Polyphenol-Rich Extract from ‘Limoncella’ Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage
Abstract
1. Introduction
2. Results
2.1. LAPE Extract Composition
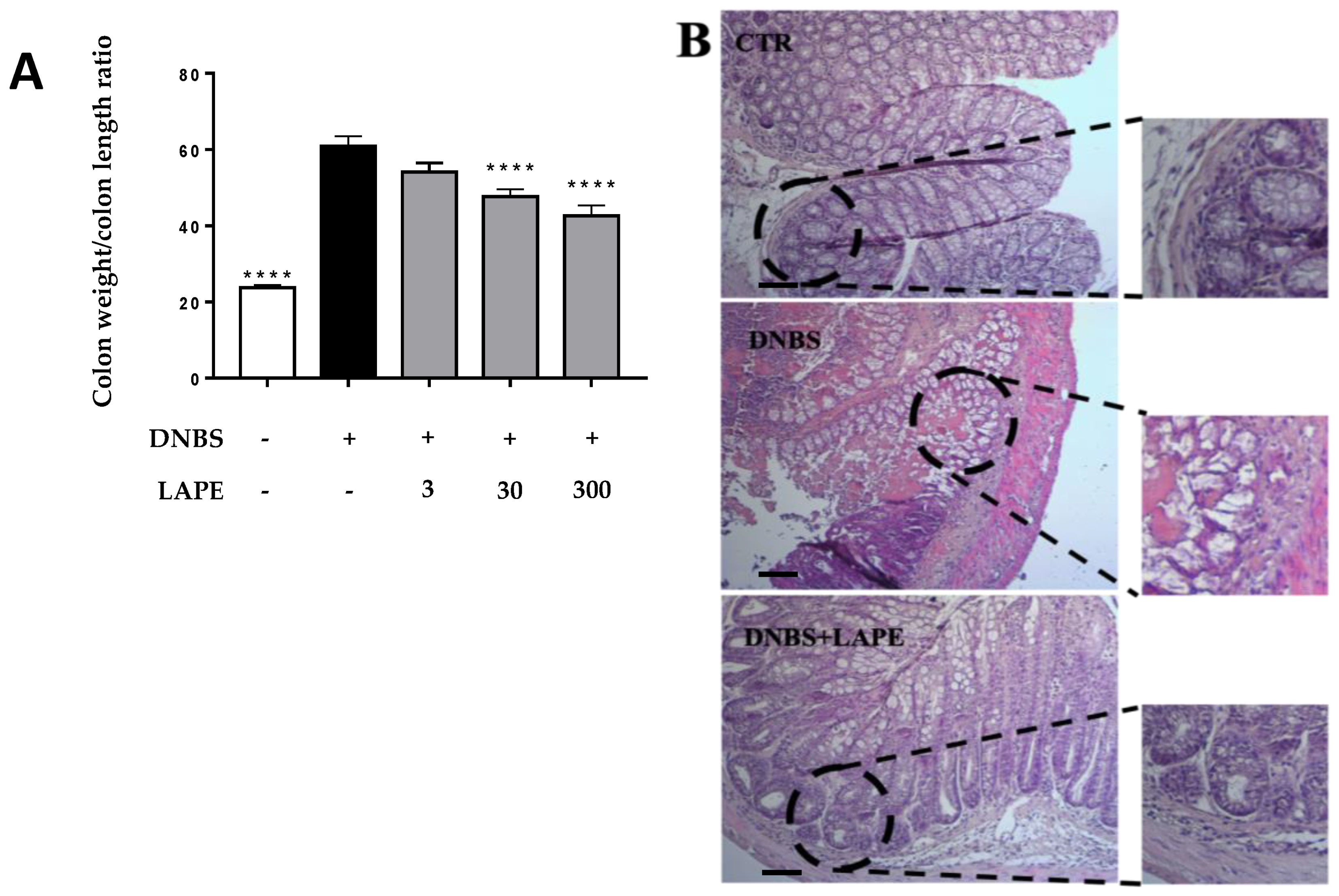
2.2. LAPE Ameliorates the Macroscopic and Histopathological Changes in the DNBS Model of Colitis
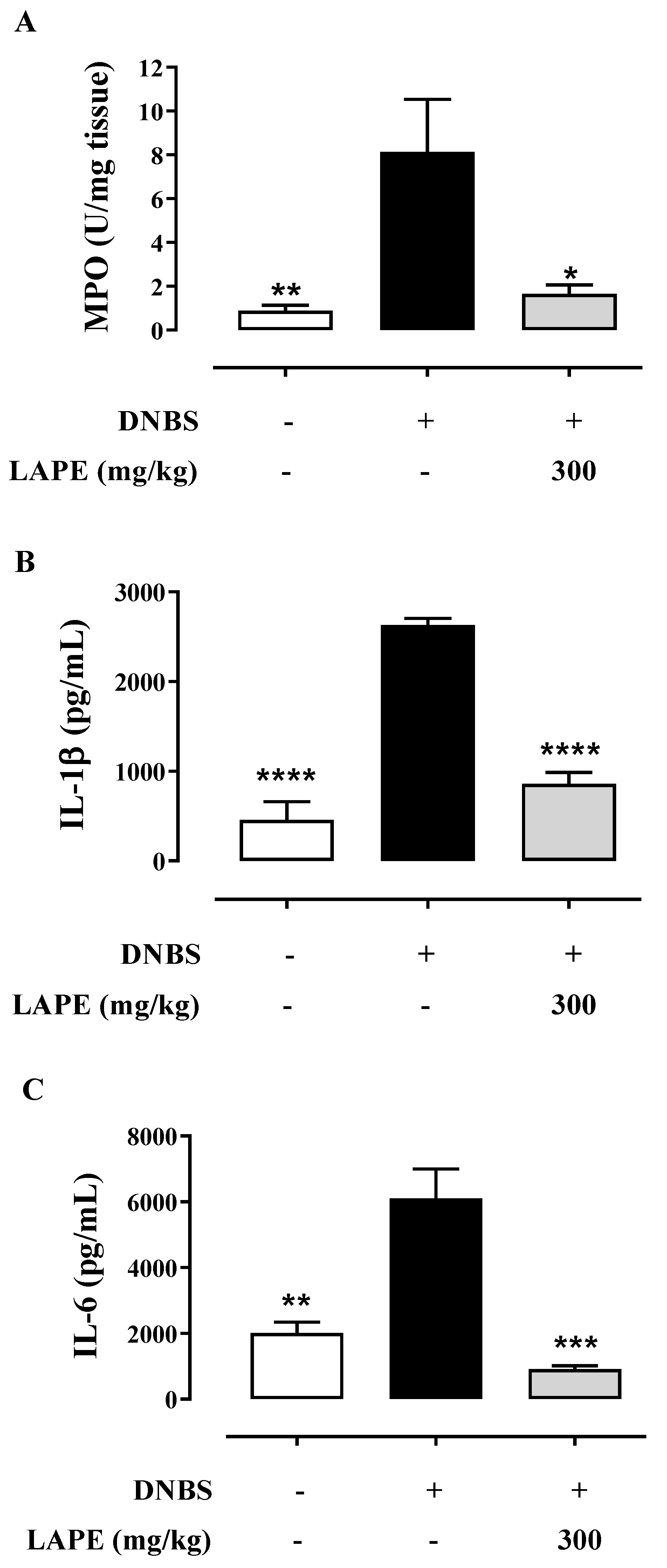
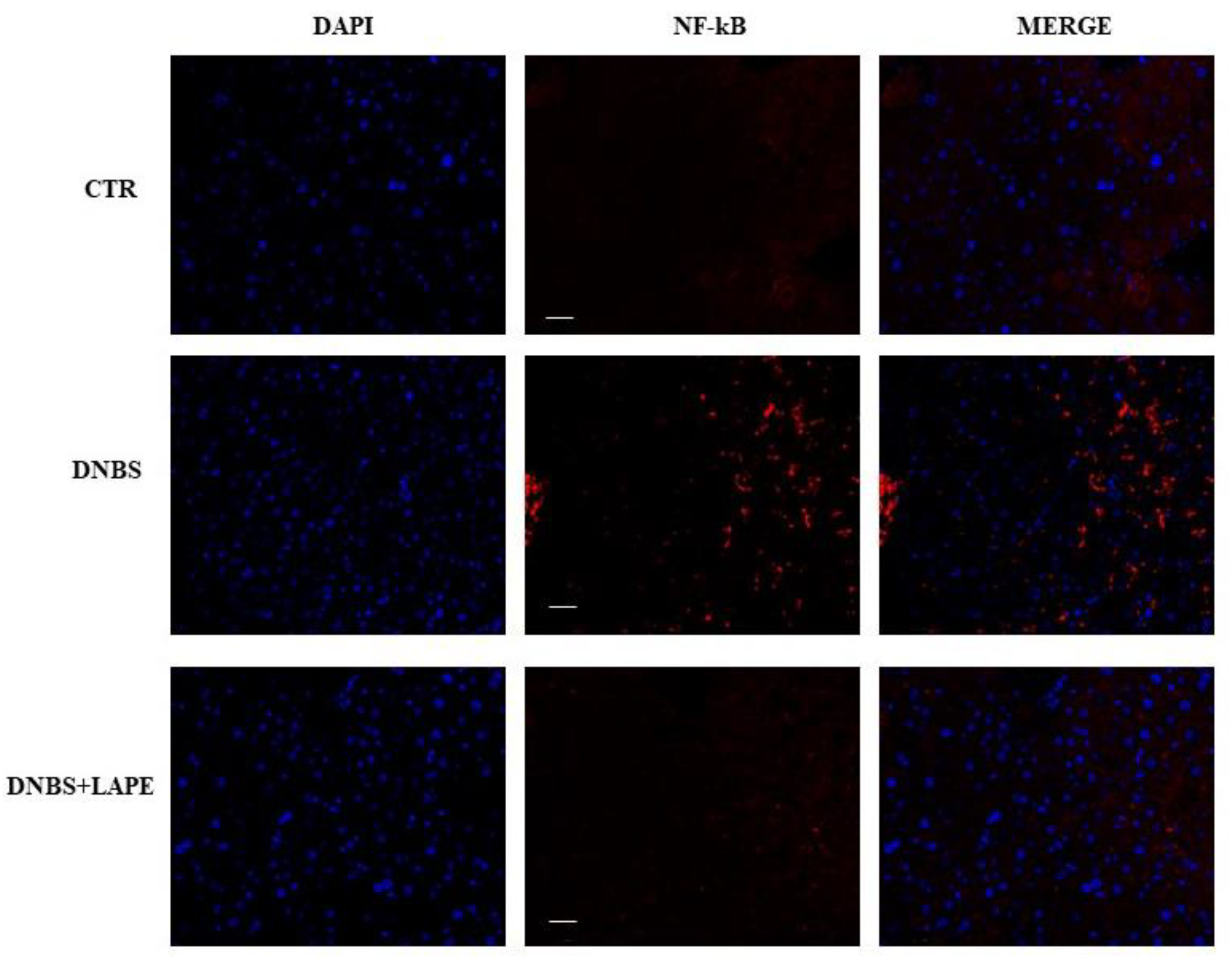
2.3. LAPE Restores Colonic Neutrophil Infiltration and Cytokine Production in DNBS-Treated Mice
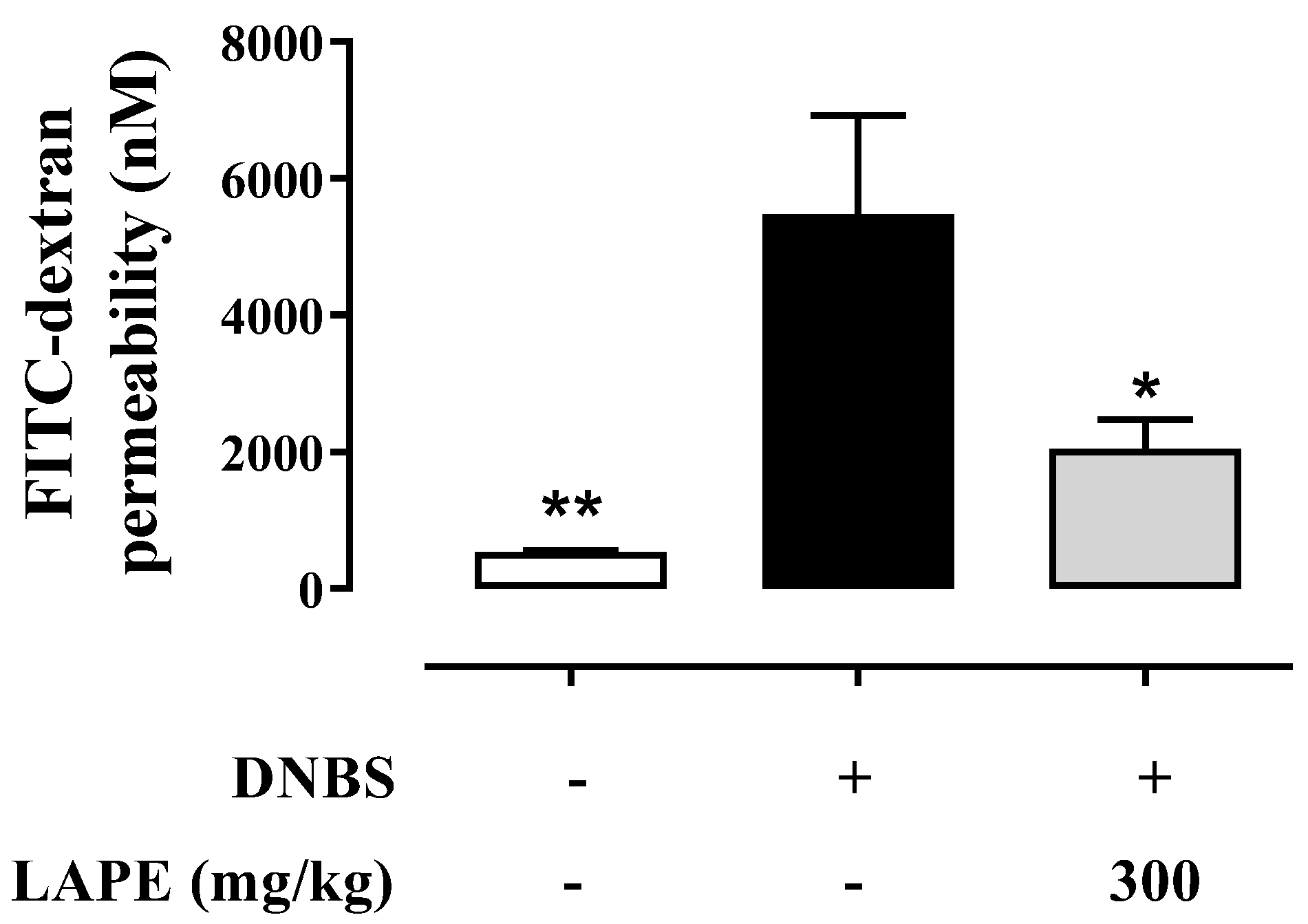
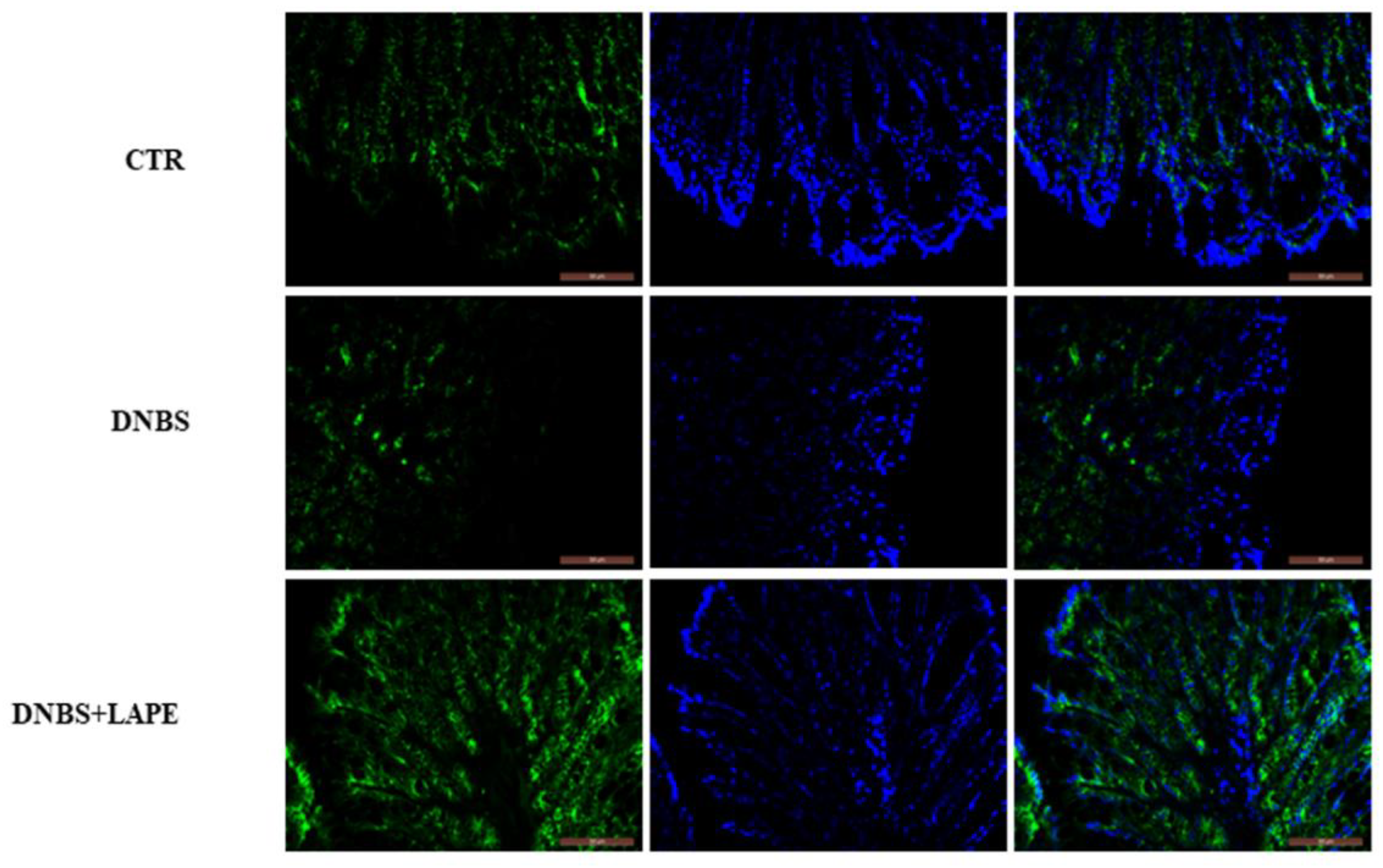
2.4. LAPE Restores the Intestinal Permeability in the DNBS Model of Colitis
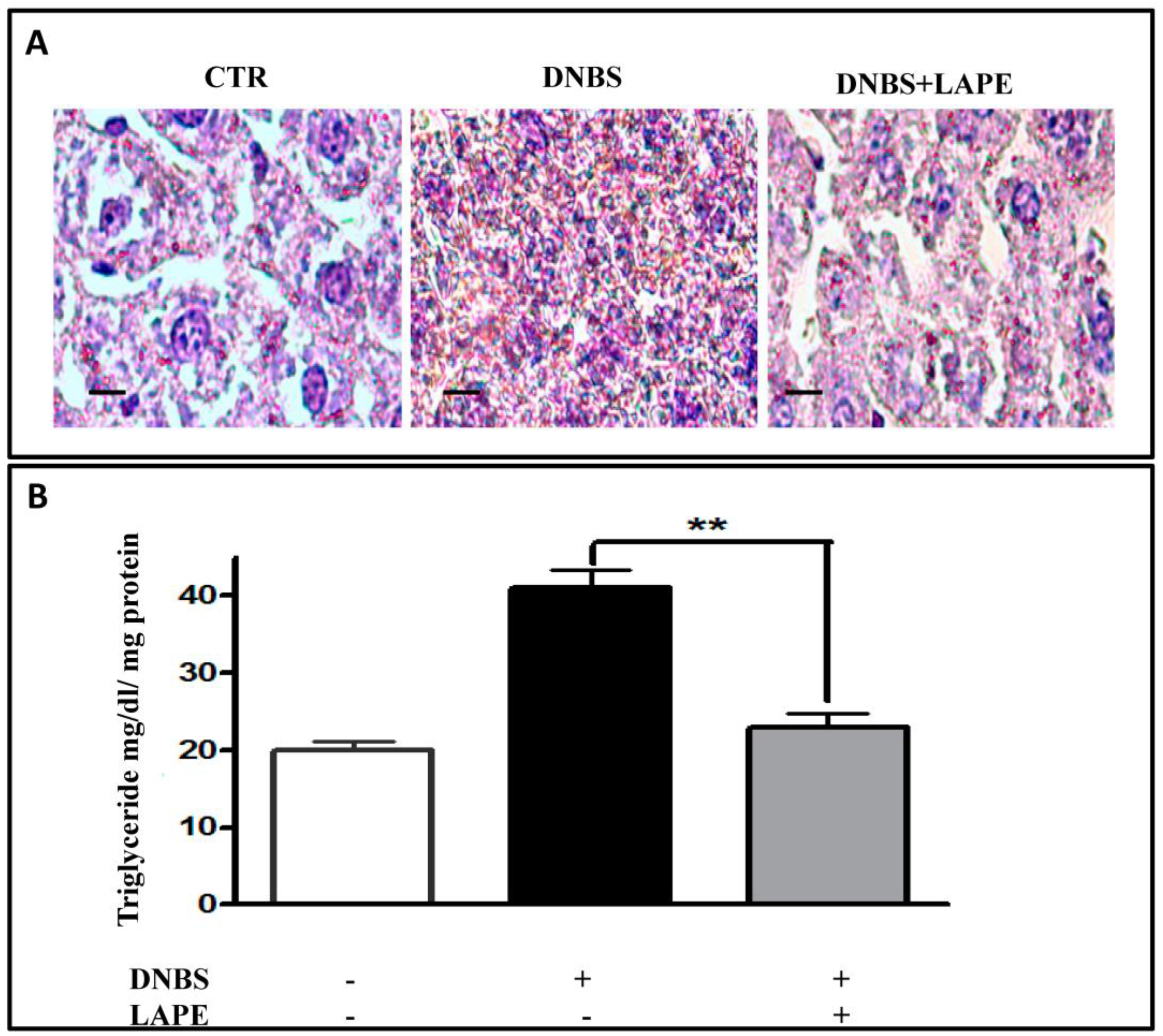
2.5. LAPE Reduces Liver Dysfunction and Hepatic Lipidic Accumulation in the DNBS Model of Colitis
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Limoncella Apple Polyphenol Extract (LAPE) Preparation
4.3. HPLC/DAD Analysis
4.4. Animals
4.5. DNBS-Induced Experimental Colitis and Pharmacological Treatments
4.6. In Vivo Intestinal Permeability
4.7. Measurements of Myeloperoxidase (MPO) Activity
4.8. Measurement of Colonic Cytokine Production
4.9. Histological Analysis
4.10. Serum ALT and AST and Lipid Peroxidation Analysis
4.11. Immuno-Fluorescence Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulkarni, N.; Manisha, P.; Girdhari, L. Role of chemokine receptors and intestinal epithelial cells in the mucosal inflammation and tolerance. J. Leukoc. Biol. 2017, 101, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxidative Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef]
- Fox-Robichaud, A.; Kubes, P. Molecular mechanisms of tumor necrosis factor α–stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology 2000, 31, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Chen, L.; You, Q.; Hu, L.; Gao, J.; Meng, Q.; Liu, W.; Wu, X.; Xu, Q. The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice. Front. Immunol. 2018, 8, 1910–1924. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Calabrese, G.; Stiuso, P.; Ritieni, A.; Giannetti, D.; Novellino, E. Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: A source of nutraceuticals potentially indicated for the metabolic syndrome. Food Res. Int. 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010, 29, 383–394. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Pacifico, S.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. “Limoncella” apple, an Italian apple cultivar: Phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Riccio, G.; Maisto, M.; Bottone, S.; Badolati, N.; Rossi, G.B.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. WNT Inhibitory Activity of Malus Pumila miller cv Annurca and Malus domestica cv Limoncella Apple Extracts on Human Colon-Rectal Cells Carrying Familial Adenomatous Polyposis Mutations. Nutrients 2017, 9, 1262. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.A.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef]
- Venditti, M.; Santillo, A.; Falvo, S.; Di Fiore, M.M.; Chieffi Baccari, G.; Minucci, S. D-Aspartate Upregulates DAAM1 Protein Levels in the Rat Testis and Induces Its Localization in Spermatogonia Nucleus. Biomolecules 2020, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Amamou, A.; Rouland, M.; Yaker, L.; Goichon, A.; Guérin, C.; Aziz, M.; Savoye, G.; Marion-Letellier, R. Dietary salt exacerbates intestinal fibrosis in chronic TNBS colitis via fibroblasts activation. Sci. Rep. 2021, 11, 15055. [Google Scholar] [CrossRef]
- Almenier, H.A.; Al Menshawy, H.H.; Maher, M.M.; Al Gamal, S. Oxidative stress, and inflammatory bowel disease. Front. Biosci. 2012, 4, 1335–1344. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, J.; Qian, W.; Zhang, L.; Hou, X. Gut inflammation exacerbates hepatic injury in the high-fat diet induced NAFLD mouse: Attention to the gut-vascular barrier dysfunction. Life Sci. 2018, 209, 157–166. [Google Scholar] [CrossRef]
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 157–165. [Google Scholar] [CrossRef]
- Farzaei, M.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef]
- Michielan, A.; D’Inca, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Nejak-Bowen, K.; Kikuchi, A.; Monga, S.P. Beta-catenin-NF-kappaB interactions in murine hepatocytes: A complex to die for. Hepatology 2013, 57, 763–774. [Google Scholar] [CrossRef]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef]

| AST International Units per Liter (IU/L) | ALT International Units per Liter (IU/L) | TBARS μM/μg Protein | |
|---|---|---|---|
| CTR | 77 ± 9 * | 39 ± 6 ** | 0.095 ± 0.01 * |
| DNBS | 418 ± 51 | 185 ± 37 | 0.38 ± 0.09 |
| DNBS+LAPE | 263 ± 25 *** | 87 ± 19 | 0.12 ± 0.05 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lama, S.; Pagano, E.; Borrelli, F.; Maisto, M.; Tenore, G.C.; Nanì, M.F.; Chacon-Millan, P.; Novellino, E.; Stiuso, P. Polyphenol-Rich Extract from ‘Limoncella’ Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage. Int. J. Mol. Sci. 2024, 25, 3210. https://doi.org/10.3390/ijms25063210
Lama S, Pagano E, Borrelli F, Maisto M, Tenore GC, Nanì MF, Chacon-Millan P, Novellino E, Stiuso P. Polyphenol-Rich Extract from ‘Limoncella’ Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage. International Journal of Molecular Sciences. 2024; 25(6):3210. https://doi.org/10.3390/ijms25063210
Chicago/Turabian StyleLama, Stefania, Ester Pagano, Francesca Borrelli, Maria Maisto, Gian Carlo Tenore, Maria Francesca Nanì, Pilar Chacon-Millan, Ettore Novellino, and Paola Stiuso. 2024. "Polyphenol-Rich Extract from ‘Limoncella’ Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage" International Journal of Molecular Sciences 25, no. 6: 3210. https://doi.org/10.3390/ijms25063210
APA StyleLama, S., Pagano, E., Borrelli, F., Maisto, M., Tenore, G. C., Nanì, M. F., Chacon-Millan, P., Novellino, E., & Stiuso, P. (2024). Polyphenol-Rich Extract from ‘Limoncella’ Apple Variety Ameliorates Dinitrobenzene Sulfonic Acid-Induced Colitis and Linked Liver Damage. International Journal of Molecular Sciences, 25(6), 3210. https://doi.org/10.3390/ijms25063210










