A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay
Abstract
:1. Introduction
2. Results and Discussion
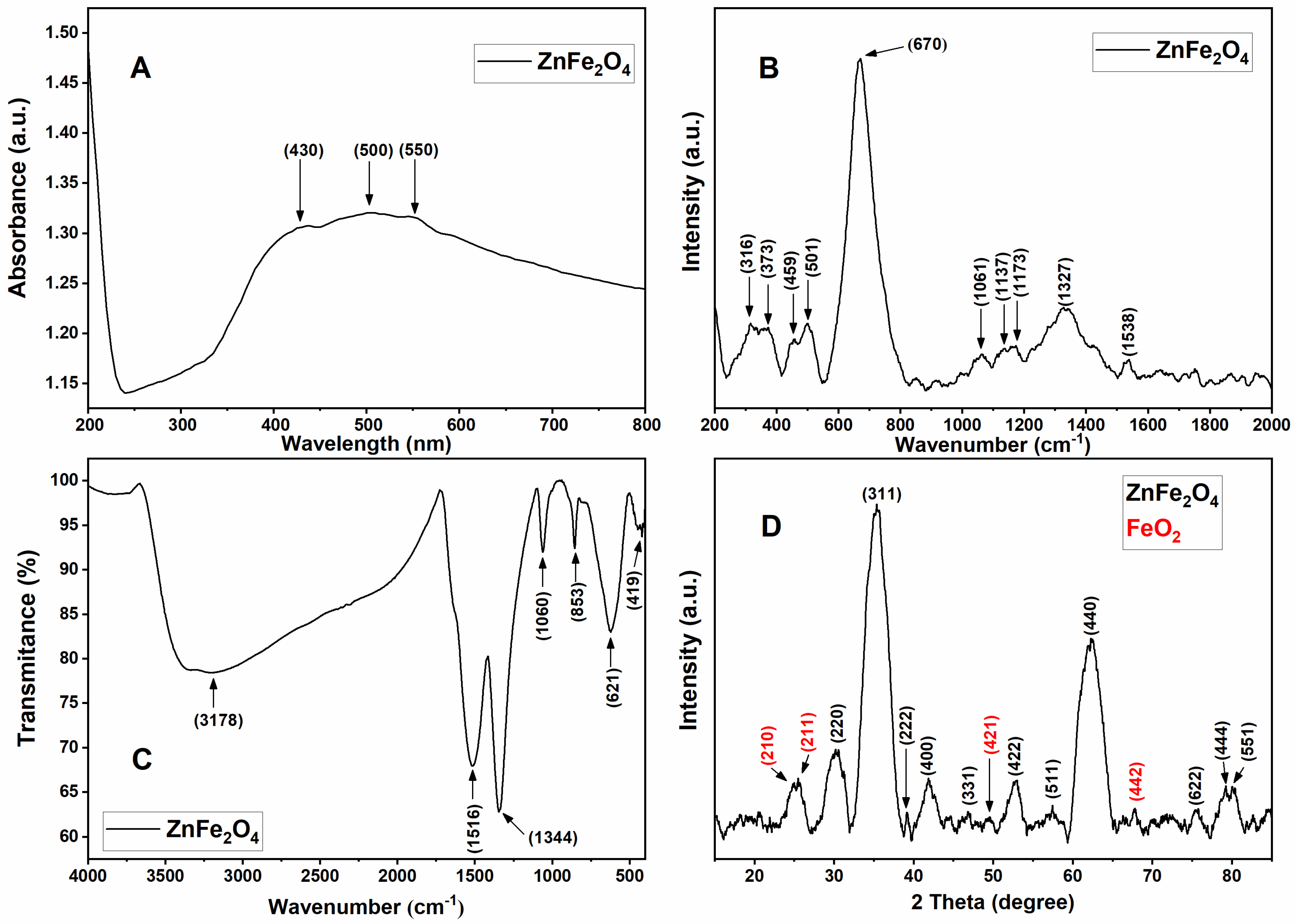
2.1. Optical and Molecular Vibrational Characterization of ZFO NPs
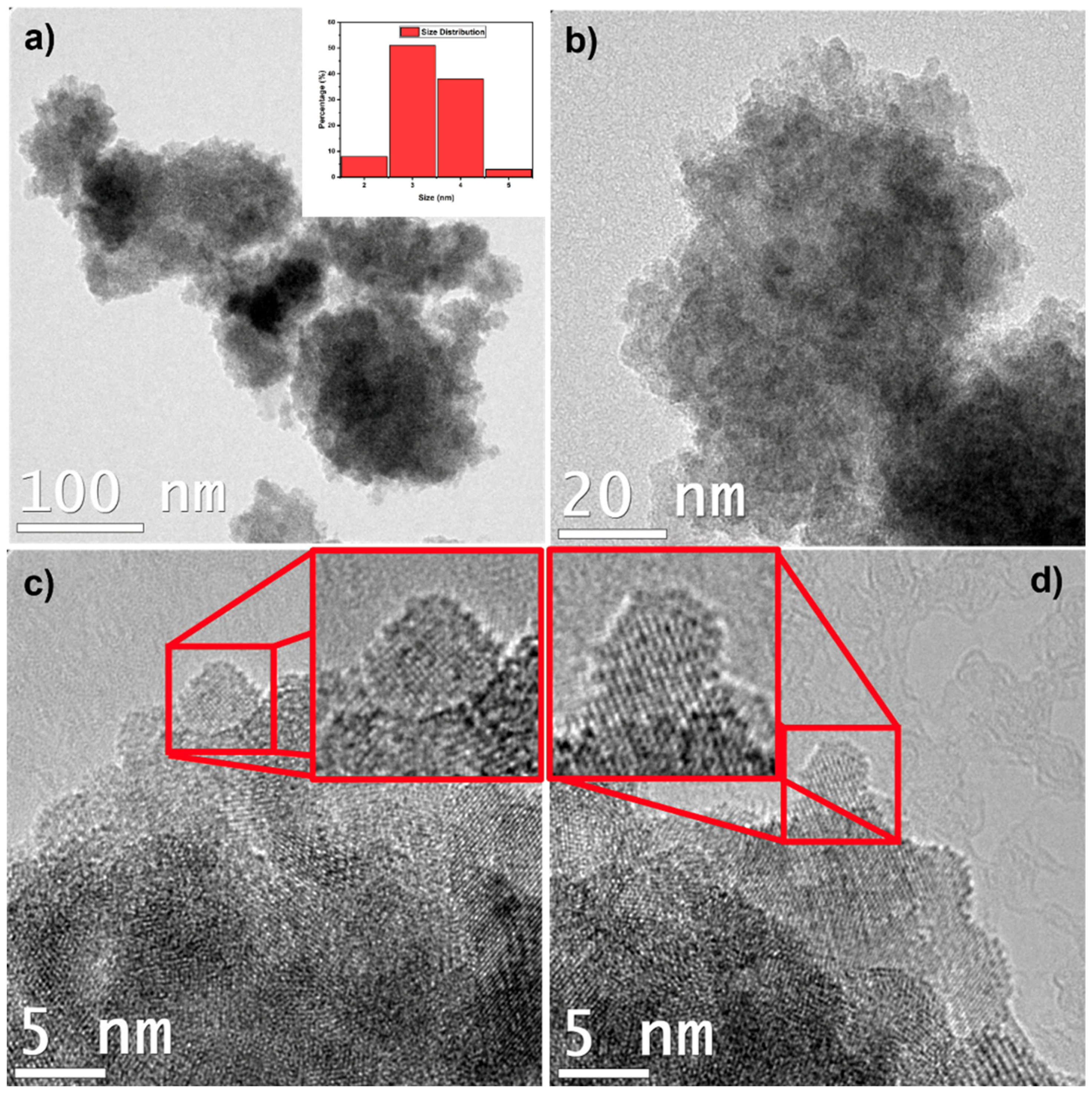
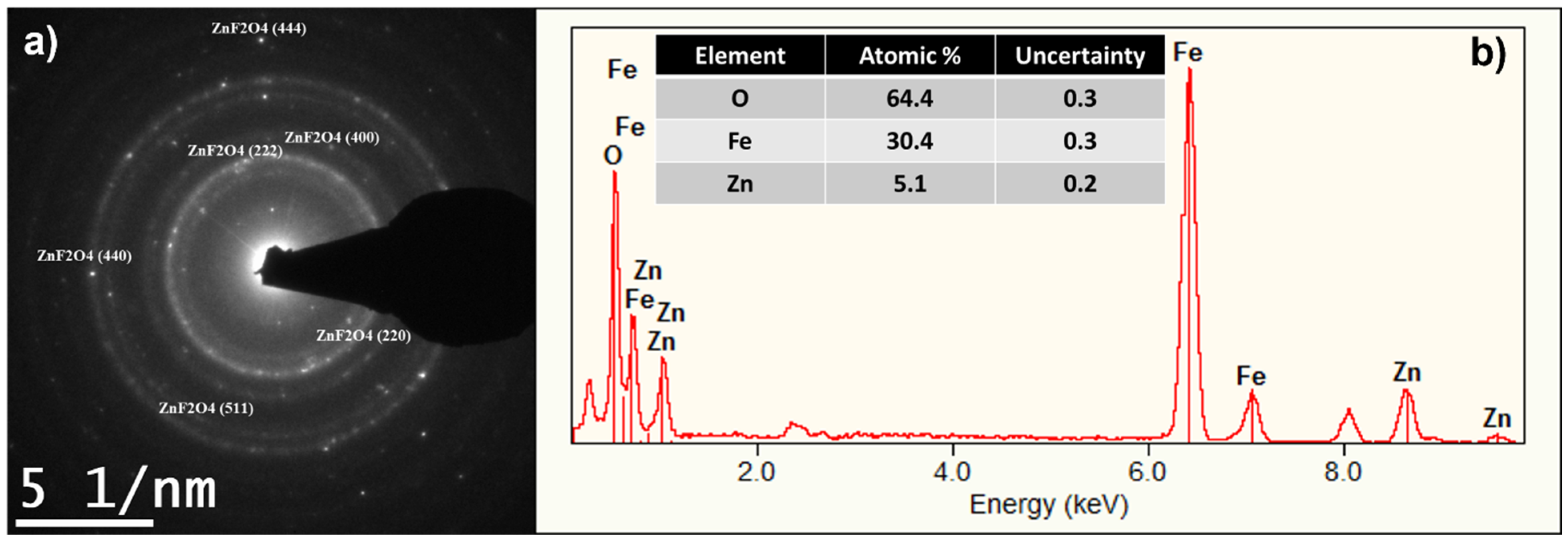
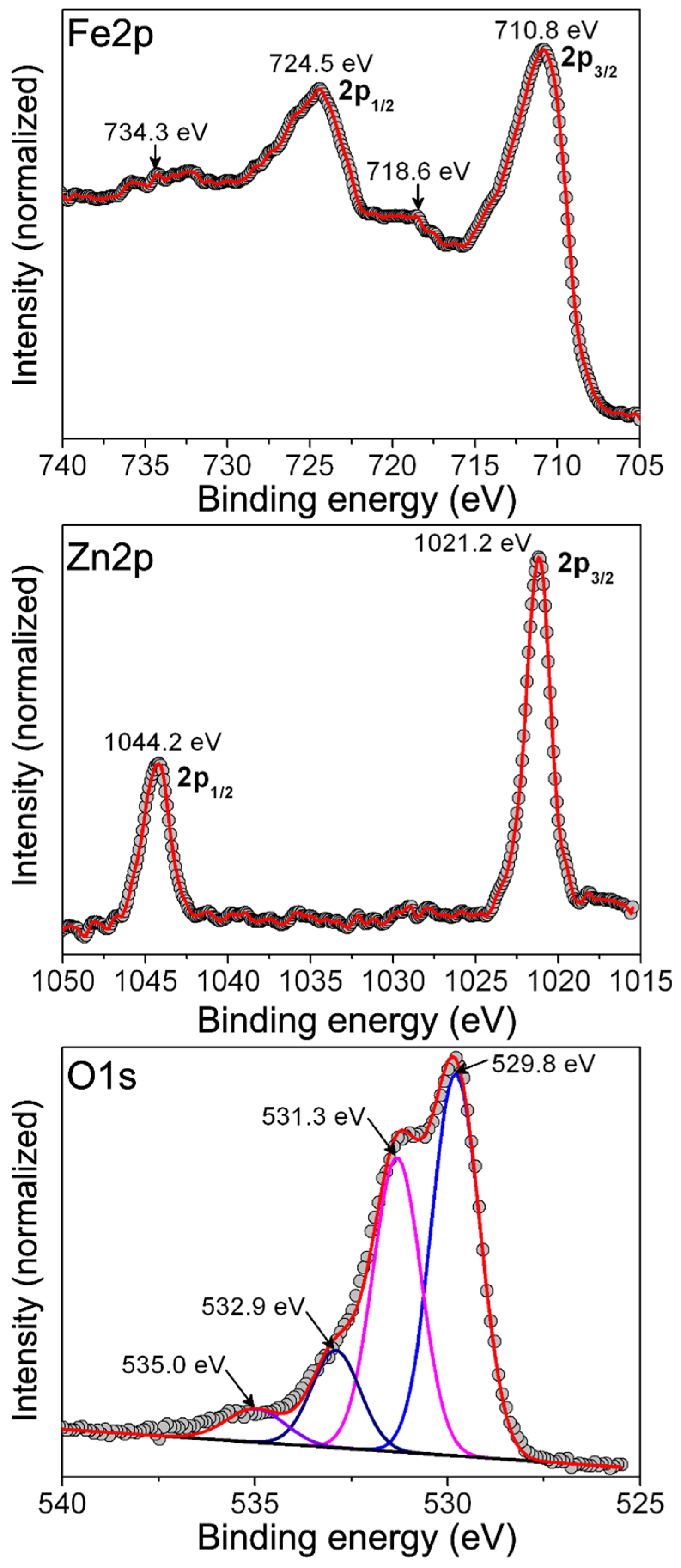
2.2. Structural Characterization of ZFO NPs
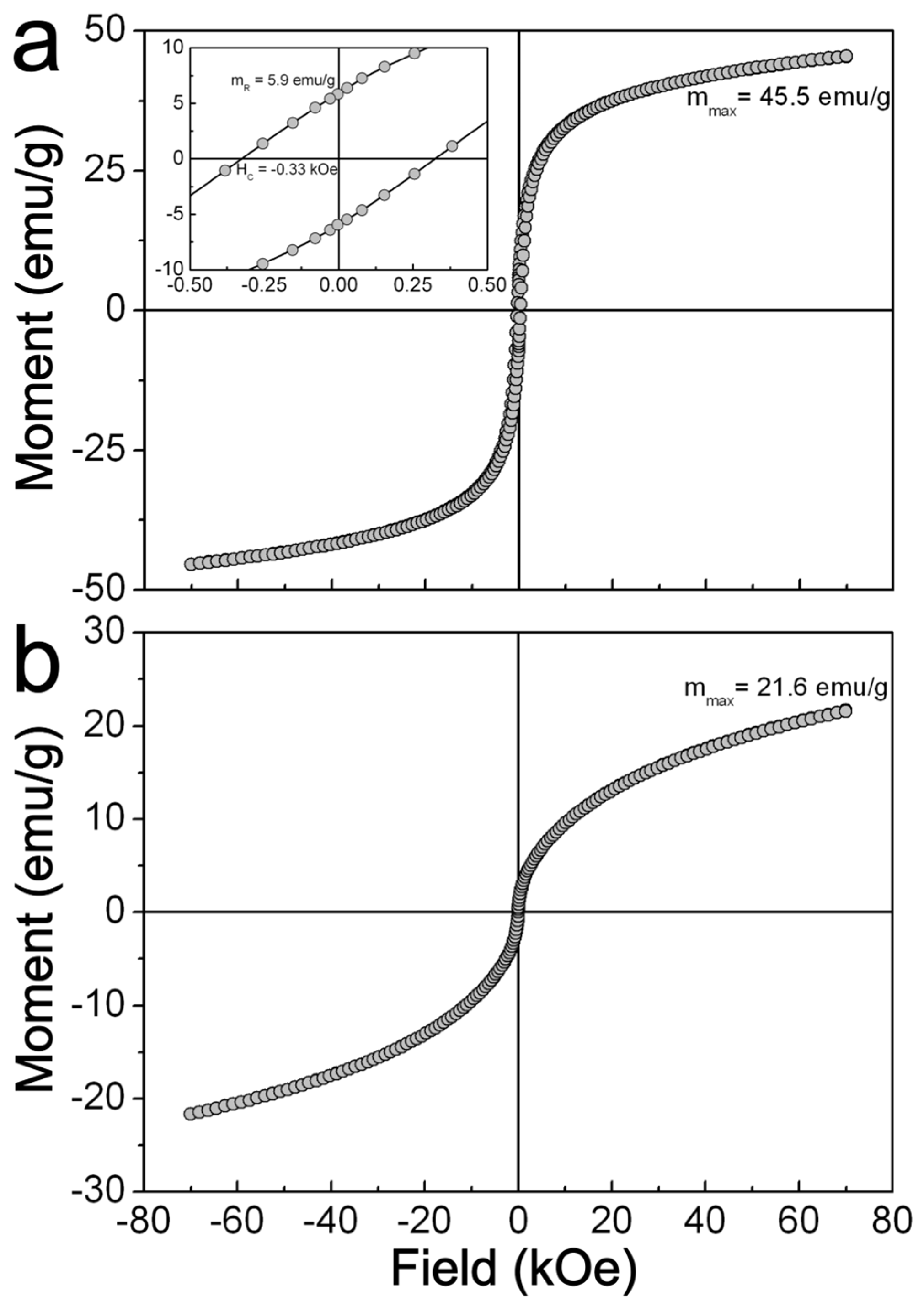
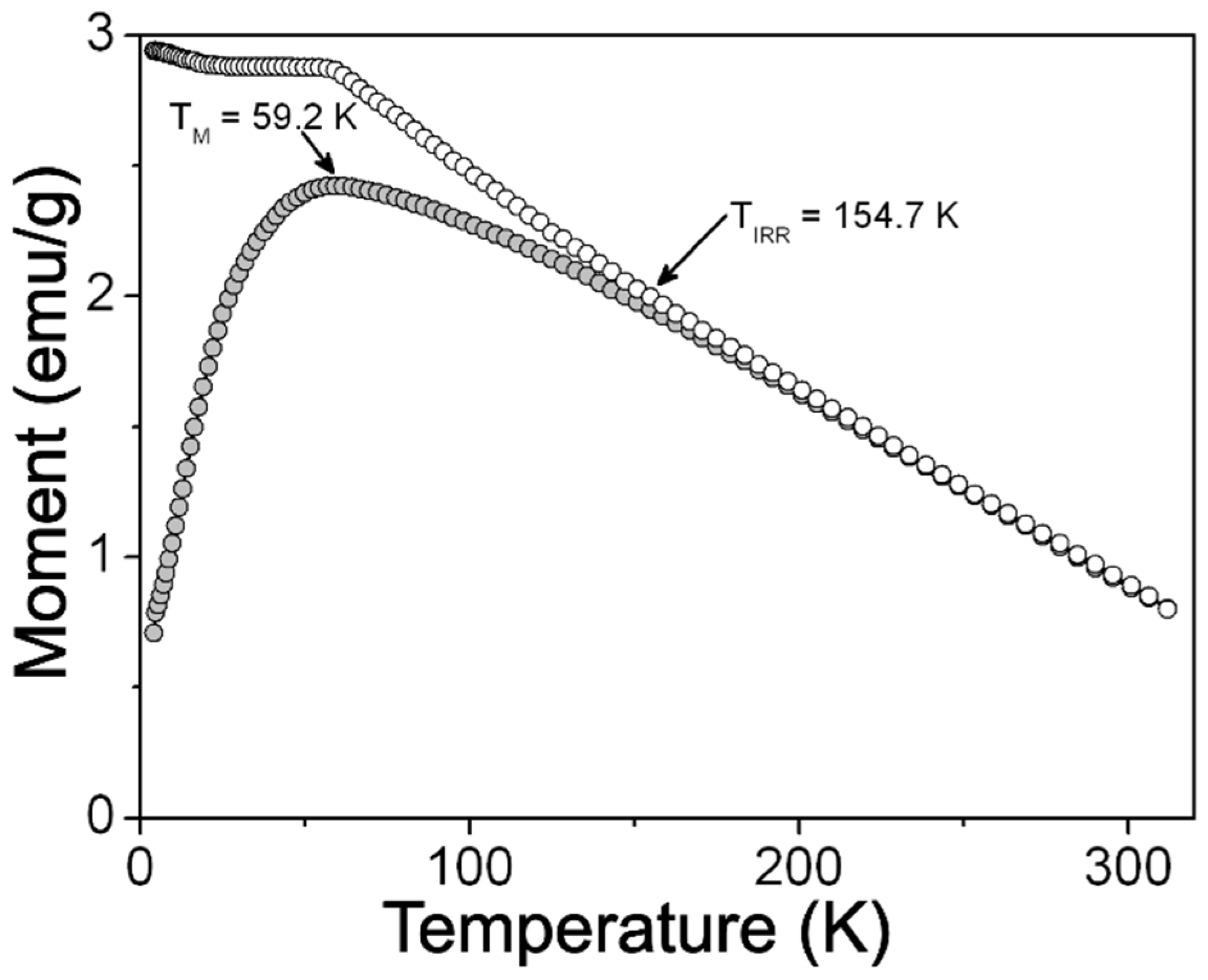
2.3. Magnetic Characterization of ZFO NPs
2.4. Comparative Cytotoxicity Evaluation Using Different Viability Assays (MTT, Crystal Violet and Lactate Dehydrogenase)
2.4.1. MTT Assay
2.4.2. Crystal Violet Assay
2.4.3. Lactate Dehydrogenase Assay
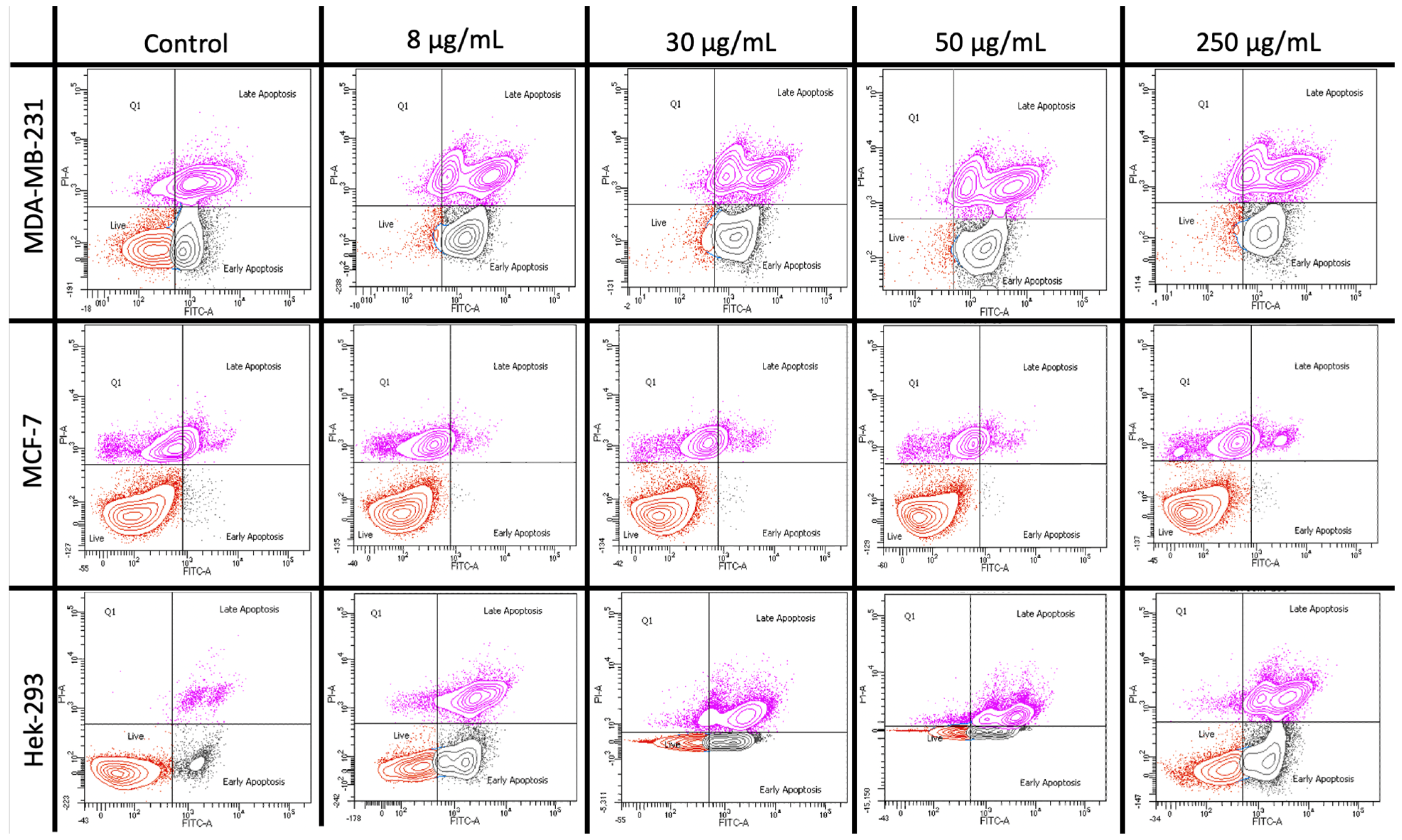
2.5. Apoptosis Assay Using Flow Cytometry
2.6. Optical Microscopy Studies for Cell Morphological Analysis
3. Materials and Methods
3.1. Synthesis of ZFO NPs
3.2. Characterization
3.3. Cell Culture and MTT, Crystal Violet, and LDH Cytotoxic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Heimke-Brinck, R.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef] [PubMed]
- Mamani, J.; Costa-Filho, A.; Cornejo, D.; Vieira, E.; Gamarra, L. Synthesis and characterization of magnetite nanoparticles coated with lauric acid. Mater. Charact. 2013, 81, 28–36. [Google Scholar] [CrossRef]
- Paz-Díaz, B.; Vázquez-Olmos, A.R.; Almaguer-Flores, A.; García-Pérez, V.I.; Sato-Berrú, R.Y.; Almanza-Arjona, Y.C.; Garibay-Febles, V. ZnFe2O4 and CuFe2O4 Nanocrystals: Synthesis, Characterization, and Bactericidal Application. J. Clust. Sci. 2021, 34, 111–119. [Google Scholar] [CrossRef]
- Shahraki, R.R.; Ebrahimi, M.; Ebrahimi, S.S.; Masoudpanah, S. Structural characterization and magnetic properties of superparamagnetic zinc ferrite nanoparticles synthesized by the coprecipitation method. J. Magn. Magn. Mater. 2012, 324, 3762–3765. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Shahraki, R.R.; Ebrahimi, S.A.S.; Masoudpanah, S.M. Magnetic Properties of Zinc Ferrite Nanoparticles Synthesized by Coprecipitation Method. J. Supercond. Nov. Magn. 2014, 27, 1587–1592. [Google Scholar] [CrossRef]
- Galinetto, P.; Albini, B.; Bini, M.; Mozzati, M.C. Raman spectroscopy in Zinc Ferrites Nanoparticles. Raman Spectrosc. 2018. [Google Scholar] [CrossRef]
- Torres-Pérez, S.A.; Ramos-Godínez, M.D.P.; Ramón-Gallegos, E. Glycosylated one-step PAMAM dendrimers loaded with methotrexate for target therapy in breast cancer cells MDA-MB-231. J. Drug Deliv. Sci. Technol. 2020, 58, 101769. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.-H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 248. [Google Scholar] [CrossRef]
- Das, P.; Dutta, A.; Bhaumik, A.; Mukhopadhyay, C. Heterogeneous Ditopic ZnFe2O4 Catalyzed Synthesis of 4H-Pyrans: Further Conversion to 1,4-DHPs and Report of Functional Group Interconversion from Amide to Ester. Green Chem. 2014, 16, 1426–1435. [Google Scholar] [CrossRef]
- Shams, S.; Sheibanizadeh, Z.; Khalaj, Z. Ternary nanocomposite of ZnFe2O4/α-Fe2O3/ZnO; synthesis via coprecipitation method and physical properties characterization. Appl. Phys. A 2021, 127, 459. [Google Scholar] [CrossRef]
- Kasparis, G.; Sangnier, A.P.; Wang, L.; Efstathiou, C.; LaGrow, A.P.; Sergides, A.; Wilhelm, C.; Thanh, N.T.K. Zn doped iron oxide nanoparticles with high magnetization and photothermal efficiency for cancer treatment. J. Mater. Chem. B 2022, 11, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Srivastava, R.C.; Agrawal, H.M.; Kumar, R. Micro-Raman investigation of nanosized zinc ferrite: Effect of crystallite size and fluence of irradiation. J. Raman Spectrosc. 2011, 42, 1510–1517. [Google Scholar] [CrossRef]
- Rivero, M.; del Campo, A.; Mayoral, Á.; Mazario, E.; Sánchez-Marcos, J.; Muñoz-Bonilla, A. Synthesis and structural characterization of ZnxFe3−xO4 ferrite nanoparticles obtained by an electrochemical method. RSC Adv. 2016, 6, 40067–40076. [Google Scholar] [CrossRef]
- Abbasian, A.R.; Afarani, M.S. One-step solution combustion synthesis and characterization of ZnFe2O4and ZnFe1.6O4 nanoparticles. Appl. Phys. A 2019, 125, 721. [Google Scholar] [CrossRef]
- Sarala, E.; Naik, M.M.; Vinuth, M.; Reddy, Y.V.R.; Sujatha, H.R. Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J. Mater. Sci. Mater. Electron. 2020, 31, 8589–8596. [Google Scholar] [CrossRef]
- Caruntu, G.; Bush, G.G.; O’Connor, C.J. Synthesis and characterization of nanocrystalline zinc ferrite films prepared by liquid phase deposition. J. Mater. Chem. 2004, 14, 2753–2759. [Google Scholar] [CrossRef]
- Singh, C.; Devika, D.; Malik, R.; Kumar, V.; Singhal, S. CdS nanorod–MFe2O4 (M = Zn, Co and Ni) nanocomposites: A heterojunction synthesis strategy to mitigate environmental deterioration. RSC Adv. 2015, 5, 89327–89337. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; El-Sayyad, G.S.; El-Bastawisy, H.S.; Fathy, R.M. Antibacterial and antibiofilm activities of silver-decorated zinc ferrite nanoparticles synthesized by a gamma irradiation-coupled sol–gel method against some pathogenic bacteria from medical operating room surfaces. RSC Adv. 2021, 11, 28361–28374. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Imani, M.; Salehi, M. ZnFe2O4 nanoparticles and a clay encapsulated ZnFe2O4 nanocomposite: Synthesis strategy, structural characteristics and the adsorption of dye pollutants in water. RSC Adv. 2015, 5, 56145–56156. [Google Scholar] [CrossRef]
- Ali, R.; Aziz, M.H.; Gao, S.; Khan, M.I.; Li, F.; Batool, T.; Shaheen, F.; Qiu, B. Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging. Ceram. Int. 2022, 48, 10741–10750. [Google Scholar] [CrossRef]
- Anooj, K.P.E.S.; Sreelekshmi, S.J.; Gopukumar, S.T.; Praseetha, P.K. Evaluation of the Zinc Ferrite Nano particles for Bio-applications. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 22–26. [Google Scholar]
- Zahi, S. Synthesis, Permeability and Microstructure of the Optimal Nickel-Zinc Ferrites by Sol-Gel Route. J. Electromagn. Anal. Appl. 2010, 02, 56–62. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, X.; Li, H.; Chen, K. Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J. Mater. Chem. 2012, 22, 13500–13505. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Hashim, M.; Shaari, A.H.; Ahangar, H.A. Synthesis and characterization of zinc ferrite nanoparticles by a thermal treatment method. Solid State Commun. 2011, 151, 1031–1035. [Google Scholar] [CrossRef]
- Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González, M.M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández Hernández, J.M.; et al. Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules 2020, 25, 4428. [Google Scholar] [CrossRef] [PubMed]
- Kahmei, R.D.R.; Seal, P.; Borah, J.P. Tunable heat generation in nickel-substituted zinc ferrite nanoparticles for magnetic hyperthermia. Nanoscale Adv. 2021, 3, 5339–5347. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Hong, S.J.; Lee, J.S.; Borse, P.H.; Jung, O.-S.; Hong, T.E.; Jeong, E.D.; Won, M.S.; Kim, H.G. Synthesis of Zinc Ferrite and Its Photocatalytic Application under Visible Light. J. Korean Phys. Soc. 2009, 54, 204–208. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; El Zowalaty, M.E.; Rasedee, A.; Flaifel, M.H.; Ahmad, S.H.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Eid, E.E.; Zulkarnain, Z.; Saeed, M.; et al. Cytotoxicity of nickel zinc ferrite nanoparticles on cancer cells of epithelial origin. Int. J. Nanomed. 2013, 8, 2497–2508. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.; Cai, B.; Fan, H. ‘One-pot’ synthesis of multifunctional GSH–CdTe quantum dots for targeted drug delivery. Nanotechnology 2014, 25, 235101. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Wang, X.; Qian, Q.; Zhang, S.; Li, Z.; Zou, Z. A facile spray pyrolysis method to prepare Ti-doped ZnFe2O4 for boosting photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 7571–7577. [Google Scholar] [CrossRef]
- Misra, R.; Gubbala, S.; Kale, A.; Egelhoff, W. A comparison of the magnetic characteristics of nanocrystalline nickel, zinc, and manganese ferrites synthesized by reverse micelle technique. Mater. Sci. Eng. B 2004, 111, 164–174. [Google Scholar] [CrossRef]
- Pem, B.; González-Mancebo, D.; Moros, M.; Ocaña, M.; Becerro, A.I.; Pavičić, I.; Selmani, A.; Babič, M.; Horák, D.; Vrček, I.V. Biocompatibility assessment of up-and down-converting nanoparticles: Implications of interferences with in vitro assays. Methods Appl. Fluoresc. 2018, 7, 014001. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2015, 36, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Pillukat, M.H.; Hahn, D.; Schnekenburger, J. Current in vitro methods in nanoparticle risk assessment: Limitations and challenges. Eur. J. Pharm. Biopharm. 2009, 72, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Vrček, I.V.; Pavičić, I.; Crnković, T.; Jurašin, D.; Babič, M.; Horák, D.; Lovrić, M.; Ferhatović, L.; Ćurlin, M.; Gajović, S. Does surface coating of metallic nanoparticles modulate their interference with in vitro assays? RSC Adv. 2015, 5, 70787–70807. [Google Scholar] [CrossRef]
- Almutary, A.; Sanderson, B.J.S. The MTT and Crystal Violet Assays: Potential Confounders in Nanoparticle Toxicity Testing. Int. J. Toxicol. 2016, 35, 454–462. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef]
- Suman, T.; Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Kumar, G.; Degheidy, H.; Casey, B.J.; Goering, P.L. Flow cytometry evaluation of in vitro cellular necrosis and apoptosis induced by silver nanoparticles. Food Chem. Toxicol. 2015, 85, 45–51. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Ali, M.; Grigalavicius, M.; Grallert, B.; Dillard, P.; Schink, K.O.; Olsen, C.E.; Wälchli, S.; Inderberg, E.M.; Kubin, A.; et al. Simultaneous defeat of MCF7 and MDA-MB-231 resistances by a hypericin PDT–tamoxifen hybrid therapy. NPJ Breast Cancer 2019, 5, 13. [Google Scholar] [CrossRef]
- Won, K.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Ketebo, A.A.; Shin, T.H.; Jun, M.; Lee, G.; Park, S. Effect of silica-coated magnetic nanoparticles on rigidity sensing of human embryonic kidney cells. J. Nanobiotechnol. 2020, 18, 170. [Google Scholar] [CrossRef]

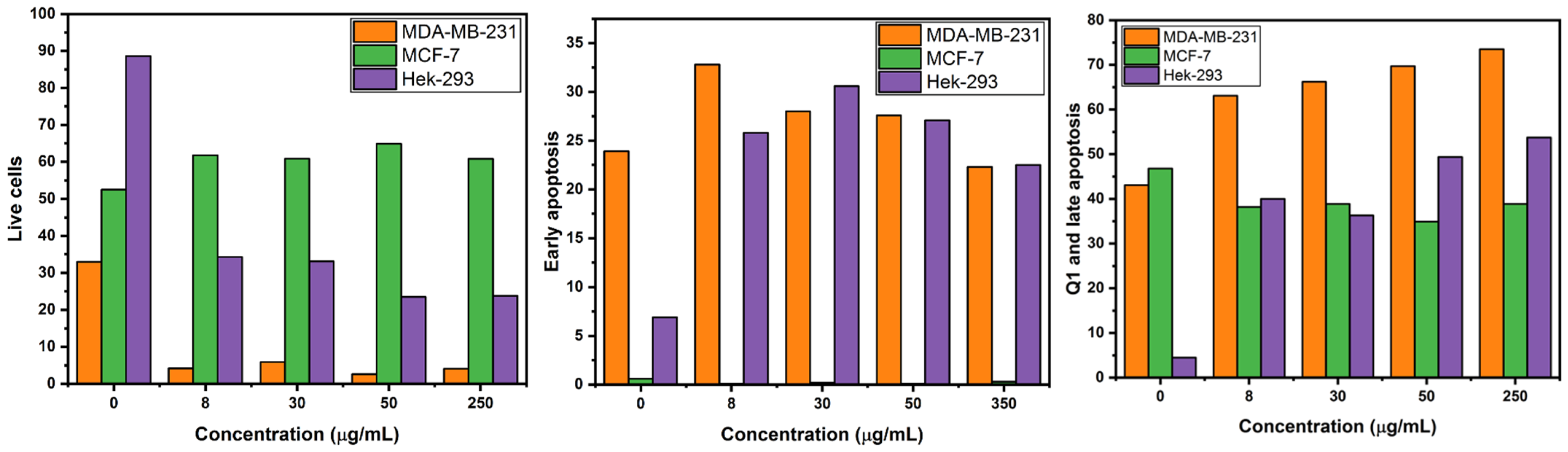

| Live (%) | Early Apoptosis (%) | Q1 and Late Apoptosis (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | MDA-MB-231 | MCF-7 | HEK-293 | MDA-MB-231 | MCF-7 | HEK-293 | MDA-MB-231 | MCF-7 | HEK-293 |
| 0 µg/mL | 33 | 53 | 89 | 24 | 1 | 7 | 43 | 47 | 5 |
| 8 µg/mL | 4 | 62 | 34 | 33 | 0 | 26 | 63 | 38 | 40 |
| 30 µg/mL | 6 | 61 | 33 | 28 | 0 | 31 | 66 | 39 | 36 |
| 50 µg/mL | 3 | 65 | 24 | 28 | 0 | 27 | 70 | 35 | 49 |
| 250 µg/mL | 4 | 61 | 24 | 22 | 0 | 23 | 74 | 39 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Fuente-Jiménez, J.L.; Rodríguez-Rivas, C.I.; Mitre-Aguilar, I.B.; Torres-Copado, A.; García-López, E.A.; Herrera-Celis, J.; Arvizu-Espinosa, M.G.; Garza-Navarro, M.A.; Arriaga, L.G.; García, J.L.; et al. A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay. Int. J. Mol. Sci. 2023, 24, 12860. https://doi.org/10.3390/ijms241612860
de la Fuente-Jiménez JL, Rodríguez-Rivas CI, Mitre-Aguilar IB, Torres-Copado A, García-López EA, Herrera-Celis J, Arvizu-Espinosa MG, Garza-Navarro MA, Arriaga LG, García JL, et al. A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay. International Journal of Molecular Sciences. 2023; 24(16):12860. https://doi.org/10.3390/ijms241612860
Chicago/Turabian Stylede la Fuente-Jiménez, Juan Luis, César Iván Rodríguez-Rivas, Irma Beatriz Mitre-Aguilar, Andrea Torres-Copado, Eric Alejandro García-López, José Herrera-Celis, María Goretti Arvizu-Espinosa, Marco Antonio Garza-Navarro, Luis Gerardo Arriaga, Janet Ledesma García, and et al. 2023. "A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay" International Journal of Molecular Sciences 24, no. 16: 12860. https://doi.org/10.3390/ijms241612860
APA Stylede la Fuente-Jiménez, J. L., Rodríguez-Rivas, C. I., Mitre-Aguilar, I. B., Torres-Copado, A., García-López, E. A., Herrera-Celis, J., Arvizu-Espinosa, M. G., Garza-Navarro, M. A., Arriaga, L. G., García, J. L., García-Gutiérrez, D. I., Dehesa, A. Z., Sharma, A., & Oza, G. (2023). A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay. International Journal of Molecular Sciences, 24(16), 12860. https://doi.org/10.3390/ijms241612860









