Performance Measures and Plasma Biomarker Levels in Patients with Multiple Sclerosis after 14 Days of Fampridine Treatment: An Explorative Study
Abstract
1. Introduction
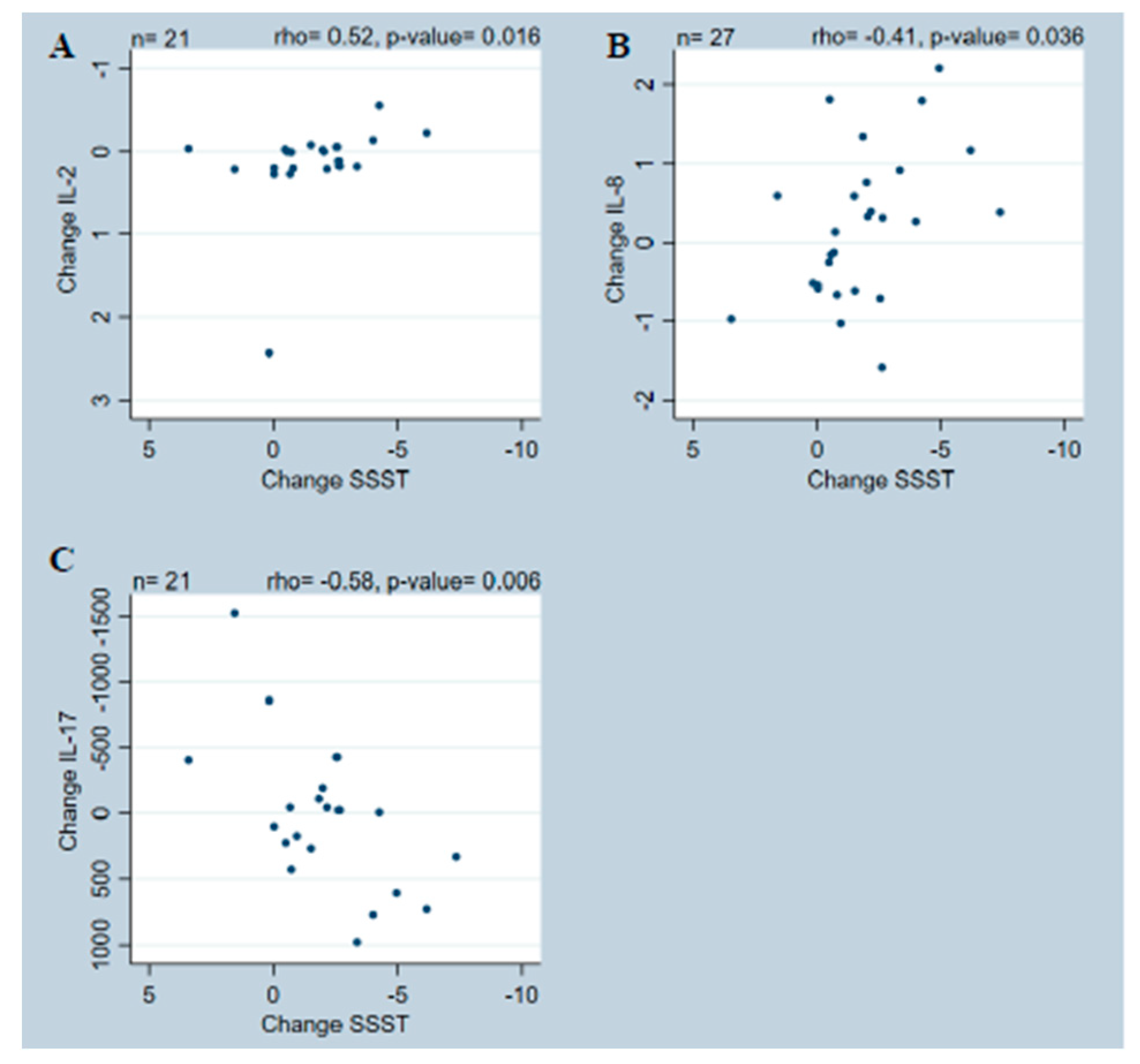
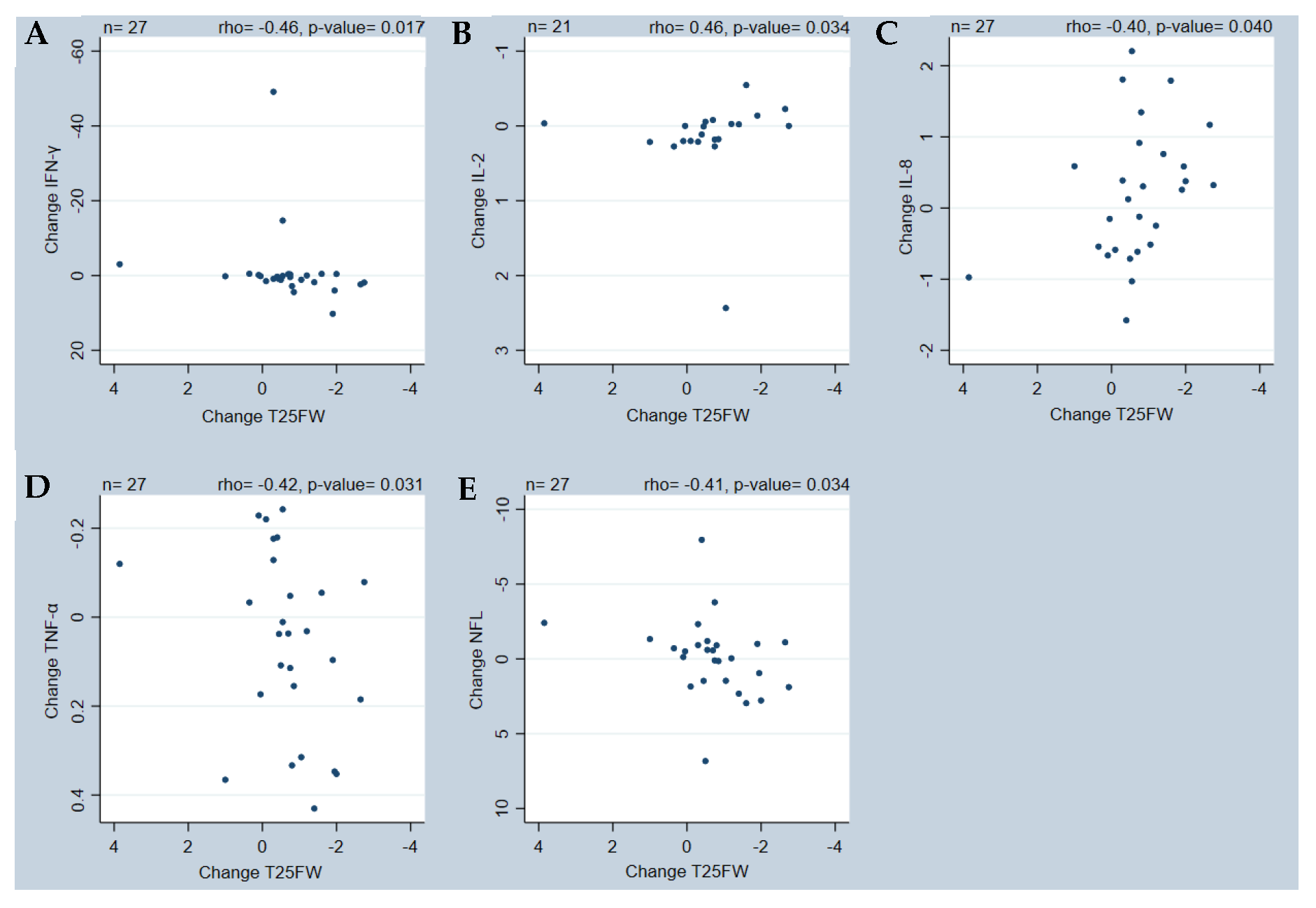
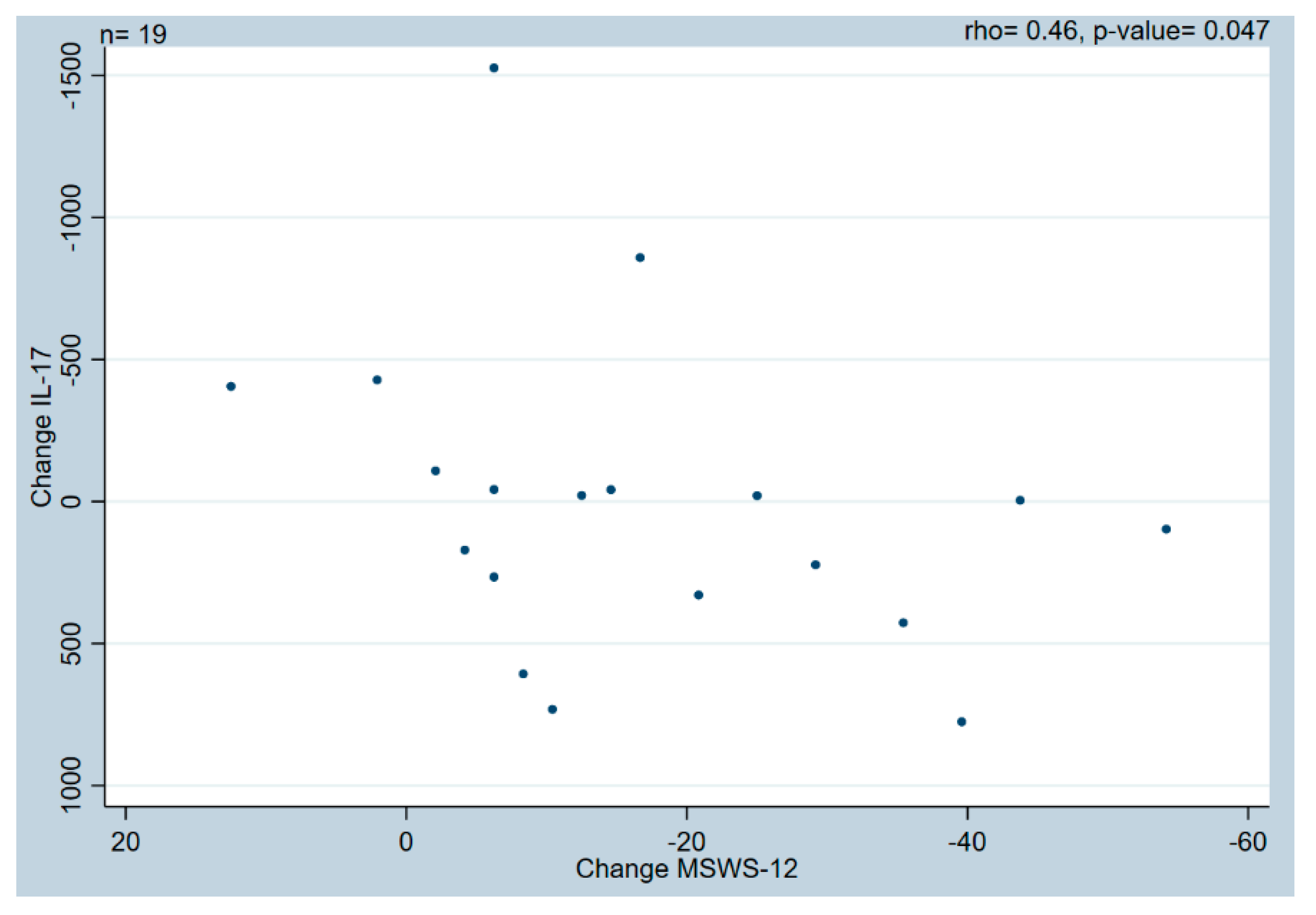
2. Results
3. Discussion
Strength and Limitations
4. Materials and Methods
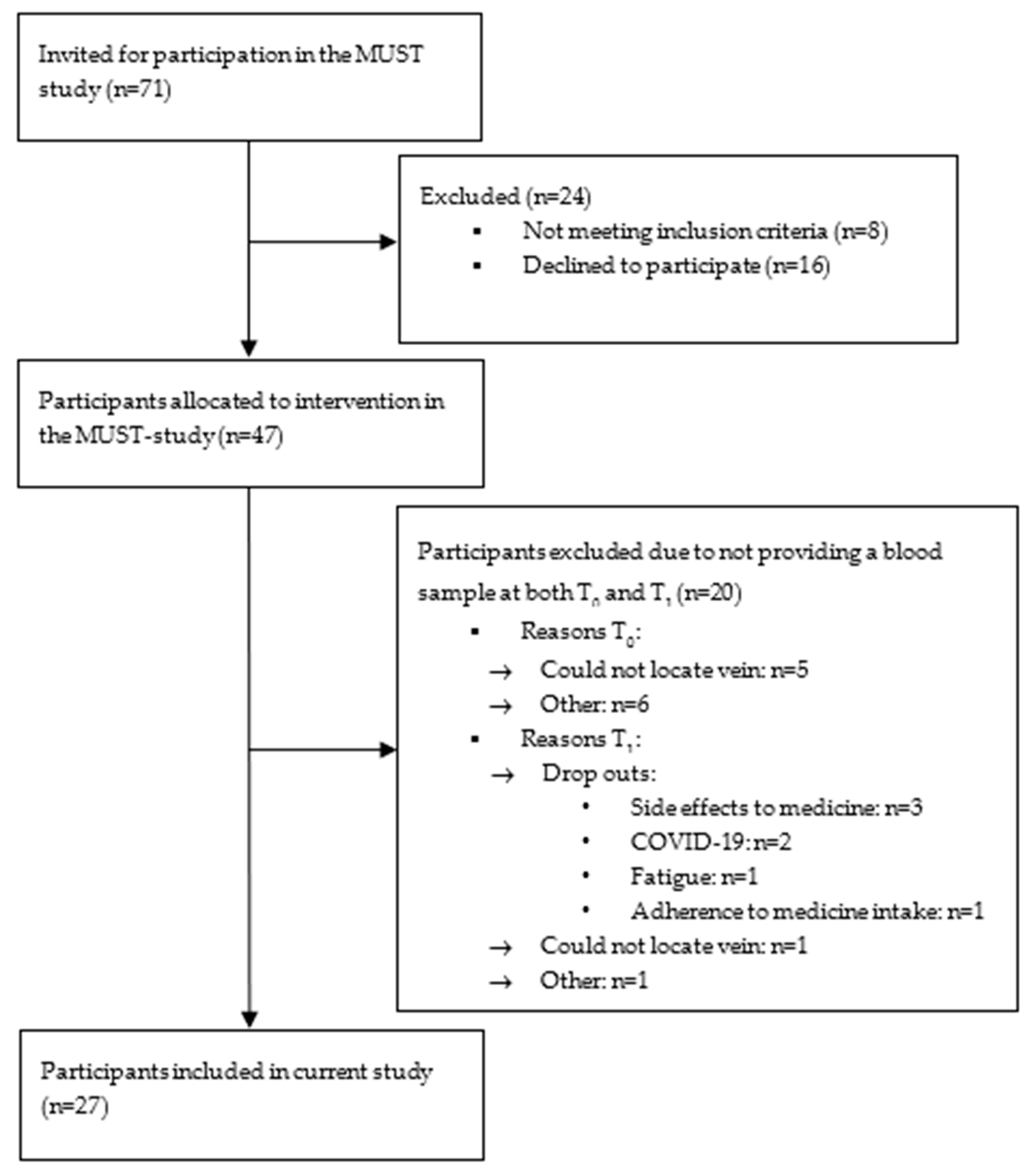
4.1. Participants and Setting
4.2. Outcome Measures
4.2.1. Performance Measures
4.2.2. Plasma Biomarkers
Chemiluminescence Analysis
Single-Molecule Array Analysis
4.3. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Bacon, T.E.; Herbert, J. Disability in multiple sclerosis: A reference for patients and clinicians. Neurology 2013, 80, 1018–1024. [Google Scholar] [CrossRef]
- Larocca, N.G. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient 2011, 4, 189–201. [Google Scholar] [CrossRef]
- Filli, L.; Sutter, T.; Easthope, C.S.; Killeen, T.; Meyer, C.; Reuter, K.; Lörincz, L.; Bolliger, M.; Weller, M.; Curt, A.; et al. Profiling walking dysfunction in multiple sclerosis: Characterisation, classification and progression over time. Sci. Rep. 2018, 8, 4984. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.D.; Brown, T.R.; Edwards, K.R.; Krupp, L.B.; Schapiro, R.T.; Cohen, R.; Marinucci, L.N.; Blight, A.R.; Investigators, M.S.F. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann. Neurol. 2010, 68, 494–502. [Google Scholar] [CrossRef]
- Goodman, A.D.; Brown, T.R.; Krupp, L.B.; Schapiro, R.T.; Schwid, S.R.; Cohen, R.; Marinucci, L.N.; Blight, A.R.; Fampridine, M.S.F.I. Sustained-release oral fampridine in multiple sclerosis: A randomised, double-blind, controlled trial. Lancet 2009, 373, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Hupperts, R.; Lycke, J.; Short, C.; Gasperini, C.; McNeill, M.; Medori, R.; Tofil-Kaluza, A.; Hovenden, M.; Mehta, L.R.; Elkins, J. Prolonged-release fampridine and walking and balance in MS: Randomised controlled MOBILE trial. Mult. Scler. 2016, 22, 212–221. [Google Scholar] [CrossRef]
- Jensen, H.; Ravnborg, M.; Mamoei, S.; Dalgas, U.; Stenager, E. Changes in cognition, arm function and lower body function after slow-release Fampridine treatment. Mult. Scler. 2014, 20, 1872–1880. [Google Scholar] [CrossRef]
- Hayes, K.C. Fampridine-SR for multiple sclerosis and spinal cord injury. Expert Rev. Neurother. 2007, 7, 453–461. [Google Scholar] [CrossRef]
- Marzal-Alfaro, M.B.; Martín Barbero, M.L.; García Domínguez, J.; Romero-Delgado, F.; Martínez Ginés, M.L.; Herranz, A.; Sanjurjo-Sáez, M. Impact of fampridine on quality of life: Clinical benefit in real-world practice. Eur. J. Hosp. Pharm. Sci. Pract. 2018, 25, 138–143. [Google Scholar] [CrossRef]
- Barry, A.; Cronin, O.; Ryan, A.M.; Sweeney, B.; Yap, S.M.; O’Toole, O.; Allen, A.P.; Clarke, G.; O’Halloran, K.D.; Downer, E.J. Impact of Exercise on Innate Immunity in Multiple Sclerosis Progression and Symptomatology. Front. Physiol. 2016, 7, 194. [Google Scholar] [CrossRef]
- Fda-Nih, B.W.G. BEST (Biomarkers, EndpointS, and other Tools) Resource. In BEST (Biomarkers, EndpointS, and other Tools) Resource; Food and Drug Administration, National Institutes of Health: Silver Spring, MD, USA; Bethesda, MD, USA, 2016. [Google Scholar]
- Schreiner, B.; Becher, B. Perspectives on cytokine-directed therapies in multiple sclerosis. Swiss Med. Wkly. 2015, 145, w14199. [Google Scholar] [CrossRef][Green Version]
- Bai, Z.; Chen, D.; Wang, L.; Zhao, Y.; Liu, T.; Yu, Y.; Yan, T.; Cheng, Y. Cerebrospinal Fluid and Blood Cytokines as Biomarkers for Multiple Sclerosis: A Systematic Review and Meta-Analysis of 226 Studies with 13,526 Multiple Sclerosis Patients. Front. Neurosci. 2019, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Ruck, T.; Meuth, S.G. Cytokine signaling in multiple sclerosis: Lost in translation. Mult. Scler. J. 2018, 24, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Hagman, S.; Raunio, M.; Rossi, M.; Dastidar, P.; Elovaara, I. Disease-associated inflammatory biomarker profiles in blood in different subtypes of multiple sclerosis: Prospective clinical and MRI follow-up study. J. Neuroimmunol. 2011, 234, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.B.; Rose, J.W.; Jaskowski, T.D.; Wilson, A.R.; Husebye, D.; Seraj, H.S.; Hill, H.R. Analysis of proinflammatory and anti-inflammatory cytokine serum concentrations in patients with multiple sclerosis by using a multiplexed immunoassay. Am. J. Clin. Pathol. 2011, 136, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.; Goyal, M.; Johri, S.; Kumar, V.; Khaibullin, T.; Rizvanov, A.A.; Verma, S.; Khaiboullina, S.F.; Baranwal, M. Serum and Cerebrospinal Fluid Cytokine Biomarkers for Diagnosis of Multiple Sclerosis. Mediat. Inflamm. 2020, 2020, 2727042. [Google Scholar] [CrossRef] [PubMed]
- Housley, W.J.; Pitt, D.; Hafler, D.A. Biomarkers in multiple sclerosis. Clin. Immunol. 2015, 161, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Momtazmanesh, S.; Shobeiri, P.; Saghazadeh, A.; Teunissen, C.E.; Burman, J.; Szalardy, L.; Klivenyi, P.; Bartos, A.; Fernandes, A.; Rezaei, N. Neuronal and glial CSF biomarkers in multiple sclerosis: A systematic review and meta-analysis. Rev. Neurosci. 2021, 32, 573–595. [Google Scholar] [CrossRef]
- Norgren, N.; Sundström, P.; Svenningsson, A.; Rosengren, L.; Stigbrand, T.; Gunnarsson, M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 2004, 63, 1586–1590. [Google Scholar] [CrossRef]
- Skov, C.D.; Sørensen, C.B.; Thorning, M.; Lambertsen, K.L.; Frich, L.H.; Jensen, H.B.; Holsgaard-Larsen, A.; Nielsen, H.H. Evaluation of functional outcome measures after fampridine treatment in patients with multiple sclerosis—An interventional follow-up study. Mult. Scler. Relat. Disord. 2022, 66, 104034. [Google Scholar] [CrossRef]
- Thorning, M.; Nielsen, H.H.; Frich, L.H.; Jensen, H.B.; Lambertsen, K.L.; Holsgaard-Larsen, A. Gait quality and function after fampridine treatment in patients with multiple sclerosis—A prospective cohort study. Clin. Biomech. 2022, 100, 105826. [Google Scholar] [CrossRef]
- Thorning, M.; Lambertsen, K.L.; Skov, C.D.; Frich, L.H.; Jensen, H.B.; Holsgaard-Larsen, A.; Nielsen, H.H. Criterion validity of muscle strain analyses of skeletal muscle function in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 70, 104478. [Google Scholar] [CrossRef]
- Brambilla, R.; Ashbaugh, J.J.; Magliozzi, R.; Dellarole, A.; Karmally, S.; Szymkowski, D.E.; Bethea, J.R. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 2011, 134 Pt 9, 2736–2754. [Google Scholar] [CrossRef]
- Kristensen, L.B.; Lambertsen, K.L.; Nguyen, N.; Byg, K.E.; Nielsen, H.H. The Role of Non-Selective TNF Inhibitors in Demyelinating Events. Brain Sci. 2021, 11, 38. [Google Scholar] [CrossRef]
- Ghorbanpour, S.; Rahimibarghani, S.; Rohani, S.; Rastkar, M.; Ghajarzadeh, M. Fampridine for gait imbalance in patients with multiple sclerosis (MS): A systematic review and meta-analysis. Neurol. Sci. 2023, 44, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Blight, A.R.; Henney, H.R., 3rd. Steady-state pharmacokinetics and tolerability of orally administered fampridine sustained-release 10-mg tablets in patients with multiple sclerosis: A 2-week, open-label, follow-up study. Clin. Ther. 2009, 31, 2215–2223. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, D.; Mimpen, M.; Damoiseaux, J. The IL-2—IL-2 receptor pathway: Key to understanding multiple sclerosis. J. Transl. Autoimmun. 2021, 4, 100123. [Google Scholar] [CrossRef] [PubMed]
- Babaloo, Z.; Aliparasti, M.R.; Babaiea, F.; Almasi, S.; Baradaran, B.; Farhoudi, M. The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: Interleukin-17A and interleukin-17F serum levels. Immunol. Lett. 2015, 164, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.; Fischer, S.K.; Townsend, M.J.; Mosesova, S.; Peng, K.; Setiadi, A.F.; Song, A.; Baruch, A. Characterization of IL-17AA and IL-17FF in rheumatoid arthritis and multiple sclerosis. Bioanalysis 2016, 8, 2317–2327. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.R.; Liu, G.J.; Feng, R.N.; Gong, F.C.; Zhong, H.; Duan, S.R.; Bi, S. Increased levels of IL-23 and osteopontin in serum and cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 2012, 244, 94–96. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef]
- Sejbaek, T.; Nielsen, H.H.; Penner, N.; Plavina, T.; Mendoza, J.P.; Martin, N.A.; Elkjaer, M.L.; Ravnborg, M.H.; Illes, Z. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1324–1330. [Google Scholar] [CrossRef]
- Dietrich, M.; Hartung, H.P.; Albrecht, P. Neuroprotective Properties of 4-Aminopyridine. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e976. [Google Scholar] [CrossRef] [PubMed]
- Allart, E.; Benoit, A.; Blanchard-Dauphin, A.; Tiffreau, V.; Thevenon, A.; Zephir, H.; Outteryck, O.; Lacour, A.; Vermersch, P. Sustained-released fampridine in multiple sclerosis: Effects on gait parameters, arm function, fatigue, and quality of life. J. Neurol. 2015, 262, 1936–1945. [Google Scholar] [CrossRef]
- Lo, A.C.; Ruiz, J.A.; Koenig, C.M.; Anderson, B.M.; Olson, K.M.; Triche, E.W. Effects of dalfampridine on multi-dimensional aspects of gait and dexterity in multiple sclerosis among timed walk responders and non-responders. J. Neurol. Sci. 2015, 356, 77–82. [Google Scholar] [CrossRef]
- Byg, K.E.; Illes, Z.; Sejbaek, T.; Lambertsen, K.L.; Ellingsen, T.; Nielsen, H.H. Inflammatory profiles in plasma and cerebrospinal fluid of patients with neurosarcoidosis. J. Neuroimmunol. 2022, 367, 577849. [Google Scholar] [CrossRef]
- Bielekova, B.; Martin, R. Development of biomarkers in multiple sclerosis. Brain 2004, 127 Pt 7, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Ann. Intern. Med. 2007, 147, W163–W194. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, A.; Chiesa, F.; Conte, S.; Bengtsson, C.; Lee, S.; Minton, N.; Niemcryk, S.; Lindholm, A.; Rosenlund, M.; Piehl, F.; et al. Infections in patients with multiple sclerosis: A national cohort study in Sweden. Mult. Scler. Relat. Disord. 2020, 45, 102420. [Google Scholar] [CrossRef]
- Fischer, J.S.; Jak, A.; Kniker, J.; Rudick, R.; Cutter, G.J. Multiple Sclerosis Functional Composite (MSFC): Administration and Scoring Manual; National Multiple Sclerosis Society: New York, NY, USA, 2001. [Google Scholar]
- Nieuwenhuis, M.M.; Van Tongeren, H.; Sorensen, P.S.; Ravnborg, M. The six spot step test: A new measurement for walking ability in multiple sclerosis. Mult. Scler. 2006, 12, 495–500. [Google Scholar] [CrossRef]
- Gijbels, D.; Eijnde, B.O.; Feys, P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult. Scler. 2011, 17, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Hobart, J.C.; Riazi, A.; Lamping, D.L.; Fitzpatrick, R.; Thompson, A.J. Measuring the impact of MS on walking ability: The 12-Item MS Walking Scale (MSWS-12). Neurology 2003, 60, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult. Scler. J. 2017, 23, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; Wirenfeldt, M.; Høgedal, S.S.; Frich, L.H.; Nielsen, H.H.; Schrøder, H.D.; Østergaard, K.; Finsen, B.; Kristensen, B.W.; Lambertsen, K.L. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.B.; Laursen, C.C.H.; Nawaz, N.; Madsen, J.S.; Nielsen, H.H.; Kruuse, C.; Møller, A.; Degn, M.; Lambertsen, K.L. Leukocyte TNFR1 and TNFR2 Expression Contributes to the Peripheral Immune Response in Cases with Ischemic Stroke. Cells 2021, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Overholser, B.R.; Sowinski, K.M. Biostatistics primer: Part 2. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2008, 23, 76–84. [Google Scholar] [CrossRef]
- Portney, L.G.W.M.P. Correlation. In Foundations of Clinical Research: Applications to Practice, 3rd ed.; F. A. Davis: Philadelphia, PA, USA; Pearsons Education, Inc.: London, UK, 2008; pp. 533–548. [Google Scholar]
| Age, Years | 52.0 (11.6) |
| Females, n (%) | 14 (51.9) |
| Disease duration, years | 17.0 (8.5) |
| Subdiagnosis, n (%): | |
| Relapsing–remitting | 11 (40.7) |
| Secondary progressive | 11 (40.7) |
| Primary progressive | 5 (18.5) |
| Disease-modifying treatment, n (%): | |
| Aubagio | 3 (11.1) |
| Tecfidera | 3 (11.1) |
| Tysabri | 2 (7.4) |
| Lemtrada | 1 (3.7) |
| Copaxone | 2 (7.4) |
| Ocrevus | 4 (14.8) |
| Rituximab | 3 (11.1) |
| Methotrexate | 1 (3.7) |
| No treatment | 8 (29.6) |
| EDSS | 6 [5.0/6.5] |
| Patients using walking aids during the following tests, n (%): | |
| T25FW | 17 (47.2) |
| SSST | 18 (50.0) |
| 2MWT | 23 (63.9) |
| Smoking status, n (%): | |
| No | 21 (77.8) |
| Yes | 5 (18.5) |
| Not answered | 1 (3.7) |
| Alcohol intake, units per week, n (%): | |
| 0 | 9 (33.3) |
| 1–3 | 12 (44.4) |
| 4–7 | 4 (14.8) |
| ≥8 | 1 (3.7) |
| Not answered | 1 (3.7) |
| (a) | ||||||
| Performance Measures | Numbers (n) | T0 | T1 | Change [95% CI] | % Change | p-Value |
| T25FW (s) | 27 | 7.05 [5.85/10.05] | 6.35 [5.25/9.95] | −0.70 [−0.85; −0.30] * | −9.93 | 0.0012 |
| SSST (s) | 27 | 13.03 [9.58/20.63] | 12.18 [8.85/16.63] | −1.80 [−2.70; −0.90] | −13.81 | 0.0003 |
| 2MWT (m) | 26 | 111.29 (38.32) | 126.76 (43.34) | 14.97 [13.26; 21.64] * | 13.45 | 0.0001 |
| 9HPT (s) | 27 | 25.55 [22.45/30.10] | 24.20 [21.10/28.95] | −1.90 [−2.80; −0.95] * | −7.44 | 0.0048 |
| MSWS-12 (points) | 25 | 68.83 (17.23) | 50.00 [41.67/72.92] | −16.25 [−22.93; −9.57] | −23.61 | <0.0001 |
| (b) | ||||||
| Biomarkers (pg/mL) | Numbers (n) | T0 | T1 | Change [95% CI] | p-Value | |
| IL-2 | 21 | 0.35 [0.13/0.62] | 0.33 [0.22/0.70] | 0.01 [−0.03; 0.18] * | 0.1981 | |
| IL-4 | 25 | 0.02 [0.01/0.05] | 0.03 [0.01/0.04] | 0.00 [0.01; 0.01] * | 0.9662 | |
| IL-8 | 27 | 2.35 [1.76/3.13] | 2.47 [1.55/3.66] | 0.19 [−0.18; 0.57] | 0.2967 | |
| IL-17 | 21 | 948.81 [540.56/1447.22] | 1168.57 [763.19/1341.31] | 45.55 [−210.55; 301.66] | 0.7145 | |
| IFN-γ | 21 | 3.36 [1.58/6.04] | 3.02 [2.41/5.90] | 0.31 [−0.02; 1.49] * | 0.1363 | |
| TNF-α | 27 | 1.12 [0.99/1.23] | 1.15 [0.96/1.35] | 0.06 [−0.02; 0.14] | 0.1456 | |
| TNF-R1 | 26 | 1640.96 [1276.69/1903.46] | 1495.61 [1126.99/1820.22] | −72.15 [−184.14; 39.85] | 0.1966 | |
| TNF-R2 | 27 | 4862.52 [4272.24/5502.56] | 4312.32 [3774.74/5675.94] | −408.34 [−810.02; −6.66] | 0.0466 | |
| NFL | 27 | 8.85 [7.04/12.09] | 8.88 [7.22/12.04] | −0.51 [−0.91; 1.46] * | 0.8288 | |
| GFAP | 27 | 97.75 [61.26/135.52] | 98.18 [77.16/133.23] | −0.77 [−9.91; 8.37] | 0.8646 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorning, M.; Lambertsen, K.L.; Jensen, H.B.; Frich, L.H.; Madsen, J.S.; Olsen, D.A.; Holsgaard-Larsen, A.; Nielsen, H.H. Performance Measures and Plasma Biomarker Levels in Patients with Multiple Sclerosis after 14 Days of Fampridine Treatment: An Explorative Study. Int. J. Mol. Sci. 2024, 25, 1592. https://doi.org/10.3390/ijms25031592
Thorning M, Lambertsen KL, Jensen HB, Frich LH, Madsen JS, Olsen DA, Holsgaard-Larsen A, Nielsen HH. Performance Measures and Plasma Biomarker Levels in Patients with Multiple Sclerosis after 14 Days of Fampridine Treatment: An Explorative Study. International Journal of Molecular Sciences. 2024; 25(3):1592. https://doi.org/10.3390/ijms25031592
Chicago/Turabian StyleThorning, Maria, Kate Lykke Lambertsen, Henrik Boye Jensen, Lars Henrik Frich, Jonna Skov Madsen, Dorte Aalund Olsen, Anders Holsgaard-Larsen, and Helle Hvilsted Nielsen. 2024. "Performance Measures and Plasma Biomarker Levels in Patients with Multiple Sclerosis after 14 Days of Fampridine Treatment: An Explorative Study" International Journal of Molecular Sciences 25, no. 3: 1592. https://doi.org/10.3390/ijms25031592
APA StyleThorning, M., Lambertsen, K. L., Jensen, H. B., Frich, L. H., Madsen, J. S., Olsen, D. A., Holsgaard-Larsen, A., & Nielsen, H. H. (2024). Performance Measures and Plasma Biomarker Levels in Patients with Multiple Sclerosis after 14 Days of Fampridine Treatment: An Explorative Study. International Journal of Molecular Sciences, 25(3), 1592. https://doi.org/10.3390/ijms25031592







