Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties
Abstract
1. Introduction
2. Results and Discussion
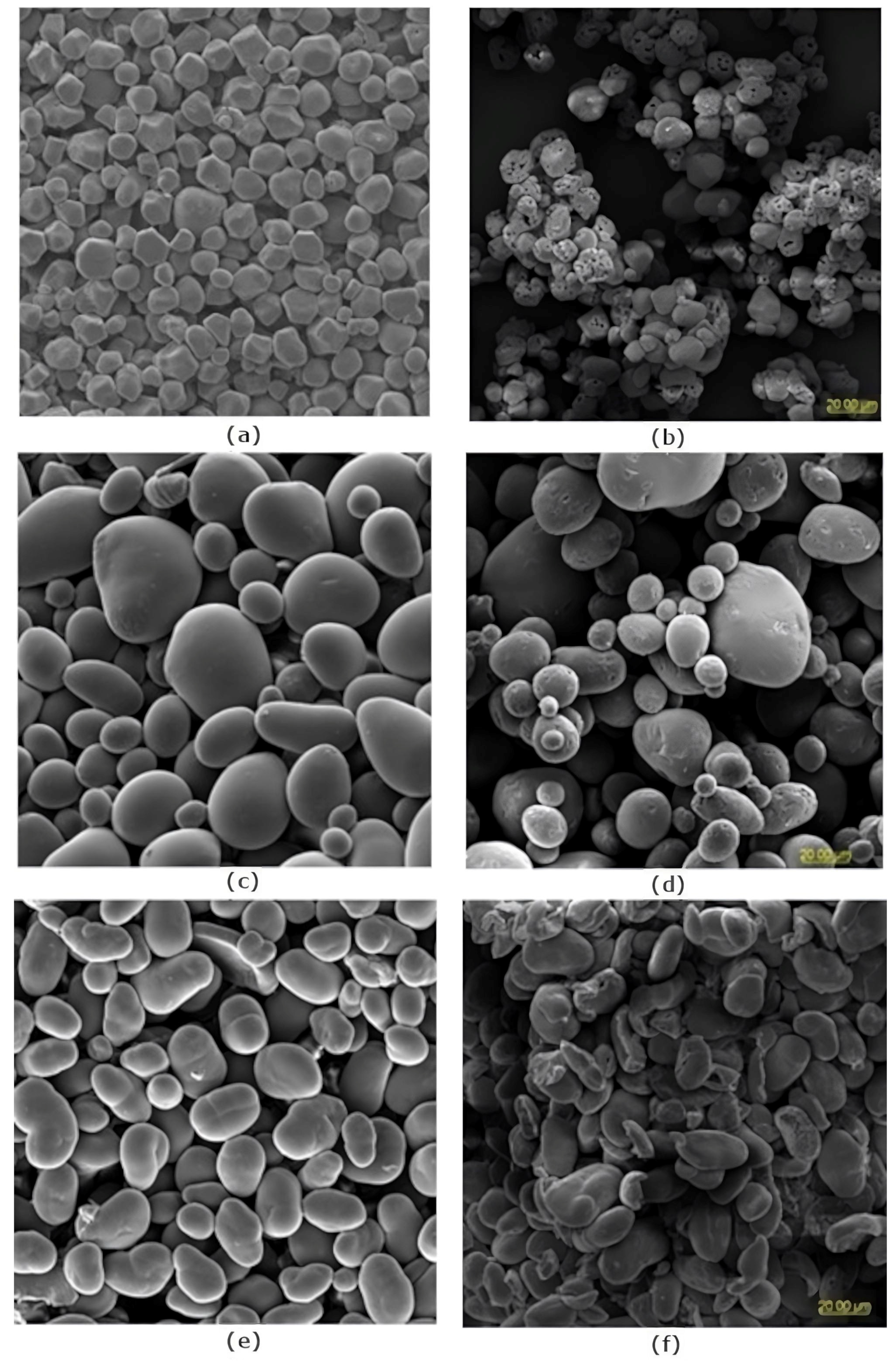
2.1. Structure and Morphology of Native and Modified Starch
2.2. Specific Surface Area and Pore Characteristics of Native and Modified Starch
2.3. Zeta Potential Calculation of the Starch Suspensions and Preliminary Wettability Test
2.4. Functional Properties of Native and Modified Starches
3. Materials and Methods
3.1. Materials
3.2. Methods
Modification of Starch with Ultrasounds, Enzymatic Hydrolysis, and Freeze-Drying
3.3. Characteristics of Native and Modified Starch
3.3.1. Analysis of Starch-Iodine Complexes
3.3.2. Scanning Electron Microscopy (SEM)
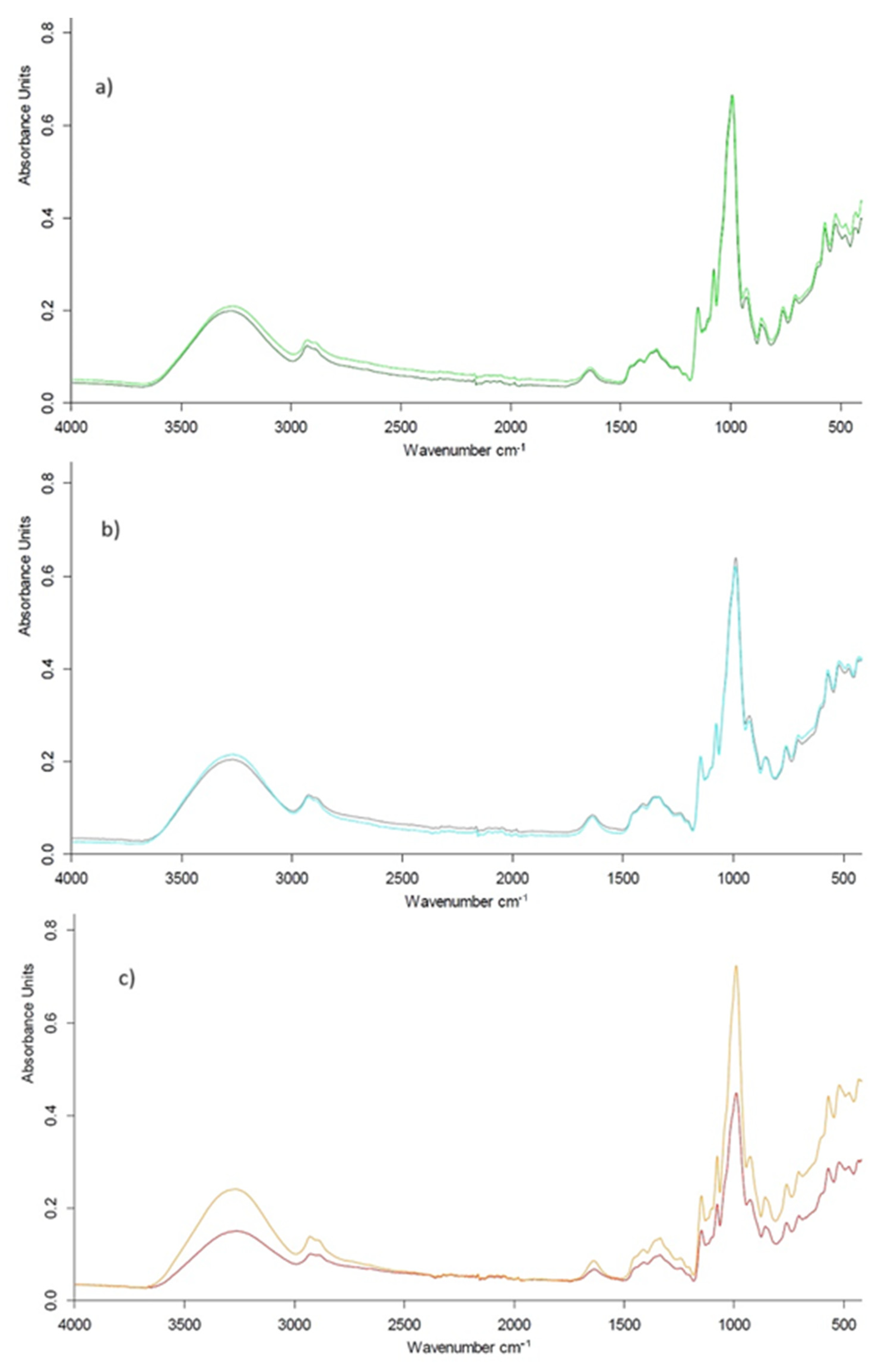
3.3.3. ATR-FTIR Analysis
3.3.4. Low-Temperature Nitrogen Adsorption
3.3.5. Determination of the Zeta Potential
3.3.6. Preliminary Wettability Tests
3.4. Functional Properties of Native and Modified Starch
3.4.1. Oil Absorption (OA)
3.4.2. Water Absorption (WA)
3.4.3. Clarity of Starch Pastes and the Minimum Concentration of Gel-Forming Starch Suspension (LGC)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Zhang, B.; Li, M.-N.; Chen, H.-Q. Effects of cross-linking with sodium trimetaphosphate on structural and adsorptive properties of porous wheat starches. Food Chem. 2019, 289, 187–194. [Google Scholar] [CrossRef]
- Gao, F.; Li, D.; Bi, C.; Mao, Z.; Adhikari, B. Application of Various Drying Methods to Produce Enzymatically Hydrolyzed Porous Starch Granules. Dry. Technol. 2013, 31, 1627–1634. [Google Scholar] [CrossRef]
- Jiang, T.; Wu, C.; Gao, Y.; Zhu, W.; Wan, L.; Wang, Z.; Wang, S. Preparation of novel porous starch microsphere foam for loading and release of poorly water soluble drug. Drug Dev. Ind. Pharm. 2014, 40, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Benavent-Gil, Y.; Rosell, C.M. Comparison of porous starches obtained from different enzyme types and levels. Carbohydr. Polym. 2017, 157, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, B. Hydrolysis of granular corn starch with controlled pore size. J. Cereal Sci. 2012, 56, 316–320. [Google Scholar] [CrossRef]
- Han, X.; Wen, H.; Luo, Y.; Yang, J.; Xiao, W.; Xie, J. Effects of chitosan modification, cross-linking, and oxidation on the structure, thermal stability, and adsorption properties of porous maize starch. Food Hydrocoll. 2022, 124, 107288. [Google Scholar] [CrossRef]
- Han, Z.; Han, Y.; Wang, J.; Liu, Z.; Buckow, R.; Cheng, J. Effects of pulsed electric field treatment on the preparation and physicochemical properties of porous corn starch derived from enzymolysis. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Ji, Y. Synthesis of porous starch microgels for the encapsulation, delivery and stabilization of anthocyanins. J. Food Eng. 2021, 302, 110552. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Yang, Q.; Pu, H.; Liu, S.; Zhu, Z. Adsorption capacity and cold-water solubility of honeycomb-like potato starch granule. Int. J. Biol. Macromol. 2020, 147, 741–749. [Google Scholar] [CrossRef]
- Qian, D.; Chang, P.R.; Ma, X. Preparation of controllable porous starch with different starch concentrations by the single or dual freezing process. Carbohydr. Polym. 2011, 86, 1181–1186. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Saravacos, G.D. Porosity and pore size distribution of starch materials. J. Food Eng. 1993, 18, 259–280. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Characteristics of pores in native and hydrolyzed starch granules. Starch Stärke 2010, 62, 229–235. [Google Scholar] [CrossRef]
- Sujka, M. Ultrasonic modification of starch—Impact on granules porosity. Ultrason. Sonochem. 2017, 37, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Keeratiburana, T.; Hansen, A.R.; Soontaranon, S.; Tongta, S.; Blennow, A. Porous rice starch produced by combined ultrasound-assisted ice recrystallization and enzymatic hydrolysis. Int. J. Biol. Macromol. 2020, 145, 100–107. [Google Scholar] [CrossRef]
- Jorge, F.-F.; Edith, C.-C.; Eduardo, R.-S.; Jairo, S.-M.; Héctor, C.-V. Hydrothermal processes and simultaneous enzymatic hydrolysis in the production of modified cassava starches with porous-surfaces. Heliyon 2023, 9, e17742. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, L.; Wang, P.; Zhan, L.; Yangzong, Y.; He, T.; Liu, Y.; Mao, D.; Ye, X.; Cui, Z.; et al. Structural and Functional Properties of Porous Corn Starch Obtained by Treating Raw Starch with AmyM. Foods 2023, 12, 3157. [Google Scholar] [CrossRef]
- Tahir, R.; Ellis, P.R.; Butterworth, P.J. The relation of physical properties of native starch granules to the kinetics of amylolysis catalysed by porcine pancreatic α-amylase. Carbohydr. Polym. 2010, 81, 57–62. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Lee, B.-H.; Yoo, S.-H. Physical structure and absorption properties of tailor-made porous starch granules produced by selected amylolytic enzymes. PLoS ONE 2017, 12, e0181372. [Google Scholar] [CrossRef]
- Wu, A.; Fang, Z.; Qin, J.; Huang, Z.; Wu, Z. Characterization and adsorption-release property of fermented porous starch as well as its bioactivity protection for guava leaf polyphenols. Food Biosci. 2023, 53, 102535. [Google Scholar] [CrossRef]
- Hu, X.; Du, X. Adsorption of Tea Polyphenols using Microporous Starch: A Study of Kinetics, Equilibrium and Thermodynamics. Molecules 2019, 24, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Cheng, Y.; Qian, H.; Yao, W. Simple microencapsulation of plant essential oil in porous starch granules: Adsorption kinetics and antibacterial activity evaluation. J. Food Process. Preserv. 2019, 43, e14156. [Google Scholar] [CrossRef]
- Belingheri, C.; Ferrillo, A.; Vittadini, E. Porous starch for flavor delivery in a tomato-based food application. LWT Food Sci. Technol. 2015, 60, 593–597. [Google Scholar] [CrossRef]
- Li, H.; van Thuy Ho, T.; Turner, M.S.; Dhital, S. Encapsulation of Lactobacillus plantarum in porous maize starch. LWT 2016, 74, 542–549. [Google Scholar] [CrossRef]
- Wiącek, A.E. Effect of surface modification on starch biopolymer wettability. Food Hydrocoll. 2015, 48, 228–237. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Dul, K. Effect of surface modification on starch/phospholipid wettability. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 351–359. [Google Scholar] [CrossRef]
- Hj Latip, D.N.; Samsudin, H.; Utra, U.; Alias, A.K. Modification methods toward the production of porous starch: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2841–2862. [Google Scholar] [CrossRef]
- Li, C.; Gong, B.; Hu, Y.; Liu, X.; Guan, X.; Zhang, B. Combined crystalline, lamellar and granular structural insights into in vitro digestion rate of native starches. Food Hydrocoll. 2020, 105, 105823. [Google Scholar] [CrossRef]
- Rundle, R.E.; French, D. The Configuration of Starch in the Starch—Iodine Complex. III. X-Ray Diffraction Studies of the Starch—Iodine Complex 1. J. Am. Chem. Soc. 1943, 65, 1707–1710. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Yu, B.; Tan, C.; Fang, Y.; Cui, B. Porous starches modified with double enzymes: Structure and adsorption properties. Int. J. Biol. Macromol. 2020, 164, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Sujka, M.; Jamroz, J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.; Hasjim, J.; Li, E.; Flanagan, B.M.; Gidley, M.J.; Dhital, S. Freeze-Drying Changes the Structure and Digestibility of B-Polymorphic Starches. J. Agric. Food Chem. 2014, 62, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D.; Leite, D.C.; Da Silveira, N.P. Relationships between enzymatic hydrolysis conditions and properties of rice porous starches. J. Cereal Sci. 2019, 89, 102819. [Google Scholar] [CrossRef]
- Sujka, M.; Cieśla, K.; Jamroz, J. Structure and selected functional properties of gamma-irradiated potato starch. Starch Stärke 2015, 67, 1002–1010. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Meng, Z.; Guo, J.; Peng, G.; Sun, W.; Zhu, X.; Gu, R.; Wu, Z.; Gan, H.; et al. Effects of enzymatic treatments on different crystal types of starch. Food Chem. Adv. 2023, 3, 100334. [Google Scholar] [CrossRef]
- Hao, J.; Lu, J.; Xu, N.; Linhardt, R.J.; Zhang, Z. Specific oxidation pattern of soluble starch with TEMPO-NaBr-NaClO system. Carbohydr. Polym. 2016, 146, 238–244. [Google Scholar] [CrossRef]
- Zhao, Y.; Huerta, R.R.; Saldaña, M.D. Use of subcritical water technology to develop cassava starch/chitosan/gallic acid bioactive films reinforced with cellulose nanofibers from canola straw. J. Supercrit. Fluids 2019, 148, 55–65. [Google Scholar] [CrossRef]
- Zhao, Y.; Saldaña, M.D. Use of potato by-products and gallic acid for development of bioactive film packaging by subcritical water technology. J. Supercrit. Fluids 2019, 143, 97–106. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Bian, X.; Guo, K.; Zhou, L.; Wei, C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018, 80, 168–176. [Google Scholar] [CrossRef]
- Ji, Y.; Lin, X.; Yu, J. Preparation and characterization of oxidized starch-chitosan complexes for adsorption of procyanidins. LWT 2020, 117, 108610. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Przykaza, K. Wettability and Stability of Naproxen, Ibuprofen and/or Cyclosporine A/Silica Delivery Systems. Colloids Interfaces 2022, 6, 11. [Google Scholar] [CrossRef]
- Han, X.; Wen, H.; Luo, Y.; Yang, J.; Xiao, W.; Ji, X.; Xie, J. Effects of α-amylase and glucoamylase on the characterization and function of maize porous starches. Food Hydrocoll. 2021, 116, 106661. [Google Scholar] [CrossRef]
- Jafari, M.; Koocheki, A. Impact of ultrasound treatment on the physicochemical and rheological properties of acid hydrolyzed sorghum starch. Int. J. Biol. Macromol. 2023, 256, 128521. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ge, X.; Guo, C.; Liu, B. Effect of Ultrasonic Treatment on Structure and Physicochemical Properties of Pea Starch. Foods 2023, 12, 2620. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.A.; Spragg, S.P. Methods in Carbohydrate Chemistry 4: Iodimetric Determination of Amylose; Academic Press: Orlando, FL, USA, 1964. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- ISO 8037-1:1986; Optics and Optical Instruments—Microscopes—Slides—Part 1: Dimensions, Optical Properties and Marking. ISO: Geneva, Switzerland, 1986.
| Starch | Degree of Hydrolysis (%) | λmax | A680/A545 | BV | |
|---|---|---|---|---|---|
| Corn | native | - | 592 ± 4 cd | 0.91 ± 0.06 ab | 0.79 ± 0.04 b |
| modified | 48.28 ± 3.83 *c | 576 ± 4 a | 0.80 ± 0.12 a | 0.65 ± 0.01 a | |
| Potato | native | - | 586 ± 2 bc | 0.96 ± 0.16 ab | 0.85 ± 0.02 b |
| modified | 5.41 ± 0.07 a | 581 ± 3 ab | 0.93 ± 0.22 ab | 0.82 ± 0.02 b | |
| Pea | native | - | 608 ± 7 e | 1.17 ± 0.15 b | 1.07 ± 0.04 c |
| modified | 24.00 ± 2.00 b | 603 ± 8 de | 1.02 ± 0.05 b | 1.05 ± 0.02 c |
| Starch | IR Ratio 1047/1022 (cm−1) | IR Ratio 1022/995 (cm−1) | |
|---|---|---|---|
| Corn | native | 0.99 ± 0.01 * | 1.01 ± 0.01 |
| modified | 1.02 ± 0.02 | 0.98 ± 0.01 | |
| Potato | native | 1.01 ± 0.02 | 0.99 ± 0.02 |
| modified | 1.05 ± 0.01 | 0.95 ± 0.01 | |
| Pea | native | 1.01 ± 0.02 | 0.99 ± 0.01 |
| modified | 1.02 ± 0.02 | 0.97 ± 0.02 |
| Starch | Specific Surface Area (m2/g) | Volume of Mesopores (cm3/g) × 10−3 | Average Pore Diameter (nm) | |
|---|---|---|---|---|
| Corn | native | 0.56 ± 0.04 * | 1.23 ± 0.06 | 8.72 ± 0.06 |
| modified | 1.66 ± 0.08 | 4.24 ± 0.12 | 10.21 ± 0.04 | |
| Potato | native | 0.02 ± 0.01 | 0.01 ± 0.01 | 33.87 ± 1.20 |
| modified | 0.22 ± 0.02 | 0.80 ± 0.06 | 14.47 ± 0.19 | |
| Pea | native | 0.16 ± 0.02 | 0.55 ± 0.04 | 14.01 ± 0.08 |
| modified | 0.40 ± 0.06 | 1.24 ± 0.05 | 12.54 ± 0.10 |
| Starch | Zeta Potential [mV] | 15 min | 30 min | 60 min | 120 min | Week | Mean |
|---|---|---|---|---|---|---|---|
| Corn | native | 7.41 ± 3.2 * | 10.58 ± 4.1 | 9.86 ± 2.6 | 7.66 ± 4.0 | 16.76 ± 3.8 | 10.45 ± 3.5 |
| modified | −13.55 ± 4.8 | −8.85 ± 0.1 | −20.14 ± 3.4 | −28.06 ± 7.2 | −17.8 ± 1.2 | −17.7 ± 3.3 | |
| Potato | native | −3.60 ± 1.8 | −3.36 ± 1.6 | −1.96 ± 0.9 | −1.56 ± 0.5 | −1.76 ± 0.7 | −2.45 ± 1.1 |
| modified | −1.16 ± 0.9 | −1.58 ± 0.7 | −1.11 ± 0.2 | −2.41 ±0.5 | −1.76 ± 0.4 | −1.60 ± 0.5 | |
| Pea | native | −0.74 ± 0.3 | −2.73 ± 1.0 | −1.89 ± 0.8 | −1.15 ± 0.2 | −1.52 ± 0.1 | −1.61 ± 0.5 |
| modified | −1.36 ± 0.5 | −1.38 ± 1.8 | −4.01 ± 1.5 | −3.14 ± 2.1 | −3.58 ± 1.8 | −2.69 ± 1.5 |
| Starch | WA (%) | OA (%) | LGC (%) | Paste Clarity (%) | |
|---|---|---|---|---|---|
| Corn | native | 194.65 ± 0.64 *c | 173.05 ± 3.46 c | 6 b | 21.50 ± 0.50 e |
| modified | 261.15 ± 1.34 e | 237.55 ± 4.03 e | 8 c | 8.30 ± 0.42 b | |
| Potato | native | 174.85 ± 3.47 a | 152.10 ± 1.70 a | 4 a | 74.85 ± 2.62 f |
| modified | 229.75 ± 2.61 d | 168.95 ± 2.05 bc | 6 b | 25.25 ± 0.92 d | |
| Pea | native | 186.20 ± 0.71 b | 163.15 ± 0.50 b | 4 a | 15.25 ± 0.21 c |
| modified | 286.95 ± 0.64 f | 206.45 ± 6.01 d | 4 a | 5.20 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujka, M.; Wiącek, A.E. Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties. Int. J. Mol. Sci. 2024, 25, 1662. https://doi.org/10.3390/ijms25031662
Sujka M, Wiącek AE. Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties. International Journal of Molecular Sciences. 2024; 25(3):1662. https://doi.org/10.3390/ijms25031662
Chicago/Turabian StyleSujka, Monika, and Agnieszka Ewa Wiącek. 2024. "Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties" International Journal of Molecular Sciences 25, no. 3: 1662. https://doi.org/10.3390/ijms25031662
APA StyleSujka, M., & Wiącek, A. E. (2024). Physicochemical Characteristics of Porous Starch Obtained by Combined Physical and Enzymatic Methods, Part 1: Structure, Adsorption, and Functional Properties. International Journal of Molecular Sciences, 25(3), 1662. https://doi.org/10.3390/ijms25031662







