Abstract
Flavonoids are a large family of polyphenolic compounds with important agro-industrial, nutraceutical, and pharmaceutical applications. Among the structural diversity found in the flavonoid family, methylated flavonoids show interesting characteristics such as greater stability and improved oral bioavailability. This work is focused on the reconstruction of the entire biosynthetic pathway of the methylated flavones diosmetin and chrysoeriol in Streptomyces albidoflavus. A total of eight different genes (TAL, 4CL, CHS, CHI, FNS1, F3′H/CPR, 3′-OMT, 4′-OMT) are necessary for the heterologous biosynthesis of these two flavonoids, and all of them have been integrated along the chromosome of the bacterial host. The biosynthesis of diosmetin and chrysoeriol has been achieved, reaching titers of 2.44 mg/L and 2.34 mg/L, respectively. Furthermore, an additional compound, putatively identified as luteolin 3′,4′-dimethyl ether, was produced in both diosmetin and chrysoeriol-producing strains. With the purpose of increasing flavonoid titers, a 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate synthase (DAHP synthase) from an antibiotic biosynthetic gene cluster (BGC) from Amycolatopsis balhimycina was heterologously expressed in S. albidoflavus, enhancing diosmetin and chrysoeriol production titers of 4.03 mg/L and 3.13 mg/L, which is an increase of 65% and 34%, respectively. To the best of our knowledge, this is the first report on the de novo biosynthesis of diosmetin and chrysoeriol in a heterologous host.
1. Introduction
Flavonoids are a large class of polyphenols, representing around 9000 compounds that are widely distributed in plants [1,2,3]. These bioactive compounds are important in the nutraceutical, pharmaceutical, cosmetic, and agro-industrial fields due to the vast variety of properties they display [4,5,6], such as antitumor [7,8,9], antimicrobial, antiangiogenic [8,9], antioxidant, and neuroprotective compounds, among other bioactivities [10]. Flavonoids undergo diverse enzymatic modifications in their core structure, such as hydroxylations, glycosylations, or methylations, and in particular, methylated flavonoids possess interesting properties, such as major stability, improved oral bioavailability, enhanced membrane transport, and better intestinal absorption [11]. In the case of the methylated flavone diosmetin, which is widely present (as a glucosylated derivative) in several Citrus fruits, it has been reported to have antioxidant, phlebotonic, oestrogenic, antimicrobial, and anti-inflammatory activities [12]. As an antimicrobial, it should be noted that in combination with erythromycin, diosmetin shows a synergistic effect against methicillin-resistant Staphylococcus aureus (MRSA) [13]. On the other hand, chrysoeriol, another luteolin methylated derivative, has been found in several plants, such as Reseda luteola, Melientha suavis, and Cardiospermum halicacabum L. This methylated flavonoid is of interest due to its potential as an anti-hyperlipidemic, antitumor, antioxidant, antimicrobial, antifungal, and neuroprotective agent, among other bioactivities [14].
Depending on their structural differences, flavonoids are classified into seven subclasses: flavanones, flavones, isoflavones, flavonols, anthocyanidins, flavanols, and chalcones [15]. For the heterologous biosynthesis of flavonoids in bacteria, several enzymatic steps must take place, starting with the conversion of L-tyrosine in coumaric acid by the tyrosine ammonia lyase (TAL). Then, coumaric acid should be activated with the molecule coenzyme-A by the action of 4-coumaroyl-CoA ligase (4CL), giving rise to 4-coumaroyl-CoA, which would be then condensed with three molecules of malonyl-CoA through chalcone synthase (CHS), generating naringenin chalcone, the basic carbon skeleton for all flavonoids known in nature [16,17,18,19]. Finally, the heterocycle closure in naringenin chalcone is carried out by a chalcone isomerase (CHI), generating the universal flavanone precursor, naringenin. For the biosynthesis of diosmetin and chrysoeriol, three extra enzymatic steps are necessary. Naringenin should be converted to apigenin by a flavone synthase. In the bacterial host S. albidoflavus, a class I flavone synthase (FNS1) is necessary, lacking the need for a cytochrome P450 membrane-bound monooxygenase, which would be difficult to express in bacteria [20]. Apigenin should then be converted to luteolin by a flavone 3′ hydroxylase. In this work, a flavone 3′ hydroxylase coupled with a soluble cytochrome P450 reductase (F3′H-CPR) has been used, providing the reducing power to the flavone 3′ hydroxylase [21]. Finally, a 4′-O-methyltransferase (4′OMT) is necessary to reach the heterologous biosynthesis of diosmetin, and a 3′-O-methyltransferase (3′OMT) is required for the heterologous production of chrysoeriol.
The biosynthesis of methylated flavonoids has been achieved using different microbial cell factories, such as Escherichia coli [22] and Saccharomyces cerevisiae [23]. Additionally, in recent work by our research group, the biosynthesis of this type of flavonoid has been achieved in the Gram-positive bacterium S. albidoflavus [24]. However, a major bottleneck in the heterologous biosynthesis of flavonoids in bacteria using synthetic biology tools is the low production titters. Several strategies have been applied to increase the intracellular pools of flavonoid precursors, such as the redirection of the carbon source for the bacteria towards the biosynthesis of malonyl-CoA [25], a key precursor in the flavonoid pathway.
So far, the biosynthesis of diosmetin and chrysoeriol had never been carried out in a heterologous host. In this work, we present the production of both methylated flavones after dissecting their biosynthetic pathways into three modules, leveraging different integration sites of the bacteriophages ϕC31 [26] and ϕBT1 [27], in addition to the integration site of the pSAM2 plasmid [28] in the chromosome of S. albidoflavus, enabling the stable incorporation of exogenous DNA into the bacterial chromosome (across three different sections, corresponding to three different steps during flavonoid biosynthesis) and avoiding the necessity for antibiotic selection to maintain plasmids.
As a further step towards the enhancement of the final production titers of these two methylated flavones in S. albidoflavus, the gene encoding a DAHP synthase from the actinomycete Amycolatopsis balhimycina [29] has been added together with the diosmetin and chrysoeriol biosynthetic pathways. This enzyme, DAHP, carries out the condensation of phosphoenolpyruvate (PEP) and erythrose 4-phosphate (E4P) [30], the first enzymatic reaction in the shikimate pathway towards the biosynthesis of aromatic amino acids, such as the flavonoid precursor L-tyrosine.
The current industrial production of diosmetin is based on semi-synthesis approaches from its glycosylated form, diosmin. This semi-synthesis makes use of concentrated sulfuric acid and several crystallization steps, which lower the final efficiency of the whole process. Other industrial alternatives include the use of hesperidin (a flavanone from orange peels), which is oxidized under hot alcoholic sodium acetate (with its sugar moiety removed chemically) to generate diosmetin [31,32]. In this work, the need for flavonoid precursors (hesperidin, diosmin, etc.) is not necessary for obtaining the final bioactive compounds, as these are generated de novo, using common metabolic intermediates available in the bacterial cytoplasm.
2. Results
2.1. Heterologous Biosynthesis of Diosmetin
For the biosynthesis of diosmetin in bacteria, the action of seven enzymes (TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR, and 4′OMT) is required (in contrast to the eight necessary genes in plants). In this work, this whole biosynthetic pathway was divided into three modules (Figure 1), and the necessary coding genes were distributed along the chromosome of the strain S. albidoflavus UO-FLAV-004 [24], taking advantage of the different prophage integration sites in this species. All the genes were codon-optimized for S. albidoflavus. TAL, 4CL, CHS, and CHI genes were integrated into the ϕC31 attb site [24], FNS1 [24] and F3′H-CPR [21] genes were integrated into the ϕBT1 attb site, and the 4′OMT gene was integrated into the pSAM2 site of the chromosome.
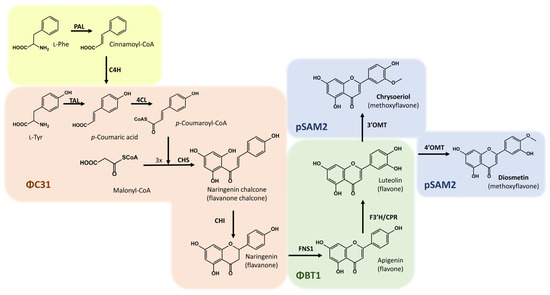
Figure 1.
Biosynthetic pathway for the biosynthesis of diosmetin and chrysoeriol. The yellow section indicates the canonical first steps in plants. Tyrosine ammonia-lyase (TAL); 4-Coumaroyl-CoA ligase (4CL); Chalcone synthase (CHS); Chalcone isomerase (CHI); Flavone synthase (FNS1); Flavonoid 3′ hydroxylase/Cytochrome P450 reductase chimera (F3′H/CPR); 4′-O-methyltransferase (4′OMT); 3′-O-methyltransferase (3′OMT). In light orange, the part of the pathway integrated into the ϕC31 attb site of the chromosome is shown; in light green, the part of the pathway integrated into the ϕBT1 attb site of the chromosome is shown; in light blue, the final part of the pathway for the heterologous biosynthesis of diosmetin or chrysoeriol is shown, with both cases integrated into the pSAM2 site of the S. albidoflavus chromosome. The yellow section indicates the naturally occurring pathway in plants, using L-phenylalanine instead of L-tyrosine.
The selected 4′OMT is a hypothetical class I SAM-dependent O-methyltransferase (M444_29925) from Streptomyces sp. Mg1 (see Section 4). This gene was assembled under the control of the SP25 promoter, which shows good transcriptional activity, being higher than the widely used PermE* promoter [33]. The gene M444_29925 was selected after a BLASTP analysis against the O-methyltransferase GerMIII, which shows a relative in vitro conversion rate of luteolin to diosmetin of 67% [34]. The differences between GerMIII and M444_29925 at the protein level lie in the amino acids 81 (A-P) and 377 (T-I).
After the corresponding fermentation experiments, the control strain S. albidoflavus UO-FLAV-004-LUT, which harbors the genes TAL, 4CL, CHS, CHI, FNS1, and F3′H-CPR, as well as an empty plasmid integrated into the pSAM2 chromosomal site, was able to produce the unmethylated precursor of diosmetin and luteolin, reaching titers of 4.61 mg/L (Figure 2A). The diosmetin-producing strain S. albidoflavus UO-FLAV-004-DIO, which additionally containins the gene coding for the M444_29925 4′-O-methyltransferase integrated into the pSAM2 chromosomal site, generated 2.44 mg/L of diosmetin and 0.73 mg/L of luteolin. Surprisingly, a small peak of chrysoeriol was also detected in the chromatogram, indicating the capability of this strain to produce the luteolin 3′-O-methyl ether (chrysoeriol) at titers of 0.38 mg/L as well (Figure 2B).
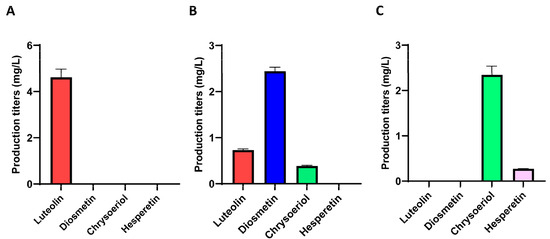
Figure 2.
Production titers of different flavonoids in different strains derived from S. albidoflavus UO-FLAV-004. (A) S. albidoflavus UO-FLAV-004-LUT; (B) S. albidoflavus UO-FLAV-004-DIO; (C) S. albidoflavus UO-FLAV-004-CHR.
2.2. Heterologous Biosynthesis of Chrysoeriol
The heterologous biosynthesis of chrysoeriol was carried out using the same distribution of genes as in the previous case. The same enzymatic activities required for the heterologous biosynthesis of diosmetin were necessary to produce the common precursor, luteolin, but a different O-methyltransferase was needed to introduce the methyl moiety at position 3′ instead of position 4′ of the ring B of luteolin (Figure 1). The 3′-O-methyltransferase used in the biosynthesis of chrysoeriol was a CCoAOMT-like enzyme from Arabidopsis thaliana (At4g26220), whose gene was optimized for S. albidoflavus (Table 1). This enzyme possessed a greater preference for introducing a methyl group in the para position (4′) in flavanones and dihydroflavonols, whereas flavones and flavonols were methylated in the meta position (3′) [35]. Using this enzyme, the biosynthesis of chrysoeriol in the strain S. albidoflavus UO-FLAV-004-CHR was achieved, reaching titers of 2.34 mg/L, with no remaining luteolin detected. Also, a small peak corresponding to hesperetin was detected in the chromatogram of this culture extract, and its production reached 0.27 mg/L (Figure 2C). The presence of this compound in this extract will be addressed in the Section 3. The strain used as control for this fermentation was S. albidoflavus UO-FLAV-004-LUT, as in the previous case.
2.3. Identification of Putative Luteolin 3′,4′-Dimethyl Ether in Both Diosmetin- and Chrysoeriol-Producing Strains
After analyzing the chromatograms of the two different producing strains, an extra differential peak was also detected at late retention times (35.4 min), with a higher intensity in the case of the strain S. albidoflavus UO-FLAV-004-DIO (Figure 3). A late retention time indicates less polarity in the used HPLC program (see Section 4), which suggested that this peak could correspond to di-methylated luteolin, a less polar compound than a single methylated luteolin. The available positions in diosmetin for O-methylation were positions 5 and 7 of ring A and position 3′ of ring B, while for chrysoeriol, the available positions were positions 5 and 7 of ring A and position 4′ of ring B. Since standards for all possible combinations were not available, a feeding assay was performed to confirm or discard our first putative candidate, which putatively was considered luteolin 3′,4′-dimethyl ether.
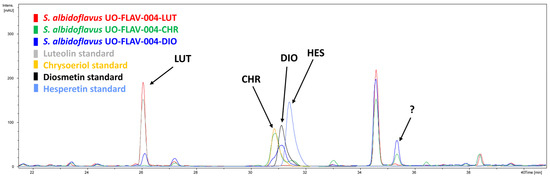
Figure 3.
HPLC-DAD chromatograms of the strains S. albidoflavus UO-FLAV-004-LUT (red), S. albidoflavus UO-FLAV-004-CHR (green), S. albidoflavus UO-FLAV-004-DIO (blue). The four commercial standards are also indicated. Luteolin (LUT); Chrysoeriol (CHR); Diosmetin (DIO); Hesperetin (HES); Unknown differential peak (?).
In the strains S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-CHR, one of the enzymes in the heterologous biosynthetic pathway was the FNS1 flavone synthase from Petroselinum crispum, which has been described as being able to transform homoeriodictyol to chrysoeriol with high efficiency [36] (Figure 4). Taking this into account, since the strain S. albidoflavus UO-FLAV-004-CHR harbored the necessary genes for the biosynthesis of hesperetin (eriodictyol 4′-O-methyl ether), such as the O-methyltransferase At4g26220 [35] (Figure 4), hesperetin could be converted to homohesperetin (eriodictyol 3′,4′-dimethyl ether) by an endogenous O-methyltransferase activity of S. albidoflavus (see Section 3). Due to the structural similarity between homoeriodictyol (eriodictyol 3′-O-methyl ether) and homohesperetin (eriodictyol 3′,4′-O-methyl ether), we hypothesize that the FNS1 enzyme could be acting on the homohesperetin flavanone to produce the luteolin 3′,4′-dimethyl ether flavone (Figure 4).
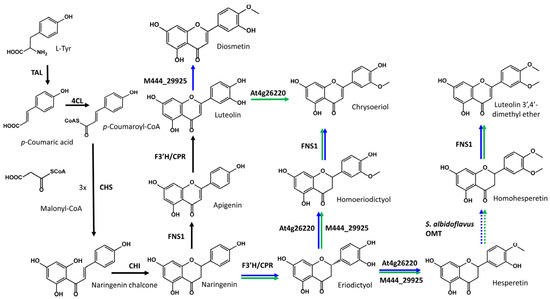
Figure 4.
Proposed heterologous biosynthetic pathway for the production of diosmetin, chrysoeriol, and luteolin 3′,4′-dimethyl ether in S. albidoflavus. Black arrows connect the necessary reactions to reach the biosynthesis of luteolin. Blue arrows represent the reactions carried out in the strain S. albidoflavus UO-FLAV-004-DIO. Finally, green arrows represent the reactions carried out in the strain S. albidoflavus UO-FLAV-004-CHR. Dashed arrows indicate a reaction carried out by an endogenous enzyme of S. albidoflavus.
With the aim of proving the presence of this extra activity in FNS1, a feeding was made with homohesperetin at a final concentration of 0.1 mM to the strain S. albidoflavus UO-FLAV-004-FNS1, previously developed by our research group [24], and to the strain S. albidoflavus UO-FLAV-004 as a control. Analysis of the HPLC-DAD chromatograms generated showed the presence of a derivative peak in the strain containing FNS1, which was co-eluting with the extra peaks observed in the diosmetin- and chrysoeriol-producing strains. This new peak was also showed the same UV absorption spectrum as the putative luteolin 3′,4′-dimethyl ether from the diosmetin- and chrysoeriol-producing strains (Figure S1). These results suggested that the strain S. albidoflavus UO-FLAV-004-CHR was able to produce luteolin 3′,4′-dimethyl ether through this pathway (Figure 4), and it indicated that homohesperetin could be a good substrate for the FNS1 enzyme.
On the other hand, the strain S. albidoflavus UO-FLAV-004-DIO, which also produced this putative luteolin di-methylated derivative, albeit at a slightly higher concentration, should not be able to produce it through the same pathway as S. albidoflavus UO-FLAV-004-CHR, since the gene At4g26220 was not present in this strain. A feasible alternative to explain the production of this compound in this strain was the conversion of eriodictyol to hesperetin by the action of the enzyme 4′OMT of Streptomyces sp. Mg1, an activity that had not been reported so far. To check the substrate flexibility of this enzyme, a feeding experiment using eriodictyol was performed on the strain S. albidoflavus UO-FLAV-004-M444_29925, harboring only the 4′OMT of Streptomyces sp. Mg1 in the pSAM2 chromosomal integration site, along with the corresponding control strain S. albidoflavus UO-FLAV-004 harboring an empty plasmid in the same chromosomal attb site. The feeding results were analyzed by HPLC-DAD and showed a good conversion of eriodictyol to both hesperetin (eriodictyol 4′-O-methyl ether) and homoeriodictyol (eriodictyol 3′-O-methyl ether) in S. albidoflavus UO-FLAV-004-M444_29925. Additionally, a peak of homohesperetin was detected after the feeding (Figure S2). No eriodictyol derivative was observed in the control strain. This suggested that hesperetin was generated in the strain S. albidoflavus UO-FLAV-004-DIO by the enzyme M444_29925 and converted to homohesperetin by the putative endogenous O-methyltransferase activity of S. albidoflavus, finally being converted to luteolin 3′,4′-dimethyl ether by the action of FNS1, like in the case observed in the strain S. albidoflavus UO-FLAV-004-CHR (Figure 4).
2.4. Use of a DAHP Synthase to Increase the Production Titers of Diosmetin and Chrysoeriol through Precursor Titer Enhancement
DAHP synthase is the first enzyme of the shikimate pathway. This enzyme condenses D-erythrose 4-phosphate and phosphoenolpyruvate to produce DAHP, a key precursor in the biosynthesis of aromatic amino acids, such as L-tyrosine (Figure 5). L-tyrosine is the first precursor in the heterologous pathway for flavonoid biosynthesis in S. albidoflavus, making the overexpression of the DAHP synthase an interesting strategy to enhance the intracellular pools of L-tyrosine and, thus, the final flavonoid titers. This strategy was followed by Thykaer and colleagues to increase the titers of the vancomycin analogue balhimycin in the natural producing strain of Amycolatopsis balhimycina, resulting in improved specific productivities of balhimycin by introducing an extra copy of the dahp gene [29].
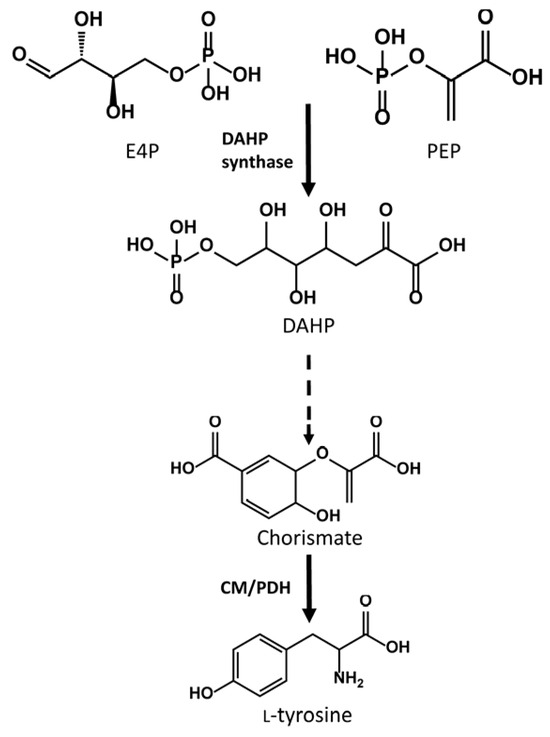
Figure 5.
Abbreviated schema of the shikimate pathway for the generation of L-tyrosine. E4P: Erythrose 4-phosphate; PEP: phosphoenolpyruvate; DAHP: 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate: CM/PDH: chorismate mutase/prephenate dehydrogenase.
In this work, a codon optimization of the dahp gene of Amycolatopsis balhimycina was conducted for S. albidoflavus, and the gene was assembled under the control of the strong SP25 promoter. Then, this gene was brought together with the different O-methyltransferases involved in diosmetin and chrysoeriol biosynthesis (see Section 4) and integrated into the pSAM2 chromosomal site of S. albidoflavus UO-FLAV-004-LUT parental strain, giving rise to the final strains S. albidoflavus UO-FLAV-004-DIO-dahp and S. albidoflavus UO-FLAV-004-CHR-dahp, respectively. These new strains were cultivated at the same time as the strains S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-CHR, used as controls, to check if the enzyme DAHP synthase was able to increase the titers of diosmetin and chrysoeriol. The resulting production titers were 4.03 mg/L of diosmetin for S. albidoflavus UO-FLAV-004-DIO-dahp and 3.13 mg/L of chrysoeriol for S. albidoflavus UO-FLAV-004-CHR-dahp, which represents a 1.65-fold and 1.34-fold increase, respectively (Figure 6). Also, as expected, the by-products chrysoeriol and hesperetin were present in the strains S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-CHR, respectively, as well as the putative luteolin 3′,4′-dimethyl ether, all in proportionally increased quantities in the strains S. albidoflavus UO-FLAV-004-DIO-dahp and S. albidoflavus UO-FLAV-004-CHR-dahp (Figures S3 and S4).
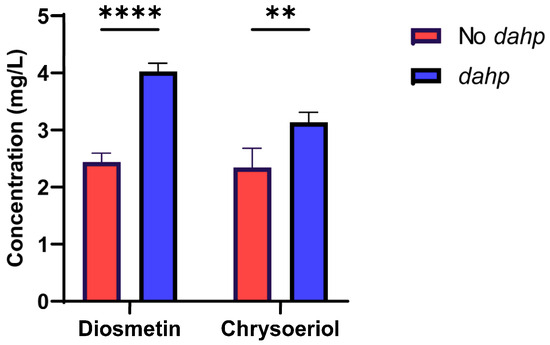
Figure 6.
Effect of the DAHP synthase on the biosynthesis of diosmetin and chrysoeriol in the strains S. albidoflavus UO-FLAV-004-DIO-dahp and S. albidoflavus UO-FLAV-004-CHR-dahp compared to the strains S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-CHR, respectively. Asterisks indicate statistically significant differences (** p <0.005; **** p <0.0001).
3. Discussion
Previous studies conducted by our research group have revealed the potential of S. albidoflavus for the biosynthesis of methylated derivatives of flavonoids [24]. To our knowledge, this work describes, for the first time, the complete biosynthesis of the methylated flavonoids diosmetin and chrysoeriol in a heterologous host.
The enzyme M444_29925 shares 98% identity with the well-studied GermIII O-methyltransferase, which shows high regiospecificity for the 4′ position of flavones [34]. Along the biosynthetic pathway of diosmetin, the reactions can also occur in a different order. If the chimeric F3′H-CPR hydroxylase uses naringenin as a substrate before FNS1, the strain will first produce eriodictyol. A difference between flavones and flavanones is the spatial configuration of their chemical structures. Flavones present a planar structure [37], while flavanones present a chair conformational structure [38]. According to this, we hypothesize that the enzyme M444_29925 may introduce a methyl group in a different position in a flavanone structure, such as eriodictyol, instead of the 4′ position, as observed in flavones. With the aim of finding out if O-methylation in a different position is possible for this enzyme, a feeding experiment with eriodictyol was performed on a strain containing only the O-methyltransferase M444_29925, named S. albidoflavus UO-FLAV-004-M444_29925, and the strain S. albidoflavus UO-FLAV-004 as a control. As shown previously, we verified that this enzyme was able to methylate eriodictyol, at least, at positions 4′ and 3′, yielding hesperetin (eriodictyol 4′-O-methyl ether) and homoeriodictyol (eriodictyol 3′-O-methyl ether) (Figure 4). The generation of homoeriodictyol through this enzyme can explain the presence of chrysoeriol in the strain S. albidoflavus UO-FLAV-004-DIO due to the action of FNS1 (Figure 4) [36].
On the other hand, the presence of hesperetin in the chrysoeriol-producing strain is easy to explain since the CcoAOMT-like enzyme At4g26220 efficiently produces hesperetin from eriodictyol in vitro (Figure 4) [35]. At4g26220 also produces homoeriodictyol [35], but again, FNS1 converts it to chrysoeriol.
Finally, as described in the Section 2, homohesperetin was detected after the administration of eriodictyol to the strain S. albidoflavus UO-FLAV-004-M444_29925. Given that luteolin 3′,4′-dimethyl ether is a direct derivative of homohesperetin following enzymatic mediation by FNS1, and this proposed compound is discernible in both S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-CHR strains, it is inferred that homohesperetin should be inherently present in both the diosmetin- and chrysoeriol-producing strains. This deduction is supported by the detection of the putative luteolin 3′,4′-dimethyl ether in both strains. Consequently, these results imply that homohesperetin is likely produced through an unknown endogenous activity of S. albidoflavus in the presence of hesperetin and/or homoeriodictyol.
Thus, if homohesperetin is found in the bacterial cytoplasm at any moment during the biosynthesis of diosmetin and chrysoeriol, it can be transformed into luteolin 3′,4′-dimethyl ether by the action of the FNS1. These results highlight the important role that substrate specificity of the selected enzymes plays in the heterologous biosynthesis of natural products, and it may be the case that these enzymes compete for different molecules found at different stages of a given pathway, generating different derivatives. However, this could also be advantageous since new unexpected products could be obtained, such as the putative luteolin 3′,4′-dimethyl ether, establishing new pathways to produce them.
Regarding the biosynthesis of diosmetin and chrysoeriol, attempts were made to increase production titers by using a DAHP synthase. The biosynthesis of aromatic amino acids is strictly regulated by feedback inhibition mechanisms, and DAHP synthases are normally feedback regulated by L-tyrosine, L-phenylalanine, or both [39]. The strategy of using a DAHP synthase to enhance the flavonoid titters has been carried out by different researchers. Koopman and colleagues achieved an increase in naringenin titters using different approaches in Saccharomyces cerevisiae, including the alleviation of feedback inhibition of yeast DAHP synthase by introducing a L-tyrosine insensitive ARO4 (ARO4G226S) allele in conjunction with the deletion of the other allele of the dahp gene [40]. In another experiment in E. coli, the overexpression of a feedback-resistant derivative of dahp (aroGfbr), together with the overexpression of chorismate mutase/prephenate dehydrogenase (tyrAfbr), led to an increase in naringenin [41,42], apigenin, and genkwanin titters [43].
Here, a DAHP synthase from the actinomycete Amycolatopsis balhimycina has been used, which has been previously proved as a useful metabolic engineering strategy to increase the biosynthesis of glycopeptide antibiotics by introducing an extra copy in its natural producer. In A. balhimycina, the gene encoding this DAHP synthase is found in a chromosomal region containing a BGC and is not involved in primary metabolism [29]. Other dahp genes have been identified in other BGCs [44,45], and in these cases, the dahp genes were like those encoding the plant type DAHP synthases, which were proven to be naturally resistant to feedback inhibition by aromatic amino acids [46,47]. Here, we have selected this gene to be assayed in the engineered strain S. albidoflavus UO-FLAV-004, as it lacks the negative feedback inhibition by the final shikimate pathway product (L-tyrosine). In this way, a significant increase in the biosynthesis of diosmetin (1.65-fold) and chrysoeriol (1.34-fold) has been achieved, proving the positive effect on flavonoid biosynthesis of this DAHP synthase gene from Amycolatopsis balhimycina, placed in this case under the regulation of a strong constitutive promoter.
This study serves as proof of the suitability of S. albidoflavus for the biosynthesis of methylated flavonoids and reveals the efficacy of using a gene coding for a DAHP synthase from a BGC of another actinomycete bacterium, which lacks feedback inhibition by aromatic amino acids (such as the primary flavonoid precursor L-tyrosine), therefore allowing the enhancement of flavonoid titers.
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
All strains used in this study are listed in Table 1. Escherichia coli TOP10 (Invitrogen, Waltham, MA, USA) was used for routine subcloning. E. coli ET12567/pUZ8002 [48] was used for conjugation. The strain used in this study for the heterologous biosynthesis of diosmetin and chrysoeriol was S. albidoflavus UO-FLAV-004-NAR [24]. To achieve the heterologous biosynthesis of these two methylated flavones, their biosynthetic pathways were divided into three parts. The first part is the BGC for naringenin biosynthesis, containing the enzymes TAL, 4CL, CHS, and CHI. The genes coding for the enzymes of this BGC were already integrated into the ϕC31 attb site of the strain S. albidoflavus UO-FLAV-004-NAR. A second plasmid containing the genes coding for the enzymes FNS1 and the chimera F3′H-CPR was integrated into the ϕBT1 attb site, giving rise to the strain S. albidoflavus UO-FLAV-004-LUT, which is able to produce luteolin. Over this last strain, a third plasmid integration was performed into the pSAM2 site, involving the plasmids pSEVAUO-M31105-M444_29925 or the plasmid pSEVAUO-M31105-At4g26220, generating the strains S. albidoflavus UO-FLAV-004-DIO or S. albidoflavus UO-FLAV-004-CHR, respectively.

Table 1.
Plasmids and strains used in this study.
Table 1.
Plasmids and strains used in this study.
| Description | Reference | |
|---|---|---|
| Plasmids | ||
| pSEVA181-At4g26220 | Source of At4g26220 (Level 0 MoClo) | This study |
| pSEVA181SP25 | Source of SP25 (Level 0 MoClo) | [21] |
| pSEVA181SP43 | Source of SP43 (Level 0 MoClo) | [21] |
| pSEVA181-M444_29925 | Source of M444_29925 (Level 0 MoClo) | This study |
| pSEVA181RiboJ-RBS | Source of RiboJ-RBS (Level 0 MoClo) | [21] |
| pIDTSMARTttsbib | Source of ttsbib (Level 0 MoClo) | [21] |
| pSEVAUO-M21102 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M31205 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M21206F3H-CPR | Level 1 MoClo harboring F3′H-CPR | [21] |
| PCR-Blunt II-TOPO-FNS1 | Source of FNS1 (Level 0 MoClo) | [24] |
| pSEVAUO-M21102-FNS1 | Level 1 MoClo harboring FNS1 | This study |
| pSEVAUO-M21503-FNS1/F3′H-CPR | Level 2 MoClo harboring FNS1 and F3′H-CPR | This study |
| pSEVAUO-M31105-At4g26220 | Level 1 MoClo plasmid harboring At4g26220 | This study |
| pSEVAUO-M31105-M444_29925 | Level 1 MoClo plasmid harboring M444_29925 | This study |
| pSEVAUO-M31105 | Level 1 MoClo receptor | [21] |
| pSEVAUO-M31205-dahp | Level 1 MoClo plasmid harboring dahp | This study |
| pSEVAUO-M31505 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M31505-At4g26220-dahp | Level 2 MoClo harboring At4g26220 and dahp | This study |
| pSEVAUO-M31505-M444_29925-dahp | Level 2 MoClo harboring M444_29925 and dahp | This study |
| Strains | ||
| E. coli TOP10 | Strain used for routine subcloning | Invitrogen (Waltham, MA, USA) |
| E. coli ET12567/pUZ8002 | Strain used for conjugation | [48] |
| UO-FLAV-004 | S. albidoflavus strain used in this work | [24] |
| UO-FLAV-004-NAR | UO-FLAV-004 harboring TAL, 4CL, CHS and CHI | [24] |
| UO-FLAV-004-LUT | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1 and F3′H-CPR | This study |
| UO-FLAV-004-DIO | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR and M444_29925 | This study |
| UO-FLAV-004-CHR | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR and At4g26220 | This study |
| UO-FLAV-004-DIO-dahp | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR, M444_29925 and dahp | This study |
| UO-FLAV-004-CHR-dahp | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR, At4g26220 and dahp | This study |
| UO-FLAV-004-FNS1 | UO-FLAV-004 harboring FNS1 | [24] |
Also, two more strains were generated over S. albidoflavus UO-FLAV-004-LUT. A MoClo level 2 plasmid was integrated into the pSAM2 chromosomal site containing the genes coding for the M444_29925 O-methyltransferase and the DAHP enzyme, generating the strain S. albidoflavus UO-FLAV-004-DIO-dahp. In the same manner, a plasmid containing the gene coding for the At4g26220 O-methyltransferase plus the DAHP enzyme was integrated in the same position, generating the strain S. albidoflavus UO-FLAV-004-CHR-dahp. In order to perform different feeding experiments, the strain S. albidoflavus UO-FLAV-004-M444_29925 was generated by transforming the strain S. albidoflavus UO-FLAV-004 with the plasmid pSEVAUO-M31105-M444_29925. Finally, the strain S. albidoflavus UO-FLAV-004-FNS1 [24] was also used for a feeding experiment.
E. coli strains were grown in tryptic soy broth (TSB, VWR, Barcelona, Spain) or on TSB agar plates, supplemented with the corresponding antibiotics (ampicillin 100 µg/mL, Sigma Aldrich, Madrid, Spain; apramycin 100 µg/mL, Thermo Fisher Scientific, Waltham, MA, USA); kanamycin 100 µg/mL (Alfa Aesar, Karlsruhe, Germany), chloramphenicol 25 µg/mL (AppliChem, Barcelona, Spain), nalidixic acid 50 µg/mL (Acros Organics, Geel, Belgium), and X-gal (AppliChem, Darmstadt, Germany) when blue-white selection was needed. S. albidoflavus was grown and sporulated at 30 °C in Bennett medium [49] supplemented with the corresponding antibiotics when necessary (thiostrepton 50 µg/mL, Cayman Chemical, Ann Arbor, MI, USA; hygromycin B 200 µg/mL, Enzo, Barcelona, Spain, or apramycin 50 µg/mL). MA medium (plus 10 mM MgCl2) was used for conjugation between S. albidoflavus and E. coli [50]. For flavonoid production, S. albidoflavus spores were quantified, and an inoculum of 106 spores/mL was performed in triplicate in shake flasks with 25 mL of NL333 medium [51] and incubated for 120 h at 30 °C and 250 rpm.
4.2. Reagents and Biochemicals
All solvents used for solid-phase extraction and HPLC-DAD analysis were LC-MS grade from either Sigma-Aldrich (Madrid, Spain) or VWR Chemicals (Barcelona, Spain). Luteolin, diosmetin, chrysoeriol, eriodictyol, hesperetin, homoeriodictyol, and homohesperetin were provided by Extrasynthese (Genay, France).
4.3. Genes and Enzymes
Restriction enzymes and T4 DNA ligase were purchased from Thermo Fisher Scientific (Madrid, Spain). Synthetic genes for the following ORFs were synthesized by Explora Biotech (Venezia, Italy), after codon optimization: a gene encoding a hypothetical class I SAM-dependent O-methyltransferase (M444_29925) from Streptomyces sp. Mg1 (Genbank accession no. OR820610); a gene encoding a 3-deoxy-7-phosphoheptulonate synthase (dahp) from Amycolatopsis balhimycina (Genbank accession no. OR820611); At4g26220 from Arabidopsis thaliana (Genbank accession no. OR820609). Other genes used in this study were WP_013066811 (for TAL), NP_628552 (for 4CL), L07647.1 (CHS), AY595413.1 (CHI), AY230247.1 (FNS), OQ674225 (3′FH/CPR).
4.4. Plasmids Construction
All the plasmids used in this study are listed in Table 1. Final constructs are depicted in Figure S5.
4.4.1. Construction of pSEVAUO-M21503-FNS1/F3′H-CPR
The gene encoding FNS1 was assembled in a level 1 MoClo reaction using the level 0 plasmids PCR-Blunt II-TOPO-FNS1 [24] pSEVA181SP43, pSEVA181RiboJ-RBS, pIDTSMARTttsbib [21], and the level 1 MoClo receptor pSEVAUO-M21102 [21], yielding the plasmid pSEVAUO- M21102–FNS1. Then, this plasmid was used in combination with the plasmid pSEVAUO-M21206F3H-CPR and the level 2 MoClo receptor pSEVAUO-M21503 [21] to generate the plasmid pSEVAUO-M21503-FNS1/F3′H-CPR.
4.4.2. Construction of pSEVAUO-M31105-At4g26220, pSEVAUO-M31105-M444_29925, and pSEVAUO-M31205-dahp
pSEVAUO-M31105-At4g26220 and pSEVAUO-M31105-M444_29925 are level 1 MoClo plasmids, and they were assembled by combining the level 1 MoClo receptor pSEVAUO-M31105 and the level 0 plasmids pSEVA181SP25, pSEVA181RiboJ-RBS, pIDTSMARTttsbib [21], and pSEVA181-At4g26220 (this study) or pSEVA181-M444_29925 (this study), respectively.
The plasmid pSEVAUO-M31205-dahp was assembled using the level 1 MoClo receptor pSEVAUO-M31205 and the level 0 plasmids pSEVA181SP25, pSEVA181RiboJ-RBS, pIDTSMARTttsbib, and pSEVA181-dahp (this study).
4.4.3. Construction of pSEVAUO-M31505-At4g26220-dahp and pSEVAUO-M31505-M444_29925-dahp
The At4g26220 and M444_29925 genes were brought together with the dahp gene in two level 2 MoClo reactions. The first reaction was performed with the plasmids pSEVAUO-M31105-At4g26220, pSEVAUO-M31205-dahp, and the level 2 MoClo receptor pSEVAUO-M31505 [21], resulting in pSEVAUO-M31505-At4g26220-dahp. The second reaction was performed using the plasmids pSEVAUO-M31105-M444_29925 and pSEVAUO-M31205-dahp, yielding the plasmid pSEVAUO-M31505-M444_29925-dahp.
4.5. Flavonoid Extraction and LC-DAD Analysis
Spores from the different S. albidoflavus strains were incubated, as described before. Flavonoids were recuperated by organic extraction with acetone (cellular pellet) and ethyl acetate (culture supernatant). A sample of 1 mL was taken from the flasks and centrifuged at 12,000 rpm for 1 min to separate the supernatant from the pellet. The pellet was extracted with 1 mL of acetone using vortex for 1 h. The supernatant was extracted with 800 µL of ethyl acetate by agitation for 10 min. Both pellet and supernatant extractions were centrifuged for 1 min at 12,000 rpm, and the organic fractions were mixed and dried in a speed-vac. A second extraction was performed using 800 µL ethyl acetate over the cellular pellet and the supernatant using vortex and agitation, respectively, as described before. Finally, these extractions (cellular pellet and supernatant) were mixed with the initial dry extract obtained in the first extraction and dried in a speed-vac.
For the identification of flavonoids using HPLC-DAD, the final dry extract obtained from each cultivation condition was dissolved in 100 µL DMSO/MeOH 1:1 (v/v), and the samples were centrifuged prior to injection into the equipment. The HPLC separation was performed on an HPLC (1260 Infinity, Agilent Technologies, Madrid, Spain) equipped with an analytical column Pursuit XRs C18 (50 × 4.0 mm, 5 μm, Agilent Technologies, Madrid, Spain). HPLC gradient was made with analytical grade solvent B (acetonitrile (VWR, Barcelona, Spain), and water as solvents (1 mL/min flow rate). All solvents contained 0.1% formic acid. Samples were run by an isocratic elution of 10% MeCN from 0 min to 5.44 min, followed by a linear gradient from 10% to 35% of MeCN from min 5.44 to min 21.77, maintaining the mobile phase composition until 27.21 min. Then, a linear gradient from 35% to 100% MeCN between 27.21 min and 43.54 min was applied, followed by an isocratic elution until 55 min. Then, a linear gradient from 100% to 10% MeCN was applied from 55 min to 56 min. Finally, this mobile phase composition was maintained until the end of the program (61). Detection and spectral characterization of peaks were carried out with a photodiode array detector, and the analysis was performed with Data Analysis 4.3 software (Bruker, Billerica, MA, USA). All chromatograms were extracted at 280 nm. The column temperature was set to 30 °C. Flavonoids luteolin, diosmetin, chrysoeriol, hesperetin, homoeriodictyol, and homohesperetin were identified using authentic commercial standards. Luteolin, diosmetin, chrysoeriol, and hesperetin were quantified by comparing the peak area with that of a known amount of an authentic compound through a calibration curve. The production titers are expressed in mg/L, and the mean value was calculated from three biological replicates.
4.6. Statistical Analysis
Two-way ANOVA (analysis of variance) with Sidak’s multiple comparisons test was used to test the differences in the biosynthesis of diosmetin among the strains S. albidoflavus UO-FLAV-004-DIO and S. albidoflavus UO-FLAV-004-DIO-dahp and the biosynthesis of chrysoeriol among the strain S. albidoflavus UO-FLAV-004-CHR and the strain S. albidoflavus UO-FLAV-004-CHR-dahp. Graphical representation of the different generated data was carried out using GraphPad Prism software (version 9.0.2, GraphPad Software, San Diego, CA, USA), with a p-value < 0.05 considered as statistically significant (* p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.0001).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25052776/s1.
Author Contributions
Funding acquisition (F.L.), investigation (Á.P.-V. and J.S.-D.); supervision (C.J.V. and F.L.); writing—original draft (Á.P.-V.); writing—review and editing (Á.P.-V., C.J.V., J.S.-D. and F.L.). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Principado de Asturias (Spain) through the program “Ayudas a organismos públicos para apoyar las actividades de I + D + I de sus grupos de investigación” (grant AYUD/2021/51347), as well as by the “Programa Severo Ochoa de Ayudas Predoctorales para la investigación y docencia” from Principado de Asturias (PhD grant PA-21-PF-BP20-150 to Á.P.-V.), “Programa de Ayudas FPI” from MICINN (PhD grant PRE2022-102792 to J.S.-D.), the research project PID2021-127812OB-I00 from MICINN (Spanish Ministry of Science and Innovation), and the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement no. 814650 for the project SynBio4Flav.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors have read and approved the final version of the manuscript and have accepted its publication in this journal.
Data Availability Statement
Data and materials can be obtained from the research group upon request. Sequences accession numbers have been included in the Section 4.
Acknowledgments
The authors thank Extrasynthese (Genay, France) for providing flavonoid standards for HPLC-MS analyses. The authors thank Explora Biotech (Venezia, Italy) for providing synthetic genes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.L.A.; Ramzi, A.B.; Baharum, S.N.; Noor, N.M.; Goh, H.H.; Leow, T.C.; Oslan, S.N.; Sabri, S. Recent Advancement of Engineering Microbial Hosts for the Biotechnological Production of Flavonoids. Mol. Biol. Rep. 2019, 46, 6647–6659. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Kaushal, N.; Singh, M.; Singh Sangwan, R. Flavonoids: Food Associations, Therapeutic Mechanisms, Metabolism and Nanoformulations. Food Res. Int. 2022, 157, 111442. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A Review on Flavones Targeting Serine/Threonine Protein Kinases for Potential Anticancer Drugs. Bioorg. Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a Newly Synthesized Flavonoid, Exhibits Potent Anti-Angiogenic Activity In Vitro and In Vivo. Gene 2018, 642, 533–541. [Google Scholar] [CrossRef]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-Angiogenic Activity of Iridoids from Galium tunetanum. Rev. Bras. Farmacogn. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New Insights into the Medicinal Importance, Physiological Functions and Bioanalytical Aspects of an Important Bioactive Compound of Foods ‘Hyperin’: Health Benefits of the Past, the Present, the Future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Diosmetin: A Concise Report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Ip, M.; Gong, H.; Lui, S.L.; See, R.H.; Jolivalt, C.; Fung, K.P.; Leung, P.C.; Reiner, N.E.; Lau, C.B.S. Synergistic Effects of Diosmetin with Erythromycin against ABC Transporter Over-Expressed Methicillin-Resistant Staphylococcus aureus (MRSA) RN4220/PUL5054 and Inhibition of MRSA Pyruvate Kinase. Phytomedicine 2013, 20, 611–614. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.E.; El Omari, N.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Zha, J.; Guleria, S.; Koffas, M.A.G. Recent Advances in the Recombinant Biosynthesis of Polyphenols. Front. Microbiol. 2017, 8, 2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yu, O. Metabolic Engineering of Flavonoids in Plants and Microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Emiliani, J.; Rodriguez, E.J.; Campos-Bermudez, V.A.; Grotewold, E.; Casati, P. The Identification of Maize and Arabidopsis Type I FLAVONE SYNTHASEs Links Flavones with Hormones and Biotic Interactions. Plant Physiol. 2015, 169, 1090–1107. [Google Scholar] [CrossRef] [PubMed]
- Magadán-Corpas, P.; Ye, S.; Pérez-Valero, Á.; McAlpine, P.L.; Valdés-Chiara, P.; Torres-Bacete, J.; Nogales, J.; Villar, C.J.; Lombó, F. Optimized De Novo Eriodictyol Biosynthesis in Streptomyces albidoflavus Using an Expansion of the Golden Standard Toolkit for Its Use in Actinomycetes. Int. J. Mol. Sci. 2023, 24, 8879. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, B.G.; Ahn, J.H. Biosynthesis of Bioactive O-Methylated Flavonoids in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 7195–7204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.; Zhu, X.; Zhang, G.; Yang, S.; Guo, X.; Jiang, H.; Ma, Y. De Novo Biosynthesis of Multiple Pinocembrin Derivatives in Saccharomyces cerevisiae. ACS Synth. Biol. 2020, 9, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valero, Á.; Ye, S.; Magadán-Corpas, P.; Villar, C.J.; Lombó, F. Metabolic Engineering in Streptomyces albidoflavus for the Biosynthesis of the Methylated Flavonoids Sakuranetin, Acacetin, and Genkwanin. Microb. Cell Factories 2023, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaur, R.; Salwan, R. Streptomyces: Host for Refactoring of Diverse Bioactive Secondary Metabolites; Springer International Publishing: Cham, Switzerland, 2021; Volume 11, ISBN 0123456789. [Google Scholar]
- Kuhstoss, S.; Rao, R.N. Analysis of the Integration Function of the Streptomycete Bacteriophage ΦC31. J. Mol. Biol. 1991, 222, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; Till, R.; Smith, M.C.M. Integration Site for Streptomyces Phage ΦBT1 and Development of Site-Specific Integrating Vectors. J. Bacteriol. 2003, 185, 5320–5323. [Google Scholar] [CrossRef] [PubMed]
- Raynal, A.; Friedmann, A.; Tuphile, K.; Guerineau, M.; Pernodet, J.L. Characterization of the AttP Site of the Integrative Element PSAM2 from Streptomyces ambofaciens. Microbiology 2002, 148, 61–67. [Google Scholar] [CrossRef]
- Thykaer, J.; Nielsen, J.; Wohlleben, W.; Weber, T.; Gutknecht, M.; Lantz, A.E.; Stegmann, E. Increased Glycopeptide Production after Overexpression of Shikimate Pathway Genes Being Part of the Balhimycin Biosynthetic Gene Cluster. Metab. Eng. 2010, 12, 455–461. [Google Scholar] [CrossRef]
- Ikeda, M. Towards Bacterial Strains Overproducing L-Tryptophan and Other Aromatics by Metabolic Engineering. Appl. Microbiol. Biotechnol. 2006, 69, 615–626. [Google Scholar] [CrossRef]
- Pandurangan, N. A New Synthesis for Acacetin, Chrysoeriol, Diosmetin, Tricin and Other Hydroxylated Flavones by Modified Baker-Venkataraman Transformation. Lett. Org. Chem. 2014, 11, 225–229. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus sinensis L. Osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valorization 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Luzhetskyy, A. Native and Engineered Promoters in Natural Product Discovery. Nat. Prod. Rep. 2016, 33, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Darsandhari, S.; Dhakal, D.; Shrestha, B.; Parajuli, P.; Seo, J.-H.; Kim, T.-S.; Sohng, J.K. Characterization of Regioselective Flavonoid O-Methyltransferase from the Streptomyces Sp. KCTC 0041BP. Enzym. Microb. Technol. 2018, 113, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wils, C.R.; Brandt, W.; Manke, K.; Vogt, T. A Single Amino Acid Determines Position Specificity of an Arabidopsis thaliana CCoAOMT-like O-Methyltransferase. FEBS Lett. 2013, 587, 683–689. [Google Scholar] [CrossRef]
- Schröder, G.; Wehinger, E.; Lukačin, R.; Wellmann, F.; Seefelder, W.; Schwab, W.; Schröder, J. Flavonoid Methylation: A Novel 4′-O-Methyltransferase from Catharanthus Roseus, and Evidence That Partially Methylated Flavanones Are Substrates of Four Different Flavonoid Dioxygenases. Phytochemistry 2004, 65, 1085–1094. [Google Scholar] [CrossRef]
- Aisa, H.A.; Izotova, L.; Karimov, A.; Botirov, E.; Mamadrahimov, A.; Ibragimov, B. Crystal, Molecular Structure and Hirshheld Surface Analysis of 5-Hydroxy-3,6,7,8-Tetramethoxyflavone. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1748–1751. [Google Scholar] [CrossRef]
- Shin, W.; Lah, M.S. Structure of (R,S)-Naringenin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 626–628. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Santos, C.N.S.; Stephanopoulos, G. Perspectives of Biotechnological Production of L-Tyrosine and Its Applications. Appl. Microbiol. Biotechnol. 2007, 77, 751–762. [Google Scholar] [CrossRef]
- Koopman, F.; Beekwilder, J.; Crimi, B.; Van Houwelingen, A.; Hall, R.D.; Bosch, D.; Van Maris, A.J.A.; Pronk, J.T.; Daran, J. De Novo Production of the Flavonoid Naringenin in Engineered Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 155. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Du, G.; Zhou, J.; Chen, J. Modular Optimization of Heterologous Pathways for de Novo Synthesis of (2S)-Naringenin in Escherichia coli. PLoS ONE 2014, 9, e101492. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, T.; Zhou, J. Fermentation and Metabolic Pathway Optimization to De Novo Synthesize (2S)-Naringenin in Escherichia coli. J. Microbiol. Biotechnol. 2020, 30, 1574–1582. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.H. Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- August, P.R.; Tang, L.; Yoon, Y.J.; Ning, S.; Müller, R.; Yu, T.-W.; Taylor, M.; Hoffmann, D.; Kim, C.-G.; Zhang, X.; et al. Biosynthesis of the Ansamycin Antibiotic Rifamycin: Deductions from the Molecular Analysis of the Rif Biosynthetic Gene Cluster of Amycolatopsis Mediterranei S699. Chem. Biol. 1998, 5, 69–79. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magarvey, N.; Piraee, M.; Vining, L.C. The Gene Cluster for Chloramphenicol Biosynthesis in Streptomyces venezuelae ISP5230 Includes Novel Shikimate Pathway Homologues and a Monomodular Non-Ribosomal Peptide Synthetase Gene The GenBank Accession Number for the Sequence Reported in This Paper is AF262220. Microbiology 2001, 147, 2817–2829. [Google Scholar] [CrossRef]
- Dyer, W.E.; Weaver, L.M.; Zhao, J.M.; Kuhn, D.N.; Weller, S.C.; Herrmann, K.M. A CDNA Encoding 3-Deoxy-D-Arabino-Heptulosonate 7-Phosphate Synthase from Solanum tuberosum L. J. Biol. Chem. 1990, 265, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.E.B.P.; Suzich, J.A.; Herrmann, K.M. 3-Deoxy-d- Arabino -Heptulosonate 7-Phosphate Synthase from Potato Tuber (Solanum tuberosum L.). Plant Physiol. 1986, 82, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Macneil, D.J.; Gewain, K.M.; Ruby, C.L.; Dezeny, G.; Gibbons, P.H.; Maeneil, T. Analysis of Streptomyces avermitilis Genes Required for Avermectin Biosynthesis Utilizing a Novel Inte-Gration Vector. Gene 1992, 111, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A.; John Innes Foundation. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; Volume 291. [Google Scholar]
- Fernández, E.; Weissbach, U.; Sánchez Reillo, C.; Braña, A.F.; Méndez, C.; Rohr, J.; Salas, J.A. Identification of Two Genes from Streptomyces argillaceus Encoding Glycosyltransferases Involved in Transfer of a Disaccharide during Biosynthesis of the Antitumor Drug Mithramycin. J. Bacteriol. 1998, 180, 4929–4937. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Tokovenko, B.; Brötz, E.; Rückert, C.; Kalinowski, J.; Luzhetskyy, A. Genome Rearrangements of Streptomyces Albus J1074 Lead to the Carotenoid Gene Cluster Activation. Appl. Microbiol. Biotechnol. 2014, 98, 795–806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).