Antimicrobial Surfaces: Stainless Steel Functionalized with the Essential Oil Component Vanillin
Abstract
1. Introduction
2. Results and Discussion
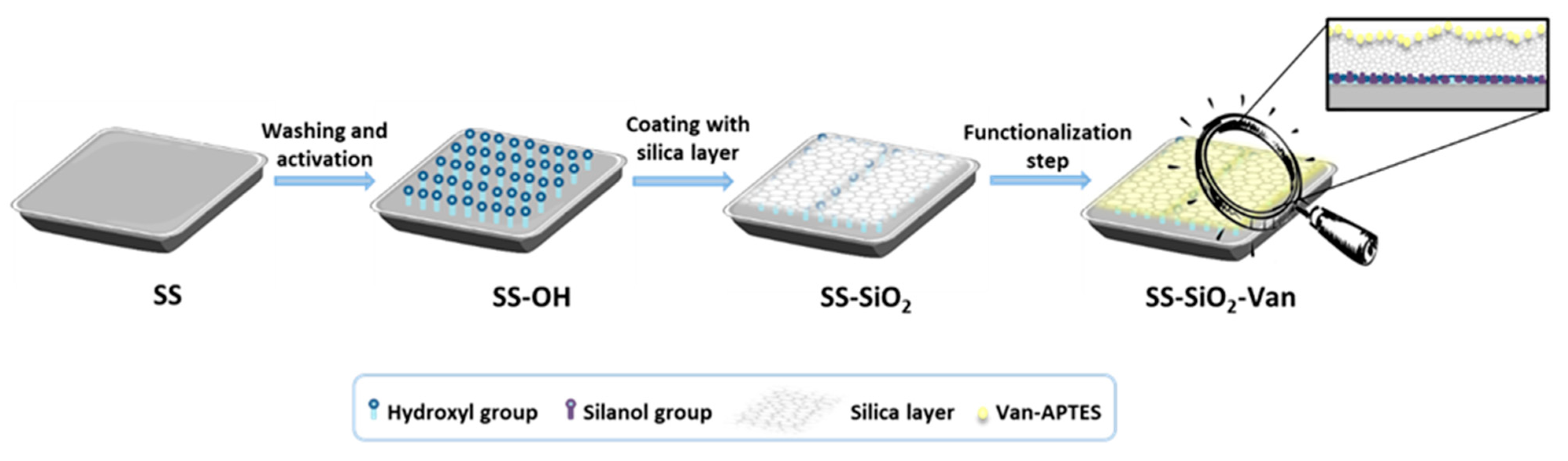
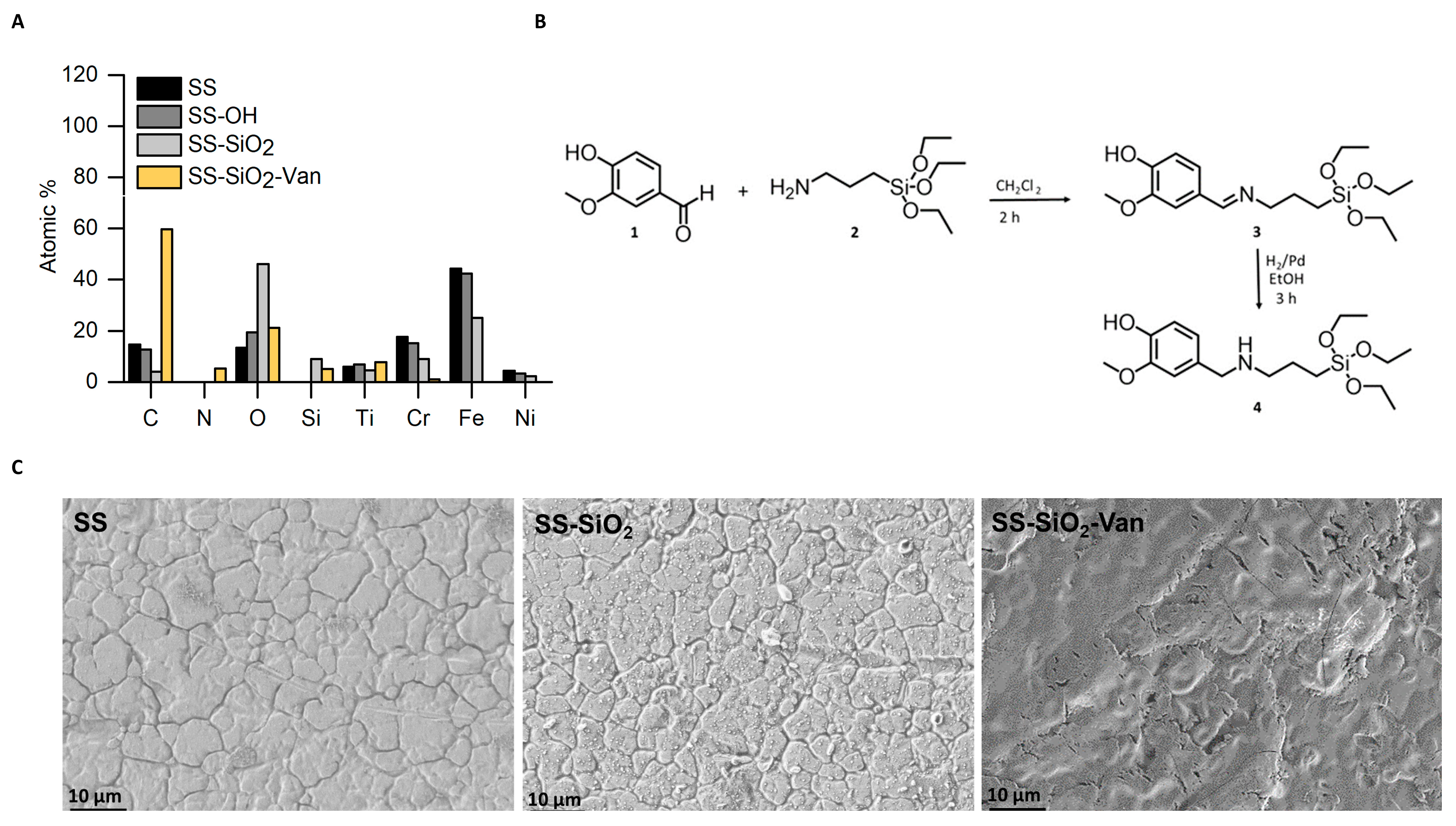
2.1. Design, Preparation, and Characterization of SS-SiO2-Van Surface
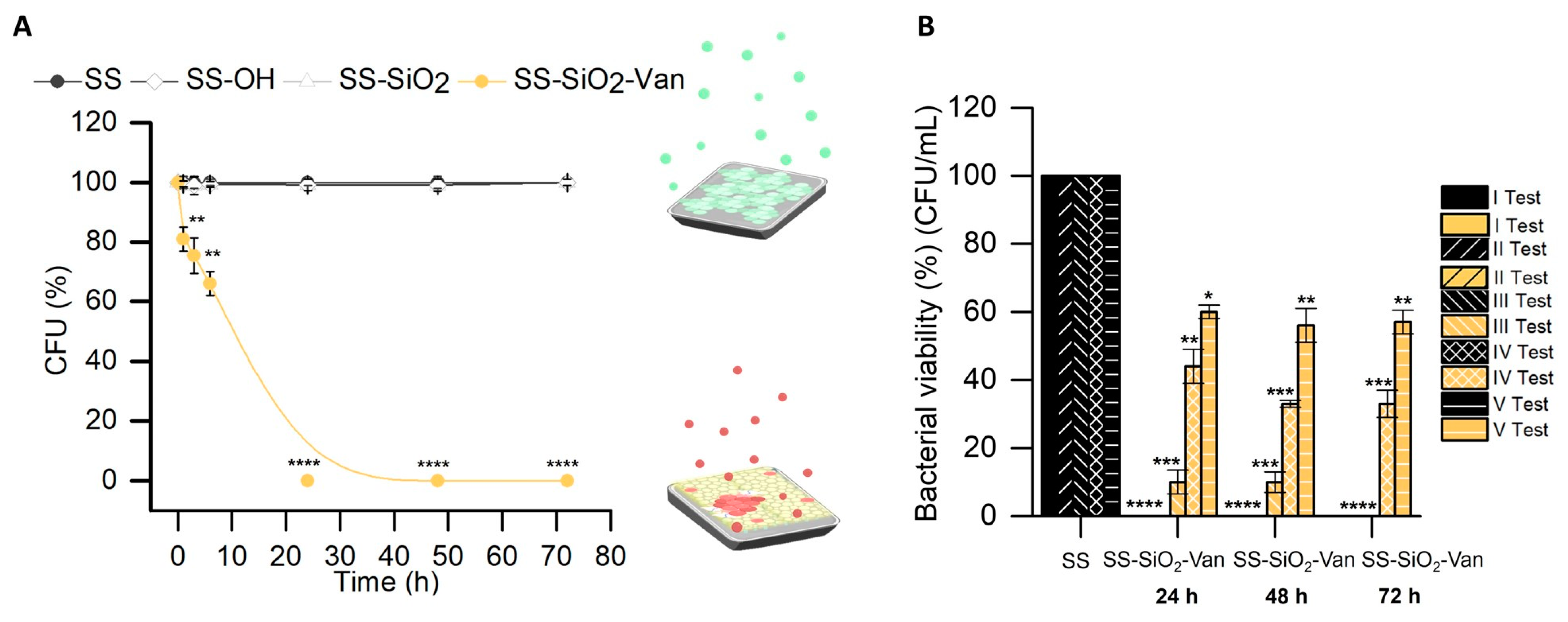
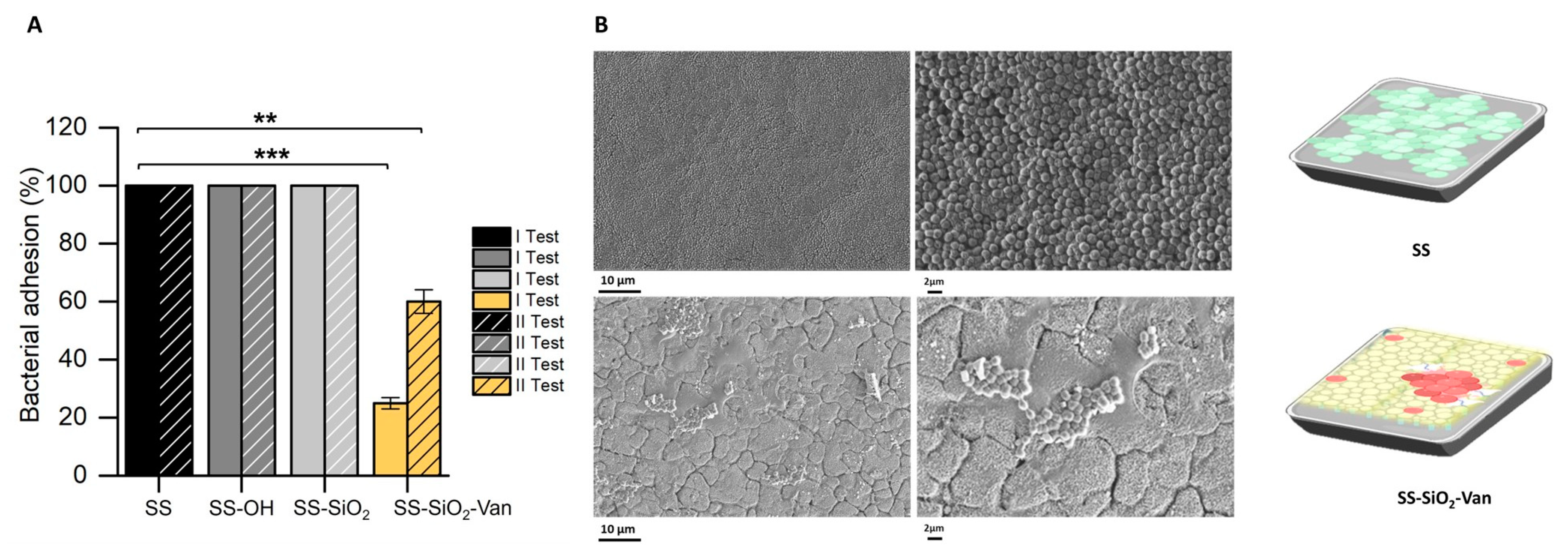
2.2. Antimicrobial Activity
3. Materials and Methods
3.1. Reagents, Bacterial Strain, and Culture Media
3.2. Synthesis of the Vanillin Derivate
3.3. Synthetic Immobilization of the EOC on the Stainless Steel Surface
3.3.1. Pretreatment of Stainless Steel Surface
3.3.2. Deposition of the Silica Layer
3.3.3. Immobilization of the Vanillin Derivative
3.3.4. Immobilization of the Vanillin Derivative Directly on the SS (Without the Silica Layer)
3.4. Characterization Methods
3.5. Quantification of the Functionalized EOC
3.6. Microbiological Analysis
3.6.1. Bacterial Viability Assays
3.6.2. Bacterial Adhesion Assays
3.6.3. Dry Bacterial Adhesion Assays
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.; Ha, S. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- França, A.; Pérez-Cabezas, B.; Correia, A.; Pier, G.B.; Cerca, N.; Vilanova, M. Staphylococcus epidermidis Biofilm-Released Cells Induce a Prompt and More Marked In vivo Inflammatory-Type Response than Planktonic or Biofilm Cells. Front. Microbiol. 2016, 7, 1530. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Oliveira, R. Mini-review: Staphylococcus epidermidis as the most frequent cause of nosocomial infections: Old and new fighting strategies. Biofouling 2014, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ziebuhr, W.; Hennig, S.; Eckart, M.; Kränzler, H.; Batzilla, C.; Kozitskaya, S. Nosocomial infections by Staphylococcus epidermidis: How a commensal bacterium turns into a pathogen. Int. J. Antimicrob. Agents 2006, 28, 14–20. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kang, S.-M.; Jeong, S.-H.; Chung, K.-H.; Kim, B.-I. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, J.; Bradley, J.W.; McKay, K.; McBride, F.; Donaghy, D.; Raval, R.; D’Sa, R.A. Linker-free covalent immobilization of nisin using atmospheric pressure plasma induced grafting. J. Mater. Chem. B 2017, 5, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Cook, J.; Griesser, H.J. Antibacterial surfaces for biomedical devices. Expert Rev. Med. Devices 2009, 6, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of antibacterial surfaces and interfaces: Polyelectrolyte multilayers as a multifunctional platform. Macromolecules 2009, 42, 8573–8586. [Google Scholar] [CrossRef]
- Duday, D.; Vreuls, C.; Moreno, M.; Frache, G.; Boscher, N.D.; Zocchi, G.; Archambeau, C.; Van De Weerdt, C.; Martial, J.; Choquet, P. Atmospheric pressure plasma modified surfaces for immobilization of antimicrobial nisin peptides. Surf. Coatings Technol. 2013, 218, 152–161. [Google Scholar] [CrossRef]
- Vreuls, C.; Zocchi, G.; Thierry, B.; Garitte, G.; Griesser, S.S.; Archambeau, C.; Van de Weerdt, C.; Martial, J.; Griesser, H. Prevention of bacterial biofilms by covalent immobilization of peptides onto plasma polymer functionalized substrates. J. Mater. Chem. 2010, 20, 8092–8098. [Google Scholar] [CrossRef]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized grafting of antimicrobial peptides on stainless steel surface and biofilm resistance tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef]
- Yuan, S.; Wan, D.; Liang, B.; Pehkonen, S.O.; Ting, Y.P.; Neoh, K.G.; Kang, E.T. Lysozyme-coupled poly(poly(ethylene glycol) methacrylate)−stainless steel hybrids and their antifouling and antibacterial surfaces. Langmuir 2011, 27, 2761–2774. [Google Scholar] [CrossRef]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Dickinson, G.H.; Teo, S.L.-M.; Rittschof, D. Biomimetic anchors for antifouling and antibacterial polymer brushes on stainless steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.; Wang, W. Bacterial adhesion and osteoblast function on titanium with surface-grafted chitosan and immobilized RGD peptide. J. Biomed. Mater. Res. Part A 2008, 86A, 865–872. [Google Scholar] [CrossRef]
- WHO. WHO Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance. Microbe Mag. 2015, 10, 354–355. Available online: www.paprika-annecy.com (accessed on 26 October 2022).
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef]
- Adelakun, O.E.; Oyelade, O.J.; Olanipekun, B.F. Use of essential oils in food preservation. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 71–84. ISBN 9780124166448. [Google Scholar]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential oils: A natural weapon against antibiotic-resistant bacteria responsible for nosocomial infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control. 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Orhan-Yanıkan, E.; da Silva-Janeiro, S.; Ruiz-Rico, M.; Jiménez-Belenguer, A.I.; Ayhan, K.; Barat, J.M. Essential oils compounds as antimicrobial and antibiofilm agents against strains present in the meat industry. Food Control. 2019, 101, 29–38. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Bernardos, A.; Bozik, M.; Alvarez, S.; Saskova, M.; Perez-Esteve, E.; Kloucek, P.; Lhotka, M.; Frankova, A.; Martinez-Manez, R. The efficacy of essential oil components loaded into montmorillonite against Aspergillus niger and Staphylococcus aureus. Flavour Fragr. J. 2019, 34, 151–162. [Google Scholar] [CrossRef]
- Bernardos, A.; Marina, T.; Žáček, P.; Pérez-Esteve, É.; Martínez-Mañez, R.; Lhotka, M.; Kouřimská, L.; Pulkrábek, J.; Klouček, P. Antifungal effect of essential oil components against Aspergillus niger when loaded into silica mesoporous supports. J. Sci. Food Agric. 2014, 95, 2824–2831. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Hallock, P.J.; Carson, R.M.; Lee, C.-C.; Mansfeld, F.B.; Wood, T.K. Inhibiting sulfate-reducing bacteria in biofilms on steel with antimicrobial peptides generated in situ. Appl. Microbiol. Biotechnol. 1999, 52, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ballarre, J.; López, D.A.; Schreiner, W.H.; Durán, A.; Ceré, S.M. Protective hybrid sol–gel coatings containing bioactive particles on surgical grade stainless steel: Surface characterization. Appl. Surf. Sci. 2007, 253, 7260–7264. [Google Scholar] [CrossRef]
- Wong, A.C.L. Biofilms in Food Processing Environments. J. Dairy Sci. 1998, 81, 2765–2770. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Pérez-Esteve, É.; Bernardos, A.; Sancenón, F.; Martínez-Máñez, R.; Marcos, M.D.; Barat, J.M. Enhanced antimicrobial activity of essential oil components immobilized on silica particles. Food Chem. 2017, 233, 228–236. [Google Scholar] [CrossRef]
- Polo, L.; de Greñu, B.D.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial activity of commercial calcium phosphate based materials functionalized with vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef]
- Nielsen, C.; Subbiahdoss, G.; Zeng, G.; Salmi, Z.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R. Antibacterial isoeugenol coating on stainless steel and polyethylene surfaces prevents biofilm growth. J. Appl. Microbiol. 2018, 124, 179–187. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Rodríguez, A.; Nerín, C.; Batlle, R. New cinnamon-based active paper packaging against Rhizopus stolonifer food spoil-age. J. Agric. Food Chem. 2008, 56, 6364–6369. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Reversible Covalent Immobilization of Cinnamal-dehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioproc. Tech. 2015, 8, 526–538. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Allizond, V.; Banche, G.; Bertea, C.; Di Confiengo, G.G.; Novara, C.; Cochis, A.; Rimondini, L.; Spriano, S. Surface Coating and functionalization of Metallic Biomaterials with Essential Oils for Antibacterial Applications. In Proceedings of the 1st Coatings and Interfaces Web Conference (CIWC 2019), Basel, Switzerland, 15–29 March 2019. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Allizond, V.; Bertea, C.; Novara, C.; Cochis, A.; Geobaldo, F.; Bistolfi, A.; Cuffini, A.; Rimondini, L.; et al. Grafting of the peppermint essential oil to a chemically treated Ti6Al4V alloy to counteract the bacterial adhesion. Surf. Coatings Technol. 2019, 378, 125011. [Google Scholar] [CrossRef]
- Getnet, T.G.; Kayama, M.E.; Rangel, E.C.; Duarte, I.C.; da Silva, G.F.; Cruz, N.C. Biofunctional coating of stainless steel surfaces with carvacrol- and eugenol-derived film using atmospheric dielectric barrier discharge plasma: Aiming for suppression of biofilm formation and corrosion protection. J. Mater. Res. Technol. 2022, 18, 2217–2231. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Guarí-Borràs, G.; Ruiz-Rico, M.; Morellá-Aucejo, Á.; Aznar, E.; Barat, J.M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A. Towards the Enhancement of Essential Oil Components’ Antimicrobial Activity Using New Zein Protein-Gated Mesoporous Silica Microdevices. Int. J. Mol. Sci. 2021, 22, 3795. [Google Scholar] [CrossRef] [PubMed]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in Controlling Listeria monocytogenes and Escherichia coli O157:H7 in Milk. Food Bioprocess Technol. 2010, 5, 2120–2131. [Google Scholar] [CrossRef]
- Kühn, J.; Considine, T.; Singh, H. Interactions of Milk Proteins and Volatile Flavor Compounds: Implications in the Development of Protein Foods. J. Food Sci. 2006, 71, R72–R82. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Dukenbayev, K.; Azevedo, H.S.; Marsili, E.; Tosi, D.; Kanayeva, D. Optimizing Silanization to Functionalize Stainless Steel Wire: Towards Breast Cancer Stem Cell Isolation. Materials 2020, 13, 3693. [Google Scholar] [CrossRef]
- Martin, H.J.; Schulz, K.H.; Bumgardner, J.D.; Walters, K.B. XPS study on the use of 3-aminopropyltriethoxysilane to bond chitosan to a titanium surface. Langmuir 2007, 23, 6645–6651. [Google Scholar] [CrossRef]
- Tamura, H.; Mita, K.; Tanaka, A.; Ito, M. Mechanism of Hydroxylation of Metal Oxide Surfaces. J. Colloid Interface Sci. 2001, 243, 202–207. [Google Scholar] [CrossRef]
- Özel, A.; Çimenoğlu, H. An Overview on Silane Based Metal Pretreatments for Powder Painting. Diffus. Found. 2016, 9, 16–29. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Wei, J.; Ravn, D.B.; Gram, L.; Kingshott, P. Stainless steel modified with poly(ethylene glycol) can prevent protein adsorption but not bacterial adhesion. Colloids Surf. B Biointerfaces 2003, 32, 275–291. [Google Scholar] [CrossRef]
- Kang, C.-K.; Lee, Y.-S. The surface modification of stainless steel and the correlation between the surface properties and protein adsorption. J. Mater. Sci. Mater. Med. 2007, 18, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Shen, D.; Wu, F.; Pleixats, R.; Pan, J. Functionalized silica nanoparticles: Classification, synthetic approaches and recent advances in adsorption applications. Nanoscale 2021, 13, 15998–16016. [Google Scholar] [CrossRef]
- Mousavi, M.; Fini, E. Silanization Mechanism of Silica Nanoparticles in Bitumen Using 3-Aminopropyl Triethoxysilane (APTES) and 3-Glycidyloxypropyl Trimethoxysilane (GPTMS). ACS Sustain. Chem. Eng. 2020, 8, 3231–3240. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Amino Acid Assay. Available online: https://user.eng.umd.edu/~nsw/ench485/lab3a.htm (accessed on 27 October 2022).
- Ninhydrin Test. Available online: https://unacademy.com/content/jee/study-material/chemistry/ninhydrin-test/ (accessed on 27 October 2022).
- Joullié, M.M.; Thompson, T.R.; Nemeroff, N.H. Ninhydrin and ninhydrin analogs. Syntheses and applications. Tetrahedron 1991, 47, 8791–8830. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Moreno, Y.; Barat, J.M. In vitro antimicrobial activity of immobilised essential oil components against Helicobacter pylori. World J. Microbiol. Biotechnol. 2019, 36, 3. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, e1900669. [Google Scholar] [CrossRef]


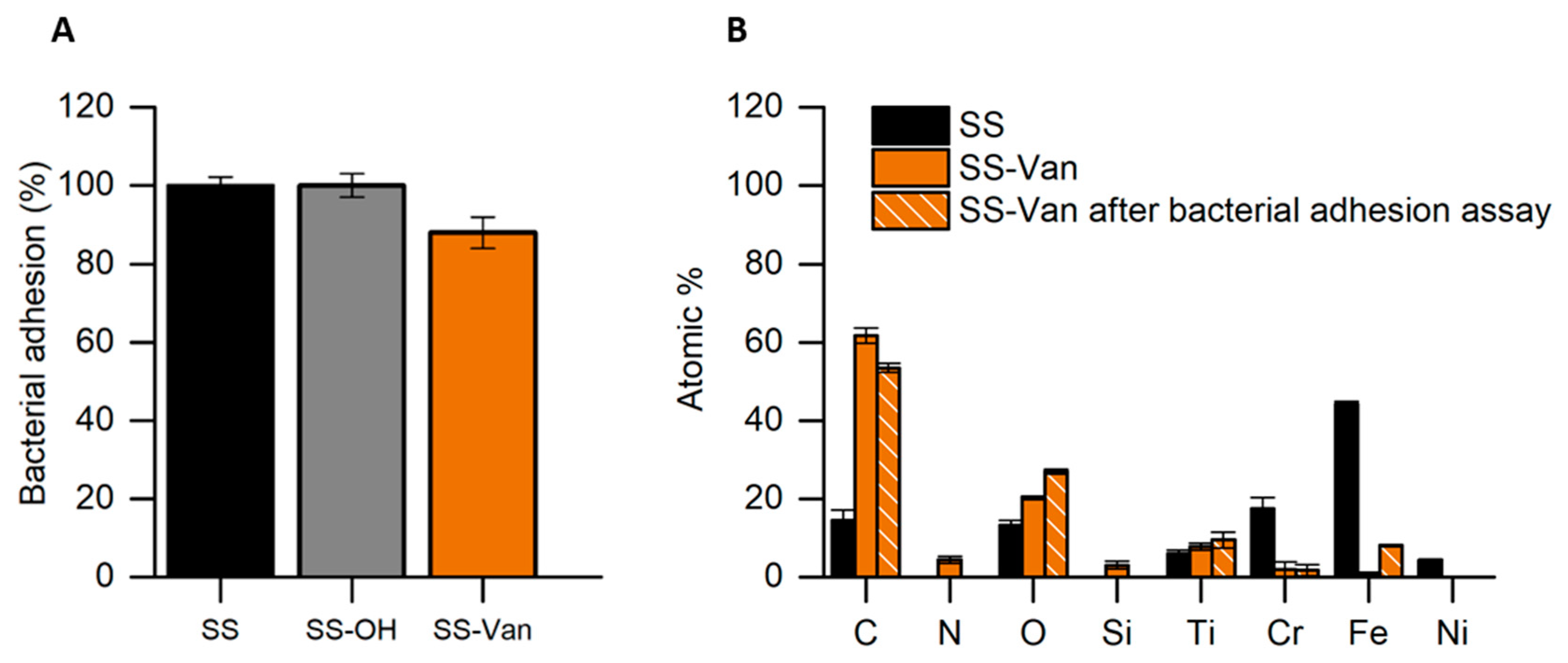
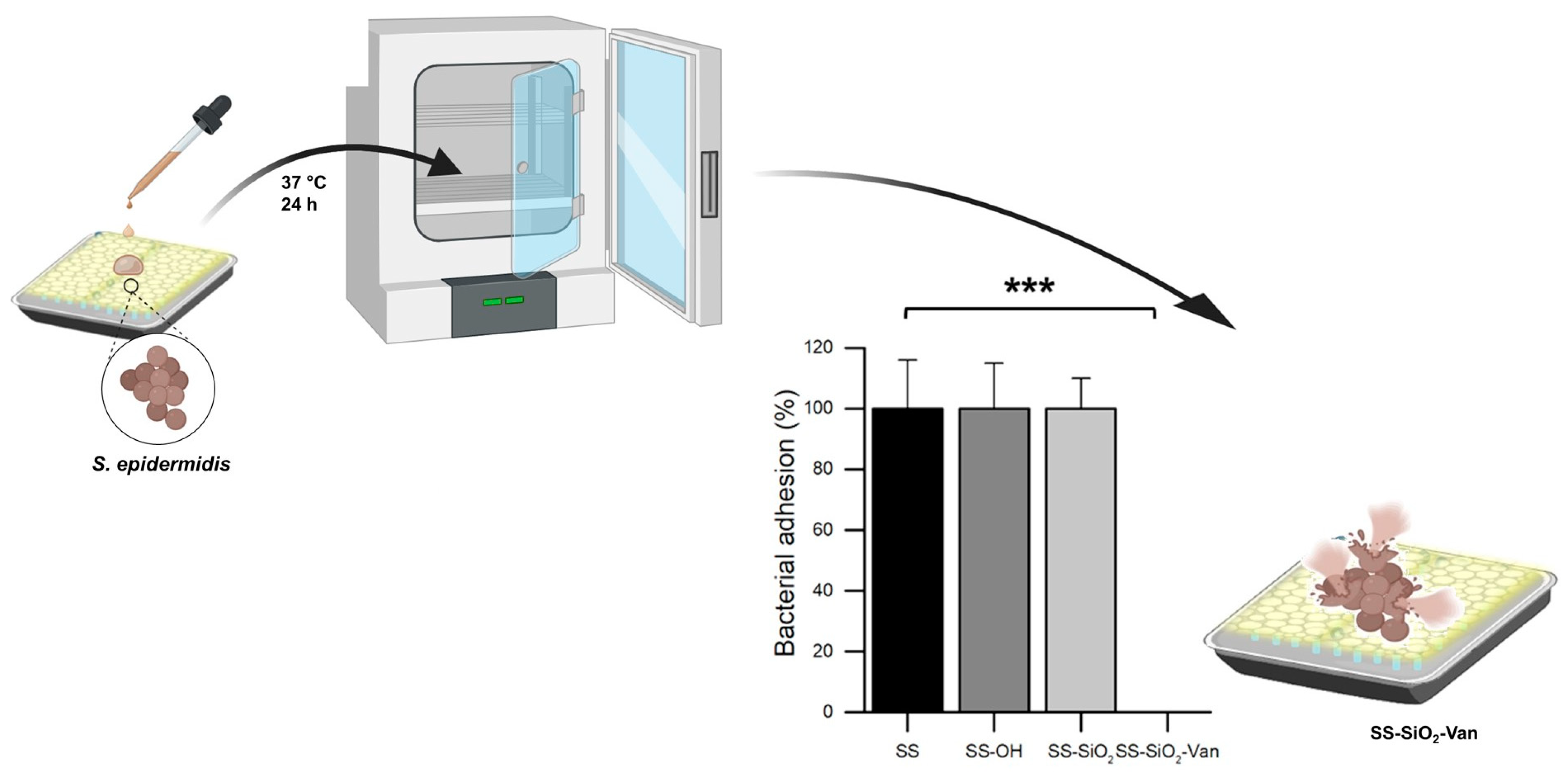
| Surface | Natural Antimicrobial | Microorganisms | Results | Durability | References |
|---|---|---|---|---|---|
| Plastic flexible films | Microcapsules containing carvacrol and thymol | E. coli, S. aureus, L. innocua, S. cerevisiae, and A. niger | Thymol and carvacrol showed significant antimicrobial activity against the studied microorganisms, with minimal inhibitory concentrations (MICs) of 125–250 ppm and 75–375 ppm for thymol and carvacrol, respectively. The concentration of the microencapsulated antimicrobial agents showed a range of inhibition zones of 4.3–11.3 mm for the microorganisms at 10% thymol and 10% carvacrol. | 28 days | [39] |
| Chitosan | Cinnamaldehyde | S. aureus and E. coli | The films’ effectiveness increased as the treatment temperature increased, and, thus, the amount of cinnamaldehyde was released. Treatment at 4 °C for 30 min showed reduced antimicrobial activity (1 log reduction). After treatment at 65 °C for 30 min, the films showed a significant log reduction of 5.66 ± 0.04 against S. aureus and 4.76 ± 0.02 against E. coli. It was also observed that the films treated at 72 °C for 15 min, 95 °C for 10 min, and 121 °C for 5 min produced a bactericidal effect. | Tested 24 h | [41] |
| Stainless steel and titanium | Mentha piperita | E. coli | The mint coating was able to reduce to 1–2 Log of CFUs after 24–48 h, demonstrating a bacteriostatic effect. | Tested 48 h | [42] |
| Titanium | Peppermint oil | Staphylococci | Bacteriostatic effect (able to reduce approximately 1–2 Log of CFUs after 24 h). | Tested 24 h | [43] |
| Stainless steel | Carvacrol and eugenol | P. aeruginosa and C. albicans | The carvacrol coating has a biofilm growth reduction rate of up to 44% for P. aeruginosa and 60% for C. albicans. Similarly, the eugenol coating exhibited up to 36% suppression for P. aeruginosa and 52% for C. albicans. Reduced in both cases approximately 1–2 log of CFUs. | Tested 24 h | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medaglia, S.; Morellá-Aucejo, Á.; Ruiz-Rico, M.; Sancenón, F.; Villaescusa, L.A.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A. Antimicrobial Surfaces: Stainless Steel Functionalized with the Essential Oil Component Vanillin. Int. J. Mol. Sci. 2024, 25, 12146. https://doi.org/10.3390/ijms252212146
Medaglia S, Morellá-Aucejo Á, Ruiz-Rico M, Sancenón F, Villaescusa LA, Martínez-Máñez R, Marcos MD, Bernardos A. Antimicrobial Surfaces: Stainless Steel Functionalized with the Essential Oil Component Vanillin. International Journal of Molecular Sciences. 2024; 25(22):12146. https://doi.org/10.3390/ijms252212146
Chicago/Turabian StyleMedaglia, Serena, Ángela Morellá-Aucejo, María Ruiz-Rico, Félix Sancenón, Luis A. Villaescusa, Ramón Martínez-Máñez, M. Dolores Marcos, and Andrea Bernardos. 2024. "Antimicrobial Surfaces: Stainless Steel Functionalized with the Essential Oil Component Vanillin" International Journal of Molecular Sciences 25, no. 22: 12146. https://doi.org/10.3390/ijms252212146
APA StyleMedaglia, S., Morellá-Aucejo, Á., Ruiz-Rico, M., Sancenón, F., Villaescusa, L. A., Martínez-Máñez, R., Marcos, M. D., & Bernardos, A. (2024). Antimicrobial Surfaces: Stainless Steel Functionalized with the Essential Oil Component Vanillin. International Journal of Molecular Sciences, 25(22), 12146. https://doi.org/10.3390/ijms252212146







