Dietary Intervention during Weaning and Development of Food Allergy: What Is the State of the Art?
Abstract
1. Introduction
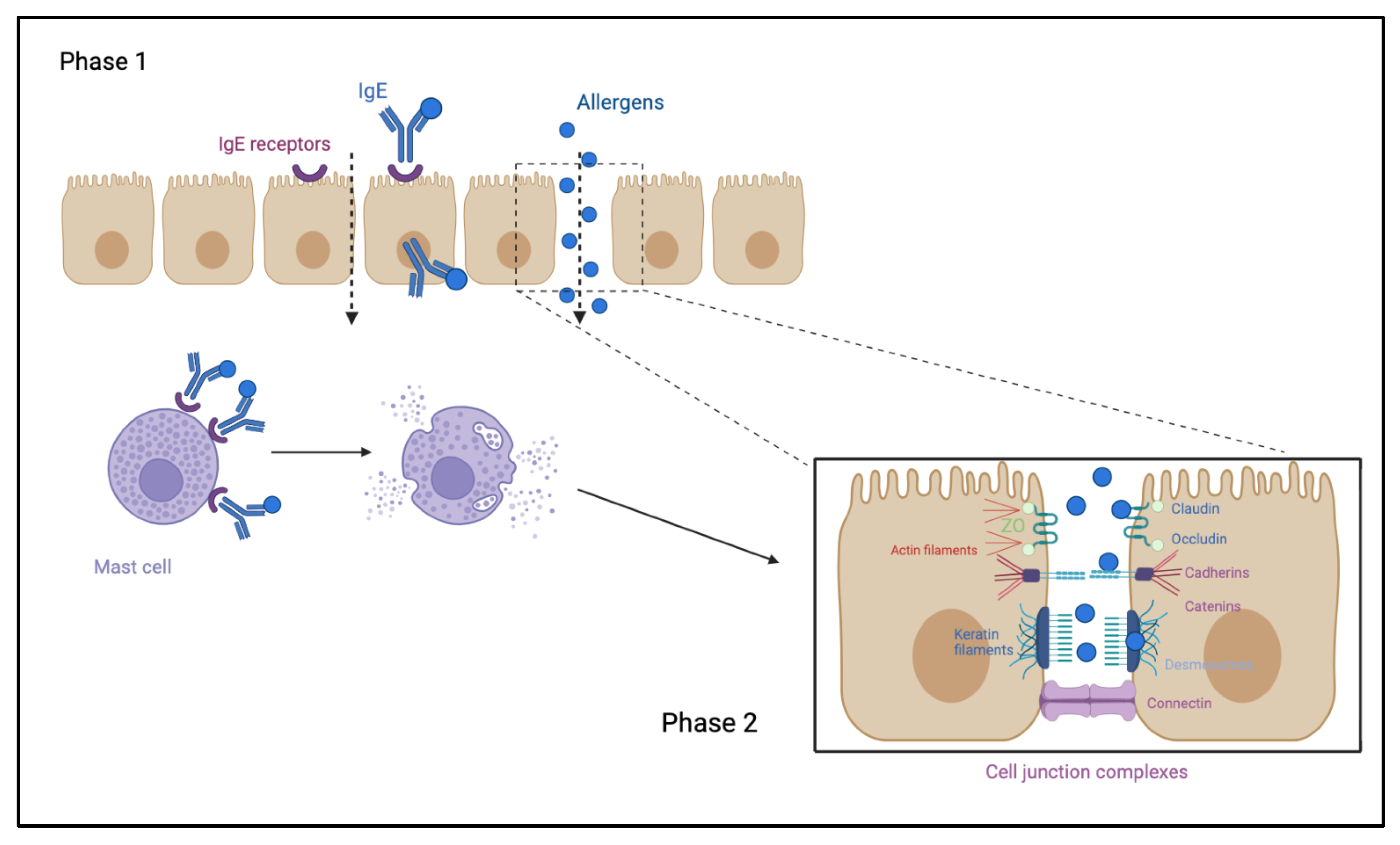
1.1. Food Allergy Immuno-Pathogenesis
1.2. Digestion and Mucosal Barrier
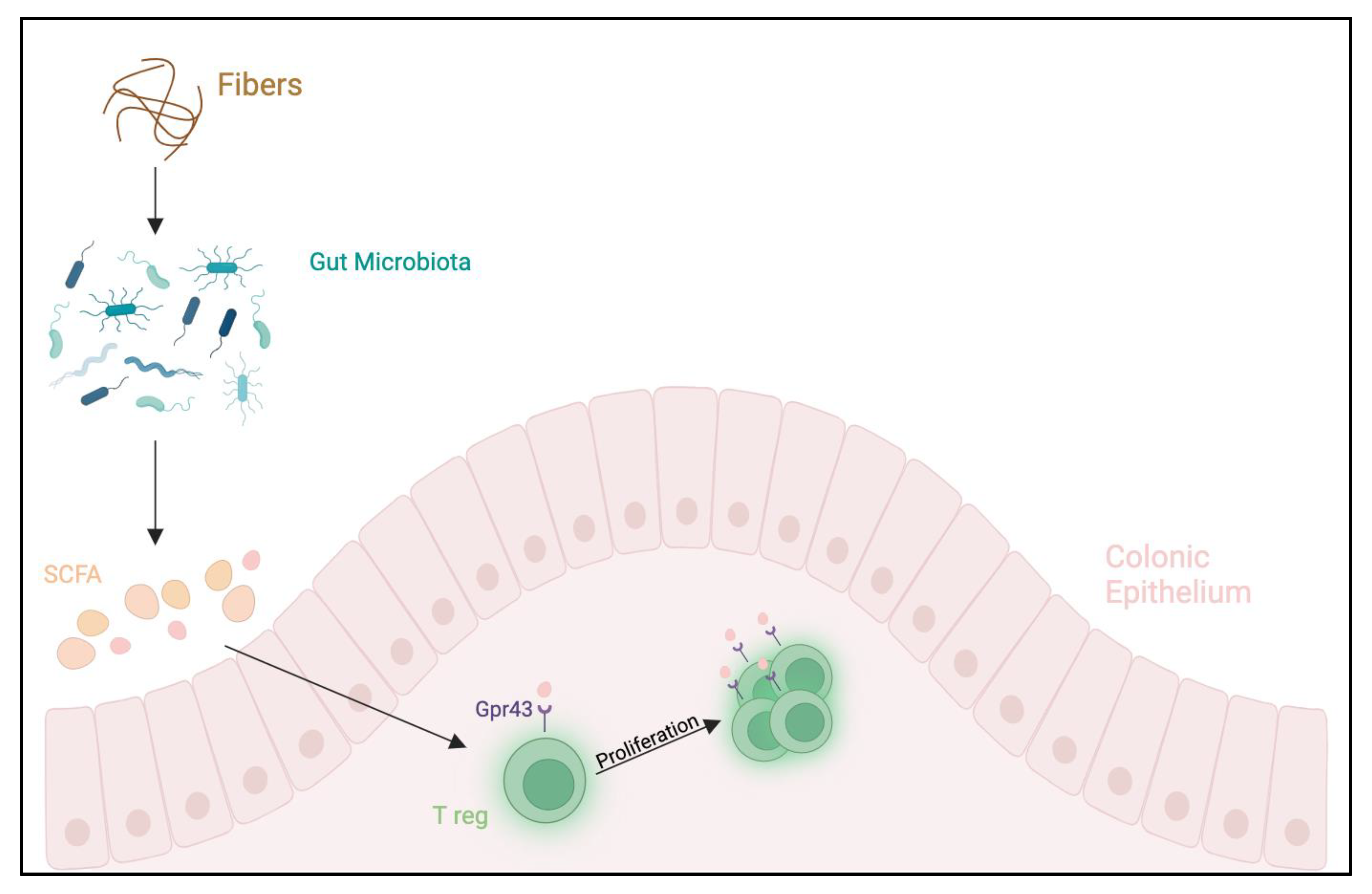
1.3. Microbiota and Food Allergies
2. Method
3. Studies
3.1. Studies Performed in High-Risk Populations
3.1.1. Egg Proteins
3.1.2. Cow’s Milk, Peanut, Hard-Boiled Hen’s Egg, Sesame, Whitefish (Cod) and Wheat
3.1.3. Rusk-like Biscuit Powder
3.1.4. Peanut
3.2. Studies Performed in Low-Risk Populations
3.2.1. Egg Proteins
3.2.2. Cow’s Milk, Peanut, Hard-Boiled Hen’s Egg, Sesame, Whitefish (Cod) and Wheat
3.3. Studies Performed in Both High-Risk and Low-Risk Populations
Cow’s Milk Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prescott, S.; Allen, K.J. Food allergy: Riding the second wave of the allergy epidemic. Pediatr. Allergy Immunol. 2011, 22, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Høst, A.; Koletzko, B.; Dreborg, S.; Muraro, A.; Wahn, U.; Aggett, P.; Bresson, J.L.; Hernell, O.; Lafeber, H.; Michaelsen, K.F.; et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch. Dis. Child. 1999, 81, 80–84. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 1989, 83, 1068–1069. [Google Scholar] [CrossRef]
- Bailey, M.; Haverson, K.; Inman, C.; Harris, C.; Jones, P.; Corfield, G.; Miller, B.; Stokes, C. The development of the mucosal immune system pre- and post-weaning: Balancing regulatory and effector function. Proc. Nutr. Soc. 2005, 64, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Bateman, B.; Sadeghnejad, A.; Gant, C.; Matthews, S.M. Prevention of allergic disease during childhood by allergen avoidance: The Isle of Wight prevention study. J. Allergy Clin. Immunol. 2007, 119, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zutavern, A.; Brockow, I.; Schaaf, B.; von Berg, A.; Diez, U.; Borte, M.; Kraemer, U.; Herbarth, O.; Behrendt, H.; Wichmann, H.-E.; et al. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: Results from the prospective birth cohort study LISA. Pediatrics 2008, 121, e44–e52. [Google Scholar] [CrossRef] [PubMed]
- Lack, G.; Fox, D.; Northstone, K.; Golding, J. Avon Longitudinal Study of Parents and Children Study Team Factors associated with the development of peanut allergy in childhood. N. Engl. J. Med. 2003, 348, 977–985. [Google Scholar] [CrossRef]
- Anvari, S.; Chokshi, N.Y.; Kamili, Q.U.A.; Davis, C.M. Evolution of Guidelines on Peanut Allergy and Peanut Introduction in Infants: A Review. JAMA Pediatr. 2017, 171, 77–82. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef]
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.J.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.C.; Hoest, A.; Lack, G.; et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food Allergy from Infancy Through Adulthood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pavón, D.; Jiménez, M.; Salinas, E. Physiopathology of food allergies. Rev. Alerg. Mex. 2020, 67, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Michelet, M.; Balbino, B.; Guilleminault, L.; Reber, L.L. IgE in the pathophysiology and therapy of food allergy. Eur. J. Immunol. 2021, 51, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A. Non-IgE Mediated Food Allergy. Curr. Pediatr. Rev. 2020, 16, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sicherer, S.; Berin, M.C.; Agyemang, A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021, 10, 431–446. [Google Scholar] [CrossRef]
- Chong, K.W.; Ruiz-Garcia, M.; Patel, N.; Boyle, R.J.; Turner, P.J. Reaction phenotypes in IgE-mediated food allergy and anaphylaxis. Ann. Allergy Asthma Immunol. 2020, 124, 473–478. [Google Scholar] [CrossRef]
- Domínguez, O.; Plaza, A.M.; Alvaro, M. Relationship Between Atopic Dermatitis and Food Allergy. Curr. Pediatr. Rev. 2020, 16, 115–122. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Blanco-Pérez, F.; Infante, S.; Zubeldia, J.M.; Pérez-Gordo, M. Non-IgE-Mediated Gastrointestinal Food Protein-Induced Allergic Disorders. Clinical Perspectives and Analytical Approaches. Foods 2021, 10, 2662. [Google Scholar] [CrossRef]
- Cafarotti, A.; Giovannini, M.; Begìn, P.; Brough, H.A.; Arasi, S. Management of IgE-mediated food allergy in the 21st century. Clin. Exp. Allergy 2023, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Bøgh, K.L.; Madsen, C.B. Food Allergens: Is There a Correlation between Stability to Digestion and Allergenicity? Crit. Rev. Food Sci. Nutr. 2016, 56, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Ha, C.-E. Chapter 11—Gastrointestinal Digestion and Absorption. In Essentials of Medical Biochemistry, 2nd ed.; Bhagavan, N.V., Ha, C.-E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 137–164. ISBN 978-0-12-416687-5. Available online: https://www.sciencedirect.com/science/article/pii/B9780 (accessed on 12 January 2024).
- Nilius, B.; Gudermann, T.; Jahn, R.; Lill, R.; Petersen, O.H.; De Tombe, P.P. (Eds.) Reviews of Physiology, Biochemistry and Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 168, ISBN 978-3-319-22502-9. [Google Scholar]
- Verhoeckx, K.; Bøgh, K.L.; Dupont, D.; Egger, L.; Gadermaier, G.; Larré, C.; Mackie, A.; Menard, O.; Adel-Patient, K.; Picariello, G.; et al. The relevance of a digestibility evaluation in the allergenicity risk assessment of novel proteins. Opinion of a joint initiative of COST action ImpARAS and COST action INFOGEST. Food Chem. Toxicol. 2019, 129, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Sanctuary, M.R.; Qu, Y.; Khajavi, S.H.; Van Zandt, A.E.; Dyandra, M.; Frese, S.A.; Barile, D.; German, J.B. Personalizing protein nourishment. Crit. Rev. Food Sci. Nutr. 2017, 57, 3313–3331. [Google Scholar] [CrossRef]
- Ballegaard, A.-S.R.; Bøgh, K.L. Intestinal protein uptake and IgE-mediated food allergy. Food Res. Int. 2023, 163, 112150. [Google Scholar] [CrossRef]
- Umar, S. Intestinal stem cells. Curr. Gastroenterol. Rep. 2010, 12, 340–348. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction With the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Steed, E.; Balda, M.S.; Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010, 20, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.; Boudaud, M.; Kuehn, A.; Ollert, M.; Desai, M.S. Intestinal mucus barrier: A missing piece of the puzzle in food allergy. Trends Mol. Med. 2022, 28, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Ermund, A.; Schütte, A.; Johansson, M.E.V.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G341–G347. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, R.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2012, 28, 124. [Google Scholar] [CrossRef]
- Brandtzaeg, P. The gut as communicator between environment and host: Immunological consequences. Eur. J. Pharmacol. 2011, 668, S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Perrier, C.; Corthésy, B. Gut permeability and food allergies. Clin. Exp. Allergy 2011, 41, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Graversen, K.B.; Ballegaard, A.-S.R.; Kræmer, L.H.; Hornslet, S.E.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Untersmayr, E.; Smit, J.J.; Bøgh, K.L. Cow’s milk allergy prevention and treatment by heat-treated whey—A study in Brown Norway rats. Clin. Exp. Allergy 2020, 50, 708–721. [Google Scholar] [CrossRef]
- So, A.L.; Pelton-Henrion, K.; Small, G.; Becker, K.; Oei, E.; Tyorkin, M.; Sperber, K.; Mayer, L. Antigen Uptake and Trafficking in Human Intestinal Epithelial Cells. Dig. Dis. Sci. 2000, 45, 1451–1461. [Google Scholar] [CrossRef]
- Yu, L.C.H.; Yang, P.-C.; Berin, M.C.; Leo, V.D.; Conrad, D.H.; Mckay, D.M.; Satoskar, A.R.; Perdue, M.H. Enhanced transepithelial antigen transport in intestine of allergic mice is mediated by IgE/CD23 and regulated by interleukin-4. Gastroenterology 2001, 121, 370–381. [Google Scholar] [CrossRef]
- Trogen, B.; Jacobs, S.; Nowak-Wegrzyn, A. Early Introduction of Allergenic Foods and the Prevention of Food Allergy. Nutrients 2022, 14, 2565. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’Orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics Function in Preventing Atopic Dermatitis in Children. Int. J. Mol. Sci. 2022, 23, 5409. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- West, C.E.; Jenmalm, M.C.; Prescott, S.L. The gut microbiota and its role in the development of allergic disease: A wider perspective. Clin. Exp. Allergy 2015, 45, 43–53. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Prince, B.T.; Mandel, M.J.; Nadeau, K.; Singh, A.M. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr. Clin. N. Am. 2015, 62, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Rachid, R.; Stephen-Victor, E.; Chatila, T.A. The microbial origins of food allergy. J. Allergy Clin. Immunol. 2021, 147, 808–813. [Google Scholar] [CrossRef]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Berni Canani, R. Specific gut microbiome signatures and the associated pro-inflamatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.-A.; Yuen, A.W.T.; Woo, E.; Chu, K.-H.; Kwan, H.-S.; Yang, G.-X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Snijders, B.E.P.; Thijs, C.; van Ree, R.; van den Brandt, P.A. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: The KOALA Birth Cohort Study. Pediatrics 2008, 122, e115–e122. [Google Scholar] [CrossRef]
- Palmer, D.J.; Metcalfe, J.; Makrides, M.; Gold, M.S.; Quinn, P.; West, C.E.; Loh, R.; Prescott, S.L. Early regular egg exposure in infants with eczema: A randomized controlled trial. J. Allergy Clin. Immunol. 2013, 132, 387–392.e1. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksünger, Ö.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599.e2. [Google Scholar] [CrossRef] [PubMed]
- Wei-Liang Tan, J.; Valerio, C.; Barnes, E.H.; Turner, P.J.; Van Asperen, P.A.; Kakakios, A.M.; Campbell, D.E. Beating Egg Allergy Trial (BEAT) Study Group A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1621–1628.e8. [Google Scholar] [CrossRef] [PubMed]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.R.; Gold, M.S.; Prescott, S.L.; Makrides, M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1600–1607.e2. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Fukazawa, M.; Fukuoka, K.; Okasora, T.; Yamada, S.; Kyo, S.; Homan, M.; Miura, T.; Nomura, Y.; Tsuchida, S.; et al. Early introduction of very small amounts of multiple foods to infants: A randomized trial. Allergol. Int. 2022, 71, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kalb, B.; Meixner, L.; Trendelenburg, V.; Unterleider, N.; Dobbertin-Welsch, J.; Heller, S.; Dölle-Bierke, S.; Roll, S.; Lau, S.; Lee, Y.-A.; et al. Tolerance induction through early feeding to prevent food allergy in infants with eczema (TEFFA): Rationale, study design, and methods of a randomized controlled trial. Trials 2022, 23, 210. [Google Scholar] [CrossRef]
| Author, Year, Country, Trial | Study Design | Sample Size | Population | Inclusion Criteria | Allergen | Outcome | Main Results |
|---|---|---|---|---|---|---|---|
| Snijders et al., 2008 Netherlands KOALA [60] | Prospective birth cohort study | 2558 infants | General population | Pregnant women with diverse lifestyles | Cow’s milk products and other solid products. | Questionnaires at 7, 12, 24 months; Specific IgE >0.3 UI/mL against eggs, cow’s milk at age 2 | Delayed introduction of CMP and other food products associated with higher risk for eczema (p = 0.01 and 0.02 for trend, respectively); no association between delayed introduction of CMP and AD; delayed introduction of other food associated with higher risk for AD (p = 0.00 trend) and increased risk of atopy development at the age of 2 years |
| Palmer et al., 2013 Australia STAR [61] | RDBPCT | 86 infants: 49 SG 37 CG | High risk | 4 months of age singleton term infants with moderate-to-severe eczema no prior egg or solid food ingestion | Hen’s egg (0.9 g/day) | OFC and SPT at 12 months | At 12 months 33% SG, 51% CG were diagnosed IgE-mediated egg allergy (relative risk, 0.65; 95% CI, 0.38–1.11; p = 0.11) |
| Du Toit et al., 2015 England LEAP [62] | RCT | 640 infants: 319 SG 321 CG | Infants 4 to 11 months of age with severe eczema, egg allergy, or both | High risk | Peanut (6 g/week) | Open OFC or DBPCFC at 12, 30 and 60 months | In the intention-to-treat population: 13.7% in the CG and 1.9% in the SG who had negative SPT developed peanut allergy (p < 0.001) |
| Perkin et al., 2016 England EAT [63] | RCT | 1303 infants: 652 SG 651 CG | Exclusively breastfed infants for ≥4–5 months, regardless of atopic status or family history of allergy | General population | Cow’s milk, peanut, hard-boiled Hen’s egg, sesame, whitefish (cod) and wheat at 3 and 6 months of age (4 g/week) | OFC at 1 and 3 years of age after allergenic food introduction | Among infants with sensitization to 1 or more foods at enrollment, EIG infants developed significantly less FA than SIG infants Intention to treat: SIG, 7.1%; EIG, 5.6% p = 0.32 Per protocol: SIG, 7.3% EIG, 2.4% p = 0.01 |
| Bellach et al., 2017 Germany HEAP [64] | RDBPCT | 383 infants: 184 SG 199 CG | GA ≥ 34 weeks and birth weight ≥ 2.5 kg Specific IgE to egg <0.35 kU/L | General population 4–6 months | Hens’ egg 2.5 g 3 times/week from 4–6 to 12 months | OFC and specific IgE ≥0.35 KU/L at 12 months after hen’s egg introduction | Sensitized to hen’s egg at age 12 months: 5.6% (6/124) in SG 2.6% (4/152) in CG (p = 0.35); allergy to hen’s egg 2.1% in SG 0.6% in CG (relative risk,3.30;95%CI, 0.35–31.32; p = 0.35); no prevention in hen’s egg sensitization nor egg allergy |
| Tan et al., 2017 Australia BEAT [65] | RDBCT | 319 infants: SG 165 CG 154 | Infants with at least 1 first-degree relative with allergic disease and SPT < 2 mm | High risk | Hens’ egg 350 mg from 4–8 months | EW SPT response of 3 mm or greater OFC to whole egg at age 12 months. | Sensitization to EW at 12 months: 20% in CG 11% in SG; allergy to EW at 12 months: 10.5% in CG 6.2% in SG (odds ratio, 0.46;95% CI, 0.22–0.95; p = 0.03) |
| Natsume et al., 2017 Japan PETIT [66] | RDBPCT | 147 infants | 4–5 months of age with eczema | High risk | Eggs 50 mg/die (3–9 months) 250 mg/die (9–12 months) | Open OFC at 12 months of age | Five (8%) of 60 participants had an egg allergy in the SG compared to 23 (38%) of 61 in the CG (risk ratio 0.221;95% CI, 0.090–0.543; p = 0.0001) |
| Palmer et al., 2017 Australia STEP [67] | RCT | 820 infants: SG 165 CG 154 | Singleton infants with atopic mothers, recruited before age 6.5 months No prior egg ingestion and allergic disease | High risk | Hens’ egg pasteurized raw whole egg powder (SG = 407) or a rice powder (CG = 413) from 6 to 10 months; introduction of egg at 10 months | OFC to egg at 12 months and SPT positive | At 12 months: IgE-mediated food allergy: SG 7.0% vs. CG 10.3% (RR(95%CI) 0.75 (0.48–1.17) p = 0.20) |
| Nishimura et al., 2022 Japan SEED [68] | RCT | 163 children: 83 SG 80 CG | 3–4 months old with atopic dermatitis | High risk | Egg, milk, wheat, soybean, buckwheat, and peanuts. Amount of powder increased at 2, 4 and 12 week. | The occurrence of FA at 18 months old | Incidence of FA episodes by 18 months: SG 7/83 vs. CG 19/80; (risk ratio 0.301 [95% CI 0.116–0.784]; p = 0.0066). Egg allergies were reduced in the SG group |
| Kalb et al., 2022 German TEFFA [69]. | RCT | 150 infants with atopic eczema at 4–8 months randomized in a 2:1 manner into an SG and CG | 4–8-month-old infants with eczema | High risk | Rusk-like biscuit powder with HE, CM, PN, HN 2 mg for 6–8 months | After 6 months of intervention they will check sensitization against hen’s egg, cow’s milk, hazelnut and peanut | At 12 months egg allergy: SG 2.1% CG 0.6% (3.30;95% CI, 0.31–3132 p = 0.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravina, A.; Olivero, F.; Brindisi, G.; Comerci, A.F.; Ranucci, C.; Fiorentini, C.; Sculco, E.; Figliozzi, E.; Tudini, L.; Matys, V.; et al. Dietary Intervention during Weaning and Development of Food Allergy: What Is the State of the Art? Int. J. Mol. Sci. 2024, 25, 2769. https://doi.org/10.3390/ijms25052769
Gravina A, Olivero F, Brindisi G, Comerci AF, Ranucci C, Fiorentini C, Sculco E, Figliozzi E, Tudini L, Matys V, et al. Dietary Intervention during Weaning and Development of Food Allergy: What Is the State of the Art? International Journal of Molecular Sciences. 2024; 25(5):2769. https://doi.org/10.3390/ijms25052769
Chicago/Turabian StyleGravina, Alessandro, Francesca Olivero, Giulia Brindisi, Antonia Fortunata Comerci, Chiara Ranucci, Cinzia Fiorentini, Eleonora Sculco, Ethel Figliozzi, Laura Tudini, Viviana Matys, and et al. 2024. "Dietary Intervention during Weaning and Development of Food Allergy: What Is the State of the Art?" International Journal of Molecular Sciences 25, no. 5: 2769. https://doi.org/10.3390/ijms25052769
APA StyleGravina, A., Olivero, F., Brindisi, G., Comerci, A. F., Ranucci, C., Fiorentini, C., Sculco, E., Figliozzi, E., Tudini, L., Matys, V., De Canditiis, D., Piccioni, M. G., Zicari, A. M., & Anania, C. (2024). Dietary Intervention during Weaning and Development of Food Allergy: What Is the State of the Art? International Journal of Molecular Sciences, 25(5), 2769. https://doi.org/10.3390/ijms25052769








