Comprehensive Assessment of CFTR Modulators’ Therapeutic Efficiency for N1303K Variant
Abstract
1. Introduction
2. Results
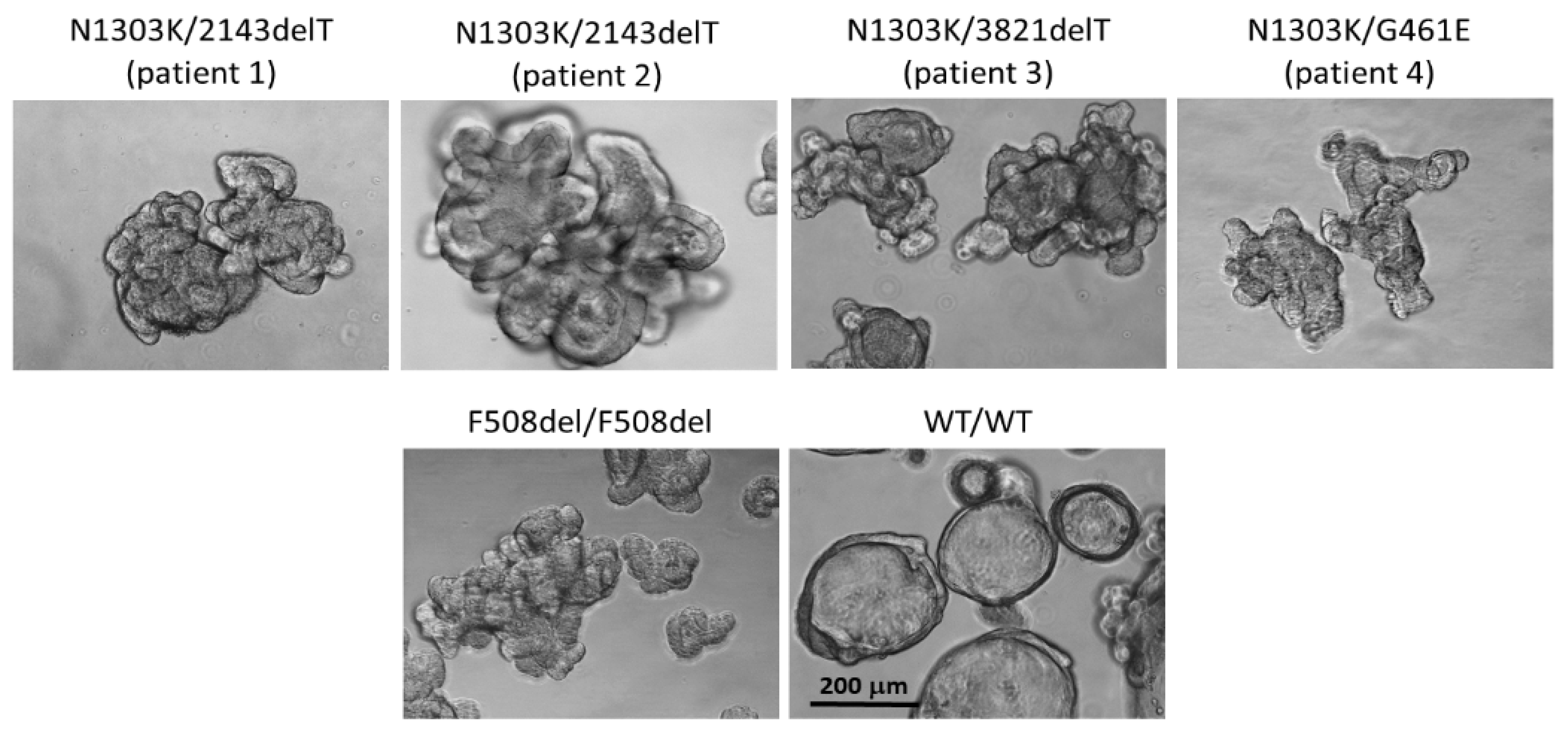
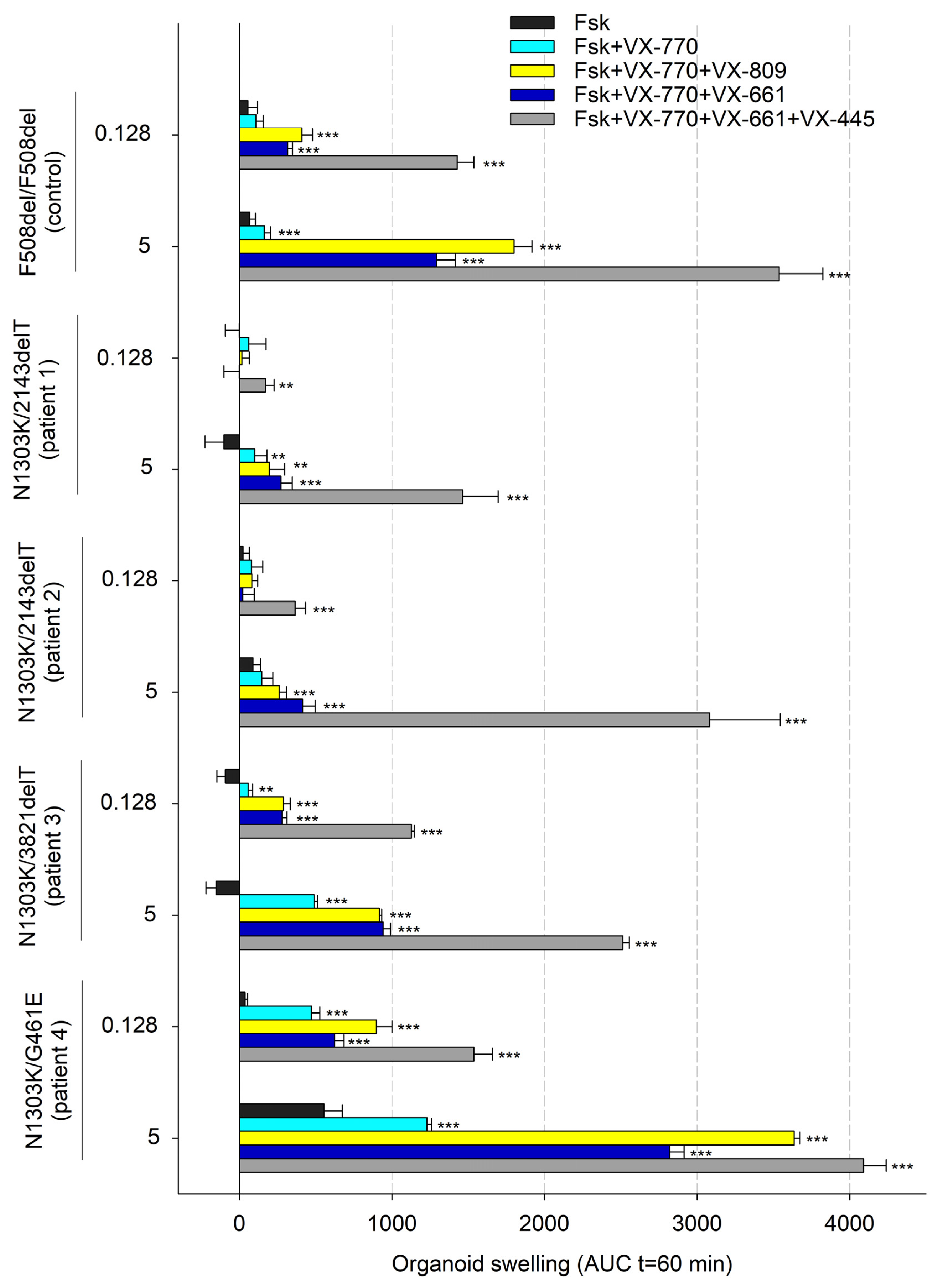
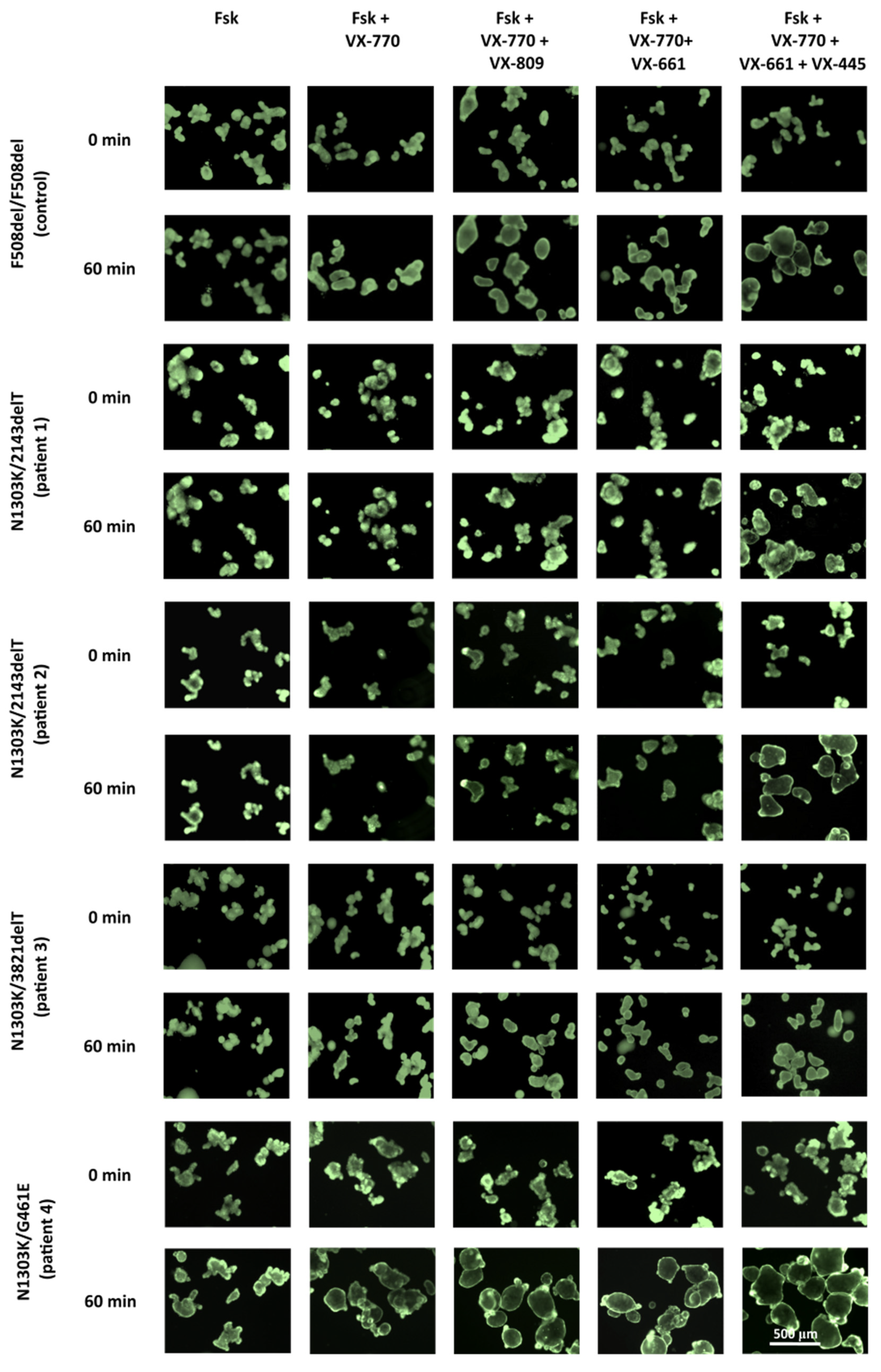
2.1. Combination of Three CFTR Modulators, VX-770, VX-661 and VX-445, Restores Chloride Channel Function in Intestinal Organoids’ Epithelium Derived from CF Patients with N1303K Variant
2.2. Elexacaftor + Tezacaftor + Ivacaftor Treatment of N1303K/2143delT Patient Produces a Positive Therapeutic Effect
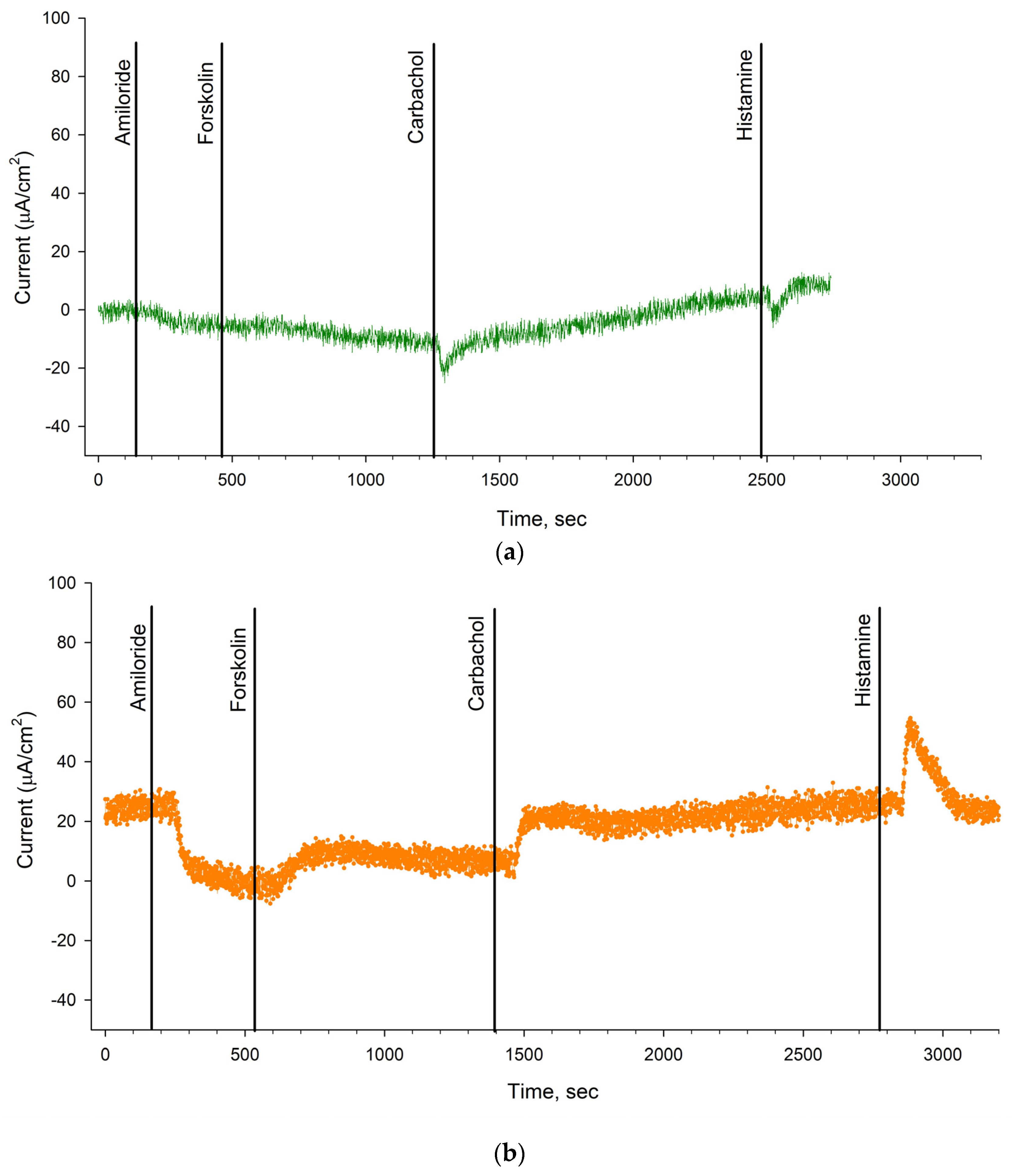
2.3. Evaluation of the Effectiveness of Elexacaftor + Tezacaftor + Ivacaftor in a Patient with the N1303K/2143delT Genotype Using the Intestinal Current Measurement (ICM) Method
3. Discussion
4. Materials and Methods
4.1. Forskolin-Induced Swelling (FIS) Assay in Intestinal Organoids
4.2. Intestinal Current Measurement (ICM)
4.3. Description of the Clinical Picture
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS Best Practice Guidelines: The 2018 Revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.; Drumond, S.; Castelletti, C.H.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundations. Available online: https://www.cff.org/managing-cf/cftr-modulator-therapies (accessed on 21 August 2023).
- Mergiotti, M.; Murabito, A.; Prono, G.; Ghigo, A. CFTR Modulator Therapy for Rare CFTR Mutants. J. Respir. 2022, 2, 59–76. [Google Scholar] [CrossRef]
- Costa, E.; Girotti, S.; Pauro, F.; Leufkens, H.G.M.; Cipolli, M. The Impact of FDA and EMA Regulatory Decision-Making Process on the Access to CFTR Modulators for the Treatment of Cystic Fibrosis. Orphanet J. Rare Dis. 2022, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninejad, M.; Asadzadeh-Aghdaei, H.; Baharvand, H.; Meyfour, A. Intestinal Organoids: A Versatile Platform for Modeling Gastrointestinal Diseases and Monitoring Epigenetic Alterations. Life Sci. 2023, 319, 121506. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A Functional CFTR Assay Using Primary Cystic Fibrosis Intestinal Organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; De Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; Van De Graaf, E.A.; Nieuwenhuis, E.E.S.; Houwen, R.H.J.; et al. Characterizing Responses to CFTR-Modulating Drugs Using Rectal Organoids Derived from Subjects with Cystic Fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef] [PubMed]
- Saini, A. Cystic Fibrosis Patients Benefit from Mini Guts. Cell Stem Cell 2016, 19, 425–427. [Google Scholar] [CrossRef]
- Kondratyeva, E.; Efremova, A.; Melyanovskaya, Y.; Voronkova, A.; Polyakov, A.; Bulatenko, N.; Adyan, T.; Sherman, V.; Kovalskaia, V.; Petrova, N.; et al. Evaluation of the Complex p.[Leu467Phe;Phe508del] CFTR Allele in the Intestinal Organoids Model: Implications for Therapy. Int. J. Mol. Sci. 2022, 23, 10377. [Google Scholar] [CrossRef]
- Furstova, E.; Dousova, T.; Beranek, J.; Libik, M.; Fila, L.; Modrak, M.; Cinek, O.; Macek, M.; Drevinek, P. Response to Elexacaf Tor/Tezacaf Tor/Ivacaf Tor in Intestinal Organoids Derived from People with Cystic Fibrosis. J. Cyst. Fibros. 2022, 21, 243–245. [Google Scholar] [CrossRef]
- Lefferts, J.W.; Bierlaagh, M.C.; Kroes, S.; Nieuwenhuijze, N.D.A.; Van Kooten, H.N.S.; Niemöller, P.J.; Verburg, T.F.; Janssens, H.M.; Muilwijk, D.; Van Beuningen, S.F.B.; et al. CFTR Function Restoration upon Elexacaftor/Tezacaftor/Ivacaftor Treatment in Patient-Derived Intestinal Organoids with Rare CFTR Genotypes. Int. J. Mol. Sci. 2023, 24, 14539. [Google Scholar] [CrossRef]
- Kondratyeva, E.; Efremova, A.; Melyanovskaya, Y.; Petrova, N.; Satsuk, N.; Bulatenko, N.; Bukharova, T.; Zodbinova, A.; Sherman, V.; Kashirskaya, N.; et al. Clinical and Genetic Characterization of Patients with Cystic Fibrosis and Functional Assessment of the Chloride Channel with the Pathogenic Variant c.831G>A (p.Trp277*), Described for the First Time. Gene 2020, 761, 145023. [Google Scholar] [CrossRef]
- Kondratyeva, E.; Bukharova, T.; Efremova, A.; Melyanovskaya, Y.; Bulatenko, N.; Davydenko, K.; Filatova, A.; Skoblov, M.; Krasovsky, S.; Petrova, N.; et al. Health Characteristics of Patients with Cystic Fibrosis Whose Genotype Includes a Variant of the Nucleotide Sequence c.3140-16t>a and Functional Analysis of This Variant. Genes 2021, 12, 837. [Google Scholar] [CrossRef]
- Kondratyeva, E.I.; Krasovsky, S.A.; Starinova, M.A.; Voronkova, A.Y.; Amelina, E.L.; Kashirskaya, N.Y.; Avdeev, S.N.; Kutsev, S.I. (Eds.) Register of Patients with Cystic Fibrosis in the Russian Federation, 2020 Year; Medpraktika-M: Moscow, Russia, 2022. [Google Scholar]
- Van Rens, J.; Prasad, V.; Fox, A.; Krasnyk, M.; Mayor, S.L.; Naehrlich, L.; Gkolia, P.; Nieto, A.E.; Bakkeheim, E.; Daneau, G.; et al. ECFSPR Annual Report 2021. 2023. Available online: https://www.ecfs.eu/sites/default/files/Annual%20Report_2021_09Jun2023_ECFSPR_final.pdf (accessed on 29 August 2023).
- Cystic Fibrosis Foundation Patient Registry 2021 Annual Data Report; Bethesda, Maryland. 2022. Available online: https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf (accessed on 29 August 2023).
- DeStefano, S.; Gees, M.; Hwang, T.-C. Physiological and Pharmacological Characterization of the N1303K Mutant CFTR. J. Cyst. Fibros. 2018, 17, 573–581. [Google Scholar] [CrossRef]
- Sadras, I.; Kerem, E.; Livnat, G.; Sarouk, I.; Breuer, O.; Reiter, J.; Gileles-Hillel, A.; Inbar, O.; Cohen, M.; Gamliel, A.; et al. Clinical and Functional Efficacy of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis Carrying the N1303K Mutation. J. Cyst. Fibros. 2023, 22, 1062–1069. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J. Atomic Structure of the Cystic Fibrosis Transmembrane Conductance Regulator. Cell 2016, 167, 1586–1597.e9. [Google Scholar] [CrossRef]
- Ramalho, A.S.; Förstová, E.; Vonk, A.M.; Ferrante, M.; Verfailli, C.; Dupont, L.; Boon, M.; Proesmans, M.; Beekman, J.M.; Sarouk, I.; et al. Correction of CFTR Function in Intestinal Organoids to Guide Treatment of Cystic Fibrosis. Eur. Respir. J. 2021, 57, 1902426. [Google Scholar] [CrossRef]
- Elson, E.C.; Capel, P.; Haynes, J.; Duehlmeyer, S.; Fischer, M.; Escobar, H. CFTR Modulator Therapy in an Individual with Cystic Fibrosis Caused by a N1303K CFTR Variant and Infected with Mycobacterium Abscessus. J. Pediatr. Pharmacol. Ther. 2022, 27, 396–399. [Google Scholar] [CrossRef]
- Ensinck, M.M.; De Keersmaecker, L.; Ramalho, A.S.; Cuyx, S.; Van Biervliet, S.; Dupont, L.; Christ, F.; Debyser, Z.; Vermeulen, F.; Carlon, M.S. Novel CFTR Modulator Combinations Maximise Rescue of G85E and N1303K in Rectal Organoids. ERJ Open Res. 2022, 8, 00716–02021. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, G.; Lee, J.; Yarlagadda, S.; McCoy, K.; Naren, A.P. Elexacaftor/Tezacaftor/Ivacaftor Improved Clinical Outcomes in a Patient with N1303K-CFTR Based on In Vitro Experimental Evidence. Am. J. Respir. Crit. Care Med. 2021, 204, 1231–1235. [Google Scholar] [CrossRef]
- Vonk, A.M.; van Mourik, P.; Ramalho, A.S.; Silva, I.A.L.; Statia, M.; Kruisselbrink, E.; Suen, S.W.F.; Dekkers, J.F.; Vleggaar, F.P.; Houwen, R.H.J.; et al. Protocol for Application, Standardization and Validation of the Forskolin-Induced Swelling Assay in Cystic Fibrosis Human Colon Organoids. STAR Protoc. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Derichs, N.; Sanz, J.; Von Kanel, T.; Stolpe, C.; Zapf, A.; Tümmler, B.; Gallati, S.; Ballmann, M. Intestinal Current Measurement for Diagnostic Classification of Patients with Questionable Cystic Fibrosis: Validation and Reference Data. Thorax 2010, 65, 594–599. [Google Scholar] [CrossRef] [PubMed]
| FEV1% Predicted | BMI (kg/m2) | Sweat Chloride, mmol/L (Conductivity) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Before | After | Change | Before | After | Change |
| 84.1 | 88.8 | 4.7 | 21.6 | 22.2 | 0.6 | 103 | 117 | 14 |
| Amiloride, 100 μM | Forskolin, 10 μM | Carbachol, 100 μM | Histamine, 500 μM | |
|---|---|---|---|---|
| N1303K/2143delT before ETI therapy | −7.0 ± 3.18 | 4.33 ± 2.51 | 10.0 ± 2.47 | 8.17 ± 1.24 |
| N1303K/2143delT 3 months after ETI therapy | −11.67 ± 6.09 | 8.33 ± 3.47 | 15.17 ± 0.2 | 15.67 ± 3.54 |
| positive control (F508del/F508del) | −15.7 ± 3.51 | 3.33 ± 0.63 | - | 17.83 ± 3.57 |
| negative control (wt/wt CFTR) | −7.67 ± 1.76 | 26.72 ± 2.66 | 117.44 ± 4.32 | 109.76 ± 8.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efremova, A.; Kashirskaya, N.; Krasovskiy, S.; Melyanovskaya, Y.; Krasnova, M.; Mokrousova, D.; Bulatenko, N.; Kondratyeva, E.; Makhnach, O.; Bukharova, T.; et al. Comprehensive Assessment of CFTR Modulators’ Therapeutic Efficiency for N1303K Variant. Int. J. Mol. Sci. 2024, 25, 2770. https://doi.org/10.3390/ijms25052770
Efremova A, Kashirskaya N, Krasovskiy S, Melyanovskaya Y, Krasnova M, Mokrousova D, Bulatenko N, Kondratyeva E, Makhnach O, Bukharova T, et al. Comprehensive Assessment of CFTR Modulators’ Therapeutic Efficiency for N1303K Variant. International Journal of Molecular Sciences. 2024; 25(5):2770. https://doi.org/10.3390/ijms25052770
Chicago/Turabian StyleEfremova, Anna, Nataliya Kashirskaya, Stanislav Krasovskiy, Yuliya Melyanovskaya, Maria Krasnova, Diana Mokrousova, Nataliya Bulatenko, Elena Kondratyeva, Oleg Makhnach, Tatiana Bukharova, and et al. 2024. "Comprehensive Assessment of CFTR Modulators’ Therapeutic Efficiency for N1303K Variant" International Journal of Molecular Sciences 25, no. 5: 2770. https://doi.org/10.3390/ijms25052770
APA StyleEfremova, A., Kashirskaya, N., Krasovskiy, S., Melyanovskaya, Y., Krasnova, M., Mokrousova, D., Bulatenko, N., Kondratyeva, E., Makhnach, O., Bukharova, T., Zinchenko, R., Kutsev, S., & Goldshtein, D. (2024). Comprehensive Assessment of CFTR Modulators’ Therapeutic Efficiency for N1303K Variant. International Journal of Molecular Sciences, 25(5), 2770. https://doi.org/10.3390/ijms25052770






