Antioxidant Activity Analysis of Native Actinidia arguta Cultivars
Abstract
1. Introduction
2. Results and Discussion
2.1. Leaf Biomass and Water Content of Kiwiberry Cultivars
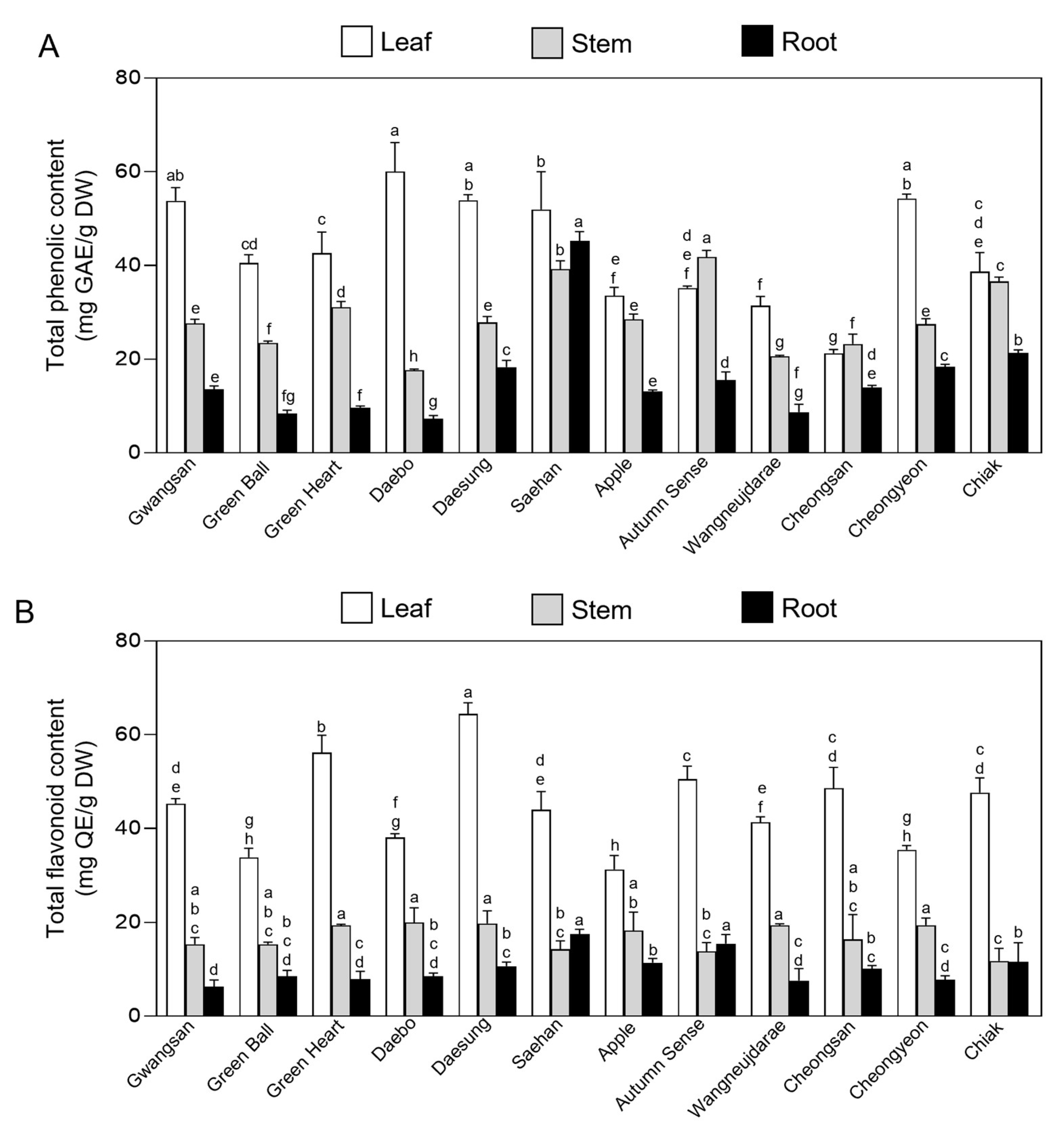
2.2. Total Phenolic and Flavonoid Content in Vegetative Tissues of Kiwiberry Cultivars
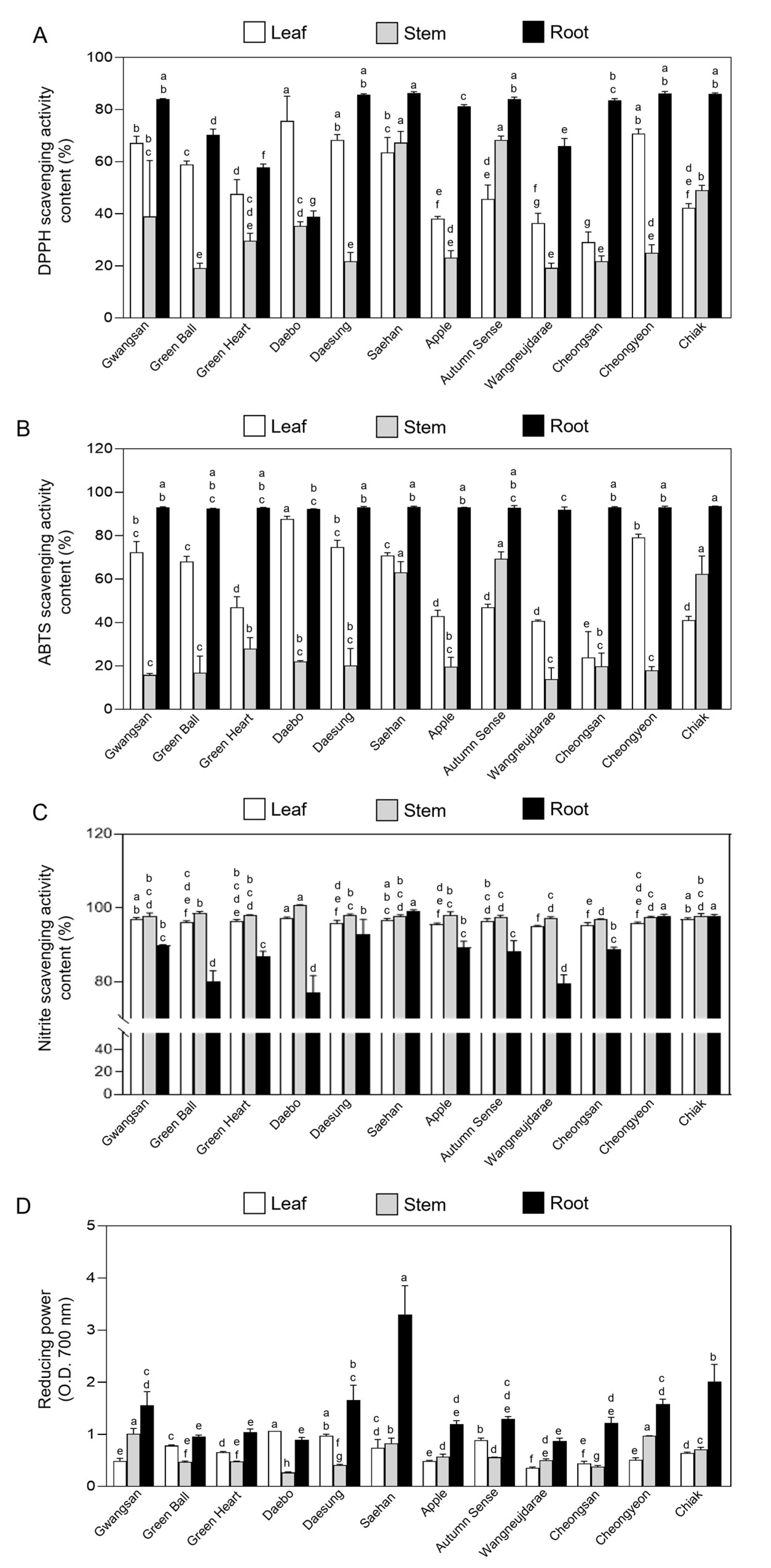
2.3. Antioxidant Activity in Vegetative Tissues of Kiwiberry Cultivars
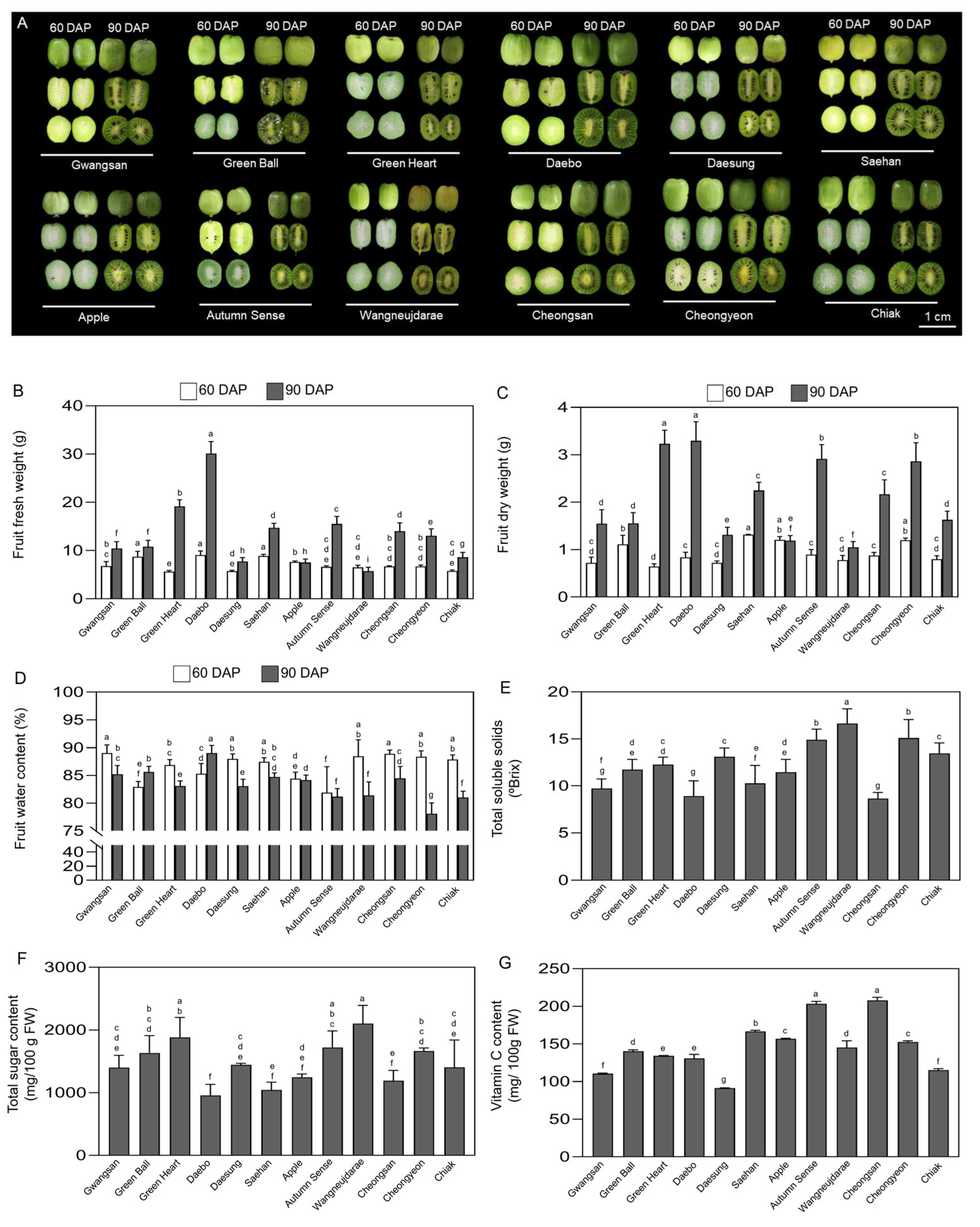
2.4. Fruit Biomass, Sugar, and Vitamin C Content of Kiwiberry Cultivars
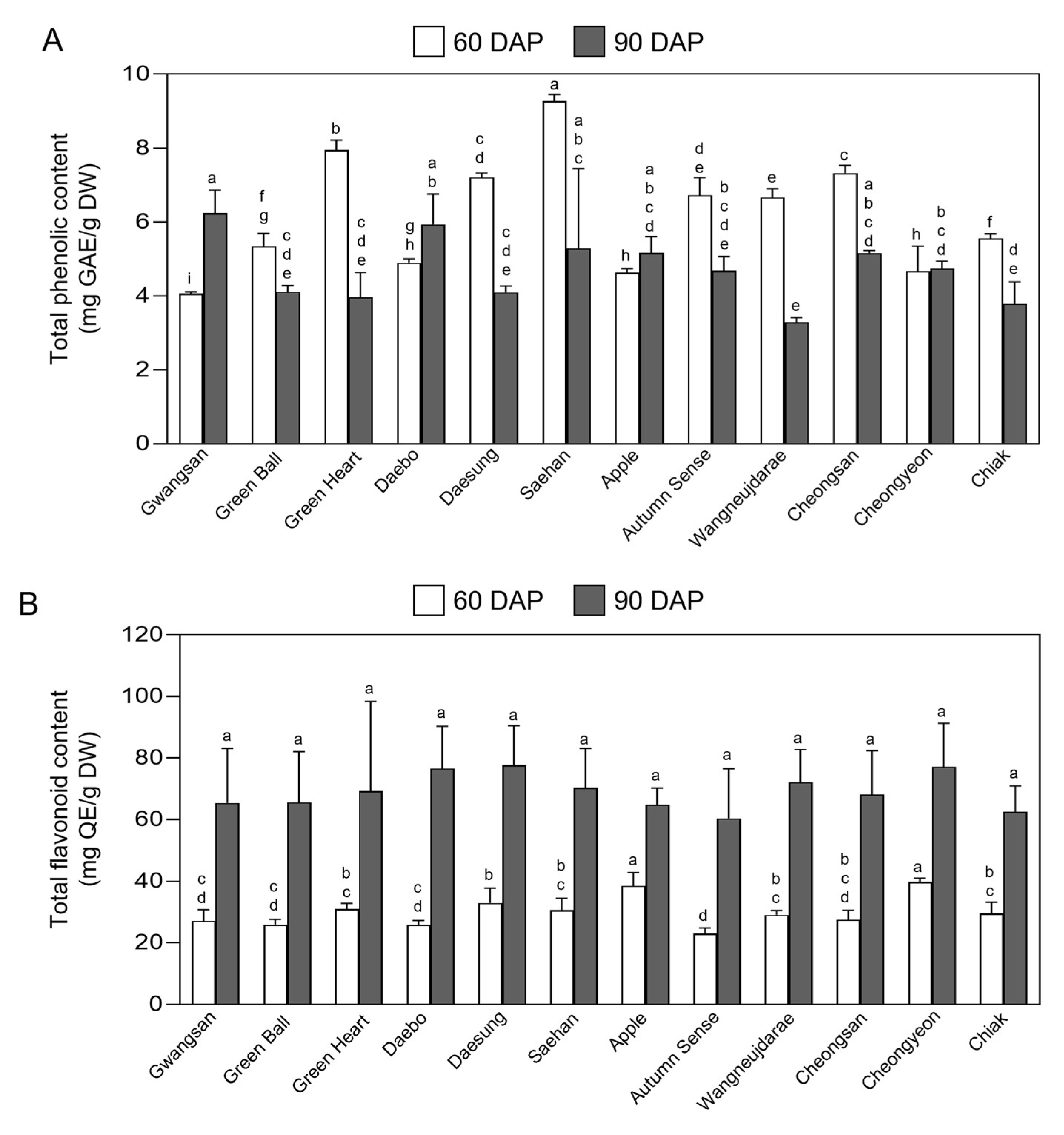
2.5. Total Phenolic and Flavonoid Content in Fruits of Kiwiberry Cultivars
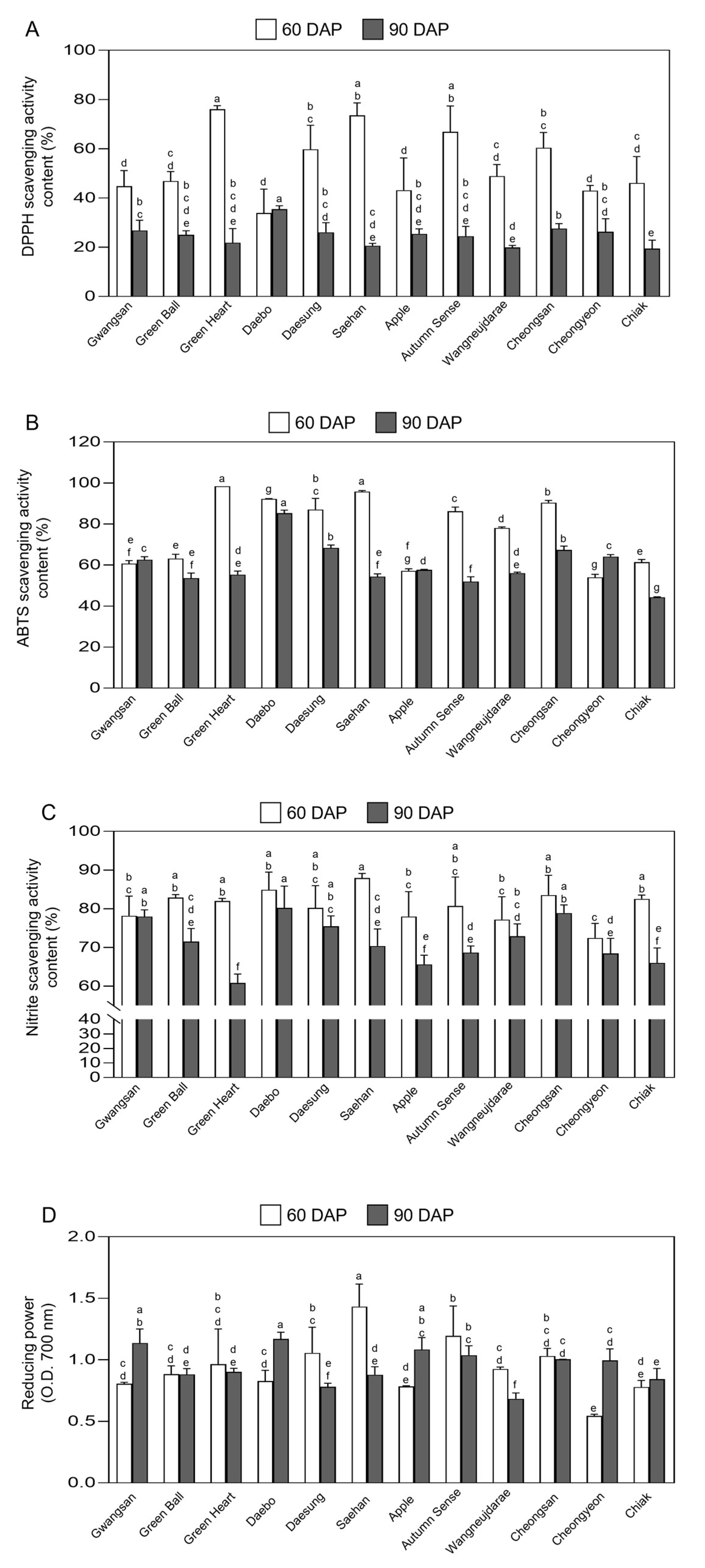
2.6. Antioxidant Activity in Fruits of Kiwiberry Cultivars
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Kiwiberry Leaf and Fruit Biomass Analysis
3.3. Kiwiberry Extracts
3.4. Total Phenolic Content
3.5. Total Flavonoid Content
3.6. Free Radical Scavenging (DPPH) Activity
3.7. Nitrite Scavenging Activity
3.8. Reducing Power Assay
3.9. ABTS Radical Cation Scavenging Activity
3.10. Brix Analysis
3.11. Total Sugar Content
3.12. Vitamin C
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FW | Fresh weight |
| DW | Dry weight |
| WC | Water content |
| cv. | Cultivar |
| TPC | Total phenolic content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| °Bx | Brix |
| ANOVA | Analysis of variance |
| RSA | Radical scavenging activity |
| HPLC | High-performance liquid chromatography |
| DAP | Day after pollination |
| GAE | Gallic acid equivalent |
| QE | Quercetin |
| TFC | Total flavonoid content |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| FC | Folin–Ciocalteu |
| NSA | Nitrite scavenging activity |
References
- Padmanabhan, G.P.P. Kiwifruit. In Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 490–494. [Google Scholar] [CrossRef]
- Oh, H.-J.; Jeon, S.-B.; Kang, H.-Y.; Yang, Y.-J.; Kim, S.-C.; Lim, S.-B. Chemical composition and antioxidative activity of kiwifruit in different cultivars and maturity. J. Korean Soc. Food Sci. Nutr. 2011, 40, 343–349. [Google Scholar] [CrossRef]
- Lee, S.-E.; Kim, D.-M.; Kim, K.-H.; Rhee, C. Several physico-chemical characteristics of kiwifruit (Actinidia chinensis Planch.) depended on cultivars and ripening stages. Korean J. Food Sci. Technol. 1989, 21, 863–868. [Google Scholar]
- Jin, D.E.; Park, S.K.; Park, C.H.; Seung, T.W.; Heo, H.J. Nutritional compositions of three traditional Actinidia (Actinidia arguta) cultivars improved in Korea. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1942–1947. [Google Scholar] [CrossRef]
- Kim, C.-W.; Oh, S.-I.; Kim, M.-J.; Park, Y. Optimal harvest time by the seasonal fruit quality and ripening characteristics of hardy kiwifruit in Korea. J. Korean For. Soc. 2014, 103, 353–358. [Google Scholar] [CrossRef]
- Park, Y.; Jang, Y.-S.; Lee, M.-H.; Kwon, O.W. Comparison of antioxidant capacity and nutritional composition of three cultivars of Actinidia arguta. J. Korean Soc. For. Sci. 2007, 96, 580–584. [Google Scholar] [CrossRef]
- Cradock-Henry, N.A. New Zealand kiwifruit growers’ vulnerability to climate and other stressors. Reg. Environ. Chang. 2017, 17, 245–259. [Google Scholar] [CrossRef]
- Jeong, Y.; Chung, U.; Kim, K.-H. Predicting future frost damage risk of kiwifruit in Korea under climate change using an integrated modelling approach. Int. J. Clim. 2018, 38, 5354–5367. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Li, K.; Wang, C.; Guna, A.; Zhang, J. Prediction of potential geographical distribution patterns of Actinidia arguta under different climate scenarios. Sustainability 2021, 13, 3526. [Google Scholar] [CrossRef]
- Debersaques, F.; Mekers, O.; Decorte, J.; Van Labeke, M.C.; Schoedl-Hummel, K.; Latocha, P. Challenges faced by commercial kiwiberry (actinidia argute planch.) Production. In Proceedings of the VIII International Symposium on Kiwifruit 1096, Chengdu, China, 18–22 September 2014; pp. 435–442. [Google Scholar]
- Latocha, P.; Łata, B.; Stasiak, A. Phenolics, ascorbate and the antioxidant potential of kiwiberry vs. common kiwifruit: The effect of cultivar and tissue type. J. Funct. Foods 2015, 19, 155–163. [Google Scholar] [CrossRef]
- Gangwon State Agricultural Research & Extention Service. Gangwon Agricultural Statistics. 2022. Available online: https://www.ares.gangwon.kr/en/statistics/economy (accessed on 8 January 2023).
- Latocha, P. The nutritional and health benefits of kiwiberry (Actinidia arguta)—A review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Latocha, P.; Debersaques, F.; Hale, I. Actinidia arguta (Kiwiberry): Botany, Production, Genetics, Nutritional Value, and Postharvest Handling. Hortic. Rev. 2021, 48, 37–152. [Google Scholar] [CrossRef]
- Kwak, C.S.; Lee, J.H. In vitro antioxidant and anti-inflammatory effects of ethanol extracts from sprout of evening primrose (Oenothera laciniata) and gooseberry (Actinidia arguta). J. Korean Soc. Food Sci. Nutr. 2014, 43, 207–215. [Google Scholar] [CrossRef]
- Jin, D.E.; Park, S.K.; Park, C.H.; Seung, T.W.; Choi, S.-G.; Heo, H.J. Nutritional components of korean traditional Actinidia (Actinidia arguta) sprout and in vitro antioxidant effect. Korean J. Food Sci. Technol. 2015, 47, 37–43. [Google Scholar] [CrossRef]
- Xin, H.L.; Wu, Y.C.; Su, Y.H.; Sheng, J.Y.; Ling, C.Q. Novel flavonoids from the leaves of Actinidia valvata dunn: Structural elucidation and antioxidant activity. Planta Med. 2011, 77, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Leong, L.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Latocha, P.; Wołosiak, R.; Worobiej, E.; Krupa, T. Clonal differences in antioxidant activity and bioactive constituents of hardy kiwifruit (Actinidia arguta) and its year-to-year variability. J. Sci. Food Agric. 2013, 93, 1412–1419. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, N.; Shu, C.; Cheng, S.; Sun, X.; Liu, C.; Xin, G.; Li, B.; Tian, J. Phenolics Profile and antioxidant activity analysis of kiwi berry (Actinidia arguta) flesh and peel extracts from four regions in China. Front. Plant Sci. 2021, 12, 689038. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Inserting cycads into global nutrient relations data sets. Plant Signal. Behav. 2018, 13, e1547578. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-Y.; Xiang, W.; Wen, Y.; Huang, D.-L.; Cao, K.-F.; Zhu, S.-D. Correlations between leaf economics, mechanical resistance and drought tolerance across 41 cycad species. Ann. Bot. 2022, 130, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, K.; Sack, L.; Li, N.; Wei, X.; Goldstein, G. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. New Phytol. 2015, 206, 817–829. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, W.T.; Lim, W.J.; Cho, K.M. Phenolic contents and antioxidant activities from different tissues of Baekseohyang (Daphne kiusiana). Food Sci. Biotechnol. 2011, 20, 695–702. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-R. Analysis of phenolic substances content in Korean plant foods. Korean J. Food Sci. Technol. 1994, 26, 310–316. [Google Scholar]
- Seo, S.J.; Kim, N.W. Antioxidant activities of extracts from leaves and stems of Achyranthes japonica. J. Korean Soc. Food Sci. Nutr. 2014, 43, 972–979. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Lee, S.-G.; Lee, E.-J.; Park, W.-D.; Kim, J.-B.; Kim, H.-J. Antioxidant activity and anti-hyperglycemic activity of medicinal herbal extracts according to extraction methods. Korean J. Food Sci. Technol. 2010, 42, 571–577. [Google Scholar]
- Guan, D.; Zhang, Z.; Yang, Y.; Sugiura, N.; Hu, H.; Xing, G.; Liu, J.; ZU, X.; Zhang, Y. Antioxidant and antitumor activities of water extracts from the root of Actinidia kolomikta. Exp. Ther. Med. 2011, 2, 33–39. [Google Scholar] [CrossRef]
- YLee, M.; Bae, J.-H.; Jung, H.-Y.; Kim, J.-H.; Park, D.-S. Antioxidant activity in water and methanol extracts from Korean edible wild plants. J. Korean Soc. Food Sci. Nutr. 2011, 40, 29–36. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, M.-L. Functional Properties of Mugwort Extracts and Quality Characteristics of Noodles Added Mugwort Powder. Korean J. Food Preserv. 2006, 13, 161–167. [Google Scholar]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Zhang, Y.; Liu, J.; Yu, J. Changes in bioactive compounds and antioxidant activities in pomegranate leaves. Sci. Hortic. 2010, 123, 543–546. [Google Scholar] [CrossRef]
- Ahn, J.H.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Turk, A.; Han, Y.K.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Aromatic Constituents from the Leaves of Actinidia arguta with Antioxidant and α-Glucosidase Inhibitory Activity. Antioxidants 2021, 10, 1896. [Google Scholar] [CrossRef] [PubMed]
- Teng, K. Antioxidant activities in vitro and components analysis of methanol extract of Actinidia arguta by HPLC-DAD-ESI-MS/MS. Chin. Tradit. Herb. Drugs 2019, 24, 4384–4388. [Google Scholar]
- Di, X.; Wang, H.B.; Zhai, Y.J.; Pan, L. Determination of oleanolic acid and ursolic acid in Radix Actinidia by RP-HPLC. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 76–78. [Google Scholar]
- Shi, Y.; Wang, H.L.; Ma, B.R. Studies on the chemical constituents of Actinidia arguta (Sieb. Et Zucc.) Planch. Ex Miquel. China J. Chin. Mater. Med. 1992, 17, 36–38+64. [Google Scholar]
- Jang, D.S.; Lee, G.Y.; Lee, Y.M.; Kim, Y.S.; Sun, H.; Kim, D.H.; Kim, J.S. Flavan-3-ols having a γ-lactam from the roots of Actinidia arguta inhibit the formation of advanced glycation end products in vitro. Chem. Pharm. Bull. 2009, 57, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Teng, K.; Zang, H. Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq.: A Review of Phytochemistry and Pharmacology. Molecules 2023, 28, 7820. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.-L.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.-L.; Hai, C.-X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Roedig-Penman, A.; Gordon, M.H. Antioxidant properties of catechins and green tea extracts in model food emulsions. J. Agric. Food Chem. 1997, 45, 4267–4270. [Google Scholar] [CrossRef]
- Kim, E.-J.; Choi, J.-Y.; Yu, M.-R.; Kim, M.-Y.; Lee, S.-H.; Lee, B.-H. total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Karlova, R.; Chapman, N.; David, K.; Angenent, G.C.; Seymour, G.B.; De Maagd, R.A. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014, 65, 4527–4541. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-H.; Lee, W.-J.; Bae, S.-H.; Choi, S.-G. Chemical components and antioxidative activity of Korean gold kiwifruit. J. Korean Soc. Food Sci. Nutr. 2007, 36, 859–865. [Google Scholar] [CrossRef]
- Drummond, L. The composition and nutritional value of kiwifruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar] [PubMed]
- Nishiyama, I.; Fukuda, T.; Shimohashi, A.; Oota, T. Sugar and organic acid composition in the fruit juice of different Actinidia varieties. Food Sci. Technol. Res. 2008, 14, 67–73. [Google Scholar] [CrossRef]
- USDA. USDA National Nutrient Database for Standard Reference, Release 27; USDA: Washington, DC, USA, 2014. Available online: https://www.ars.usda.gov/ARSUserFiles/80400535/DATA/sr27/sr27_doc.pdf (accessed on 7 January 2024).
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S. The Concise New Zealand Food Composition Tables, 11th ed.; The New Zealand Institute for Plant & Food Research Limited and Ministry of Health: Auckland, New Zealand, 2014; ISBN 978-0-9876680-5-9. Available online: https://www.health.govt.nz/publication/concise-new-zealand-food-composition-tables-11th-edition (accessed on 7 January 2024).
- Kim, J.-H.; Park, Y.; Kim, S.-H. Morphological characteristics of leaves and fruits of Actinidia arguta as honey plant. J. Apic. 2017, 32, 69–75. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Zhang, Z.; Jiang, Z.; Wang, S. Actinidia germplasm resources and kiwifruit industry in China. HortScience 2004, 39, 1165–1172. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef]
- D’evoli, L.; Moscatello, S.; Lucarini, M.; Aguzzi, A.; Gabrielli, P.; Proietti, S.; Battistelli, A.; Famiani, F.; Böhm, V.; Lombardi-Boccia, G. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa Planch.; cv. Hayward) grown in Italy. J. Food Compos. Anal. 2015, 37, 25–29. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The nutritional value and vitamin C content of different raspberry cultivars from organic and conventional production. J. Food Compos. Anal. 2020, 87, 103429. [Google Scholar] [CrossRef]
- Massot, C.; Génard, M.; Stevens, R.; Gautier, H. Fluctuations in sugar content are not determinant in explaining variations in vitamin C in tomato fruit. Plant Physiol. Biochem. 2010, 48, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Society, G.N. New reference values for vitamin C intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef]
- Fisk, C.L.; Mcdaniel, M.R.; Strik, B.C.; Zhao, Y. Physicochemical, sensory, and nutritive qualities of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) as affected by harvest maturity and storage. J. Food Sci. 2006, 71, S204–S210. [Google Scholar] [CrossRef]
- Park, Y.-S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivars. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Ma, T.; Ge, Q.; Fang, Y.; Sun, X. Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Honisch, C.; Osto, A.; de Matos, A.D.; Vincenzi, S.; Ruzza, P. Isolation of a tyrosinase inhibitor from unripe grapes juice: A spectrophotometric study. Food Chem. 2020, 305, 125506. [Google Scholar] [CrossRef] [PubMed]
- Jančářová, I.; Jančář, L.; Náplavová, A.; Kubáň, V. Changes of organic acids and phenolic compounds contents in grapevine berries during their ripening. Open Chem. 2013, 11, 1575–1582. [Google Scholar] [CrossRef]
- Zheng, H.-Z.; Kim, Y.-I.; Chung, S.-K. A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem. 2012, 131, 106–110. [Google Scholar] [CrossRef]
- Labbe, M.; Ulloa, P.A.; Lopez, F.; Saenz, C.; Pena, A.; Salazar, F.N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (‘wonderful’, ‘chaca’ and ‘codpa’) at different maturity stages. Chil. J. Agric. Res. 2016, 76, 479–486. [Google Scholar] [CrossRef][Green Version]
- Jiao, Y.; Chen, D.; Fan, M.; Quek, S.Y. UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 2019, 281, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-N.; Nee, C.-C.; Ou, A.S.-M.; Chou, T.-H.; Chien, C.-C. Characterization of the physico-chemical and antioxidant properties of Taiwanese kiwifruit (Actinidia setosa). Bot. Stud. 2008, 49, 215–224. [Google Scholar]
- Pal, R.S.; Kumar, V.A.; Arora, S.; Sharma, A.K.; Kumar, V.; Agrawal, S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015, 58, 262–271. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tao, J.-J.; Liao, G.-L.; Xie, M.; Qu, X.-Y.; Chen, L.; Xu, X.-B. Dynamic changes of phenol and antioxidant capacity during fruit development of three Actinidia species (kiwifruit). Sci. Hortic. 2020, 273, 109571. [Google Scholar] [CrossRef]
- He, J.-G.; Cheng, Y.-D.; Guan, J.-F.; Ge, W.-Y.; Zhao, Z. Changes of chlorogenic acid content and its synthesis-associated genes expression in Xuehua pear fruit during development. J. Integr. Agric. 2017, 16, 471–477. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in phenolic compounds and antioxidant activity during development of ‘Qiangcuili’ and ‘Cuihongli’fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Liu, Z.; Fang, Y.; Wang, D.; Huang, T.; Zhang, L.; Liang, J.; Kou, L. Difference of resistance to postharvest blue mold between Hongyang and Qihong kiwifruits. Food Chem. 2019, 285, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kato, H.; Lee, I.E.; Van Chuyen, N.; Kim, S.B.; Hayase, F. Inhibition of nitrosamine formation by nondialyzable melanoidins. Agric. Biol. Chem. 1987, 51, 1333–1338. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yuan, Q.; Yang, Y.-L.; Han, Q.-H.; He, J.-L.; Zhao, L.; Zhang, Q.; Liu, S.-X.; Lin, D.-R.; Wu, D.-T.; et al. Phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of different kiwifruits. Molecules 2018, 23, 2957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, D.; Wang, S.; Cao, X.; Ye, Y.; Suo, Y. Comparison of phenols content and antioxidant activity of fruits from different maturity stages of Ribes stenocarpum maxim. Molecules 2018, 23, 3148. [Google Scholar] [CrossRef] [PubMed]
- Korea Meteorological Administration. Available online: https://www.kma.go.kr/neng/index.do (accessed on 8 January 2023).
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Ntanos, E.; Tsafouros, A.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P.; Roussos, P.A. Mitigation of high solar irradiance and heat stress in kiwifruit during summer via the use of alleviating products with different modes of action—Part 1 effects on leaf physiology and biochemistry. Agriculture 2022, 12, 2121. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of temperature on phenolic and osmolyte contents in in vitro cultures and micropropagated plants of two mediterranean plant species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef] [PubMed]
- Koppen, W. Das geographische System de Klimate. In Handbuch der Klimatologie; Gebrüder Borntraeger: Berlin, Germany, 1936; Available online: https://koeppen-geiger.vu-wien.ac.at/pdf/Koppen_1936.pdf (accessed on 7 January 2024).
- Trewartha, G.; Horn, L. Köppen’s Classification of Climates. An Introduction to Climate; McGraw-Hill: New York, NY, USA, 1980; pp. 397–403. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 7 January 2024).
- Kao, Y.-T.; Lu, M.-J.; Chen, C. Preliminary analyses of phenolic compounds and antioxidant activities in tea pollen extracts. J. Food Drug Anal. 2011, 19, 3. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, C.Y. Anti browning and antioxidant properties of Foeniculum vulgare seed extracts. Korean J. Food Preserv. 2020, 27, 188–196. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lim, S.D.; Yim, W.C.; Liu, D.; Hu, R.; Yang, X.; Cushman, J.C. A Vitis vinifera basic helix–loop–helix transcription factor enhances plant cell size, vegetative biomass and reproductive yield. Plant Biotechnol. J. 2018, 16, 1595–1615. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Non-thermal treatment of postharvest strawberry and establishment of its optimal freezing condition. J. Appl. Biol. Chem. 2015, 58, 55–60. [Google Scholar] [CrossRef][Green Version]
- Bland, J.M.; Altman, D.G. Statistics notes: Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.K.; Kim, S.J.; Jang, C.S.; Lim, S.D. Antioxidant Activity Analysis of Native Actinidia arguta Cultivars. Int. J. Mol. Sci. 2024, 25, 1505. https://doi.org/10.3390/ijms25031505
Hu YK, Kim SJ, Jang CS, Lim SD. Antioxidant Activity Analysis of Native Actinidia arguta Cultivars. International Journal of Molecular Sciences. 2024; 25(3):1505. https://doi.org/10.3390/ijms25031505
Chicago/Turabian StyleHu, Yu Kyong, Soo Jae Kim, Cheol Seong Jang, and Sung Don Lim. 2024. "Antioxidant Activity Analysis of Native Actinidia arguta Cultivars" International Journal of Molecular Sciences 25, no. 3: 1505. https://doi.org/10.3390/ijms25031505
APA StyleHu, Y. K., Kim, S. J., Jang, C. S., & Lim, S. D. (2024). Antioxidant Activity Analysis of Native Actinidia arguta Cultivars. International Journal of Molecular Sciences, 25(3), 1505. https://doi.org/10.3390/ijms25031505






