 2015, 65, 257–264. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Wang, C.; Tabl, K.M.; Khatab, A.; Kuai, J.; Wang, J.; Wang, B.; et al. Mitigation of the salinity stress in rapeseed (Brassica napus L.) productivity by exogenous applications of bio-selenium nanoparticles during the early seedling stage. Environ. Pollut. 2022, 310, 119815–119831. [Google Scholar] [CrossRef]
- Kannur, D.; Dongre, P.; KosambiyaBina, U.; Desai, D. Significant role of hydrotropes in extraction of phytoconstituents—A review. Int. J. Pharm. Sci. Res. 2011, 2, 730–734. [Google Scholar]
- Zhang, Z.J.; Li, H.Z.; Zhou, W.J.; Takeuchi, Y.; Yoneyama, K. Effect of 5-aminolevulinic acid on development and salt tolerance of potato (Solanum tuberosum L.) microtubers in vitro. Plant Growth Regul. 2006, 49, 27–34. [Google Scholar]
- Yuan, G.-f.; Sun, B.; Yuan, J.; Wang, Q.-m. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, S.-w.; Tao, Y.; Ye, J.-z.; Liang, Y.; Peng, X.-x.; Yang, L.-f.; Li, H. A glucose-mediated antibiotic resistance metabolic flux from glycolysis, the pyruvate cycle, and glutamate metabolism to purine metabolism. Front. Microbiol. 2023, 14, 1267729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, L.; Ding, T.; Tian, E.; Yan, X.; Wang, X.; Zhang, J.; Yu, K.; Chen, Z. Comparative transcriptome analysis reveals the molecular basis of Brassica napus in response to aphid stress. Plants 2023, 12, 2855. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.A.; Shalby, N.; El-Badri, A.M.A.; Saleem, M.H.; Khan, M.N.; Nawaz, M.A.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B.; et al. Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. Plants. Agronomy 2020, 10, 1186. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Xiao, J.; Wang, S. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 2012, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.A.; Shalby, N.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Salah, A.; Rady, M.M.; Jie, K.; Wang, B.; et al. Rna-seq analysis revealed key genes associated with salt tolerance in rapeseed germination through carbohydrate metabolism, hormone, and mapk signaling pathways. Ind. Crops Prod. 2022, 176, 114262–114280. [Google Scholar] [CrossRef]
- Lou, H.; Zhao, B.; Peng, Y.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Huang, W.; Wang, T.; Li, Z.; et al. Auxin plays a key role in nitrogen and plant density-modulated root growth and yield in different plant types of rapeseed. Field Crops Res. 2023, 302, 109066–109078. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, X.; El-Badri, A.M.; Batool, M.; Lou, H.; Gao, G.; Bai, C.; Wang, Z.; Jiang, C.; Zhao, X.; Wang, B.; et al. Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed. Int. J. Mol. Sci. 2024, 25, 3308. https://doi.org/10.3390/ijms25063308
Ai X, El-Badri AM, Batool M, Lou H, Gao G, Bai C, Wang Z, Jiang C, Zhao X, Wang B, et al. Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed. International Journal of Molecular Sciences. 2024; 25(6):3308. https://doi.org/10.3390/ijms25063308
Chicago/Turabian StyleAi, Xueying, Ali Mahmoud El-Badri, Maria Batool, Hongxiang Lou, Gengdong Gao, Chenyang Bai, Zongkai Wang, Chunji Jiang, Xinhua Zhao, Bo Wang, and et al. 2024. "Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed" International Journal of Molecular Sciences 25, no. 6: 3308. https://doi.org/10.3390/ijms25063308
APA StyleAi, X., El-Badri, A. M., Batool, M., Lou, H., Gao, G., Bai, C., Wang, Z., Jiang, C., Zhao, X., Wang, B., Kuai, J., Xu, Z., Wang, J., King, G. J., Yu, H., Zhou, G., & Fu, T. (2024). Morpho-Physiochemical Indices and Transcriptome Analysis Reveal the Role of Glucosinolate and Erucic Acid in Response to Drought Stress during Seed Germination of Rapeseed. International Journal of Molecular Sciences, 25(6), 3308. https://doi.org/10.3390/ijms25063308