Carotenoid Cleavage Dioxygenase Gene CCD4 Enhances Tanshinone Accumulation and Drought Resistance in Salvia miltiorrhiza
Abstract
1. Introduction
2. Results
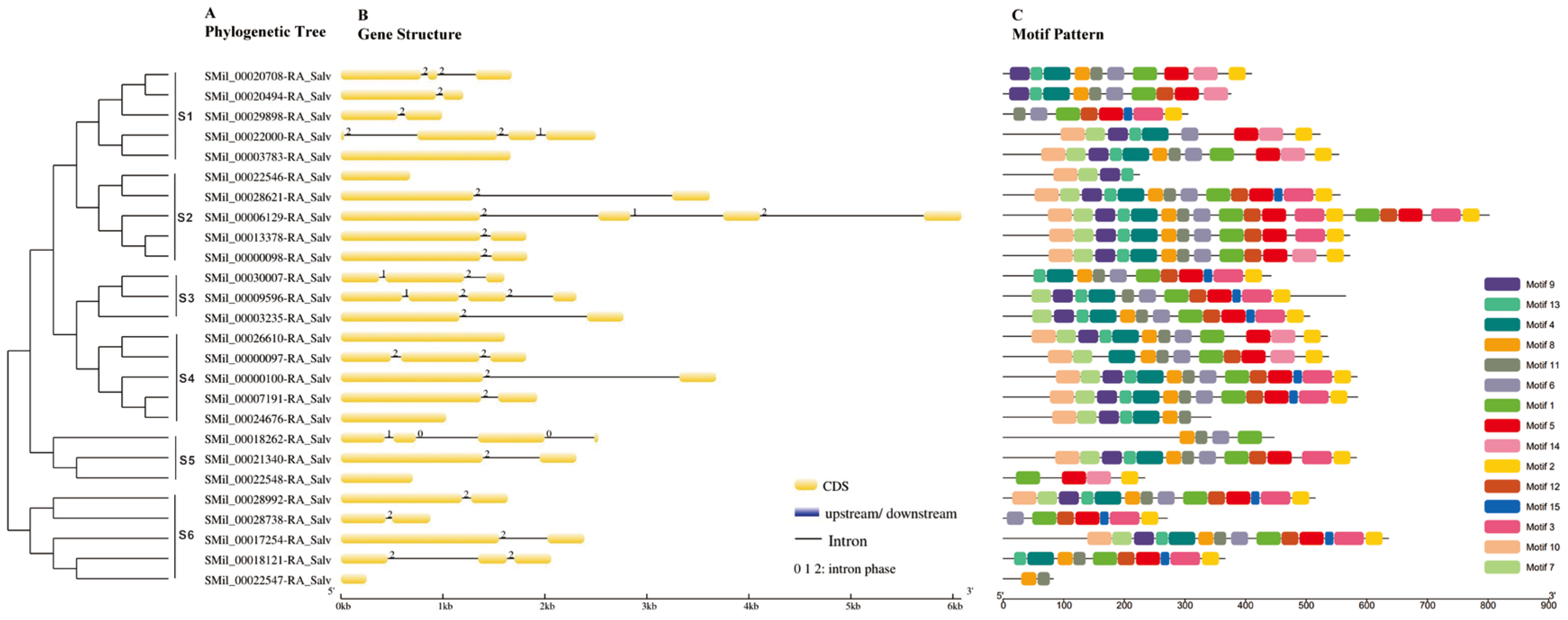
2.1. Identification and Sequence Analysis
2.2. Cis-Acting Elements Analysis
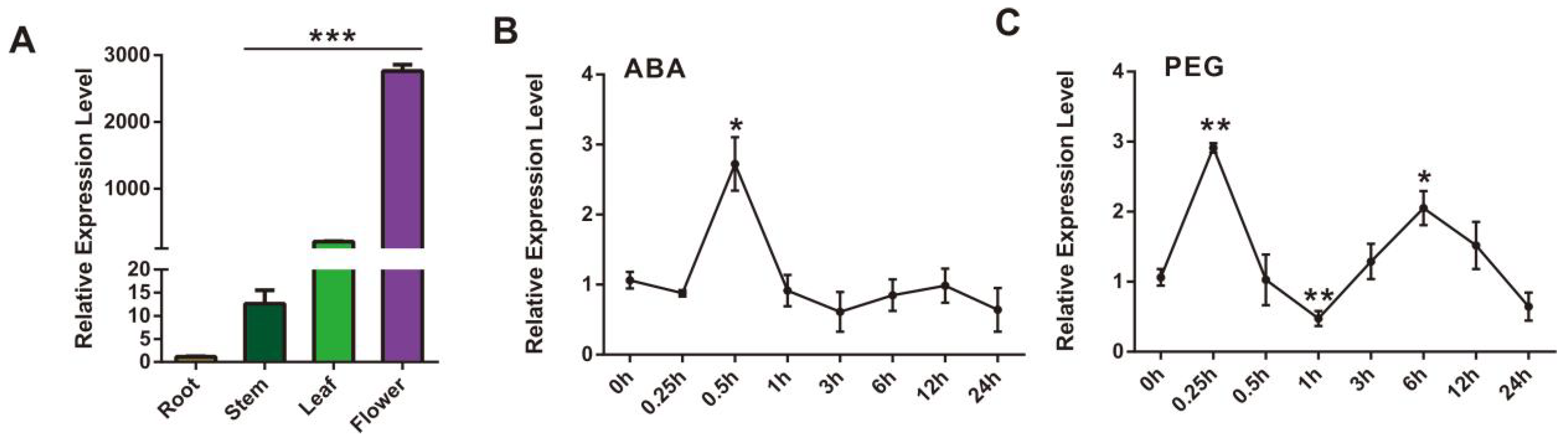
2.3. Characterization and Expression Profile of SmCCD4
2.4. Acquisition and Validation of SmCCD4 Transgenic Strain
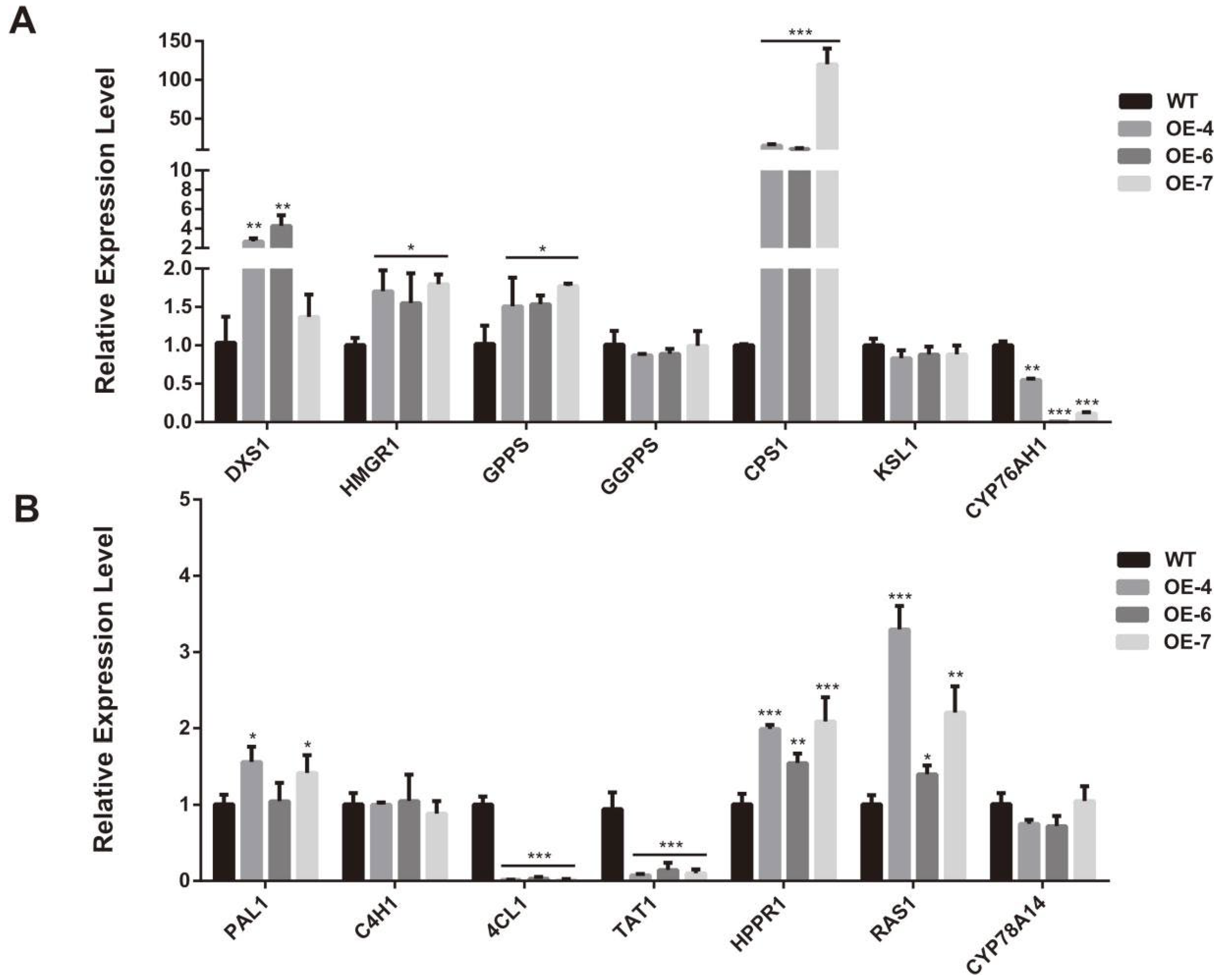
2.5. SmCCD4 Regulates Tanshinone and Phenolic Acid Biosynthesis in S. miltiorrhiza
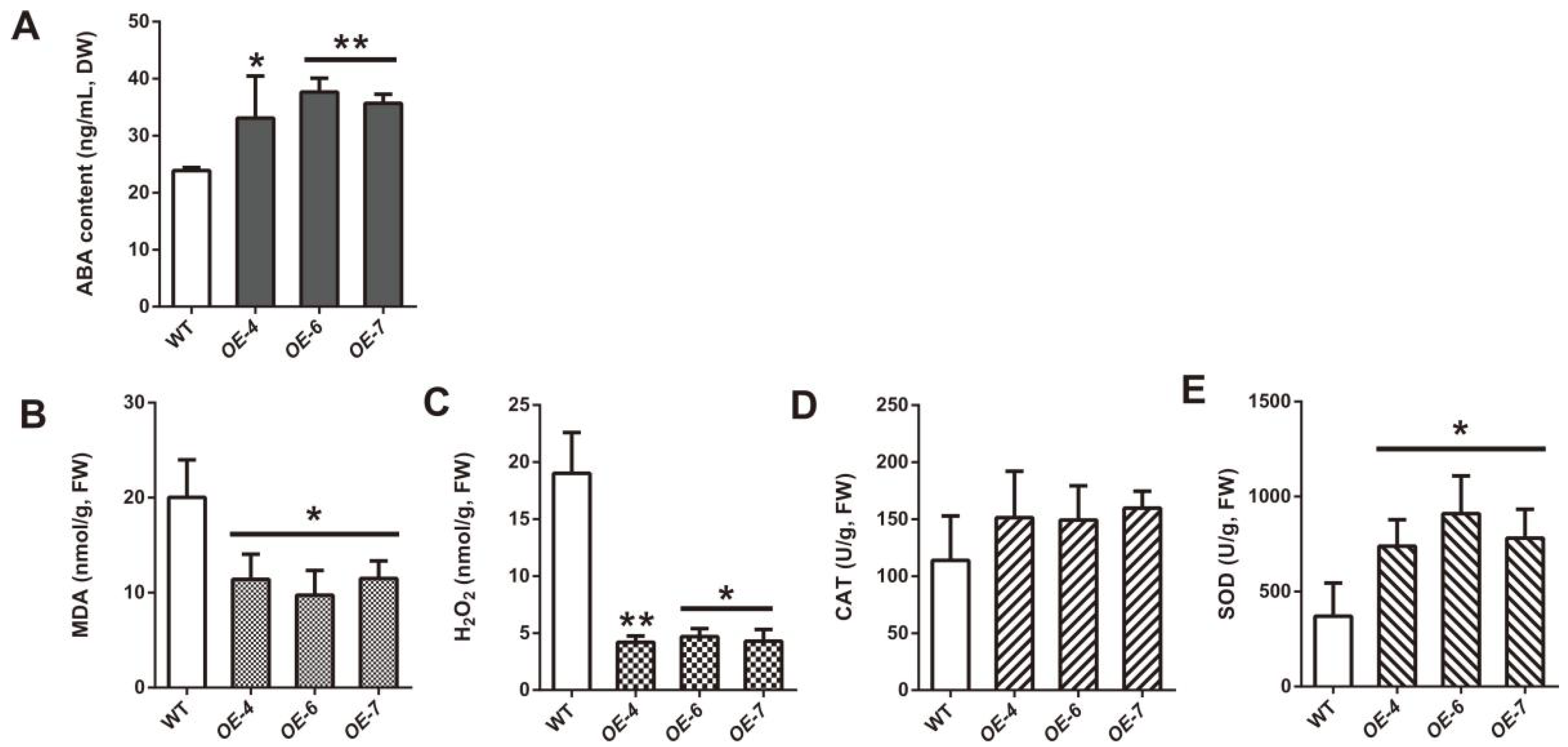
2.6. SmCCD4 Overexpression Increases ABA Accumulation and Improves Drought Tolerance in S. miltiorrhiza
3. Discussion
4. Materials and Methods
4.1. Identification of SmCCO Family and Sequence Analysis
4.2. Plant Materials and Treatments
4.3. Expression Pattern Analysis
4.4. SmCCD4 Gene Cloning and Vector Construction
4.5. Genetic Transformation and Transformant Selection
4.6. Determination of Tanshinone and Phenolic Acid Contents
4.7. Physiological Assays
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, T.O. Danshen: A versatile Chinese herbal drug for the treatment of coronary heart disease. Int. J. Cardiol. 2006, 113, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peters, R.J. Tanshinones: Leading the way into Lamiaceae labdane-related diterpenoid biosynthesis. Curr. Opin. Plant Biol. 2022, 66, 102189. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Chen, Q.; He, B.; Cai, J.; Yao, Y.; Cai, Y.; Xu, S.; Rengasamy, K.R.R.; Gowrishankar, S.; Pandian, S.K.; et al. Tanshinone IIA attenuates TNF-α induced PTX3 expression and monocyte adhesion to endothelial cells through the p38/NF-κB pathway. Food Chem. Toxicol. 2018, 121, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Yin, J.; Yang, Y.; Chen, S.; Gao, X. Renoprotection of Danshen Injection on streptozotocin-induced diabetic rats, associated with tubular function and structure. J. Ethnopharmacol. 2014, 151, 667–674. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Tan, W.; Wang, S.; Liu, J.; Liu, X.; Wang, X.; Gao, X. A Review of Danshen Combined with Clopidogrel in the Treatment of Coronary Heart Disease. Evid.-Based Complement. Altern. Med. 2019, 2019, 2721413. [Google Scholar] [CrossRef]
- Lin, S.; He, X.; Zhai, G.; Wang, C.; Xue, H.; Lin, S. Prospective study of the effect of sulfotanshinone sodium combined with tirofiban on vascular endothelial function and indicators of plaque stability in elderly patients with acute coronary syndrome. J. Clin. Pharm. Ther. 2021, 46, 319–327. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Yan, X.; Wang, Q.; Tao, Y.; Li, J.; Peng, Y.; Liu, P.; Liu, C. Salvianolic Acid B Attenuates Rat Hepatic Fibrosis via Downregulating Angiotensin II Signaling. Evid.-Based Complement. Altern. Med. 2012, 2012, 160726. [Google Scholar] [CrossRef]
- Li, Y.-G.; Song, L.; Liu, M.; Zhi Bi, H.; Wang, Z.-T. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen). J. Chromatogr. A 2009, 1216, 1941–1953. [Google Scholar] [CrossRef]
- Wang, X.; Morris-Natschke, S.L.; Lee, K.-H. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med. Res. Rev. 2007, 27, 133–148. [Google Scholar] [CrossRef]
- Giuliano, G.; Al-Babili, S.; von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Bai, H.; Zhang, X.; Wang, R. Genome-wide identification and expressional analysis of carotenoid cleavage oxygenase (CCO) gene family in Betula platyphylla under abiotic stress. BMC Genom. 2024, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Farquhar, E.R.; Hill, H.E.; von Lintig, J.; Shi, W.; Kiser, P.D. Preparation and characterization of metal-substituted carotenoid cleavage oxygenases. J. Biol. Inorg. Chem. 2018, 23, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Fukamatsu, Y.; Tamura, T.; Hihara, S.; Oda, K. Mutations in the CCD4 Carotenoid Cleavage Dioxygenase Gene of Yellow-Flesh Peaches. Biosci. Biotechnol. Biochem. 2013, 77, 2514–2516. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.; Dubey, N.K.; Yahyaa, M.; Abu-Nassar, J.; Ibdah, M. Gene silencing of CCD7 and CCD8 in Phelipanche aegyptiaca by tobacco rattle virus system retarded the parasite development on the host. Plant Signal. Behav. 2014, 9, e29376. [Google Scholar] [CrossRef]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Jia, K.-P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.; McCarty, D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef]
- Tan, B.C.; Joseph, L.M.; Deng, W.T.; Liu, L.J.; Li, Q.B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Wang, R.-K.; Wang, C.-E.; Fei, Y.-Y.; Gai, J.-Y.; Zhao, T.-J. Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol. Biol. Rep. 2013, 40, 4737–4745. [Google Scholar] [CrossRef]
- Wei, Y.; Wan, H.; Wu, Z.; Wang, R.; Ruan, M.; Ye, Q.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y. A Comprehensive Analysis of Carotenoid Cleavage Dioxygenases Genes in Solanum Lycopersicum. Plant Mol. Biol. Report. 2016, 34, 512–523. [Google Scholar] [CrossRef]
- Ilg, A.; Beyer, P.; Al-Babili, S. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 2009, 276, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Hans, J.; Walter, M.H.; Matusova, R.; Beekwilder, J.; Verstappen, F.W.A.; Ming, Z.; van Echtelt, E.; Strack, D.; Bisseling, T.; et al. Cloning and characterisation of a maize carotenoid cleavage dioxygenase (ZmCCD1) and its involvement in the biosynthesis of apocarotenoids with various roles in mutualistic and parasitic interactions. Planta 2008, 228, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Schwartz, S.H.; Auldridge, M.; Taylor, M.G.; Klee, H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004, 40, 882–892. [Google Scholar] [CrossRef]
- Ohmiya, A.; Kishimoto, S.; Aida, R.; Yoshioka, S.; Sumitomo, K. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 2006, 142, 1193–1201. [Google Scholar] [CrossRef]
- Yoshioka, S.; Aida, R.; Yamamizo, C.; Shibata, M.; Ohmiya, A. The carotenoid cleavage dioxygenase 4 (CmCCD4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica 2012, 184, 377–387. [Google Scholar] [CrossRef]
- Ledger, S.E.; Janssen, B.J.; Karunairetnam, S.; Wang, T.; Snowden, K.C. Modified CAROTENOID CLEAVAGE DIOXYGENASE8 expression correlates with altered branching in kiwifruit (Actinidia chinensis). New Phytol. 2010, 188, 803–813. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, T.; Xu, B.; Jia, L.; Xiao, B.; Liu, H.; Liu, L.; Yan, H.; Xia, Q. CRISPR/Cas9-Mediated Mutagenesis of Carotenoid Cleavage Dioxygenase 8 (CCD8) in Tobacco Affects Shoot and Root Architecture. Int. J. Mol. Sci. 2018, 19, 1062. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.Q.; Loewen, M.C. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J. Biol. Chem. 2004, 279, 46940–46945. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, I.; Jung, H.-J.; Park, J.-I.; Kang, J.-G.; Nou, I.-S. Genome-Wide Classification and Abiotic Stress-Responsive Expression Profiling of Carotenoid Oxygenase Genes in Brassica rapa and Brassica oleracea. J. Plant Growth Regul. 2016, 35, 202–214. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Luis Rambla, J.; Fernandez-de-Carmen, A.; Trapero-Mozos, A.; Ahrazem, O.; Orzaez, D.; Granell, A.; Gomez-Gomez, L. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol. Biol. 2014, 86, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Koshiba, T. Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 2002, 7, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Molnar, P.; Schwab, W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009, 60, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000, 23, 363–374. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef]
- Leung, J.; Giraudat, J. Cloning genes of Arabidopsis thaliana by chromosome walking. Methods Mol. Biol. 1998, 82, 277–303. [Google Scholar]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef]
- Liu, B.; Du, Y.; Cong, L.; Jia, X.; Yang, G. Danshen (Salvia miltiorrhiza) Compounds Improve the Biochemical Indices of the Patients with Coronary Heart Disease. Evid.-Based Complement. Altern. Med. 2016, 2016, 9781715. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Gao, F.; Jia, W.; Li, C. Salvia miltiorrhiza and Tanshinone IIA reduce endothelial inflammation and atherosclerotic plaque formation through inhibiting COX-2. Biomed. Pharmacother. 2023, 167, 115501. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, D.; Bi, H.; Duan, J.; Liang, W.; Jing, Z.; Liu, M. Salvia miltiorrhiza Bunge (Danshen) based nano-delivery systems for anticancer therapeutics. Phytomedicine 2024, 128, 155521. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Wang, H.; Yang, C.; Zhang, J.; Zhu, M.; Yang, R. Variation in microbial community structure in the rhizosphere soil of Salvia miltiorrhiza Bunge under three cropping modes. Acta Ecol. Sin. 2019, 39, 4832–4843. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Rambla, J.L.; Santaella, M.; Gomez, M.D.; Orzaez, D.; Granell, A.; Gomez-Gomez, L. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in β-ionone release. J. Biol. Chem. 2008, 283, 24816–24825. [Google Scholar] [CrossRef]
- Song, J.-Y.; Luo, H.-M.; Li, C.-F.; Sun, C.; Xu, J.; Chen, S.-L. Salvia miltiorrhiza as medicinal model plant. Yao Xue Xue Bao = Acta Pharm. Sin. 2013, 48, 1099–1106. [Google Scholar]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Zhong, M.; Yu, H.; Jiang, Y.; Liao, J.; Li, G.; Chai, S.; Yang, R.; Jiang, H.; Wang, L.; Deng, X.; et al. Physiological and molecular mechanisms of carbon quantum dots alleviating Cu2+ toxicity in Salvia miltiorrhiza bunge. Environ. Pollut. 2024, 358, 124521. [Google Scholar] [CrossRef]
- Deng, H.; Li, Q.; Cao, R.; Ren, Y.; Wang, G.; Guo, H.; Bu, S.; Liu, J.; Ma, P. Overexpression of SmMYC2 enhances salt resistance in Arabidopsis thaliana and Salvia miltiorrhiza hairy roots. J. Plant Physiol. 2023, 280, 153862. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, X.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Zhao, C.; Yan, X.; Liu, X.; Han, M. Genome-wide analysis of carotenoid cleavage oxygenase genes and their responses to various phytohormones and abiotic stresses in apple (Malus domestica). Plant Physiol. Biochem. 2018, 123, 81–93. [Google Scholar] [CrossRef]
- Haider, M.Z.; Sami, A.; Shafiq, M.; Anwar, W.; Ali, S.; Ali, Q.; Muhammad, S.; Manzoor, I.; Shahid, M.A.; Ali, D.; et al. Genome-wide identification and in-silico expression analysis of carotenoid cleavage oxygenases gene family in Oryza sativa (rice) in response to abiotic stress. Front. Plant Sci. 2023, 14, 1269995. [Google Scholar] [CrossRef]
- Yao, Y.; Jia, L.; Cheng, Y.; Ruan, M.; Ye, Q.; Wang, R.; Yao, Z.; Zhou, G.; Liu, J.; Yu, J.; et al. Evolutionary Origin of the Carotenoid Cleavage Oxygenase Family in Plants and Expression of Pepper Genes in Response to Abiotic Stresses. Front. Plant Sci. 2022, 12, 792832. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Deng, Y.; Yu, W.; Gao, H.; Jin, J.; Wang, N.; Wang, F.; Li, H. Cloning and expression analysis of GmNCED1 from Glycine max. Chin. J. Oil Crop Sci. 2014, 36, 455–460. [Google Scholar]
- Iuchi, S. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis (vol 27, pg 325, 2001). Plant J. 2002, 30, 611. [Google Scholar]
- Bouvier, F.; Dogbo, O.; Camara, B. Biosynthesis of the food and cosmetic plant pigment bixin (annatto). Science 2003, 300, 2089–2091. [Google Scholar] [CrossRef]
- Bouvier, F.; Suire, C.; Mutterer, J.; Camara, B. Oxidative remodeling of chromoplast carotenoids: Identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in crocus secondary metabolite biogenesis. Plant Cell 2003, 15, 47–62. [Google Scholar] [CrossRef]
- Chernys, J.T.; Zeevaart, J.A.D. Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol. 2000, 124, 343–353. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Adami, M.; De Franceschi, P.; Brandi, F.; Liverani, A.; Giovannini, D.; Rosati, C.; Dondini, L.; Tartarini, S. Identifying a Carotenoid Cleavage Dioxygenase (ccd4) Gene Controlling Yellow/White Fruit Flesh Color of Peach. Plant Mol. Biol. Report. 2013, 31, 1166–1175. [Google Scholar] [CrossRef]
- Jonathan, D.-C.; Rosa, U.-C.; Yair, C.-C.; Manuel, C.-U.V.; Diana, S.-U.; Margarita, A.-E.; Renata, R.-M. Carotenoid cleavage dioxygenases 4 from woodiness plant and their relationships with herbaceous plants. Ind. Crops Prod. 2023, 205, 117529. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Zhao, J.; Zhou, H.; Ren, F.; Wang, L.; Gu, C.; Liao, L.; Han, Y. Inactivation of a Gene Encoding Carotenoid Cleavage Dioxygenase (CCD4) Leads to Carotenoid-Based Yellow Coloration of Fruit Flesh and Leaf Midvein in Peach. Plant Mol. Biol. Report. 2014, 32, 246–257. [Google Scholar] [CrossRef]
- Ureshino, K.; Takara, H.; Miyajima, I. Comparison of Expression CCD4 Gene Levels in Petals of Evergreen Azalea Species. Hortic. J. 2019, 88, 535–540. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, J.; Golczak, M.; Palczewski, K.; Kiser, P.D. Key Residues for Catalytic Function and Metal Coordination in a Carotenoid Cleavage Dioxygenase. J. Biol. Chem. 2016, 291, 19401–19412. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Z. Genetic transformation of the medicinal plant Salvia miltiorrhiza by Agrobacterium tumefaciens-mediated method. Plant Cell Tissue Organ Cult. 2007, 88, 175–184. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, S.; Cui, G.; Chen, S.; Wei, J.; Huang, L. Characterization of reference genes for quantitative real-time PCR analysis in various tissues of Salvia miltiorrhiza. Mol. Biol. Rep. 2010, 37, 507–513. [Google Scholar] [CrossRef]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.M.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006, 45, 616–629. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Zhou, L.; Yu, X.; Yan, X.; Zhang, Q.; Li, B.; Liu, Y.; Zhou, W.; Cao, X.; et al. JA-Responsive Transcription Factor SmMYB97 Promotes Phenolic Acid and Tanshinone Accumulation in Salvia miltiorrhiza. J. Agric. Food Chem. 2020, 68, 14850–14862. [Google Scholar] [CrossRef]
- Wang, B.; Niu, J.; Li, B.; Huang, Y.; Han, L.; Liu, Y.; Zhou, W.; Hu, S.; Li, L.; Wang, D.; et al. Molecular Characterization and Overexpression of SmJMT Increases the Production of Phenolic Acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 3788. [Google Scholar] [CrossRef]


| Liposoluble Components (Tanshinones) | Water-Soluble Components (Phenolic Acids) | ||
|---|---|---|---|
| Name | Molecular Structure | Name | Molecular Structure |
| Tanshinone I | 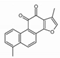 | Rosmarinic acid | 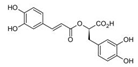 |
| Tanshinone IIA |  | Salvianolic acid A |  |
| Tanshinone IIB |  | Salvianolic acid B | 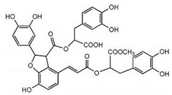 |
| Cryptotanshinone | 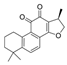 | Salvianolic acid C |  |
| Isotanshinone I |  | Protocatechuic acid |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Han, W.; Zhou, S.; Yang, L.; Wang, D.; Zhou, W.; Wang, Z. Carotenoid Cleavage Dioxygenase Gene CCD4 Enhances Tanshinone Accumulation and Drought Resistance in Salvia miltiorrhiza. Int. J. Mol. Sci. 2024, 25, 13223. https://doi.org/10.3390/ijms252313223
Tian Q, Han W, Zhou S, Yang L, Wang D, Zhou W, Wang Z. Carotenoid Cleavage Dioxygenase Gene CCD4 Enhances Tanshinone Accumulation and Drought Resistance in Salvia miltiorrhiza. International Journal of Molecular Sciences. 2024; 25(23):13223. https://doi.org/10.3390/ijms252313223
Chicago/Turabian StyleTian, Qian, Wei Han, Shuai Zhou, Liu Yang, Donghao Wang, Wen Zhou, and Zhezhi Wang. 2024. "Carotenoid Cleavage Dioxygenase Gene CCD4 Enhances Tanshinone Accumulation and Drought Resistance in Salvia miltiorrhiza" International Journal of Molecular Sciences 25, no. 23: 13223. https://doi.org/10.3390/ijms252313223
APA StyleTian, Q., Han, W., Zhou, S., Yang, L., Wang, D., Zhou, W., & Wang, Z. (2024). Carotenoid Cleavage Dioxygenase Gene CCD4 Enhances Tanshinone Accumulation and Drought Resistance in Salvia miltiorrhiza. International Journal of Molecular Sciences, 25(23), 13223. https://doi.org/10.3390/ijms252313223





